?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Concentrations of humic substance (HS) in deep groundwater from sedimentary formations were determined by the carbon concentration-based DAX-8 resin isolation technique. The groundwater samples were collected from test galleries at depths of 140, 250 and 350 m at the Horonobe Underground Research Laboratory (URL) in Hokkaido, and two observation wells with different depths of 560 and 1050 m in Niigata, Japan. The analytical condition was optimized for the groundwater samples with a high salinity and a high concentration of dissolved organic matters (DOMs). The analytical results showed that the HS concentrations varied from 7.8 to 23.5 mg-C dm−3, depending on the depth and the area. The HS proportions to DOM (%HS) were evaluated to be 58 – 67% and slightly decreased in the deeper groundwater. The regression analysis showed that the HS concentrations are positively correlated with the DOM concentrations. In addition, the low deviation of the %HS values from the slope in the regression equation indicated that the slight variation of %HS can be trivial in the prediction of the concentration of HS. In previous studies, HS concentrations in the groundwater had been determined with a large uncertainty and potential usage of the regression equation for the prediction had not been elucidated. Therefore, the results in this study can provide useful information on the HS concentration and its prediction from the DOM concentration in deep groundwater from sedimentary formations.
1. Introduction
In geological disposal system of high-level radioactive waste, effects of dissolved organic matters (DOMs) in groundwater on facilitated migration of radionuclides (RNs) are of concern, because DOMs have an ability to form a stable complex with RNs to suppress their sorption and diffusion to host rocks [Citation1–Citation4]. Among such DOMs, humic substance (HS) is a major fraction, and can be a factor determining the effect of DOMs [Citation5,Citation6]. Then, concentration of HS in groundwater as well as its metal-binding constant are essential. In previous studies, the concentration of HS in groundwater has been determined by a gravimetry after the conventional isolation using the hydrophobic resin such as XAD-8 [Citation7–Citation10] and DAX-8 [Citation11–Citation14], or in conjunction with preconcentration techniques such as reverse osmosis (RO) [Citation15] and diethylaminoethyl (DEAE)-cellulose [Citation16–Citation18]. Because, however, these methods had not proved to be quantitative, the concentrations determined could include an uncertainty.
Recently, the determination method for aquatic HS, i.e. the carbon concentration-based DAX-8 resin isolation technique, has been developed by optimizing the extraction conditions for HS in terms of ratio of resin to water, salinity and structural feature [Citation19,Citation20]. In this method, the HS concentration is determined as the difference between carbon concentrations of DOM and non-HS, which are measured before and after the DAX-8 treatment, respectively. This method without an elution procedure has an advantage for the determination of HS at low concentration compared to that with an elution procedure [Citation21], because the carbon contamination from the resin can be kept low. Furthermore, the analysis can be easy and simple, and then have an advantage in work in a grove-box of low-oxygen atmospheric condition. Based on such advantages, the method has widely applied into a variety of natural waters such as lake [Citation22,Citation23], bog [Citation23] and river [Citation24–Citation26].
On the other hand, the analysis using the method may lead to the development of model to predict concentration of HS from those of DOM, because the HS concentrations obtained by the method can be linearly correlated with the DOM concentration [Citation23,Citation25–Citation27]. Since there is a case that a volume of groundwater is insufficient to analyse both concentrations of HS and DOM and a lot of data on the DOM effect without an information on HS concentration has been presented, the development of the model can be useful. Because, however, the carbon concentration-based DAX-8 resin isolation technique has not been applied for the determination of HS in the groundwater, the linearity and predictability of the relationship between HS and DOM concentrations is still unclear.
In this study, the carbon concentration-based DAX-8 resin isolation technique is firstly applied to determine the concentration of HS in groundwater from sedimentary formations. The groundwater has relatively high concentration of DOM, and then has a larger impact for the effect of HS. Analytical condition is optimized for the dilution, which can be a factor changing the degree of the HS extraction. The HS concentrations or HS proportions to DOM in the groundwater were compared in terms of the geological conditions. The relationship between the concentrations of DOM and HS and its application into the prediction of HS concentration from DOM concentration are presented.
