?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The feasibility of NiO-MoO3 reaction as a complement for the self-healing reaction proposed in our previous study is assessed in high-temperature aqueous environment. NiMoO4·nH2O and crystal α-NiMoO4 are produced in an aqueous environment by Ni(OH)2-MoO3 and NiO-MoO3 reactions, of which the starting temperature is about 100°C. NiMoO4·nH2O is completely dehydrated into β-NiMoO4 when annealed at about 600°C in air. Therefore, NiO-MoO3 reaction is expected to self-heal the cracks as a supplement of Fe2O3-MoO3 reaction in the high-temperature aqueous environment.
1. Introduction
As the inherent defect with the zirconium-based (Zr-based) cladding, the Zr-steam reaction in the lost-of-coolant accident (LOCA) condition is the largest threat to nuclear safety. A coating on the Zr-based cladding can inhibit the high-temperature steam oxidation and preserve cladding mechanical properties. Therefore, to develop a reliable coating technique for the current cladding has been one of the research focuses since the Fukushima-Daiichi accident. As a branch of coating technology, various self-healing coatings have been widely developed for protection against corrosion [Citation1], but there are rarely reports about the self-healing coating on the fuel cladding for LWRs to date. Carr et al. employed aluminum as the surface dopant to increase the oxidation resistance of Zircaloy cladding, and expected that aluminum embedded in the Zircaloy cladding could migrate to the surface and form a renewed alumina layer in case that the outer most layer spall during a high-temperature event [Citation2]. Another self-healing mechanism was proposed by Yeom et al. [Citation3] that the ZrSi2 can naturally evolve into a tenacious oxide coating of ZrO2 and SiO2 sandwiched layers under high-temperature oxidative condition, thus potentially provide the necessary protection.
In our previous study [Citation4], a novel scheme of self-healing coating with the inner layer and outer layer was proposed. The basis of this idea is to create a bilayer coating on the surface of cladding tubes. The outer layer must protect the inner layer from undesirable reactions with substances in the LWR water. Once a crack is introduced, the inner layer is exposed to the water and the self-healing reaction takes place. The self-healing mechanism involves Fe2O3 (a corrosion product in the coolant) diffusion into a crack on the coating and subsequent reaction with MoO3 (inner layer) produces insoluble Fe2(MoO4)3 which deposits and repairs the cracks under normal condition. NiO was found to account for 70% and 25% of the CRUD (Chalk River unidentified deposit: corrosion products of the core structural materials) at the bottom and top of a fuel rod in PWRs (pressurized water reactors), respectively [Citation5], indicating that a certain amount of NiO exists in the coolant. Accordingly, the NiO-MoO3 reaction is hopeful to work as a complementary method for Fe2O3-MoO3 in PWRs and the feasibility was investigated in this study.
2. Experimental
2.1. Specimens
Specimens were noted as the following four forms: (1) As-received powders: NiO (99.8%), Ni(OH)2 (99.9%) and MoO3 (99.9%) powders; (2) As-mixed NiO-MoO3 and Ni(OH)2-MoO3 composites: as-received NiO and Ni(OH)2 powders were mixed with the as-received MoO3 powder at a molar ratio of 2:1, respectively; (3) Immersed powders and composites: after autoclave experiments, the as-received and mixed composites were dried below 50°C in air; (4) Annealed composites: the immersed composites were further annealed in the furnace.
2.2 Experiments
The autoclave experiments were conducted with a batch autoclave. The as-received powders or as-mixed composites were dispersed in pure water to form the suspensions, and then, the immersed powders and composites were obtained by drying the suspensions below 50°C in air after the autoclave experiments. To confirm the stability of NiO and Ni(OH)2 powders in the normal condition, the as-received NiO and Ni(OH)2 powders were immersed under the normal condition (360°C with the saturated pressure of 18.5 MPa) for 28 h. To investigate the effects of the exposure temperature and time on the NiO-MoO3 and Ni(OH)2-MoO3 reactions, the as-mixed NiO-MoO3 and Ni(OH)2-MoO3 composites were immersed at different temperature (under the corresponding saturated pressure) for various time intervals.
Thermogravimetry-differential thermal analysis (TG-DTA) was also conducted in flow of Ar (50 cm3/min) on an instrument (DTG-60H, SHIMADZU). The sample was heated from room temperature to 800°C with a heating rate of 10°C/min.
The amorphous and/or nanocrystalline phase(s) (noted as AMNA hereafter) and/or hydride compounds were detected in the immersed NiO-MoO3 and Ni(OH)3-MoO3 composites, of which some were annealed at 200°C, 450°C, 600°C for 1 h, respectively, and then cooled down to room temperature in air.
