?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A new amidoxime polymer gel for uranium extraction from seawater was successfully prepared by photoinitiated crosslinking polymerization. It involves UV-induced radical polymerization of acrylonitrile (AN) and methacrylic acid (MAA) monomers with crosslinking agent and photoinitiator: an alternative synthesis pathway to gamma radiation. Characteristic peaks of Fourier transform infrared spectroscopy (FTIR) indicated that nitrile group was completely converted into amidoxime group. Eight hours of 60 W UV curing was enough for polymer gel formation. For the 4 to 1 ratio of AN to MAA, crosslinking agent concentration of 1 g/100 mL-monomers and photoinitiator content of 60 mL/100 mL-monomer, maximum uranium adsorption capacity of 17.02 mg/g adsorbent in seawater was achieved for sample spiked with 10 ppm uranium. For seawater sample spiked with 2,245 ppm uranium, the adsorption capacity reached 432.41 mg/g adsorbent. The amidoxime polymer gel could be regenerated for at least eight cycles while retaining about 50% of the uranium uptake capacity. Thus, the present amidoxime polymer adsorbent is a high-efficiency seawater uranium recovery agent for both high and low uranium concentrations.
1. Introduction
Uranium, a naturally occurring heavy metal, is used as a raw material to produce fuels for nuclear power reactors and nuclear research reactors. It has a natural abundancy of 2–4 ppm, totalling 5.5 million metric tons, while being in the oceans at 3–3.3 ppb [Citation1]. As any consumed resource it cannot be mined to perpetuity. Thus, it is of interest to look for non-land–based occurrences such as seawater. The total quantity of dissolved uranium in seawater is estimated to be about 4.5 billion metric tons [Citation1]. This rather large quantity can supply fuels for nuclear power plants worldwide for approximately 60,000 years, thus, it is a sustainable source for uranium.
The partitioning of dissolved uranium ion species in seawater is a strong function of pH. Uranyl tricabonate ion, [UO2(CO3)3]4-, is the major species at pH of natural seawater (about 8), whereas UO22+ is the major species at pH < 4 and UO2(OH)+ is mostly formed at pH between 4 and 5 [Citation2]. Recovery of uranium from seawater involves facing many challenges such as the extremely low uranium concentration in seawater, the presence of other competing ionic species at much higher concentrations, biological fouling of adsorbents, and the high recovery cost. Therefore, uranium extraction from seawater requires methods with high efficiency, as well as high selectivity toward uranyl ions.
From the past to the present, several methods have been developed. For example, ion exchange resins were the first method researched. Although resins exhibit fast kinetics, they offer very poor selectivity for uranium. Solvent extraction is a well-confirmed method for specific metals, but the solvent may be lost due to entrainment or solubility [Citation3]. The adsorption method is preferred due to its selectivity, fast adsorption kinetics, and easy operation [Citation4–Citation8]. Many adsorbents have been studied such as activated carbon [Citation9–Citation11], mesoporous materials [Citation12–Citation16], titanium dioxide [Citation3,Citation17], fibers [Citation2,Citation5,Citation18–Citation21], and hydrogel [Citation22,Citation23]. Recently, modified adsorbents with chemical functional groups have been studied, which include amine, amide, dopamine, oxime, hydroxamic acid, and amidoxime [Citation2,Citation24]. The amidoxime group is composed of the oxime group and the amine group which can coordinate with [UO2(CO3)3]4- in seawater. At present time, it is the most promising functional group exhibiting high selectivity and fast adsorption rate [Citation5,Citation6]. Ideally, amidoximated adsorbents with high uranium loading capacity should exhibit the following desirable characteristics: high hydrophilicity, large surface-to-volume ratio, abundant amidoxime group, and positively charged surface which helps bond with [UO2(CO3)3]4- and repel cations [Citation25,Citation26].
According to the research of Badawy et al. [Citation18], radiation grafting of AN/MAA onto cotton cloth was performed followed by the amidoximation process for the purpose of recovering uranium from radioactive waste. Results demonstrated the uranium uptake capacity of 662 µg/g filter. Seko et al. [Citation21] tested fibrous adsorbents containing the amidoxime group with column test at room temperature and the flow rate of filtered seawater of 3 L/min. It was found that after 7 days, uranium adsorption reached 300 mg U/kg adsorbent. Furthermore, synthesized adsorbents containing the amidoxime group could also be applied to study the removal of copper in aqueous solutions. The maximum adsorption capacity of amidoxime sepiolite nanohybrid material (AO-RGS) was found to be 278 mg/g adsorbent within 30 minutes [Citation27]. Testing the adsorbents in real natural seawater is an important experiment to demonstrate the actual ability of the adsorbents, which also allows performance comparison among the adsorbents under a realistic marine condition. Lately, the AF series adsorbents from Oak Ridge National Laboratory exhibited a high adsorption capacity in marine testing of 3.9 g-U/kg-adsorbent after 56 days [Citation28] Also, adsorbent fibers prepared by atom-transfer radical polymerization from amidoximated fibers were tested in natural seawater by Pacific Northwest National Laboratory. Results show a high uranium uptake of 5.22 g-U/kg-adsorbent after 49 days [Citation29]. In addition, Oak Ridge National Laboratory achieved a very high uranium uptake (6.56 mg-U/g-adsorbent after 56 days) using high-surface-area amidoxime-based polymeric adsorbents [Citation30].
