?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The radiation-induced surface activation (RISA) effect will be applied to the core design in supercritical light water reactor (SCWR) in order to achieve a high performance with excellent economy and safety. The purpose of the present study is to investigate the RISA effect in the candidate fuel cladding materials in SCWR such as PNC1520. The change of weldability due to RISA effect and the related microstructure analysis were performed in oxidized PNC1520 and 304 stainless steel with various oxidization periods. The phases contained in the surface oxide layer of the present specimen were identified as Fe2CrO4, γ-Fe2O3, and Fe2O3. The lifetime of 13.8 days for wettability improving factor was confirmed in the ultraviolet (UV) irradiation. Meanwhile, the long life of 13.8 days and short life of 0.8 days for wettability improving factors were identified in the γ-ray irradiation. Based on the fact that the band gap energies of Fe2CrO4, γ-Fe2O3, and Fe2O3 were, respectively, 2.1, 2.0, and 2.2 eV, and the photo energies of UV and γ-ray irradiation were 4.48 eV and 13.3 MeV, it is therefore clarified that the hydrophilization on the oxide layer is ascribed to the RISA effect.
1. Introduction
The radiation-induced surface activation (RISA) phenomenon was first studied and proposed by Takamasa et al. more than a decade ago [Citation1–Citation3]. The authors reported the surface wettability on metal oxide pieces of titanium, zircaloy, stainless-304, and copper increased greatly by γ-ray irradiation [Citation4,Citation5]. This RISA phenomenon is described as follows [Citation1–Citation5]: (1) the bending of the band gap in the oxide layer is introduced to align the Fermi-level between oxide layer and atmospheric condition such as water and air; (2) the electron (e−) and hole (h±) pairs are formed by irradiation into the oxide layer, and the e− and h± diffuse to the matrix and surface sides, respectively; (3) cathodic and anodic reactions are enhanced by the diffusion of e− and h±. Finally, the hydrophilization by adsorption of OH radicals at the sample surface is caused by an electrochemical reaction. Such RISA phenomenon is believed to contribute to increasing the wettability and heat-transfer properties at the surface of oxide layers. Hence, the concept of the RISA effect can be applied to the core design of various reactors so as to get a higher performance with excellent economy and safety.
The supercritical water-cooled reactor (SCWR) is one of the promising Generation IV advanced nuclear reactor concepts with a high thermal efficiency (45%) and considerable plant simplification compared to the current light water reactors [Citation6]. The typical operating temperature and pressure of the coolant in SCWR are 583–689 K and 25 MPa, respectively [Citation7]. In the current SCWR design, austenitic stainless steel and Ni-based alloy are candidate materials for fuel cladding tubes [Citation8,Citation9]. The material of PNC1520, which is an improved material of 316 stainless steel, has been studied to exhibit an excellent swelling resistance under neutron irradiation at a temperature range of 600–900 K compared with some other commercialized austenitic stainless steels. Thus, PNC1520 has been considered as one of the candidate materials for fuel cladding materials in SCWR [Citation10,Citation11].
With regards to the RISA effect in stainless steels, previous studies could only be found in BWR conditions. Therefore, study related to the RISA effect in SCWR is still limited. Takamasa et al. previously reported that the critical heat flux of zirconia-coated stainless steel after neutron irradiation increased in comparison to the un-irradiated condition, which was revealed from the boiling heat transfer experiment under boiling water reactor (BWR) flow field in japan material test reactor (JMTR) [Citation12]. It is understood that the mechanism involves increasing the heat-transfer efficiency from the fuel cladding material to the coolant, which is achieved by improving the wettability of the surface oxide layer due to the RISA effect.
Therefore, the purpose of the present study is to investigate the RISA effect in SCWR candidate materials under ultraviolet (UV) light and γ-ray irradiations. The wettability and microstructure analysis of PNC1520 and 304 stainless steel (SS304) were systematically evaluated. Attempts were also made to supplement the basic knowledge of the RISA effect in the application of SCWR core design.