The X-ray diffraction (XRD) patterns of the as-received, as-mixed, immersed as well as annealed powders/composites were measured. All XRD measurements were performed using an Ultima IV instrument (Rigaku). A 1°/min scanning speed with Cu Kα radiation and the 2θ angle scan range was set from 10° to 70°.
3. Results
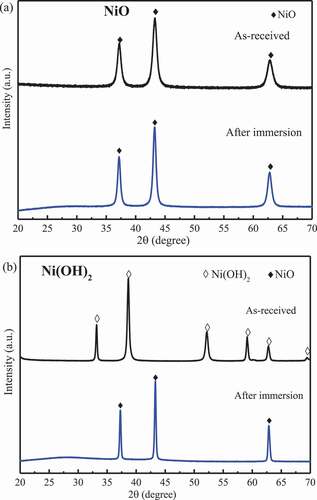
Prior to investigate the as-mixed composites, the stability of the as-received NiO and Ni(OH)2 powders were assessed in the 360°C aqueous environment. As shown in after immersion, no obvious changes occurred on the NiO (International Centre for Diffraction Data (ICDD) #47–1049) while Ni(OH)2 (ICDD #14–0117) was dehydrated into NiO, thus, NiO is the stable form for the Ni(OH)2 under the normal condition.
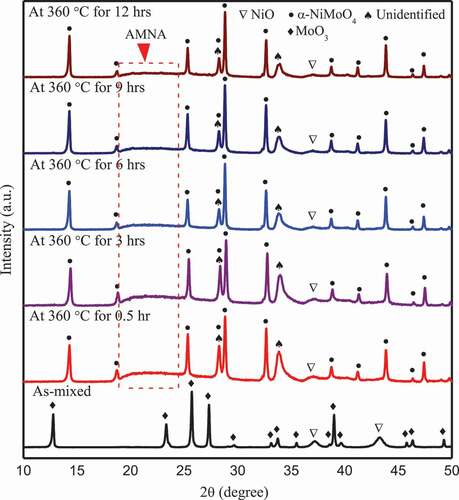
shows the changes in XRD patterns for NiO-MoO3 and Ni(OH)2-MoO3 composites with the increasing immersion temperature. In ), AMNA was detected in the composites immersed above 100°C. At 100°C, a peak around 25°corresponding to the α-NiMoO4 (ICDD #33–0948) can be found, and then became stronger at higher temperature, indicating that the critical starting temperature of the following reaction in aqueous environment is around 100°C, and the reaction rate became faster at higher temperature.
Figure 2. XRD patterns for NiO-MoO3 powders (a) and Ni(OH)2-MoO3 powders before and after immersion at 100, 200, 300, 360 °C for 3 h.
In addition, many peaks (noted as the unidentified compound(s) hereafter) in the spectra of the powders immersed at 200°C could not be matched well with the current database, and there were so much peaks that it was nearly impossible to clarify the new compound(s) formed at 200°C. However, it can be sure that the unidentified compound(s) was/were rich in Mo-Ni-O-H because the weaker spectra of MoO3 and NiO indicated the concentrations of MoO3 and NiO decreased at this temperature. At higher temperature, some peaks of the unidentified compound(s) disappeared but some remained and even became stronger at 300°C, and then became weaker at 360°C. Therefore, it was reasonable to deduce that the unidentified compound(s) was/were favored to form at about 200°C and then decreased with increasing the temperature. As shown in ), the similar spectrum was detected in the Ni(OH)2-MoO3 composite immersed at 200°C, which confirmed that the unidentified compound(s) could form at 200°C. However, above 200°C, the spectrum corresponding to the unidentified compound(s) nearly disappeared, which means that the compound(s) did not form above 300°C in the case of the Ni(OH)2-MoO3 composite. The α-NiMoO4 was detected in the immersed Ni(OH)2-MoO3 composite immersed at 100°C, confirming the Reaction (1) could occur from around 100°C.
shows the effects of exposure time on the NiO-MoO3 reactions. After immersion at 360°C, MoO3 disappeared, thus, MoO3 reacted completely with Ni(OH)2 in 0.5 h. AMNA was observed in all spectra, but its intensity became gradually weaker with the increasing time. Like in , similar unidentified peaks were detected under all conditions, and their intensities became weaker when immersed for a longer time.