However, past researches used fibers as a supporting substrate for chemical grafting of the functional group. Thus, the mass of the fiber is a part of the mass of the adsorbent. When expressing the adsorption capacity in mg-U adsorbed/g adsorbent, the result would be higher if no mass of the substrate is present.
Study of Hara et al. [Citation22] illustrated a hydrogel adsorbent prepared from acrylamide (AM) and AN. A crosslinking agent was N,N’-Methylene bisacrylamide (MBA) and an initiator of polymerization was ammonium peroxodisulfate. The produced hydrogel was tested in a solution with 4 ppb of uranium and other elements for 3 days. Results concluded that the hydrogel could adsorb uranium even in an extremely low concentration. Moreover, a research of Wongjaikham et al. [Citation23] prepared a polymer gel with the amidoxime functional group from the mixture of AN and MAA monomers. MBA was used as a crosslinking agent and polymerization was promoted by gamma radiation from Co-60. Results revealed the highest adsorption capacity in seawater spiked with 300 ppb uranium of 6.96 mg/g adsorbent with the following optimum synthesis parameters: gamma ray dose of 40 kGy, AN:MAA ratio of 80:20 and crosslinking agent concentration of 0.8 g/100 mL.
From the reviewed literature, researchers performed either chemical or radiation polymerization. There has been no experimental work on synthesis of uranium adsorbent agent using UV polymerization. UV-assisted polymerization can be a desirable alternative to gamma ray polymerization for synthesis of uranium adsorbent because of easy equipment setup with low maintenance, lower operating cost and no concern for safety of ionizing radiation. Most importantly, this alternative allows researchers without access to gamma irradiation facility to synthesize a uranium adsorbing agent.
UV polymerization is widely carried out through photoinitiated reactions and many studies demonstrated that polymerization reactions can occur rapidly by photoinitiated crosslinking of monomers and oligomers. Moreover, because of low energy consumption, UV curing technology has become an environmentally friendly process. Wan et al. [Citation31] prepared a superabsorbent material from kaolinite and poly (acrylic acid - acrylamide) by photopolymerization using Michler’s ketone and benzophenone as photoinitiators. Ruan et al. [Citation32] synthesized superabsorbent resin from acrylic acid (AA) monomer using 700 W UV light with various crosslinking agents and photoinitiators. Results indicated high water absorbency when MBA (crosslinking agent) and Irgacure 1800 (initiator) were used. Dolat et al. [Citation33] synthesized a high-capacity water absorbent agent by UV polymerization without any crosslinker or initiator and result showed high water absorption rate which might be good for industrial applications.
The purpose of this study is to employ UV-C irradiation to synthesize the high-efficiency amidoxime polymer gel to extract uranium from seawater. As no gamma radiation will be utilized and as no substrate is required, a solution mixture of AN and MAA monomers, hydrogen peroxide as a photoinitiator and MBA as a crosslink agent was selected for radical polymerization by UV-C. UV-C will break the bonding of hydrogen peroxide to become OH free radicals to effectively assist in polymerization and crosslinking. This synthetic technique can be performed in a laboratory and no researcher has synthesized amidoxime polymer gel by UV-C curing, thus, this work is genuine and will provide new knowledge for the scientific community.
2. Experimental section
2.1. Materials
Reagent grade MBA, dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), hydroxylamine hydrochloride (NH2OH.HCl), methanol, hydrochloric acid (HCl), and potassium hydroxide (KOH) were purchased from Merck-Schuchardt company. Industrial grade AN and MAA monomers with purity of 98 and 99.9%, respectively, were procured in Thailand. All chemicals were used with no further purification. UV-C lamps of 15 W output (Philips TUV 36W/G36 T8) were purchased from Vasuthip Co., Ltd. in Thailand. Lamps were combined with reflectors to form a rectangular box with UV sources emanating from each side of the box toward the solution.
2.2. Polymer gel preparation
Several volume ratios of AN and MAA monomers were dissolved in 50 (w/w)% DMSO. For each volume ratio, different concentrations of MBA and hydrogen peroxide were added. The solution mixture was polymerized by the 4 UV-C lamps (60 W total) for 4, 6, 8, and 10 h at 45°C (318 K). The studied conditions were summarized in . After completion of the UV-C irradiation, the resulting polymer gel was crushed into tiny particles and DMF was added overnight to remove homopolymer and unreacted monomers. The polymer particles were dried in a forced-convection oven at 50°C (323 K) for 24 h.
Table 1. Ratio of chemicals for polymer gel preparation
2.3. Amidoximation of nitrile group
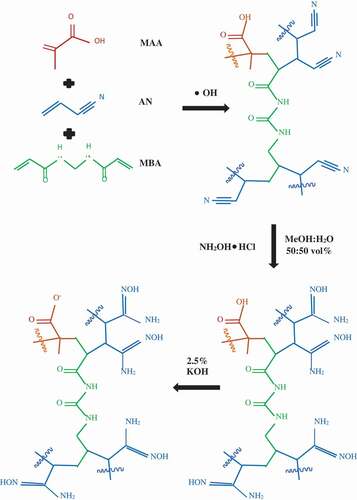
The nitrile group in the polymer particles was converted into the amidoxime group (AO) by immersion in a 3% hydroxylamine hydrochloride solution at 73°C (346 K) with stirring. The solution was prepared by dissolving 54 g hydroxylamine hydrochloride in 1:1 (v/v) DI water-methanol solution and neutralized to pH of 7 by 20 M KOH solution. After 90 min, the polymer particles were washed with DI water-methanol and dried in a forced-convection oven at 50°C for 24 h.
