Abstract
For prepubertal youth, sexual stimuli elicit disgust and avoidance, yet in adolescence this avoidance shifts to sexual approach. One explanation could be that disgust declines in adolescence. This project examined whether disgust is indeed lower in adolescence compared to preadolescence, and whether this difference across age groups would be restricted to sex-relevant disgust elicitors. We also examined whether the strength of disgust would depend on familiarity between participant and source. To examine disgust responses in youths, two cross-sectional studies (N = 248, ages six to 17 years) were conducted using scenario-based measurements. Disgust was overall higher in early adolescence than in preadolescence and relatively weak when the source of disgust was a familiar person. Specifically, when parents were the source, sex-relevant disgust was higher in the groups of early and middle adolescents than in the group of preadolescents. Sex-relevant disgust elicited by a stranger or best friend, however, was lower in middle than in early adolescence. The latter is consistent with the view that repeated confrontation with disgusting stimuli might attenuate disgust, which could contribute to healthy sexual functioning. The heightened sex-relevant disgust in middle adolescents when parents were the source might reflect a functional avoidance mechanism of inappropriate sex mates.
The first kiss I had was the most disgusting thing in my life.
—Leonardo DiCaprio, Movieline, 1995
There is anecdotal evidence that prepubertal children find sexual activities, such as French kissing, disgusting; however, this response to sexual stimuli generally shifts to sexual appetite later on in life. From an evolutionary stance, disgust and sex are both relevant and hold important functions. Disgust has been conceptualized as a defense mechanism that evolved to protect organisms from contamination in the outside world (Curtis, de Barra, & Aunger, Citation2011). Via eliciting the urge to avoid the disgusting cue, disgust-induced avoidance helps prevent exposure to pathogens, thereby promoting health and survival (Oaten, Stevenson, & Case, Citation2009). Consistent with the view that disgust serves disease-avoidance functions, it has been found that people’s inclination to respond with disgust (i.e., disgust propensity) increases with lower immune status (e.g., Ersche et al., Citation2014). In a similar vein, it has been shown that women’s disgust propensity varies as a function of the menstrual cycle, with highest scores during the luteal phase when the vulnerability to disease is relatively high due to a down-regulation of inflammatory immune responses (Fleischman & Fessler, Citation2011). Furthermore, it appears that disgust propensity is relatively high during the first trimester of pregnancy, when mother and fetus are most vulnerable to disease (Fessler, Eng, & Navarrete, Citation2005).
Engaging in sexual behaviors involves pathogen exposure and can be considered a high-risk enterprise. From a disease-avoidance perspective, it may then not come as a surprise that people in general find body parts involved in sexual behavior—the mouth, the vagina, and the penis—to be especially sensitive to disgust (Rozin, Nemeroff, Horowitz, Gordon, & Voet, Citation1995) and that, due to their contamination potency, sex-relevant stimuli like saliva, semen, and sweat are considered among the strongest disgust elicitors (Rozin & Fallon, Citation1987). It may also help explain why in some adults feelings of disgust may arise during sex (de Jong & Peters, Citation2009) and why prepubertal children are typically disgusted by sex-relevant stimuli. The latter can be explained by the absence of sexual maturity and the sensitivity for sexual excitation, allowing for the default disgust characterizing these stimuli to be more salient. However, what remains unexplained, is how, at some point, youth start to become attracted to sexual stimuli and (even) get involved in interpersonal sexual behaviors.
One possible explanation for this remarkable transition might be that disgust generally decreases around puberty, allowing a more positive appraisal for sexual stimuli and consequently facilitating initial sexual contacts. However, this explanation would go against the disease-avoidance function of disgust (Oaten et al., Citation2009). In fact, from a disease-avoidance perspective, one would predict that disgust would escalate as a protective factor against the increased autonomy and the potential health risks involved in sexual behaviors that evolve in early adolescence (Curtis et al., Citation2011). An alternative explanation could therefore be that disgust specifically toward sex-relevant stimuli would decrease around puberty, whereas for other stimuli (i.e., sex-irrelevant stimuli) disgust remains stable or increases. This specific decrease in sex-relevant disgust could be a general developmental variation in disgust responding.
Taken together, both of these perspectives would suggest that disgust increases before puberty or until the beginning of puberty. However, the disease-avoidance notion would indicate that disgust continues to increase, whereas the alternative hypothesis would be that sex-relevant disgust in particular declines around puberty.