2. Materials and methods
2.1. Sampling sites
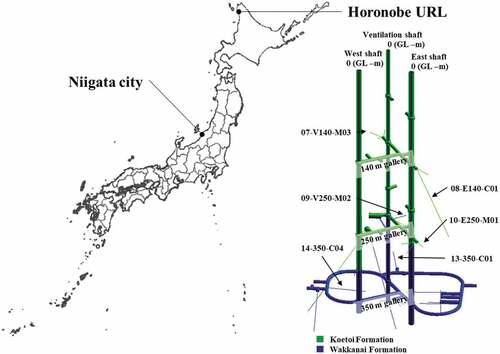
Groundwater samples were taken from boreholes at galleries in the Horonobe Underground Research Laboratory (URL), Hokkaido, Japan, in September 2016. The depths of the galleries are −140, −250 and −350 m, which are corresponding to the Koetoi Formation, the transition zone between the Koetoi and Wakkanai formations, and the Wakkanai Formation, respectively. The Koetoi and Wakkanai formations are composed of diatomaceous and siliceous mudstones, respectively. The detailed geology and geochemistry of the URL are described elsewhere [Citation28]. Other groundwater samples in sedimentary formations were collected from observation wells for subsidence in Niigata city, Japan, on February and August 2017. The observation wells are located in Nuttari and Nishikambara areas, which are in the Niigata gas field [Citation29]. The depths of the Nuttari and Nishikambara observation wells are −560 and −1010 m, which are at the Kanbara and Uonuma formations, respectively. These formations are found in alternate layer composed of sand gravel layer being natural gas bed and mud layer mainly including sand and silt. The details of the geology are described elsewhere [Citation29–Citation33]. The locations of the sampling sites are shown in . The geological conditions of the sampling sites are summarized in .
Table 1. Geological conditions at the Horonobe boreholes and the Niigata observation wells.
2.2. Groundwater sampling
A packer type sampling device was used for the collections of the groundwater from the boreholes in the Horonobe URL. The groundwater sample was taken into a brown glass bottle and then that was immersed into a larger bottle filled with same groundwater to prevent the oxidation during the transportation. The groundwater samples in the observation wells in the Niigata city were taken by means of water pumping after the water discharge followed by the stabilization of the water quality. The sample bottles were immediately brought into a grove box (<1 ppm O2) and stored for the analysis. The pH and electronic conductivity (EC) in the groundwater samples were measured by a pH/EC meter (DKK-TOA, WM-32EP) after the calibration with the pH standard and EC cell check solutions (DKK-TOA). The groundwater qualities measured are summarized in . The details on the groundwater qualities are found elsewhere [Citation34–Citation36].
Table 2. Water qualities and concentrations of DOM and HS in the groundwater at the Horonobe boreholes and Niigata observation wells.
2.3. Quantitative analysis of HS
Concentration of HS in the groundwater was determined by the carbon concentration-based DAX-8 isolation technique [Citation19]. The groundwater samples were passed through a glass fiber filter with pore size of 0.3 μm (ADVANTEC, GF-75). A portion of the filtrate was transferred into a glass vial for a measurement of total organic carbon (TOC), and the rest was acidified to below pH 2 with 2.5 mol dm−3 H2SO4 aqueous solution for the DAX-8 treatment. The acidified samples were mixed with a purified DAX-8 resin in the water-resin volume ratio of 50:1. The mixtures were shaken below 4°C for 24 h in the dark. After the equilibration, the mixture was passed through the glass fiber filter to separate the sample solution from the DAX-8 resin. The carbon concentration in the filtrate (CnonHS) was measured by a TOC analyser (Shimazu, TOC-Lcph). The procedure described here was conducted under a low-oxygen atmospheric condition (<1 ppm O2), except for TOC measurement. The concentration of HS (CHS) was calculated by the subtraction of CnonHS from the total carbon concentration of DOM (CDOM). The detection limit (3 μ) and the minimum limit of determination (10 μ) in this analysis were 0.7 and 2.4 mg-C dm−3 (n = 40), respectively. The analysis was conducted in duplicate or triplicate.