2.4. Alkaline conditioning
The aim of the alkaline treatment is to transform the carboxylic group (COOH) of MAA to carboxylate (
COO−) so that the polymer particles become hydrophilic [Citation34]. Prior to alkaline conditioning, the amidoxime polymer particles were soaked in 1 M hydrochloric acid solution for 15 min and washed with DI water. The alkaline conditioning was performed with 2.5% (w/v) KOH solution at 80°C (353 K) for 60 min. The particles were then washed with DI water until the pH of the drain water reached 9 and were dried in a forced-convection oven at 50°C. demonstrates the most probable reactions taking place during the amidoxime polymer gel synthesis.
2.5. Uranium adsorption test
Approximately 0.15–0.2 g of the amidoxime polymer particles was packed in a fine cloth filter, and the polymer particles in the fine cloth filter were packed inside another layer of polyester coarse-mesh bag for submersion in seawater. Batches of the polymer particles were submersed in 100 liters of seawater sample spiked with 10 ppm uranium (confirmed by ICP-OES). A small fish tank water pump was submerged at the bottom of the seawater sample container to generate continuous current. In addition, the adsorbent exhibiting the highest adsorption determined from the test in seawater sample spiked with 10 ppm uranium was also tested in seawater sample spiked with 86.3 and 2,245 ppm uranium. The seawater sample was collected near the port of Mab Ta Phut, Rayong Province, Thailand. The soaking time was 1 week, and the experiment was carried out at room temperature in the laboratory.
2.6. Uranium elution
The amidoxime polymer particles with fine cloth filter were removed from the seawater sample and were soaked in DI water for 2 days to remove seawater and uranium adhering to the polymer particles. The particles were subsequently immersed in 1 M HCl solution at 50°C for 2 h (except for one batch that underwent the elution study, which were submerged in the HCl solution for 1, 2, and 4 h). After the elution process, the polymer particles were removed from the HCl solution and the solution was slowly boiled until only yellowish residue remained at the bottom of the beaker. To liquefy the residue, a 1% HNO3 solution was added to the beaker at room temperature. Uranium concentration in the HNO3 solution was evaluated by the ICP-OES technique.
2.7. Water absorbency
Approximately 0.2–0.3 g of the amidoxime polymer particles was soaked in DI water and seawater to determine the water absorption kinetic. The adsorbent was removed from DI water or seawater after every 5 or 10 min to determine the weight gain. Excess water surrounding the absorbent was taken up by tissue papers. Similar to evaluation of superadsorbent materials, the swelling ratio is conventionally expressed as:
where w1 and w2 are the weight of the dry and swollen adsorbent, respectively.
2.8. Measurement of gel fraction
To determine the gel fraction of irradiated polymer gel, after finishing the UV-C irradiation, the dried polymer gel was cut into 2 × 2 mm pieces. The small pieces of polymer gel were immersed in DMF (150 mL) for 5 h at room temperature. The soluble fraction was washed away and the remaining insoluble fraction was allowed to dry at room temperature overnight and was further dried in a forced convection oven for 0.5 h. The gel fraction can be calculated from EquationEquation (2)(2)
(2) as follows:
where wi and wf represent the weights of dried polymer gel before and after DMF treatment, respectively.
2.9. Reusability test
Firstly, the amidoxime polymer particles were submerged in the seawater sample spiked with 500 ppb uranium (confirmed by ICP-OES). This chosen initial concentration is the same as that in our previous work [Citation23] to allow direct comparison. Then, after 1, 2, and 3 days representing 1 cycle, the particles were removed from the seawater followed by the elution process discussed in Section 2.6. Subsequently, the particles underwent the alkaline conditioning procedure detailed in Section 2.4 and were submerged in the new seawater sample spiked with 500 ppb uranium to ensure the same initial uranium concentration in the aqueous solution. The experiment was performed for eight cycles.
2.10. Characterization and measurement
The characteristic bands of the nitrile group and the amidoxime group were investigated by the Fourier-transform infrared spectroscopy (FTIR) technique (Perkin Elmer model Spectrum one) at Scientific and Technological Research Equipment Centre Chulalongkorn University, Thailand. Uranium concentration in the HNO3 solution was evaluated by the ICP-OES technique at Elemental Analysis Unit, Department of Chemistry, Faculty of Science, Silpakorn University, Thailand.
3. Results and discussion
3.1. Effect of UV-C exposure time
The preparation condition of AN:MAA of 80:20, crosslinking agent of 1 g and H2O2 of 60 mL was selected to study the effect of UV-C irradiation time, as it will be shown in a later section that this condition resulted in the amidoxime polymer particles with the highest uranium adsorption capacity. As can be seen in ), after 4 h of UV-C irradiation, the solution became very soft gel but remained a liquid phase in a beaker. After 6 h of exposure, according to ), the solution became solid polymer gel almost completely. After 8 h as shown in ), the solution solidified completely, but was still soft. Sufficient softness was required to allow the gel to be crushed into tiny particles. After 10 h of irradiation as shown in ), the gel became rigid and hard to mash into small particles.
Figure 2. Effect of UV-C exposure time: (a) 4 h, (b) 6 h, (c) 8 h, and (d) 10 h (AN:MAA of 80:20, crosslinking agent of 1 g/100 mL and H2O2 of 60 mL).