2. Experiment procedures
2.1. Materials
PNC1520 and SS304 were used as experimental materials in this study, and their chemical compositions are shown in . Specimens with the sizes of 25 × 25 × 2 mm (length, width, and thickness) or 20 × 20 × 2 mm for PNC1520 and SS304 were prepared. After machining, these specimens were first mechanically polished up to a 0.05 μm colloidal silica suspension to achieve a high-quality mirror surface. For both materials, oxidation treatment under atmospheric conditions at 773 K for 43.2, 86.4, and 172.8 ks were carried out, so as to create an oxide layer on the surface. After, the surface was carefully re-polished to produce a smooth surface. The surface roughness after re-polishing of the specimen was confirmed to be ≤0.1 μm.
Table 1. Chemical compositions of PNC1520 and SS304 (wt.%)
2.2. UV and γ-ray irradiation
In order to clarify the RISA effect in PNC1520 and SS304, UV and γ-ray irradiations were independently performed using the oxidized PNC1520 and SS304 specimens. UV irradiation with a peak wavelength, photon energy, and radiation intensity being 254 nm, 4.48 eV, and 5 mW/cm2, respectively, was performed at atmospheric conditions and room temperature up to 277.2 K. The Co-60 γ-ray irradiations were performed at the Kyoto University Research Reactor (KUR) and National Institutes for Quantum and Radiological Science and Technology in Takasaki (QST) under atmospheric conditions and at room temperature. The wavelength and photon energy of the γ-ray source in KUR were 1.06 × 10−3 nm and 1.17 MeV, whilst at QST were 9.32 × 10−4 nm and 1.33 MeV, respectively. At KUR, the irradiation intensity was controlled by varying the distance from the γ-ray source, and the irradiation damage was performed up to 710 kGy. At QST, the irradiation was performed up to 685 kGy by controlling the irradiation time, and the irradiation dose rate was set as 10 kGy/h. It must also be noted that the γ-ray irradiation at KUR was performed under at higher humidity in comparison to that at QST.
2.3. Wettability measurement
The wettability of the specimens was evaluated based on a half-angle (θ/2) method [Citation13–Citation15]. A 5.0 μl of pure water was dropped from a height of <5 mm above the specimen surface using a micropipette. The size of the spherical water droplet was 0.35–0.40 mm. The contact angle, θ, was measured by the lateral side view of the water droplet as depicted in the standard half-angle method. By assuming that the water droplet shape is a part of a sphere, the θ is then obtained from the following equation.
where, h is the height of the water droplet and r is the radius of the water droplet. Since the volume of the water droplet was fixed at 5.0 μl in this study, the following equation can be derived.
Thus, θ can be evaluated by measuring the r of the droplet water from the photograph taken from directly above the water droplet. Moreover, some of the irradiated specimens were kept in a dark-room at ~298 K and 50–60 RH% with various holding periods, so as to evaluate the wettability recovery behavior after irradiation. The contact angle measurement after irradiation and/or holding under darkness was conducted within 300 s. The measurement of the contact angle was also performed at ~298 K and 50–60 RH%.
2.4. Microstructure analysis
Raman measurements of the oxide layer were performed at room temperature using a RamanRxn System manufactured by Kaiser Optical System, in which a green-laser with the wavelength of 532 nm was used. The spot size of the Raman laser was ~2 μm. It is generally believed that the vibration mode for the oxygen-sublattice can be detected in ceramic materials by Raman. Honjo et al. reported that from the XPS analyses of zirconia and titania before and after UV and γ-ray irradiation [Citation15], the bridging oxygen of the surface became smaller due to irradiation, and the magnitude of chemisorbed hydroxyl groups increased with increasing irradiation damage. Thus, in this study, the Raman analyses were performed in the oxidized specimens to investigate the bonding state of oxygen in the oxide layer, based on which the hydrophilicity can be elucidated.
3. Results and discussion
3.1. Wettability
The contact angle (θ) of the water droplet as a function of irradiation damage under (1) UV and (2) γ-ray irradiation is summarized and shown in . As-received and oxidized specimens at 773 K up to 43.2, 86.4, and 172.8 ks were subjected to UV irradiation. These specimens are hereafter designated after their heating times, e.g. for the 86.4 ks heated specimen, it is called as 86.4 ks. It is also noted that only the 86.4 ks specimens were subjected to γ-ray irradiations. For the γ-ray irradiated specimens, they are named according to the irradiation facilities, i.e. KUR and QST. It is also noted that the γ-ray irradiations under KUR and QST were shown together although the γ-ray flux was varied in both irradiation conditions.