3. Results and discussion
3.1. Validations of the analysis
In the carbon concentration-based DAX-8 isolation technique, the sample with the CDOM over 30 mg-C dm−3 needs to be diluted within the range 10–20 mg-C dm−3 [Citation19]. Groundwater in sedimentary formations sometimes had a high CDOM over the upper limit, then needed to dilute. However, such groundwater also have a high salinity (). The dilution may lead to a suppressed sorption of HS to DAX-8 resin due to the reduced salting-out effect [Citation37,Citation38]. On the other hand, Kida et al. show that the variation of salinity is negligible on the analysis, because no sorption of aromatic and aliphatic HS to DAX-8 resin were affected by the salinity [Citation20]. In this study, however, only HSs isolated from the lake and river waters were used for the validation. It is not true that the finding is also available for the more aliphatic HS in groundwater from sedimentary formation [Citation13].
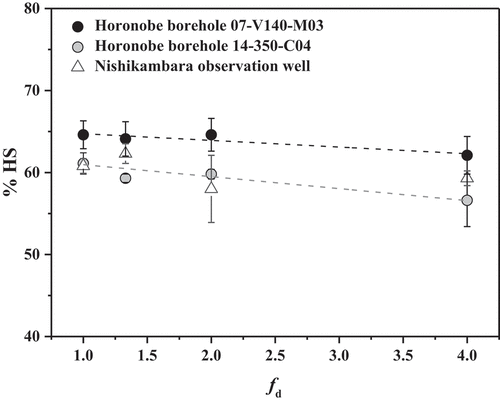
To verify this, we investigated the HS proportion (%HS = 100 × CHS/CDOM) as a function of dilution factor (fd = Cinitial/Cdiluted) in the groundwater. The relationships between %HS and fd in the Horonobe groundwater at the different depths and the Nishikambara groundwater are shown in . In the Horonobe groundwater, the %HS values seemed to decrease with increasing the fd value but were within the error (e.g. %HS for the borehole 07-V140-M03 = 64.6 ± 1.7 at fd = 1, 62.1 ± 2.3 at fd = 4; %HS for the borehole 14-350-C04 = 61.1 ± 1.3 at fd = 1, 56.6 ± 3.2 at fd = 4). The Nishikambara groundwater showed the similar result (e.g. %HS = 60.8 ± 0.9 at fd = 1, 59.3 ± 0.9 at fd = 4). These results indicate that the dilution effect in the fd range from 1 to 4 is negligible. Thus, this shows that the proposed method is also available for the HS in groundwater from sedimentary formations. Since the Nishikambara groundwater has a high concentration over 30 mg-C dm−3, this sample was diluted by a factor of 2 for the analysis.
Figure 2. Relationships between the HS proportion (%HS) and the dilution factor (fd) in the different groundwater samples.
In the Horonobe groundwater at the borehole 09-V250-M02, the CHS value was determined to be 7.8 mg-C dm−3 by the carbon concertation-based DAX-8 resin isolation technique (), while the value obtained by the gravimetry was 1.0 mg-C dm−3 [Citation13]. This shows that the DAX-8 isolation technique provides higher CHS. In the gravimetry, the groundwater sample was treated in the water-resin ratio of 1500 to 1, excessing the optimized ratio of 125 to 1 [Citation7]. Because the excessed volume of water leads to the overcapacity of the DAX-8 resin, the gravimetry in the previous study could have resulted in the lower CHS. Thus, the higher CHS determined by the carbon concentration-based DAX-8 isolation technique can be reasonable.
3.2. Concentrations of DOM and HS
The CDOM and CHS values in the groundwater samples are summarized in . The CDOM values varied within a range from 12.5 to 40.5 mg-C dm−3, depending on the depth and area. The CDOM values in the Horonobe groundwater were comparable with that in the Nuttari groundwater, while the value in the Nishikambara groundwater is remarkably high. The observation wells in the Nuttari and Nishikambara are located in the Niigata gas field [Citation29], where sedimentary organic carbon (SOC) can be widely distributed. Such SOC can have been subjected to the microbiological-mediated mineralization to enhance the CDOM in groundwater [Citation39–Citation41]. Moreover, the Nishikambara groundwater can be less influenced by the dilution with the recharge of meteoric water, because it has the highest EC value (). Thus, the high CDOM in the Nishikambara groundwater can be due to the high degree of the mineralization of SOM and/or the less influence of recharge water. On the other hand, the CDOM values in the Horonobe groundwater varied with the depth: the CDOM values are relatively high at the depth of 140 and 350 m, while low at the depth of 250 m. It is known that the transition zone at the depth of 250 m has a relatively high degree of permeability [Citation42]. Thus, the relatively low CDOM at the depth can be due to the dilution with the recharge of meteoric water.