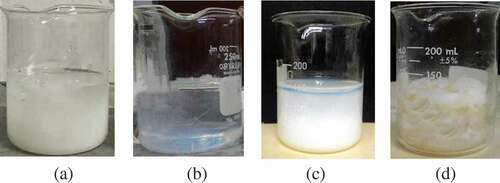
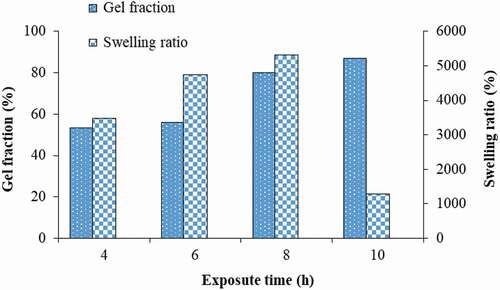
The effect of exposure time can be explained by gel fraction and swelling ratio at equilibrium as shown in . The gel fraction increased as the exposure time rose from 4 to 10 h. At 8 and 10 h of UV curing, the reaction yield reached approximately 80 and 87%, respectively. For the swelling ratio at equilibrium (after soaking in DI for 90 min), the swelling also increased with increasing curing time up to 8 h. However, at 10 h, the swelling ratio dropped considerably. This is because the polymer gel becomes so rigid that water could not effectively diffuse into the polymer particles. Therefore, the optimal UV-C irradiation time for further experiments was determined to be 8 h. These behaviors can be explained as follows. For short exposure time, the amount of generated OH free radicals was still low resulting in a small degree of polymerization and crosslinking. For a too long exposure time, polymerization was completed and crosslinking became too intensive causing the polymer to become very rigid. This inflexible polymer cannot be used because the structure has no elasticity to accommodate diffusion of water inside.
Figure 3. Effect of exposure time on gel fraction and swelling ratio at equilibrium of polymer gel (AN:MAA of 80:20, crosslinking agent of 1 g/100 mL and H2O2 of 60 mL).
Dolat’s research [Citation33] on synthesis of superabsorbent from AA monomer found a similar behavior in the formation of polymer gel with respect to the exposure time. The optimal exposure time for synthesis was determined to be 45 min with 1,000 W of UV radiation power with a small amount of the monomer contained in a dish (5 ml plus water). The obtained gel content was 80%. Thus, the energy efficiency was 0.15 kWh/ml monomer. For the present work, at the gel fraction of 80%, 160 mL of the solution was irradiated with the UV power of 60 W for 8 h (energy efficiency = 0.003 kWh/ml monomer). Thus, when comparing the energy efficiency in running the UV equipment to obtain 80% gel fraction, the present work is higher than the Dolat’s work. However, it is important to note that Dolat’s UV polymerization technique utilized water as photoinitiator and required no crosslinker.
3.2. Characterization of nitrile and amidoxime groups
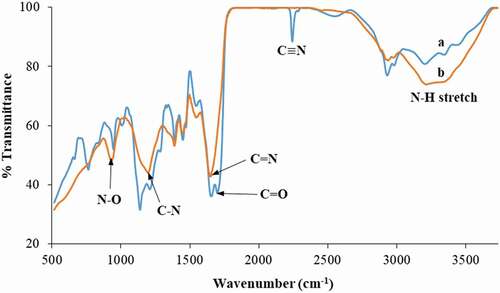
Relevant characteristic absorption bands for the determination of the functional groups present in the studied polymer gel is summarized in . displays FTIR spectra of the polymer gel before and after amidoximation. The absorption bands of AN/MAA prior to amidoximation is illustrated in ) and can be interpreted as follows: the characteristic peak at 2,243 cm−1 representing the CN group confirmed the presence of poly(AN) in the polymer particles, and the split small sharp peaks at 1,653 and 1,700 cm−1 represented the carbonyl group (C
O) of poly(MAA) [Citation4]. As shown in ), after amidoximation, the C
N peak vanished completely and the presence of C
N (1,652 cm−1), N
O (934 cm−1), C
N (1,200 cm−1) [Citation4] and N
H vibration (3,200–3,500 cm−1) [Citation2,Citation18,Citation23] indicated the conversion of nitrile group into the amidoxime group.
Table 2. Relevant characteristic absorption bands for determination of functional groups present in studied polymer gel
3.3. Effect of monomer ratio, photoinitiator content, and crosslinking agent concentration on uranium adsorption
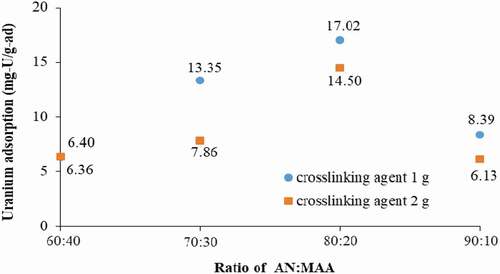
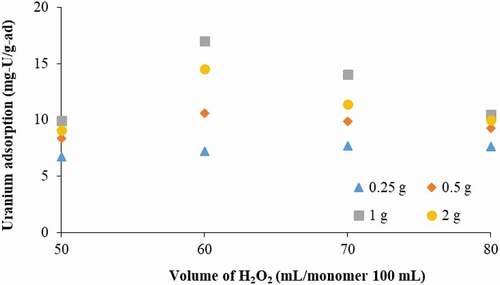
The crosslinking agent of 1 and 2 g and hydrogen peroxide of 60 mL was chosen to examine the effect of monomer ratio on uranium adsorption. Lower crosslinking agent concentrations were not considered in this experiment because it will be shown later that crosslinking agent concentrations of 0.25 and 0.5 g resulted in a low uranium adsorption capacity. The ratio of AN:MAA studied was 60:40, 70:30, 80:20, and 90:10. UV-C exposure time was 8 h. The resulting adsorbent for each condition was submerged in the seawater sample with spiked 10 ppm of U for 1 week. shows the results of uranium adsorption.