Figure 1. The irradiation damage dependency of the contact angle of the water droplet in PNC1520 and SS304 specimens under (a) UV and (b) γ-ray irradiation. The dotted lines are guides.
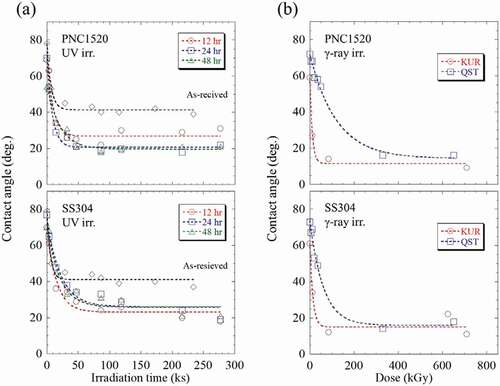
Regarding As-received PNC1520 and SS304 specimens, it is found from ) that the contact angle of the water droplet for both specimens was approximately 70–80° before UV irradiation. In both cases, θ decreased quickly at an irradiation time below 15 h, and then it was saturated above. No obvious difference was observed between PNC1520 and SS304 specimens, revealing that the chemical composition does not exert a significant influence on the hydrophilic property in these two similar Fe-based materials.
In comparison to the As-received specimens, the oxidized specimens exhibited a lower contact angle at the same irradiation time for both PNC1520 and SS304 samples. The tendency shown in the oxidized specimens is akin to that of As-received samples, i.e. the contact angle of the water droplet decreased with increasing irradiation time. Besides, it is seen that the contact angle of the water droplet decreased from ~50–70° to ~30° after 4 h irradiation, then it gradually saturated at about 20° after 43.2 ks irradiation in all the oxidized specimens. Additionally, it seems there is no obvious difference observed among 43.2, 86.4, and 172.8 ks specimens in both PNC1520 and SS304.
Similar to UV irradiation, it is seen from ) that the contact angle decreased with increasing irradiation damage in the γ-ray irradiated PNC1520 and SS304, revealing the wettability enhanced with increasing irradiation damage in both specimens. Furthermore, the minimum contact angle of the water droplet obtained within this study was 9.5° and 15.8° for PNC1520, and 9.8°and 18.7° for SUS304 irradiated at KUR and QST, respectively. It is noted that the decreasing contact angles are different between KUR and QST specimens. For the PNC1520 specimen under KUR irradiation, the contact angle of the water droplet was 26.1° at 13 kGy, but it was 58.7° even at 30 kGy under QST irradiation. Thus, it is confirmed that a more significant RISA effect occurred under KUR irradiation compared to QST irradiation. The reason for this difference is unclear but believed to be influenced by the irradiation environments such as humidity and temperature because the formation of the oxide layer is susceptible to these factors. For example, the irradiation periods are different from each other, and the irradiation was conducted in winter at QST and in summer at KUR. It is commonly believed that the generation of OH radicals during irradiation is an important factor for controlling the RISA phenomenon, and the results shown from ) indicate that the formation of OH radicals is influenced by the irradiation environment. It is unfortunate that the γ-ray irradiation environments involved within this study were not elaborately designed. Thus, it is difficult to discuss the irradiation environment effects of surface wettability by the RISA effect, which will be revealed in our future works. In addition, all these γ-ray-irradiated values are much smaller relative to UV irradiated specimens, indicating a more significant RISA effect by γ-ray irradiation. The higher radiation energy of γ-ray compared with UV radiation is considered to be the reason for this as the bonding of metal and oxygen is more likely to be broken by the higher radiation energy of γ-rays other than the formation of electron-hole pairs [Citation15]. A similar tendency is also observed in the SS304 specimen.