The CHS also fluctuated with changes in the CDOM, depending on the depth and area (). The CHS values in the groundwater of both areas ranged from 7.8 to 23.5 mg-C dm−3, and were accounted for 58–67% of CDOM. This clearly shows that HS is a dominant fraction of DOM in the deep groundwater from sedimentary formations. When the %HS values were compared between different areas, it was found that the %HS values in the Niigata groundwater fall within the range of those in the Horonobe groundwater. On the other hand, the %HS values in both areas slightly decreased in the deeper groundwater. For example, the %HS values in the Horonobe groundwater ranged from 62.8% to 67.0% over the depths of 140 and 250 m, but from 60.9% to 61.1% at the depth of 350 m. The similar trend was also observed in the Niigata groundwater at the depth of 560 and 1050 m. Because it is suggested that the release of HS from SOC can be a dominant factor determining the CHS in the groundwater [Citation15], the variations of %HS among the different depths might be brought by the differences in characteristics of SOC and/or its mineralization process by a microbial activity at each depth.
3.3. Prediction of CHS from CDOM
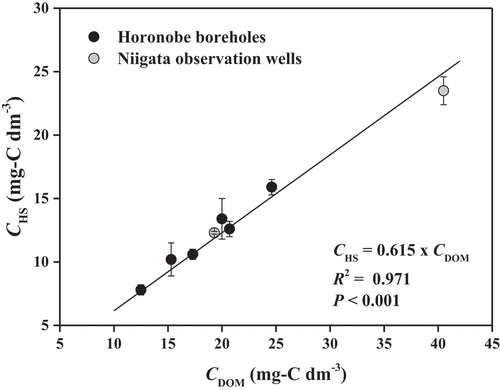
shows the relationship between the CHS and CDOM in the groundwater together with the results of regression analysis. The CHS increased with increasing the CDOM, and the regression analysis for the relationship showed a good positive correlation (CHS = 0.615 × CDOM, r2 = 0.971, P < 0.001). This suggests a possibility that the linear regression can be used to predict CHS from CDOM. On the other hand, it has been mentioned that the CHS in surface waters cannot necessarily be predicted from the CDOM by using a fixed regression equation [Citation23], because the %HS may be variable even in a given aquatic environment. Because, however, the %HS could be altered by photodegradation and microbial processes highly activated in the surface of the water [Citation22,Citation23], the variations of %HS may occur only in the surface water such as lake and bog.
Figure 3. Relationships between CDOM and CHS in the groundwater at the Horonobe boreholes and Niigata observation wells and the result of the linear regression analysis.
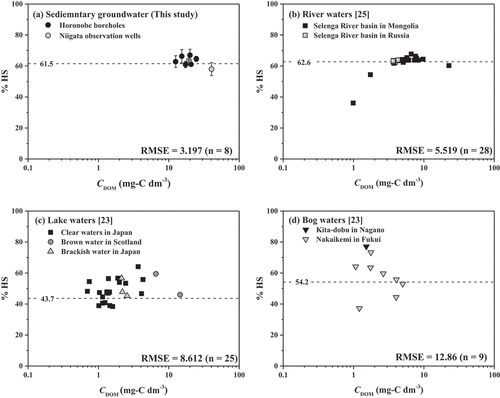
To clarify this, the measured %HS were compared with the constant %HS defined as the slope of the linear regression equation. The degree of the deviation from the constant %HS was also compared with those in the surface waters found in the literatures, in which the carbon concertation-based DAX-8 resin isolation technique was used for the analysis. The results of the comparisons are shown in . The measurements of the groundwater in this study agreed well with the constant value ()), while those in the lakes and bogs were widely distributed (). The measurements in the river waters well agreed with the constant value at higher CDOM, but were largely deviated at lower CDOM ()). The root mean square error (RMSE) for %HS measurements of the groundwater in this study was calculated to be 3.197, and was obviously small compared to those for the rivers (5.519), the lakes (8.612) and the bogs (12.86). This shows that, in the range of CDOM from 12.5 to 40.5 mg-C dm−3, the %HS in the groundwater from sedimentary formations can be almost constant, while those in the surface waters are variable. The groundwater used in this study are a fossil seawater whose compositions are altered by diagenetic processes in marine sediments. In the fossil seawater, DOM may have similar characteristics in the composition and structure due to the similarities of origins, e.g. marine planktons and humification process [Citation43]. In addition, such similar characteristics are likely to be maintained by the negligible percolation of organic carbon from surface to deep underground [Citation44,Citation45] and the low microbial activity induced by the consumption of available nutrients and energy sources [Citation46]. Thus, the narrower deviation of the %HS supports the suggestion that the prediction of CHS from CDOM by the linear regression equation can be possible in the deep groundwater with higher CDOM from 10 to 40 mg-C dm−3 from sedimentary formations. However, it should be noted that no the equation can be applied into other groundwater in different geological conditions, because %HS in the other groundwater may be variable due to different characteristics and fate of DOM. Therefore, for the prediction of CHS from CDOM in other groundwater, the equation needs to be verified by the determination of HS in a target groundwater and be modified if necessary.