Except for the monomer ratio of 60:40, the adsorbent performance was higher with 2 g of the crosslinking agent concentration than 1 g for every AN:MAA ratio. Also, the AN:MAA ratio of 80:20 was the optimal one. A higher AN portion provides a higher amidoxime group concentration, which is desirable. However, this results in a lower MAA portion and less hydrophilic group in the adsorbent. Seawater needs to diffuse into the interior of each particle for the uranyl ions to be captured by the amidoxime groups inside, besides being captured only on the surface of each particle. Thus, each particle must be able to accommodate water diffusion and have a flexible structure to hold water inside. The competing effect of AN and MAA portions resulted in the 80:20 ratio being the optimal one.
Photoinitiator is the most crucial factor affecting photopolymerization. To evaluate the effect of photoinitiator content on uranium adsorption, the concentration of hydrogen peroxide of 50, 60, 70 and 80 mL/100 mL monomer was investigated based on the AN:MAA ratio of 80:20 with different crosslinker concentrations.
As illustrated in , the trend of uranium uptake rises as the photoinitiator content increases from 50 to 60 mL but drops with a further increase in the photoinitiator content. The trend from 60 to 80 mL of photoinitiator can be explained as follows: Increasing the photoinitiator content resulted in a reduced molecular weight of the macromolecule because the increasing number of generated OH free radicals started to destroy the polymer chains with the corresponding increase in the concentration of polymer chain ends. These polymer chain ends do not lead to the absorption capacity [Citation31,Citation35]. According to the equation of Seymour and Carraher [Citation35,Citation36], the number-averaged degree of polymerization () is inversely proportional to the square root of the concentration of the initiator and it can be expressed by the following Equation:
where [I] and [M] are the concentrations of the initiator and the monomer, respectively; is a combination constant;
,
, and
are the rate constants for propagation, initiation, and termination, respectively. Thus, for a given monomer concentration, temperature of polymerization, and crosslinking agent concentration, the higher the photoinitiator content the lower the molecular weight of polymers [Citation35].
For the photoinitiator content below the optimum volume of 60 mL, the generated free radicals are not enough for network formation and polymerization [Citation31,Citation37–Citation39], giving low crosslinking density [Citation40] and resulting in a low adsorption capacity.
The crosslinking agent is another crucial factor that affects uranium adsorption characteristics, as well as mechanical properties of the amidoxime polymer. The crosslinking reaction of MBA is copolymerization with AN and MAA monomers [Citation32,Citation41] The uranium adsorption capacity as a function of crosslinking agent concentration was investigated and the results are also displayed in . The uranium adsorption increased with increasing MBA concentration from 0.25 to 1 g, and then decreased with a further increase in MBA concentration. The maximum uranium uptake was 17.02 mg/g-adsorbent with 1 g crosslinking agent concentration.
For the crosslinking agent concentration less than 1 g, the amidoxime polymer has lower crosslinking density and crosslinking point [Citation40]. Even though the homopolymer and unreacted monomers have been washed by the DMF solution, AN and MAA monomers have been polymerized to became a long chain copolymer. The resulting polymer gel will be physically softer than that synthesized from the condition of higher crosslinking concentration. Therefore, the swelling capacity will be lower resulting in low uranium adsorption. For the crosslinking agent concentration of 2 g, the tighter network is formed, causing difficulty for water to diffuse into the interior of the polymer resulting in a reduction in the uranium adsorption capacity [Citation37]. These results conform with Flory’s theory [Citation42] and previous studies [Citation37,Citation39,Citation41].
In summary, the optimal parameters of synthesis of the adsorbent are: AN:MAA = 80:20, crosslinking agent concentration = 1 g, and photoinitiator content = 60 mL.
3.4. Elution duration
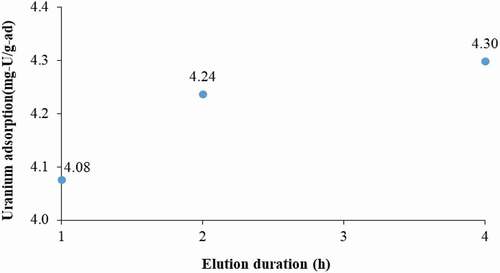
Hydrochloric acid is typically employed to remove uranium from amidoxime-based polymer adsorbents. displays the amount of recovered uranium from the amidoxime polymer particles with different elution durations. Results indicate the required elution duration of at least 2 h in 1 M HCl at 50°C. Furthermore, by allowing more elution time from 2 to 4 h, only about 1.4% additional uranium was obtained. For this reason, the elution duration of 2 h is considered sufficient and, thus, all experiments in the present study followed the 2 h elution duration.
3.5. Swelling ratio in DI water and seawater
As shown in , the adsorbent prepared from 60 mL of photoinitiator exhibited a higher swelling ratio than that prepared from 50 mL of photoinitiator for both DI water and seawater. This result supports the finding shown in and . For the case of 60 mL, the equilibrium swelling ratio was reached at 3,800% after 15 min and 1,400% after 20 min in DI water and seawater, respectively. When compared to the previous finding of Wongjaikham et al. [Citation23] where the swelling ratio of the amidoxime polymer gel synthesized from gamma radiation reached 1,000%, the present polymer particles appear to perform better as they are able to absorb water almost four times higher.