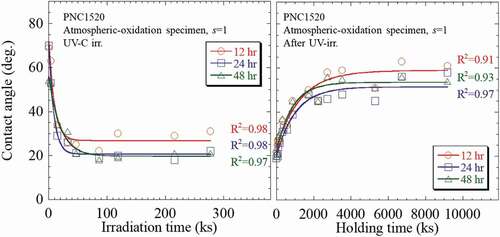
After irradiation, the recovery behavior of wettability in both (1) UV and (2) γ-ray irradiated PNC1520 and SS304 specimens was investigated. The contact angle was measured after holding under darkness with room temperature up to ~9000 ks. The result is presented in . Note that the specimens of 277 ks-irradiated with UV were employed in the recovery behavior measurements, and the 710 kGy in KUR and 685 kGy in QST-irradiated specimens were used in this measurement. In the UV irradiation, the oxidation time dependency of the recovery behavior of wettability was the same in both specimens. From ), the contact angle of the water droplet was found to gradually increase with increasing holding time; the contact angle of ~20° right after irradiation increased quickly to 60° after 3600 ks holding, then it saturated approximately at this value after a longer holding time.
Figure 2. The contact angle of the water droplet plotted as a function of holding time in PNC1520 and SS304 specimens after (a) UV and (b) γ-ray irradiation. Note that the specimens of 277.2 ks irradiated under UV irradiation were employed in the recovery behavior measurements, and the 710 kGy in KUR and 685 kGy in QST irradiated specimens were, respectively, used in this measurement. The dotted lines are guides.
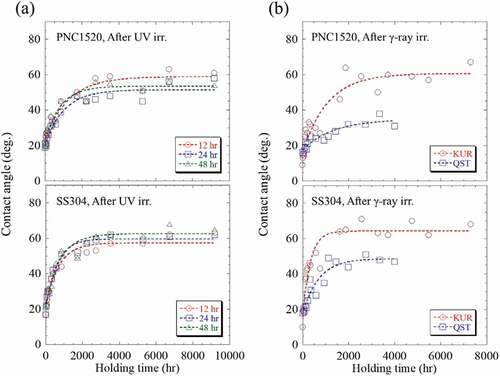
In , the contact angle of the water droplet of the KUR-irradiated PNC1520 specimen rapidly increased just after holding began, and it increased from 9.5° to ~30° after 130 ks holding time, and then kept increasing to ~60° after 1908 ks (>22 days) at a relatively slower rate. For the longer holding time of ≥1908 ks, the contact angle approximately stabilized at the same value. The QST-irradiated PNC1520 specimen showed a more moderate recovery behavior in comparison to the KUR-irradiated specimen. The contact angle of the water droplet was ~25° after 360 ks holding, and it was 30° even after 4320 ks (50 days). The moderate recovery behavior occurred in QST irradiation is also confirmed in SS304 specimens. The contact angle of the water droplet of KUR-irradiated SS304 specimen was 40° after 86.4 ks holding, and it increased to 60° after 1620 ks (19 days). Whereas, it required 936 ks holding to recover to 40° in the QST-irradiated specimen, and the contact angle reached at ~50° even after holding of 3960 ks (46 days). Additionally, compared with γ-ray irradiation, the recovery behavior in wettability after UV irradiation exhibited a relatively more gentle tendency in both the PNC1520 and SS304 specimens.
From the previous studies, it is pointed out that the possible factors influencing the wettability change (s) in a certain surface include annihilation and abundance factors, and the ratio of these characteristics are factor for wettability, i.e. annihilation rate (kb,i) and abundance ratio (gi = g1 +g2 + …+ gi +…+ gs = 1) in the state i, can then be related to the contact angle (θ) using the following equation [Citation14,Citation15].
where kf is the factor of generation rate caused by wettability, c is fraction of hydrophilic region by radioactively irradiation (0≦c≦1), t is holding time, f is the force vectors between the solid-liquid-gas acting on the droplet water, and f1 and f2 are the surface tensions in the region of hydrophilic and the non-hydrophilic [Citation17]. The generation factor is assumed to be inoperative in the current study as the measurements were made in darkness. Thus the kf is considered as 0, and the following equation is accordingly derived.