Figure 4. Comparisons between the %HS measured by the carbon concentration-based DAX-8 resin isolation technique and the constant %HS defined as the slope of the regression analysis. The constant %HS from the literatures was calculated by the linear regression analysis as an intercept of zero. , where n is a number of sample. yobs,i and ypred,i arethe experimentally determined and predicted %HS (i.e. the slope of the linear regression), respectively.
4. Conclusion
In previous studies, the concentration of HS in groundwater from sedimentary formations had been estimated with a large uncertainty, because the gravimetry unestablished for a quantitative analysis was applied for the determination. In this study, the carbon concentration-based DAX-8 resin isolation technique, which has been well established for the analysis, was applied for the determination of HS in the groundwater. As a result, the concentrations of HS in the groundwater from sedimentary formations in Japan were determined to be 7.8–23.5 mg-C dm−3 and the HS proportions were then calculated to be 58 – 67%. Because the HS proportions in our previous studies were evaluated to be 1.0–9.4% [Citation12–Citation14], our result improves the knowledge on the proportion. Moreover, the linear relationship between the concentrations of HS and DOM and the low variability in %HS suggest that the linear regression equation can be useful for the prediction of CHS from CDOM in the groundwater in sedimentary formations. Although the application of the equation can be limited to the groundwater in the similar geological condition, it is expected that the applicability can be improved by the modification of the equation via the analysis of HS in groundwater in different geological conditions. Therefore, the findings obtained in this study can provide useful information on the HS concentration and its prediction from the DOM concentration in deep groundwater from sedimentary formations.
Acknowledgments
We thank Dr. Isao Machida at the Advanced Industrial Science and Technology (AIST) for groundwater sampling from the two observation wells in Niigata city. We also thank Prof. Nobuhide Fujitake at the Kobe University for the advice on the carbon concentration-based DAX-8 isolation technique and useful discussion on the analytical values. This study has been conducted as a part of the public offered project, a commission investigation of geological disposal technology (Development of enhancing the disposal system in the coastal region) under the contract with Agency for Natural Resources and Energy (ANRE), Ministry of Economy, Trade and Industry, Japan.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Choppin GR. The role of natural organics in radionuclide migration in natural aquifer systems. Radiochim Acta. 1992;58–59(1):113–120.
- McCarthy JF, Czerwinski KR, Sanford WE, et al. Mobilization of transuranic radionuclides from disposal trenches by natural organic matter. J Contam Hydorol. 1998;30(1–2):49–77.
- McCarthy JF, Sanford WE, Stanfford PL. Lanthanide field tracers demonstrate enhanced transport of transuranic radionuclides by natural organic matter. Environ Sci Technol. 1998;32(24):3901–3906.
- Reiller PE, Buckau G. Impacts of humic substances on the geochemical behaviour of radionuclides. In: Poinssot C, Geckeis H, editors. Radionuclide behavior in the natural environment. Oxford: Woodhead Publishing Limited; 2012. p. 103–160.
- Choppin GR. Humics and radionuclide migration. Radiochim Acta. 1988;44/45(1):23–28.
- Moulin V, Moulin C. Fate of actinides in the presence of humic substances under conditions relevant to nuclear waste disposal. Appl Geochem. 1995;10(5):573–580.