Figure 8. Result of water and seawater absorption kinetic of amidoxime polymer particles (AN:MAA of 80:20 crosslinking agent of 1 g/100 mL).
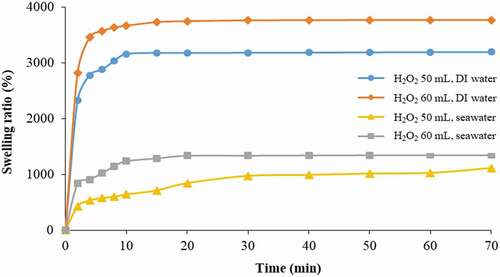
Figure 6. Effect of photoinitiator and crosslinking agent concentrations on uranium adsorption (AN:MAA of 80:20).
Even though these maximum water absorbencies are not higher than those of other studies which focus directly on synthesizing superabsorbent materials [Citation31,Citation39], the present amidoxime particles was prepared with the objective to adsorb uranium in seawater and good water absorbance helps promote uranium adsorption.
3.6. Reusability test
The usage repeatability of the adsorbent is important in terms of actual applications and economic worthiness. The adsorption capacity of the amidoxime polymer particles for up to 8 adsorption cycles is illustrated in . The adsorbent could be reused up to at least eight cycles while retaining about 50% of the uranium uptake capacity compared to the first cycle (interpreted from the 3-day adsorption data in each cycle). This result is comparable to the findings from the works of Yu [Citation1] and Seko [Citation21] that the adsorption capacity fairly dropped after five cycles.
Figure 9. Repeatability test of amidoximed polymer particles (AN:MAA of 80:20, crosslinking agent of 1 g/100 mL, H2O2 of 60 mL).
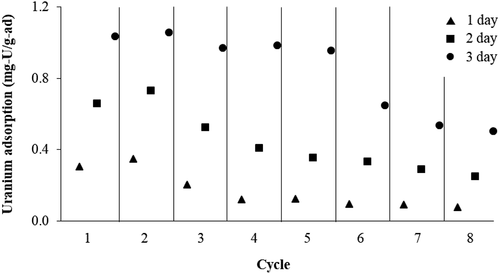
The adsorption capacity of a similar polymer gel adsorbent prepared by gamma irradiation detailed in Ref. [Citation23] did not decrease with the elution cycle for up to eight cycles. The downward trend of the present adsorbent might be due to the fact that gamma radiation has higher penetration than UV radiation, resulting in denser crosslinking of polymer chains and a stronger bonding among monomers. Therefore, when the present polymer was repeatedly exposed to strong acid and alkaline solutions for elution and reconditioning, respectively, this caused polymer degradation and lowered the adsorption efficiency.
3.7. Comparison with other works
The synthesized amidoxime polymer particles were submerged in the seawater sample with spiked uranium of 86.3 and 2,245 ppm (confirmed by ICP-OES) at room temperature for 2 weeks and results were compared with other studies as shown in . At the high uranium concentration of 2,245 ppm, the polymer particles can absorb uranium very well. For conditions of low uranium concentrations, the adsorption capacity of this polymer is superior to many other adsorbents such as AO-MWCNTs, Pal/PAO, and cloth filter. Thus, the present amidoxime polymer adsorbent prepared by UV-C irradiation is an excellent adsorbent for recovery of uranium from seawater for both high and low uranium concentrations.
Table 3. Comparison of uranium adsorption capacity of various studies based on amidoxime adsorbents
3.8. Production cost analysis
In terms of cost and economic aspects, the present adsorbent has a lower production cost than that prepared by gamma radiation [Citation23]. shows the cost components for synthesis of the same amount of uranium adsorbent with different radiation sources based on the optimal synthesis parameter for each case. For the case of UV lamp, the four UV lamps with reflectors costed approximately US$ 331.35 and have a useful life of 9,000 h [Citation47]. The normal experiment based on 8 h, thus, the experiment can run for 1,125 times (over 1 year). With 1.86 kg of solution that can be irradiated for each batch (9 full 200-ml beakers in the irradiation rig), the equipment cost for each batch was evaluated to be US$ 0.29. If employing gamma radiation, the cost of irradiating 1.86 kg of solution based on Thailand Institute of Nuclear Technology, Thailand, is US$ 120.01. As a comparison of the two cases, the cost of adsorbent synthesis by using gamma radiation is approximately 2.5 times higher than using UV lamps (excluding installation, maintenance, reaction apparatus, and electricity costs for both cases). In addition, in terms of workplace safety, working with UV lamps is safer and more convenient than working with gamma irradiator, which requires qualified safety personnel as well as staff oversight while irradiating. UV-C irradiation can also be performed essentially in any laboratory with minimal safety precaution. Finally, for an organization with no access to a gamma irradiator, the gamma irradiation technique cannot be an option to pursue. In conclusion, due to above reasons, the amidoxime polymer particles synthesized from UV-C irradiation technique could be considered as an alternative adsorbent for uranium extraction from seawater.