Based on the EquationEquation (4)(4)
(4) , the s can be determined by fitting the result of the recovery behavior of the contact angle of the water droplet in the dark place holding experiment, as shown in , as well as the kb. In this study, the f1 and f2 as shown in EquationEquation (4)
(4)
(4) were assumed as experimental constants, and they were determined from the contact angles of the water droplet when t = 0, i.e. right after irradiation and when t = ∞, i.e. the wettability was completely recovered. Moreover, the kf and kb,i were determined by fitting the irradiation damage dependence of the contact angle of the water droplet as shown in , using EquationEquation (3)
(3)
(3) . An analysis example for these parameters in the UV-irradiated PNC1520 (43.2 ks oxidization) specimen is shown in . The specimen was used in the sufficiently hydrophilized due to the UV-irradiation in this experiment, and the corresponding parameter in the cases of s = 1 and s = 2 were respectively evaluated. It is seen from that no clear difference was found in the R2 linear regression from the approximate curves obtained using s = 1 and s = 2. The abundance ratio (g2) was as low as 5%, and the lifetime was approximately 30 s. From the comparison result as shown in , it is considered that the experimental data can be well-explained as s = 1 in the UV irradiation.
Figure 3. An example to show the fitting results of the related parameters in the UV-irradiated PNC1520 (43.2 ks oxidization) specimen.
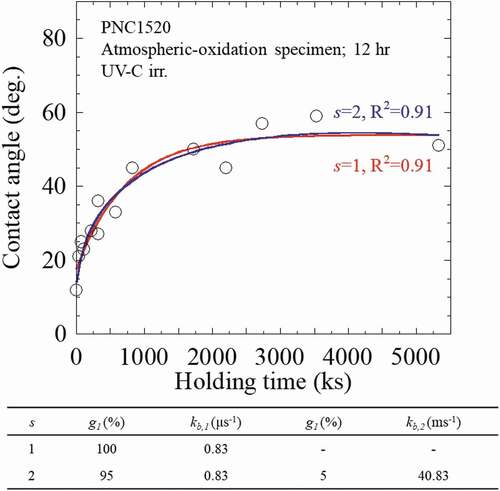
The numerical analyses for all the oxidized PNC1520 specimens after UV irradiation and dark place holding experiment were then analyzed, and the results are summarized and shown in . As a summary to our numerical analyses, the characteristic parameters for wettability improving factor in both PNC1520 and SS304 specimens after UV irradiation are indicated in . The kb was estimated in the range of 0.83–1.11 μs−1 in all the specimens; that is to say, the time required for a saturated wettability (~20°) in oxidized PNC1520 under UV irradiation is 10.5–13.8 day. In addition, the dependence of the kb on the oxidation period is not confirmed here, probably because the occurrence of a wetting reaction requires only a superficial layer of oxides, which could be obtained even within the shortest-term oxidation, i.e. 43.2 ks. The kf showed a dependence on oxidization time: the kf decreased with increasing oxidation time. The kf of the 43.2 ks specimen is 1.5 times greater in comparison to the 86.4 ks specimen, and a similar tendency was also observed in the SS304 specimens.
Table 2. The characteristics for the wettability improving factor in PNC1520 and SS304 specimen under UV irradiation. The kb denotes the lifetime of wettability control factor
Figure 4. The numerical analysis results of PNC1520 specimens before and after UV irradiation. Specimens exposed to UV irradiation were kept in darkness after irradiation for various holding time.
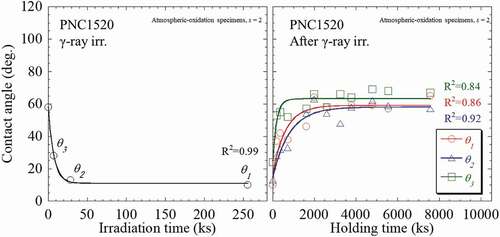
Similarly, the numerical analysis was also conducted on the γ-ray irradiated specimens. An example is given using the 710 kGy-irradiated SS304 specimen at KUR, as shown in , in which the s was evaluated from the recovery behavior of wettability. Obviously, the application of s = 1 could not well describe the experimental results especially at the holding time <3600 ks. In contrast, application of s = 2 and s = 3 gives rise to a better fitting to describe the results, and the g3 was estimated as 1%. Thus, in the γ-ray-irradiated specimens, s = 2 was used to numerically analyses their experimental results in order to achieve an appropriate fitting and precise parameters. The numerical analysis results of the SS304 specimens after γ-ray irradiation at KUR and dark place holding experiment are then summarized and shown in . Furthermore, the behavior of wettability change in QST irradiation specimen was greatly different from the UV irradiation for which the mechanism is also unclear. The typical behavior of γ-ray irradiation was thus set as the KUR irradiation specimen. The recovery experiments were conducted at irradiation doses of 14, 84, and 697 kGy, which are, respectively, designated as θ1, θ2, and θ3. It was found that the experimental data can be sufficiently explained by the kinetics equation. The characteristic parameters for wettability improving factors of γ-ray irradiation are summarized in .