- Thurman EM, Malcolm RL. Preparative isolation of aquatic humic substances. Environ Sci Technol. 1981;15(4):463–466.
- Wassenaar L, Aravena R, Fritz P, et al. Isotopic composition (13C, 14C, 2H) and geochemistry of aquatic humic substances from groundwater. Org Geochem. 1990;15(4):383–396.
- Grøn C, Wassenaar L, Krog M. Origin and structures of groundwater humic substances from three Danish aquifers. Environ Int. 1996;22(5):519–534.
- Kovács K, Gáspár A, Sajgó C, et al. Comparative study on humic substances isolated in thermal groundwaters from deep aquifers below 700 m. Geochem J. 2012;46(3):211–224.
- Mäkelä J, Manninen P. Humic and fulvic acids in groundwater. Olkiluoto: POSIVA; 2007. (Posiva working report 2007–23).
- Terashima M, Nagao S, Iwatsuki T, et al. Europium-binding abilities of dissolved humic substances isolated from deep groundwater in Horonobe area, Hokkaido, Japan. J Nucl Sci Technol. 2012;49(8):804–815.
- Saito T, Terashima M, Aoyagi N, et al. Physicochemical and ion-binding properties of highly aliphatic humic substances extracted from deep sedimentary groundwater. Environ Sci. 2015;17(8):1386–1395.
- Kimuro S, Kirishima A, Nagao S, et al. Characterization and thermodynamic study of humic acid in deep groundwater at Horonobe, Hokkaido, Japan. J Nucl Sci Technol. 2018;55(5):503–515.
- Artinger R, Buckau G, Geyer S, et al. Characterization of groundwater humic substances: influence of sedimentary organic carbon. Appl Geochem. 2000;15(1):97–116.
- Pettersson C, Arsenie I, Ephraim J, et al. Properties of fulvic acids from deep groundwaters. Sci Total Environ. 1989;81/82:287–296.
- Pettersson C, Ephraim J, Allard B. On the composition and properties of humic substances isolated from deep groundwater and surface waters. Org Geochem. 1994;21(5):443–451.
- Nagao S, Iwatsuki T, Hama K. Characteristics of dissolved humic substances isolated from groundwater in Tono area, Gifu Prefecture, Japan. J Nucl Fuel Cycle Environ. 2009;15(2):77–86. Japanese.
- Tsuda K, Takata A, Shirai H, et al. A method for quantitative analysis of aquatic humic substances in clear water based on carbon concentration. Anal Sci. 2012;28(10):1017–1020.
- Kida M, Ohtsuka T, Kato T, et al. Evaluation of salinity effect on quantitative analysis of aquatic humic substances using nonionic DAX-8 resin. Chemosphere. 2016;146:129–132.
- van Zomeren A, Comans RNJ. Measurement of humic and fulvic acid concentrations and dissolution properties by a rapid batch procedure. Environ Sci Technol. 2007;41(19):6755–6761.
- Kida M, Maki K, Tanaka A, et al. Quantitative monitoring of aquatic humic substances in Lake Biwa, Japan, using the DAX-8 batch method based on carbon concentration. Org Geochem. 2015;83–84:153–157.
- Tsuda K, Kida M, Aso S, et al. Determination of aquatic humic substances in Japanese lakes and wetlands by the carbon concentration-based resin isolation technique. Limnology. 2016;17(1):1–6.
- Kida M, Kinjo K, Ohtsuka T, et al. Dynamics of dissolved organic matter in the mangrove area of the Fukido River, Ishigaki Island. Jap J Ecol. 2017;67(2):85–93.
- Kida M, Myangan O, Oyuntsetseg B, et al. Dissolved organic matter distribution and its association with colloidal aluminum and iron in the Selenga River Basin from Ulaanbaatar to Lake Baikal. Environ Sci Poll Res. 2018;25(1):11948–11957.
- Sato H, Kida M, Yamano S, et al. Variable relationships between the hydrophobic fraction of dissolved organic matter and metals in Scottish freshwater before the estuarine mixing zone. Limnology. 2019;20(2):215–224.
- Watanabe A, Moroi K, Sato H, et al. Contributions of humic substances to the dissolved organic carbon pool in wetlands from different climates. Chemosphere. 2012;88(10):1265–1268.