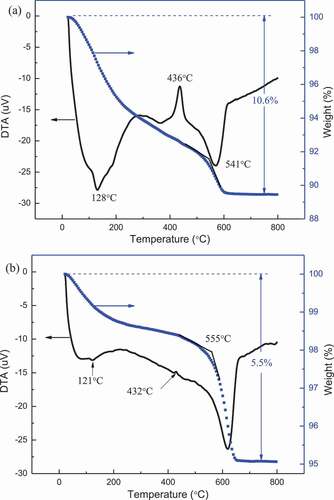
To clarify the unidentified compound(s), the TG-DTA experiments were conducted. The immersed NiO-MoO3 composites at 200 and 360°C for 3 h were chosen as the specimens for the TG-DTA experiments since the relative intensity of the unidentified compound(s) formed at 200°C was the highest while some peaks of the unidentified compound(s) disappeared at 360°C. Rodriguez et al. [Citation6]. and Eda et al. [Citation7] reported that upon heating NiMoO4.nH2O (n = 0.75 or 1) to high temperature in TG-DTA experiments, water desorption can be divided into three steps: The first step appears at 100 − 200°C and is associated with water molecules reversibly bounded to the hydrate; The second one appears from 200°C to 400°C and corresponds to desorption of water molecules that form part of the crystal structure of the hydrate; Third one starts from 490°C to 520°C, which is an additional water desorption. Considering the weight loss rate (TG curve) in current research, weight loss process could be roughly divided into three steps according to as shown in ): Step1, below 128°C; Step 2, 128°C- 541C; Step 3, above 541°C. In Step1, the weight loss was assigned to loosely bound molecular water and adsorbed water. In Step 2, a strongly exothermic loss occurred at around 436°C although the DTA curve shows a downward tendency. Thus, the weight loss in Step 2 was associated with the decomposition of the complex, and crystallization caused the exothermic peak. In Step 3, the curve of weight shows a final loss between 541°C and 600°C, which might be ascribed to a dehydration reaction. There was no significant weight loss above 600°C, indicating the formation of the stable products. The weight loss of the NiO-MoO3 composite immersed at 200°C for 3 h was about 10.6% in total. In ), the temperature turning points for weight loss and crystallization are close to that observed in ), thus, the NiO-MoO3 composite, immersed at different temperature for 3 h, consists of similar chemical components. In addition, compared with the curves in ), weight loss in total, and the exothermic peak at 432°C were smaller in ). Therefore, the concentration of each component of the immersed NiO-MoO3 composite changed with increasing temperature. Consequently, based on the two figures, it could be deduced that the immersed composite powders contained hydrated products, of which the concentration decreased as the temperature increased.
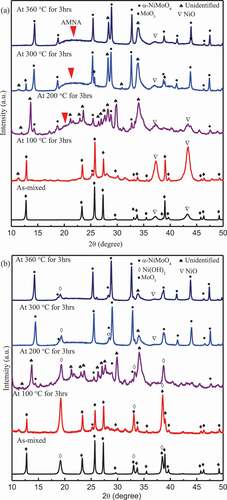
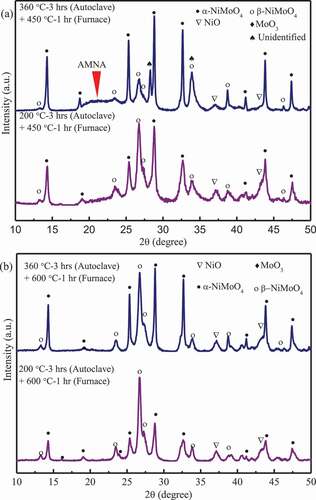
To further identify the chemical components at each step described above, the NiO-MoO3 composites immersed at different temperature were further annealed at 200°C, 450°C and 600°C for 1 h, respectively. No obvious changes in all XRD spectra were observed after annealed at 200°C (not show here for avoiding redundancy), which confirms that only the absorbed water disappeared and no chemical reactions occurred when annealed below 200°C. The XRD spectra of the immersed NiO-MoO3 composites annealed at 450°C for 1 h are shown in ). Compared with the spectra in ), after annealed at 450°C for 1 h the unidentified peaks were replaced by the peaks corresponding to β-NiMoO4 (ICDD #45–0142) in the NiO-MoO3 composite immersed at 200°C and β-NiMoO4 also was detected in the composite immersed at 360°C. However, AMNA was still obvious in the two samples. Thus, results of TG-DTA could be further confirmed that the unidentified peaks were associated with the hydrated compound (NiMoO4·nH2O) which was dehydrated into β-NiMoO4 during 200–450°C.
As shown in ), the unidentified peaks and AMNA in and disappeared completely while β-NiMoO4 was identified in all annealed composites. AMNA could be NiMoO4·nH2O which was dehydrated into α-NiMoO4 and/or β-NiMoO4 from 450°C to 600°C since weight loss still was found above 450°C (as shown in ) and AMNA disappeared during 450–600°C.