Table 3. The characteristics for the wettability improving factor in KUR-irradiated PNC1520 and SS304 specimens under γ-ray irradiation. The kb and g denote the lifetime of wettability control factor and abundance ratio, respectively
Figure 5. The numerical analysis results of the recovery behavior of wettability in the 710 kGy irradiation SS304 specimen in KUR.
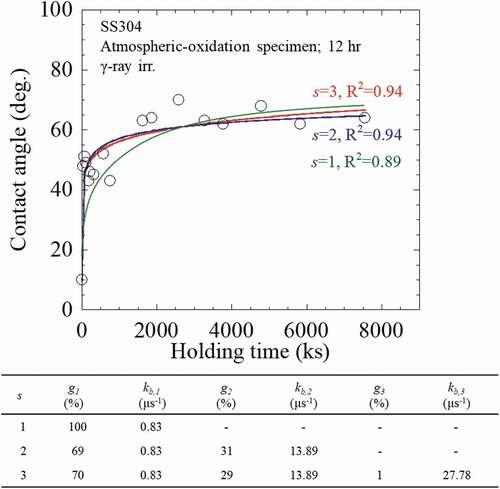
Figure 6. The numerical analysis results of PNC1520 specimens after γ-ray irradiation at KUR and after dark place holding experiment.
As aforementioned, s = 1 was applied to fit the experimental results from UV-irradiated specimens. The kb decreased with increasing oxidation time in both PNC1520 and SS304 specimens, and the lifetime of the wettability improving factor was estimated as 10.5–13.8 days. On the other hand, the s = 2 was applied for the γ-ray-irradiated specimens. As a result, the long-life and short-life factors due to the irradiation were yielded from the numerical analyses. The comparison of the effects of UV and γ-ray irradiations on wettability behavior change has been scarcely reported so far. Kariyazaki et al. suggested that the surface crystal structure might change due to γ-ray irradiation [Citation18]; however, the relevant knowledge to explain this mechanism has not yet been presented. A thorough understanding of the differences in the wettability changes by UV and γ-ray irradiations is lacking, and it will be a future research subject.
3.2. Characterization of oxide layer
Laser microscope photographs of oxidized and non-oxidized SS304 specimens are shown in . Note that the micrographs of oxidized specimen were taken after re-polishing the oxidized specimen. For each specimen and condition, two measurements were conducted. The arithmetic average roughness (Ra) of each sample measured via the confocal imaging technique is also shown on the upper left corner of the figure. Scratches were not observed in the As-received (non-oxidized) specimen, revealing that the qualified surface was provided to the oxidation and following contact angle measurements. On the other hand, in the oxidized specimens, small-sized contrast with the diameter of about 20–50 nm were observed, which is considered to be the massive oxides formed during high temperature annealing. Overall, the Ra was less than 1 μm in all the samples. No change in surface roughness was measured before and after contact the angle measurement, indicating that the contact angle measurement does not exert an obvious influence on the oxide surface morphology. Thus, specimens with a smooth surface could be provided for the evaluation of the contact angle of water droplets in this study, the surface roughness of PNC1520 specimens was almost same as SS304 specimens.
Figure 7. Laser microscope photographs of oxidized and non-oxidized SS304 specimens. The photographs of oxidized specimens were taken after re-polishing the oxidized layer.