Table 4. Cost components for synthesis of the same amount of uranium adsorbent with different radiation sources
4. Conclusion
The amidoxime polymer adsorbent for uranium extraction from seawater was successfully synthesized by radical photopolymerization technique. AN and MAA monomers were utilized with MBA as the crosslinking agent and hydrogen peroxide as the photoinitiator. The optimum parameters to obtain high uranium adsorption capacity are: ratio of AN:MAA of 80:20, crosslinking agent concentration of 1 g/100 mL-monomers, and photoinitiator content of 60 mL/100 mL-monomers. Eight hours of 60 W UV-C curing was suitable for polymer gel formation. The elution duration using 1 M HCl solution at 50°C to extract uranium from the adsorbent should be at least 2 h. When submerging in seawater samples spiked with 10 and 2,245 ppm uranium, the adsorption capacity reached 17.02 and 432.41 mg/g adsorbent, respectively. Moreover, the synthesized amidoximated polymer particles could be regenerated for at least 8 cycles while retaining about 50% of the uranium uptake capacity. Thus, the present amidoxime polymer adsorbent is an excellent seawater uranium recovery agent for both high and low uranium concentrations.
Acknowledgments
The authors would like to express sincere gratitude to The Royal Golden Jubilee (RGJ) Ph.D. programme [funding number PHD/0225/2558]; and Faculty of Engineering, Chulalongkorn University [funding number 006/2560].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Yu HW, Yang SS, Ruan HM, et al. Recovery of uranium ions from simulated seawater with palygorskite/amidoxime polyacrylonitrile composite. Appl Clay Sci . 2015;111:67–75.
- Ratnitsai V, Wongsawaeng D, Chankow N, et al. Enhancement of uranium extraction from seawater using chromic-acid-treated amidoxime adsorbent prepared by simultaneous irradiation grafting technique. J Nucl Sci Technol. 2015;52(9):1151–1161.
- Sodaye HN, Nisan S, Poletikoc C, et al. Extraction of uranium from the concentrated brine rejected by integrated nuclear desalination plants. Desalination. 2009;235(1):9–32.
- Das S, Brown S, Mayesa RT, et al. Novel poly(imide dioxime) sorbents: development and testing for enhanced extraction of uranium from natural seawater. Chem Eng J. 2016;298:125–135.
- Zhang A, Asakura T, Uchiyama G. The adsorption mechanism of uranium(VI) from seawater on a macroporous fibrous polymeric adsorbent containing amidoxime chelating functional group. React Funct Polym. 2003;57(1):67–76.
- Wu J, Tian K, Wang J. Adsorption of uranium (VI) by amidoxime modified multiwalled carbon nanotubes. Prog Nucl Energ. 2018;106:79–86.
- Gamal KM. Radioactive contamination of groundwater, special aspects and advantages of removal by reverse osmosis and nanofiltration. Desalination. 2013;321:47–54.
- Santos EA, Ladeira ACQ. Recovery of uranium from mine waste by leaching with carbonate-based reagents. Environ Sci Technol. 2011;45(8):3591–3597.
- Ismail AF, Yim MS. Investigation of activated carbon adsorbent electrode for electrosorption-based uranium extraction from seawater. Nucl Eng Technol. 2015;47(5):579–587.
- Mellah A, Chegrouche S, Barkat M. The removal of uranium(VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interface Sci. 2006;296(2):434–441.
- Kütahyalı C, Eral M. Selective adsorption of uranium from aqueous solutions using activated carbon prepared from charcoal by chemical activation. Sep Purif Technol. 2004;40(2):109–114.
- Zhang Z, Dong Z, Wang X, et al. Ordered mesoporous polymer–carbon composites containing amidoxime groups for uranium removal from aqueous solutions. Chem Eng J. 2018;341:208–217.
- Jamali MR, Assadi Y, Shemirani F, et al. Synthesis of salicylaldehyde-modified mesoporous silica and its application as a new sorbent for separation, preconcentration and determination of uranium by inductively coupled plasma atomic emission spectrometry. Anal Chim Acta. 2006;579(1):68–73.
- Tian G, Geng J, Jin Y, et al. Sorption of uranium(VI) using oxime-grafted ordered mesoporous carbon CMK-5. J Hazard Mater. 2011;190(1–3):442–450.
- Yuan LY, Liu YL, Shi WQ, et al. A novel mesoporous material for uranium extraction, dihydroimidazole functionalized SBA-15. J Mater Chem. 2012;22(33):17019–17026.
- Vivero-Escoto J, Carboni M, Abney CW, et al. Organo-functionalized mesoporous silicas for efficient uranium extraction. Microporous Mesoporous Mater. 2013;180:22–31.
- Jaffrezic-Renault N, Andrade-Martins H. Study of the retention mechanism of uranium on titanium oxide. J Radioanal Nucl Chem. 1980;55(2):307–316.
- Badawy SM, Sokker HHH, Othman S, et al. Cloth filter for recovery of uranium from radioactive waste. Radiat Phys Chem. 2005;73(2):125–130.
- Kabay N, Katakai A, Sugo T. Preparation of amidoxime-fiber adsorbents by radiation-induced grafting. Radiat Phys Chem. 1995;46(4 Part 1):833–836.
- Saito K, Uezu K, Hori T, et al. Recovery of uranium from seawater using amidoxime hollow fibers. AIChE J. 1988;34(3):411–416.
- Seko N, Katakai A, Tamada M, et al. Fine fibrous amidoxime adsorbent synthesized by grafting and uranium adsorption–elution cyclic test with seawater. Sep Sci Technol. 2004;39(16):3753–3767.