Perhaps more likely, however, is that the hypothesized decrease of sex-relevant disgust during adolescence occurs as the consequence and not as the antecedent of repeated engagement in sexual behaviors. There is increasing evidence indicating that sexual excitation may temporarily reduce the experience of disgust. For example, two studies that tested male participants have shown that prior exposure to erotica diminished disgust toward subsequently presented disgust elicitors (Ariely & Loewenstein, Citation2006; Stevenson, Case, & Oaten, Citation2011). In a similar vein, it has been shown that in women sexual excitation can have an acute attenuating effect on both the experience of disgust and disgust-induced avoidance (Borg & de Jong, Citation2012). In apparent contrast, Fleischman, Hamilton, Fessler, and Meston (Citation2015) did not find an influence of film-induced sexual excitation on subjective disgust in women. However, in the latter study, disgust was elicited by pictures instead of actual disgust-eliciting stimuli and relied on passively viewing stimuli instead of handling stimuli, which both might have reduced the sensitivity of the design to detect an influence of sexual arousal on disgust.
Sexual excitation has also been found to promote approach behavior toward initially disgusting stimuli (Borg & de Jong, Citation2012). At least in the short term, this mechanism may help to engage in “disgusting” sexual behavior. Because prolonged contact with initially disgusting stimuli has been shown to be an efficient strategy to reduce disgust (Bosman, Borg, & de Jong, Citation2016), repeated sexual behavior might well result in weakening disgust response toward sex-relevant stimuli, which in turn may further lower the threshold for sexual approach behavior, which then may lead to further weakening of disgust.
There are other competing or complementing hypotheses possibly explaining this observation. However, as a first step to explore the mechanisms underlying the transition, the current two cross-sectional studies were primarily designed to examine the variation in subjective disgust responding toward sex-relevant and sex-irrelevant disgust elicitors in youth, before and after having reached puberty.
Disgust and Familiarity
Besides the dynamics with sexual excitation, disgust intensity is partially dependent on the extent to which an individual has already been exposed to a stimulus (Stevenson & Repacholi, Citation2005). In other words, our intensity of disgust is supposed to depend on familiarity, including familiarity of persons. In particular, the people we have regular contact with are more likely to share the same pool of germs that understandably are more defendable by our shared physical immunity. From this perspective, familiar people are less harmful than unknown people with another pool of bacteria that might be new to our immune system. Thus, sharing of the environment results in a reduction of disgust intensity toward familiar people (Peng, Chang, & Zhou, Citation2013; Stevenson & Repacholi, Citation2005).
From an evolutionary stance, it makes sense that our response to disgusting stimuli is more intense to unfamiliar rather than to familiar stimuli (Curtis, Citation2013). However, when it comes to sexual excitation the relationship between disgust and familiarity seems to be reversed. Due to inbreeding aversion, disgust is especially elicited toward close relatives and less toward evolutionarily more optimal mates, at least when it comes to sexual behaviors (Antfolk, Lieberman, & Santtila, Citation2012). From an evolutionary point of view, incest aversion is explained by high reproductive costs (Westermarck, Citation1891). The latter is supported with evidence indicating increased risk of disease and death for children born to close relatives (Bittles, Citation1983; May, Citation1979). However, this innate aversion is debated by several others who emphasize the relevance of other factors that might be at play in avoiding sex with close family members (e.g., Shor & Simchai, Citation2009). Therefore, in the current study, we tested if the impact of familiarity on disgust in various age groups varies between sex-relevant and sex-irrelevant disgust elicitors. Familiarity was disentangled between familiarity that is more a representation for sharing genes (e.g., parents) and familiarity related to friends (e.g., best friend) selected later on in life, which may promote a weakened disgust response. In addition, we added a category of nonfamiliar persons (strangers) demonstrating neither shared genes nor shared environment.
Aims and Hypotheses
These two cross-sectional studies examined the variation in subjective disgust responses toward sex-relevant and sex-irrelevant disgust, as a function of age group (1 = preadolescence, nine to 11 years; 2 = early adolescence, 12 to 14 years; and 3 = middle adolescence, 15 to 17 years) and level of familiarity (parent, best friend, and stranger).
Despite the competing theories explaining the development of disgust at different age groups (as explained earlier on), we predicted that during preadolescence sex-irrelevant disgust would increase but that disgust specifically for sex-relevant stimuli would decrease. In terms of disgust responding as a function of familiarity level, we anticipated that in early adolescence, when sexual stimuli become motivationally salient (Fortenberry, Citation2013), sex-relevant disgust would be relatively high in the context of “familial” familiarity (parents) as a functional adjustment/adaptation to help avoid potential sex mates of strong genetic similarity. Whereas independent of developmental stage, sex-irrelevant disgust would be relatively low when parents (or best friends) are the source of the disgust elicitors because of the lowered threat for contamination.