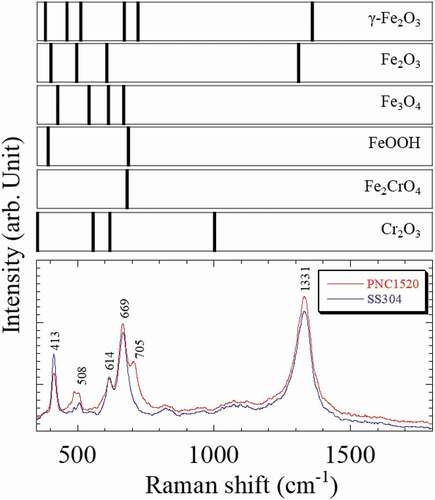
Raman spectra of 86.4 ks oxidized PNC1520 and SS304 specimens are shown in . Raman spectra of Fe-oxide, Fe-hydroxide, and Cr-oxide are also indicated as reference data [Citation17,Citation18]. Clearly, six bands are present in the range of 350–1800 cm−1. From the previous study, it is generally known that a Fe2CrO4 and Cr2O3-rich passive layer was formed in the austenitic stainless steels [Citation19]. It is also reported the Raman band of Fe2CrO4 was observed at 680 cm−1 [Citation20], and the Raman bands of Cr2O3 were observed at 385, 540, 598, and 1001 cm−1 [Citation21], approximately. The Raman band of the Fe2CrO4 was confirmed at 669 cm−1, whereas the peak of Cr2O3 was not observed. The bands at 413, 508, 614, and 1331 cm−1 were identified as Fe2O3 [Citation22], and the band at 705 cm-1 was assumed to be γ-Fe2O3. Furthermore, the band gap energies of Fe2CrO4, Fe2O3, and γ-Fe2O3 are 2.1 [Citation23], 2.0 [Citation24,Citation25], and 2.2 eV [Citation26], respectively. The photon energies of UV and γ-ray were 4.48 eV and 13.3 MeV, respectively. This result indicates that the occurrence of hydrophilization of the oxide layer, which is closely linked with the adsorption OH radicals at the surface, is caused by electrochemical reactions due to the irradiation; i.e. irradiation is capable of facilitating the electrochemical catalysis via the localized electron and hole at the oxide layer. In other words, the decrease of the contact angle of the water droplet is ascribed to the occurrence of RISA effect.
4. Conclusion
In order to conduct a fundamental study of the RISA effect on SCWR candidate fuel cladding materials, i.e. PNC1520 and SS304 specimen, the wettability change behavior and microstructural analysis of the oxide layers of these materials under the UV and γ-ray irradiations were evaluated. The main results in this study are summarized as follows:
(1) Surface roughness in the oxidized specimens were <1 μm, sufficient for the measurement of the contact angle of the water droplets, and Raman spectrum analysis revealed that the surface oxide layer contained Fe2CrO4, γ-Fe2O3, and Fe2O3 in 86.4 ks oxidized PNC1520 and SS304 specimens.
(2) The contact angle of droplet water measurements was conducted in both UV and γ-ray irradiated PNC1520 and SS304 steels, and the RISA effect was confirmed in both irradiation conditions and both materials. Further numerical analyses were conducted to reach a quantitative understanding on the RISA effect in these specimens. The lifetime of 13.8 days of wettability improving factor was produced by the UV irradiation, meanwhile, the γ-ray irradiation gave rise to the long-life (13.8 days) and short-life (0.8 days) wettability improving factors. Also, the abundance ratio of the long-life factor tended to increase with increasing irradiation damage.
(3) On the account of the fact that the band gap energy of Fe2CrO4, γ-Fe2O3, and Fe2O3 is, respectively, 2.1, 2.0, and 2.2 eV, and the photo energy of UV and γ-ray irradiation present study is 4.48 eV and 13.3 MeV, it is indicated that the occurrence of hydrophilization on the oxide layer, in other words decreasing of contact angle of droplet water, is ascribed to the RISA effect.
Acknowledgments
This study was supported by the JST Innovative Nuclear Research and Development Project. A part of study was carried out in a collaborative research project at Nuclear Professional School, School of Engineering, The University of Tokyo.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Takamasa T, Hazuku T, Tujimura N, et al. Radiation Detector Using Radiation InducedSurface Activation. JNST. 2004;41:152–155.
- Kato T, Hazuku T, Motoda S, et al. Crevice corrosion control for stainless steel using radiation-induced surface activation. Proceedings of 2009 International Congress on Advances in Nuclear Power Plants. 2009;43:2572–2579.