- Hara K, Fujiwara S, Fujii T, et al. Attempts to capturing ppb-level elements from sea water with hydrogels. Prog Nucl Energ. 2016;92:228–233.
- Wongjaikham W, Wongsawaeng D, Hoseman P, et al. Enhancement of uranium recovery from seawater using amidoximated polymer gel synthesized from radiation-polymerization and crosslinking of acrylonitrile and methacrylic acid monomers. J Environ Chem Eng. 2018;6(2):2768–2777.
- Wu F, Pu N, Ye G, et al. Performance and mechanism of uranium adsorption from seawater to poly(dopamine)-inspired sorbents. Environ Sci Technol. 2017;51(8):4606–4614.
- Zeng Z, Wei Y, Shen L, et al. Cationically charged poly(amidoxime)-grafted polypropylene nonwoven fabric for potential uranium extraction from seawater. Ind Eng Chem Res. 2015;54(35):8699–8705.
- Abney CW, Mayes RT, Saito T, et al. Materials for the recovery of uranium from seawater. Chem Rev. 2017;117(23):13935–14013.
- Taimur S, Hassan MI, Yasin T. Removal of copper using novel amidoxime based chelating nanohybrid adsorbent. Eur Polym J. 2017;95:93–104.
- Das S, Oyola Y, Mayes RT. Extracting uranium from seawater: promising AF series adsorbents. Ind Eng Chem Res. 2016;55(15):4110–4117.
- Brown S, Yue Y, Kuo LJ, et al. Uranium adsorbent fibers prepared by atom-transfer radical polymerization (ATRP) from poly(vinyl chloride)-co-chlorinated poly(vinyl chloride) (PVC-co-CPVC) fiber. Ind Eng Chem Res. 2016;55(15):4139–4148.
- Gill G, Kuo L-J, Strivens J, et al. Summary of adsorption capacity and adsorption kinetics of uranium and other elements on amidoxime-based adsorbents from time series marine testing at the Pacific Northwest National Laboratory. Sequim, WA: Pacific Northwest National Laboratory, Marine Sciences Laboratory; 2016. (Report No. PNNL-25899)
- Wan T, Wang X, Yuan Y, et al. Preparation of a kaolinite–poly(acrylic acid acrylamide) water superabsorbent by photopolymerization. J Appl Polym Sci. 2006;102(3):2875–2881.
- Ruan W, Qiao J, Huang Y, et al. Synthesis of superabsorbent resin by ultraviolet photopolymerization. J Appl Polym Sci. 2003;92(3):1618–1624.
- Dolat B, Sawut A, Yimit M, et al. Ultraviolet photopolymerization and performances of fast-water absorbing sodium polyacrylate. J Appl Polym Sci. 2015;132(46):42787.
- Pan HB, Kuo LJ, Wai CM, et al. Elution of uranium and transition metals from amidoxime-based polymer adsorbents for sequestering uranium from seawater. Ind Eng Chem Res. 2016;55(15):4313–4320.
- Liu ZS, Rempel GL. Preparation of superabsorbent polymers by crosslinking acrylic acid and acrylamide copolymers. J Appl Polym Sci. 1998;64(7):1345–1353.
- Seymour RB, Carraher CE. Polymer chemistry: an introduction. Brit Polym J. 1982;14(1):36–37.
- Li A, Wang A, Chen J. Studies on poly(acrylic acid)/attapulgite superabsorbent composite. I. Synthesis and characterization. J Appl Polym Sci. 2004;92(3):1596–1603.
- Wan T, Wang X, Yuan Y, et al. Preparation of bentonite–poly[(acrylic acid)‐acrylamide] water superabsorbent by photopolymerization. Polym Int. 2006;55(12):1413–1419.
- Wan T, Liao L, Huang R, et al. Synthesis and swelling behaviors of microcrystal muscovite composite superabsorbent by photopolymerization. J Wuhan Univ Technol Mat Sci Edit. 2016;31(1):151–156.
- Mohan YM, Sudhakar K, Murthy PSK, et al. Swelling properties of chemically crosslinked poly(acrylamide-co-maleic acid) hydrogels. Int J Polym Mater Po. 2006;55(7):513–536.
- Ruan W, Wang X, Lian Y, et al. Superabsorbent hydrogel of acrylic acid/potassium acrylate copolymers by ultraviolet photopolymerization: synthesis and properties. J Appl Polym Sci. 2006;101(2):1181–1187.
- Flory PJ. Principles of polymer chemistry. Ithaca, United States: Cornell University Press; 1953.
- Yi X, Xu Z, Liu Y, et al. Highly efficient removal of uranium(vi) from wastewater by polyacrylic acid hydrogels. RSC Adv. 2017;7(11):6278–6287.
- Hamza MF, Roux JC, Guibal E. Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem Eng J. 2018;344:124–137.
- Wang X, Zhu G, Guo F. Removal of uranium (VI) ion from aqueous solution by SBA-15. Ann Nucl Energy. 2013;56:151–157.
- Ling C, Liu X, Yang X, et al. Uranium adsorption tests of amidoxime-based ultrahigh molecular weight polyethylene fibers in simulated seawater and natural coastal marine seawater from different locations. Ind Eng Chem Res. 2017;56(4):1103–1111.
- Philips. TUV T8. [cited 2018 Oct 1]. Available from: http://www.assets.lighting.philips.com/is/content/PhilipsLighting/fp 928048604003-pss-global