Method
Participants
For Study 1, a group of children between ages six and 17 was recruited from a small private school near Hamburg, Germany. The group was equally distributed across the various age groups (see ), and participants were recruited from all available classes in the school. In total, 148 children completed the study and, after the inclusion criteria were met (e.g., no moderate to severe learning disabilities), 139 participants (female, n = 74; male, n = 65) could be included in the analysis.
Table 1. Groups as a Function of Age
For Study 2, a group of 110 children between ages nine and 17 was recruited from a primary and secondary school in Purda, Poland (see ). After the inclusion criteria were met, 109 participants (female, n = 63; male, n = 46) were included in the analysis. Thus, in total, 248 participants were included in the study. Power analysis using GPower (Erdfelder, Faul, & Buchner, Citation1996), with power = .80 and an alpha level of .05, indicated that to reliably detect differences with a large effect size (Cohen’s f = .40) in a mixed analysis of variance (ANOVA) with 12 groups (3 [age] × 2 [sex] × 2 [country]) and six repeated measurements (2 [type of disgust] × 3 [familiarity]), we needed a total sample size of at least 204 participants. Thus, the current study had sufficient power to reliably detect differences with a large effect size.
For both studies, all students in the class could fill in the measurements for inclusion purposes; however, the teachers indicated which of the participants were indexed as suffering from moderate to severe learning difficulties, which could have conflicted with the required capacities for completing the measurements. Their data were not included in the current study (i.e., n = 9 from Study 1). For Study 2, the data of one participant were removed from analysis because this participant did not satisfy the inclusion criteria (was not yet nine years old).
Differences in Age Range Between Study 1 and Study 2
Following the preliminary analysis of Study 1, it was noted that children younger than nine years of age tended to give rather extreme answers, namely 0 or 100, compared to older children. For this reason, the results of these younger children were quite complex for interpretation. To prevent this anchoring effect of answers and possible influences on results, in Study 2 we did not include children aged eight or younger. Moreover, the data of Study 1 was restricted to Group 1 to 3 for more direct comparisons with Study 2. That means that for both studies, we included the data of the three groups with children from ages nine to 17.
For the interested reader, we included the additional descriptive analysis of group 0 (i.e., the group including children from ages six to eight from Study 1; n = 24) and the results of the main analysis with the inclusion of group 0 as Appendix A. The main findings remained the same when group 0 was included in the analysis. Furthermore, Appendix A provides the reader with a description of how data collection was done for group 0.
The Ethical Committee of Psychology at the University of Groningen (ECP: ppo-014–037; ECP: ppo-015–065) approved these studies, and all procedures were kept in line with protocols. In addition, for both studies the schools gave written permission of their participation and the parents gave active informed consent. Besides the parents’ consent, children ages 12 and up were also asked to sign an informed consent.
Measurements
For the purpose of these two studies the research team built the Disgust Development Scenario-Based Assessment (DD-SBA). The DD-SBA consisted of 48 scenario questions and was based on six pathways of contamination as categorized by Curtis (Citation2013). These contamination pathways included (a) sex-relevant disgust (e.g., kissing another person on the mouth) as a main pathway of interest for this study; (b) atypical appearance disgust (e.g., shaking hands with a person who has a deformed hand); (c) lesions disgust (e.g., reusing a towel previously used by a person with an open wound); (d) hygiene disgust (e.g., eating food from a person who has touched a used diaper without washing hands afterward); (e) food and animal disgust (e.g., biting into rotten fruit); and (f) fomite disgust (e.g., using a toothbrush which was previously used by another person). In this project, sex-relevant disgust was compared to the other five pathways of disgust (atypical appearance, lesions, hygiene, food and animal, and fomite disgust) merged together as sex-irrelevant pathogen disgust. See Appendix B for the actual scenario-question items of the DD-SBA. Moreover, for the interested reader, the actual DD-SBA is provided (see Link to https://www.researchgate.net/publication/323662617_Disgust_Toward_Sex-Relevant_and_Sex-Irrelevant_Stimuli_in_Pre-_Early_and_Middle_Adolescence_Data_disgust_questionnaire_merged_All_group) for replicability.
In addition, 18 general questions were included to examine the sociodemographic aspects of the children and to examine whether children learned to differentiate/disentangle various feelings. To control for priming and order effects, six randomized versions of the DD-SBA were created, each starting with a question of one of the six pathways of contamination and with a different person (to indicate varying levels of familiarity). During the experiment, these different versions also functioned to prevent children from being tempted to copy answers from one another.