4. Discussions
As described in the previous part, AMNA and unidentified peaks appeared in the immersed NiO-MoO3 and Ni(OH)2-MoO3 composites. In addition, it was reported that MoO3 · 2H2O was dehydrated into β-MoO3·H2O at 60–80°C, and further into α-MoO3 at 110–140°C [Citation8]. The XRD patterns for the immersed composites did not change after annealed at 200°C for 1 h. Therefore, AMNA and unidentified compound(s) did not contain MoO3·nH2O. On the other hand, the unidentified peaks were replaced by the spectra for β-NiMoO4 as the immersed composites were further annealed at 450°C for 1 h, and then, the disappearance of AMNA was accompanied by the formation of β-NiMoO4 after annealed at 600°C for 1 h. In addition, TG curves in show that it is a weight loss process from room temperature to 600°C. Accordingly, AMNA and unidentified compound(s) should be composed of amorphous and/or crystal NiMoO4·nH2O, which started to transform into β-NiMoO4 at about 450°C and the dehydration finished at about 600°C. The similar results [Citation6] were also reported that NiMoO4·nH2O started to transfer into β-NiMoO4 from 250°C and finally only β-NiMoO4 was detected when heated up to 600°C. Consequently, the following reaction occurred at 600°C in air.
α-NiMoO4 and NiMoO4·nH2O were formed in the aqueous environment while at 600°C in air, the as-mixed composite produced α-NiMoO4 and β-NiMoO4, suggesting phase transformation may occur during heating and cooling process.
Three compounds of NiMoO4 are known under atmospheric pressure: α-NiMoO4 at low temperature, β-NiMoO4 at high temperature, and NiMoO4·nH2O hydrate at high temperature [Citation9]. α-NiMoO4 and β-NiMoO4 have a monoclinic crystal structure, and the main difference between them is in the coordination of the Mo6+ ions, octahedral in the α-NiMoO4, and tetrahedral in the β-NiMoO4 [Citation10]. α-NiMoO4 → β-NiMoO4 transformation during heating process occurs at approximately 700°C [Citation11]. It also was reported that pure β-NiMoO4 could be derived by heating the α-NiMoO4 at temperature above 760°C [Citation10]. Thus, in this study, minor α-NiMoO4 may transform into β-NiMoO4 at 600°C for 1 h. β-NiMoO4 transforms into α-NiMoO4 between 140°C and 180°C depending on the cooling rate [Citation6]. However, β-NiMoO4 could be stabilized at lower temperature either by an excess of NiO or by supporting the active phase on Al2O3 [Citation12]. Extra NiO powder was observed in all resulting powders, which should be responsible for the existence of β-NiMoO4 at low temperature. In addition, the annealed powders at 600°C were cooled to room temperature in 10 min, hence, the fast cooling rate may also contribute to the existence of β-NiMoO4 at low temperature. Accordingly, β-NiMoO4 shown in mainly came from the decomposition of NiMoO4·nH2O via Reaction (2). Further, the concentration of β-NiMoO4 can reflect that of NiMoO4·nH2O to a certain extent. As shown in ), the relative intensity of the strongest peak (2θ = 26.6°) for β-NiMoO4 is strongest at the immersion temperature of 200°C and decreased with increase in the immersion temperature and time. Therefore, NiMoO4·nH2O was favored to form at 200°C and slightly dehydrated as the immersion temperature increased or the immersion time was extended at the temperature of 360°C.
Figure 5. XRD patterns for NiO-MoO3 powders immersed at 200 °C and 360 °C for 3 h (in autoclave), and then annealed at 450 °C and 600 °C for 1 h (in furnace).
In light of the chemical stability, NiMoO4 is readily formed by heating a mixture of NiO and MoO3 above 500°C [Citation13], and starts decomposing at 1100°C (phase transformation at about 700°C), while the decomposition rate is quite sluggish at 1100°C [Citation11]. Therefore, NiMoO4·nH2O and α-NiMoO4 formed in the aqueous environment will experience dehydration and/or phase transformation, and finally decompose above 1100°C under accident conditions, releasing MoO3. Thus, the crack, which has been healed by NiMoO4·nH2O and α-NiMoO4 under normal condition, almost maintain healed state until about 1100°C under accident conditions. In the reactor, the stable nuclei Ni58 could be activated into unstable nuclei Co58 of which half-life is 70.86d [Citation14], and decays into stable Co59 [Citation15]. Hence, the unstable nuclei Co58 would decay completely in the spent fuel pool. The Ni58→Co58 activation reactions are accompanied by the NiMoO4→CoMoO4 transformation. Similar with NiMoO4, CoMoO4 has two phase forms: α phase at low temperature and β phase at high temperature; α→β transition occurs at 330–400°C while β phase would not transfer to α phase when cooled down from high temperature to room temperature [Citation6]. Accordingly, NiMoO4→CoMoO4 transformation make nearly no effects on the self-healing process. The NiO-MoO3 reaction can work as a complementary method for Fe2O3-MoO3reaction in PWR.