- Sayano A, Kano F, Saito N, et al. Improvement of the wettability of metal oxide surface by radiation induced surface activation. Proceedings of the International Conference on Nuclear Energy Systems for Future Generation and Global Sustainability. 2005;43:2562–2568.
- Takamasa T, Abe H, Akiba M, et al. Development of innovative nuclear technology based on radiation induced surface activation. J Atom Energy Soc Jpn. 2007;49:45–50..
- Takamasa T, Hazuku T, Okamoto K, et al. Radiation induced surface activation on Leidenfrost and quenching phenomena. Exp Therm Fluid Sci. 2005;29:267–274.
- Oka Y, Koshizuka S, Jevremovic T, et al. Supercritical-pressure light-water-cooled reactors for improving economy, safety, plutonium utilization and environment. Prog Nucl Energy. 1995;29:431–438.
- Oka Y, Koshizuka S, Yamasaki T. Direct cycle light water reactor operating at supercritical pressure. Jnst. 1992;29:585–588.
- Oka Y, Koshizuka S, Ishiwatari Y, et al. Super light water reactors and super fast reactors. France: Springer; 2010.
- OECD Nuclear Energy Agency for the Generation IV International Forum, Technology Roadmap Update for Generation IV Nuclear Energy Systems. 2014 Jan.
- Katsuragawa M, Kashihara H, Akebi M. Status of liquid metal fast breeder reactor fuel development in Japan. J Nucl Mater. 1993;204:14–22.
- Shikakura S, Ukai S, Sato Y, et al. Development of advanced austenitic stainless steel for fast reactor core material. J At Energy Soc Jpn. 1994;5:441–455.
- Takamasa T, Hazuku T, Mishima K, et al. Surface wettability caused by radiation induced surface activation. Therm Sci Eng Appl. 2004;12:39–44.
- Honjo Y, Furuya M, Takamasa T, et al. Mechanism of hydrophilicity by radiation-induced surface activation. J Power Energy Syst. 2009;3:216–227.
- Seki K, Tachiya M. Kinetics of photoinduced hydrophilic conversion processes of TiO2 surfaces. J Phys Chem B. 2004;108:4806–4810.
- Kariyazaki M, Abe H, Akio S, et al. Ray type dependence of radiation induced surface activation phenomenon. J Jpn Inst Metals. 2007;71:423–426.
- Young T. An essay on the cohesion of fluids. Philos Trans. 1805;95:65–87.
- Farrow RL, Mattern PL, Nagelberg AS. Characterization of surface oxides by Raman spectroscopy. Appl Phys Lett. 1980;36:212–214.
- Gardiner DJ, Littleton CJ, Thomas KM, et al. Distribution and characterization of high temperature air corrosion products on iron-chromium alloys by Raman microscopy. Oxid Met. 1987;27:57–72.
- Inoue S, Uchida H, Morii M, et al. Characterization by Raman spectroscopy of oxide layer formed on stainless steels. JJpn Inst Metals. 1990;54:1376–1381.
- Matsuda Y, Hinotani S, Yamanaka K. Characterization of oxide layers on SUS304L stainless steel by Raman spectroscopy. Tetsu-to-Hagane. 1993;79:48–54.
- Fan J, Cheng Y, Xie Z, et al. Hydrogen adsorption on high surface area Cr2O3 materials. Phys Status Solidi A. 2013;210:1920–1924.
- Colomban P, Cherifi S, Despert G. Raman identification of corrosion products on automotive galvanized steel sheets. J Raman Spectrosc. 2008;39:881–886.
- Andersson D, Stanek C. Mixing and non-stoichiometry in Fe–Ni–Cr–Zn–O spinel compounds: density functional theory calculations. Phys Chem Chem Phys. 2013;15:15550–15564.
- Degradation I, Liang C, Liu H, et al. One-step synthesis of spherical γ-Fe2O3 nano powders and the evaluation of their photocatalytic activity for orange. J Chem. 2015;791829:8.
- Leland J, Bard A. Photochemistry of colloidal semiconducting iron oxide polymorphs. J Phy Chem. 1987;91:5076–5083.
- Mochizuki S. Electrical conductivity of α-Fe2O3. Phys Status Solidi. 1977;41:591–594.