- Hama K, Kunimaru T, Metcalfe R, et al. The hydrogeochemistry of argillaceous rock formations at the Horonobe URL site, Japan. Phys Chem Earth. 2007;32(1–7):170–180.
- Makiyama T. On the stratigraphy and geological structure of the Niigata gas field. J Jpn Pet Inst. 1963;6(9):684–687. Japanese.
- Kazaoka O, Tateishi M, Kobayashi I. Stratigraphy and facies of the Uonuma group in the Uonuma district, Niigata prefecture, central Japan. J Geol Soc Japan. 1986;92(12):829–853. Japanese.
- Kobayashi I. Quaternary geology of the Echigo Plain, Niigata, Japan. Quat Res (Daiyonki-Kenkyu). 1996;35(3):191–205. Japanese.
- Kobayashi I, Takano O. Records of major and minor transgression and regression events in the Paleo-Sea of Japan during late Cenozoic. Revista Mexicana De Ciencias Geológicas. 2002;19(3):226–234.
- Sekiguchi H, Yoshimi M, Horikawa H, et al. 3D subsurface structure model of the Niigata sedimentary basin. Tsukuba: AIST; 2009. (Annual Report on Active Fault and Paleoearthquake Researches No.9).
- Miyakawa K, Mezawa T, Mochizuki A, et al. Data of groundwater chemistry obtained in the Horonobe underground research laboratory project (FY2014–2016). Horonobe: JAEA; 2017. (JAEA-Data/Code 2017–012).
- Advanced Industrial Science and Technology (AIST), Japan Atomic Energy Agency (JAEA), Radioactive Waste Management Funding and Research Center (RWMC), et al. A commission investigation of geological disposal technology: development of enhancing the disposal system in the coastal region. Tokyo: METI; 2017. Japanese.
- Advanced Industrial Science and Technology (AIST), Japan Atomic Energy Agency (JAEA), Radioactive Waste Management Funding and Research Center (RWMC), et al. A commission investigation of geological disposal technology: development of enhancing the disposal system in the coastal region. Tokyo: METI; 2018. Japanese.
- Thurman EM, Malcolm RL, Aiken GR. Prediction of capacity factors for aqueous organic solutes adsorbed on a porous acrylic resin. Anal Chem. 1978;50(6):775–779.
- Aiken GR, McKnight DM, Thorn KA, et al. Isolation of hydrophilic organic acids from water using nonionic macroporous resins. Org Geochem. 1992;18(4):567–573.
- Murphy EM, Davis SN, Long A, et al. Characterization and isotopic composition of organic and inorganic carbon in the Milk River aquifer. Water Resour Res. 1989;25(8):1893–1905.
- Aravena R, Wassenaar LI. Dissolved organic carbon and methane in a regional confined aquifer, southern Ontario, Canada: carbon isotope evidence for associated subsurface sources. Appl Geochem. 1993;8(5):483–493.
- Buckau G, Artinger R, Geyer S, et al. Groundwater in-site generation of aquatic humic and fulvic acids and the mineralization of sedimentary organic carbon. Appl Geochem. 2000;15(6):819–832.
- Teramoto M, Shimada J, Kunimaru T. Evidence of groundwater regime in impermeable rocks by stable isotopes in porewaters of drilled core. J Japan Soc Eng Geol. 2006;47(2):68–76.
- Endo R, Tamamura S, Omi Y, et al. Depositional environment in the aquifer, and lignin concentrations and organic C/ N rations in the groundwater in Horonobe town, Hokkaido. Chikyukagaku (Geochemistry). 2013;47(1):21–40. Japanese.
- Routh J, Grossman EL, Murphy EM, et al. Characterization and origin of dissolved organic carbon in Yegua ground water in Brazos Country, Texas. Groundwater. 2001;39(5):760–767.
- Pabich W, Valiela I, Hemond HF. Relationship between DOC concentration and vadose zone thickness and depth below water table in groundwater of Cape Cod, U.S.A. Biogeochemistry. 2001;55(3):247–268.
- Fredrickson JK, Balkwill DL. Geomicrobial processes and biodiversity in the deep terrestrial subsurface. Geomirobiol J. 2006;23(6):345–35.