5. Summary
In this study, the feasibility of NiO-MoO3 reaction as a complement for the self-healing reaction (Fe2O3-MoO3 reaction) proposed in our previous study was investigated by means of autoclave experiments. Ni(OH)2 was dehydrated into NiO under normal condition. NiMoO4·nH2O and crystal α-NiMoO4 were produced in an aqueous environment by Ni(OH)2-MoO3 and NiO-MoO3 reactions, of which the starting temperature was around 100°C. NiMoO4·nH2O was completely dehydrated into β-NiMoO4 when annealed at about 600°C. Therefore, NiO-MoO3 reaction exhibited potential ability to self-heal the cracks as a supplement of Fe2O3-MoO3 reaction.
Acknowledgments
This study is supported by the project of “The development of self-healing intelligence on nuclear fuel cladding” carried out under the Center of World Intelligence Project for Nuclear S&T and Human Resource Development by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Stankiewicz A, Barker MB. Development of self-healing coatings for corrosion protection on metallic structures. Smart Mater Struct. 2016;25(8):084013.
- Carr J, Vasudevamurthy G, Snead L, et al. Investigations of aluminum-doped self-healing Zircaloy surfaces in context of accident-tolerant fuel cladding research. J Mater Eng Perform. 2016;25(6):2347–2355.
- Yeom H, Lockhart C, Mariani R, et al. Evaluation of steam corrosion and water quenching behavior of zirconium-silicide coated LWR Fuel claddings. J Nucl Mater. 2018;499:256–267.
- Duan Z, Yang H, Kano S, et al. Application of chemical interaction between (Fe, Cr) oxides and Mo oxide at high temperature for self-healing intelligence on nuclear fuel cladding in LWRs. J Nucl Sci Technol. 2018;55(12):1402–1411.
- Riess R. Chemistry experience in the primary heat transport circuits of Kraftwerk Union pressurized water reactors. Nucl Technol. 1976;29(2):153–159.
- Rodriguez JA, Chaturvedi S, Hanson JC, et al. Electronic properties and phase transformations in CoMoO4 and NiMoO4: XANES and time-resolved synchrotron XRD studies. J Phys Chem B. 1998;102(8):1347–1355.
- Eda K, Kato Y, Ohshiro Y, et al. Synthesis, crystal structure, and structural conversion of Ni molybdate hydrate NiMoO4·nH2O. J Solid State Chem. 2010;183(6):1334–1339.
- Alexei K, Juris P. Dehydration of the molybdenum trioxide hydrates MoO3·nH2O: in situ x-ray absorption spectroscopy study at the Mo K edge. J Phys: Condens Matter. 2000;12(9):1959–1970.
- Hassani HO, Al Wadaani FT. Preparation, characterization and catalytic activity of nickel molybdate NiMoO4 nanoparticles. Molecules. 2018;23(273):1–12.
- De Moura AP, De Oliveira LH, Rosa IL, et al. Structural, optical, and magnetic properties of NiMoO4 nanorods prepared by microwave sintering. Sci World J. 2015;2015:1–8.
- PK R, Akinc M, Kramer MJ. Formation of multilayered scale during the oxidation of NiAl–mo alloy. Appl Surf Sci. 2014;301:107–111.
- Zăvoianu R, Pavel OD, Cruceanu A, et al. Characterization of silica supported NiMoO4 doped with Ce, Cr and Zr using thermodesorption techniques. Prog Catal. 2003;12:83–92.
- Brenner SS. Oxidation of iron-molybdenum and nickel-molybdenum alloys. J Electrochem Soc. 1955;102(1):7–15.
- Bouhaddane A, Farkas G. Calculation of induced activity in the V-230 reactor. Proceedings of the 19th International Conference on Applied Physics of Condensed Matter Physics; 2013 Jun 19-21; Spisska Nova Ves, Slovakia; 2013.
- Morgan. WC. Long-term neutron activation products of nickel-58. Washington: Hanford Atomic Products Operation (US); 1963. p. HW–79734.