Disgust
To measure the intensity of disgust, for each scenario participants were asked to rate disgust on a visual analogue scale (VAS), from 0 (Not disgusting at all) to 100 (Very disgusting). Two scenarios were designed per disgust pathway. For the sex-irrelevant disgust items of the questionnaire the Cronbach’s alpha was .98, implying high internal consistency, whereas the Cronbach’s alpha for sex-relevant disgust items was .69.
Familiarity
To investigate whether the intensity of disgust is dependent on the level of familiarity, all children were asked to imagine the scenarios involving four different people: (a) a parent (one scenario involving mother, referred to as “your mom”; one scenario involving father, referred to as “your dad”), (b) the best friend (depending on whether the child had a female or male friend, thus it could either be a boy or a girl, referred to as “best friend”), (c) an unknown person (one scenario involving a man, one scenario involving a woman, referred to as “stranger”), and (d) a sibling (referred to as “sister” and/or “brother”).
If children had no mother, father, or siblings, they skipped the questions involving those persons. The sibling-related questions were eventually excluded because of many missing data points, i.e., n = 39 did not have a sibling or had difficulties filling out the sibling-related questions. This was not deemed problematic for the study as the three levels of familiarity (parents, best friend, and stranger) seemed sufficient to differentiate between these different levels that we were interested in investigating.
Control emotional dimension
To balance the survey-based questionnaire with a positive question, for all of the 48 scenarios the children were also asked to rate their response on a dimension labeled as “good feeling.” This rating had to be done on an additional VAS scale, from 0 (Not a good feeling at all) to 100 (Very much a good feeling). The data collected on the dimension of “good feeling” were not analyzed, as its aim was strictly to balance the questionnaire. In addition, to disentangle fear from disgust (because these two emotions can be difficult to distinguish) and to optimize our design sensitivity, in both studies we first had a practice session in which youth could experience the “yuck” feeling, fear, and a positive emotion “good feeling.” In these practice sessions we did not collect data, as these questions had as their sole purpose to teach the youth the boundaries of fear and disgust.
Still stimuli
To make the scenarios more explicit and easy to understand, 12 pictures representing scenario-relevant content were included in the DD-SBA. The presentation of these pictures differed for children aged six to eight (i.e., age group 0; see Appendix A).
In addition to the previously mentioned aspects that were included in both studies, in Study 2 we also included a measure to index the values one could indicate as being devoted to. However, these data were not used in this article.
Translation
For Study 1, to translate the measures from English to German, the standard back-translation was done by the two researchers who conducted Study 1 (Brislin, Citation1970). For Study 2, the researcher herself did the Polish translation, and then a second native speaker back-translated the questionnaire to English. Any differences in translation were resolved and a third party was involved when necessary.
Procedure
The data collection of Study 1 took place November 4 through 7, 2014, in the first through fifth school lessons (8:00 a.m. to 12:40 p.m.). Two researchers conducted the study; both were German natives studying at the University of Groningen. For Study 2, the data collection took place December 9 through 11, 2015, during the first and the sixth lesson (8:00 a.m. to 1:20 p.m.). The researcher, a native Polish student at the University of Groningen, led the data collection.
For children of eight years and older, the study took place in a classroom setting (see Appendix A for a description for children younger than age eight). All children were asked to keep only writing materials on their desks. The experimenter introduced the questionnaire to the children and explained the scale on the board by using simple examples, such as “Imagine a barking dog offleash running toward you. How would you feel about that?” (fear).
Following the explanation, all children received the questionnaire and were instructed to fill it in on their own. To ensure good task engagement, children were told that they could spend the whole school lesson (45 minutes) filling in the questionnaire, and their teachers explained that they could work on their schoolwork if they finished earlier than their classmates. The researcher was present in the class throughout the study, just in case the children raised any questions or concerns.
In Study 1, participants were thanked for their participation after they filled in the questionnaire. The children did not receive a thank-you gift, as this did not fit the pedagogical ideas of that school. In Study 2, at the end of the study all participants received a cereal bar as a thank-you gift.
Data Reduction and Analysis
All data were processed, screened, and analyzed in IBM SPSS, Version 24. For the reasons outlined in the Method section, for Study 1 the data represented in the Results section excludes the youngest group (age group 0: six to eight years old).
The disgust ratings (dependent variable) were subjected to a mixed-factor ANOVA, with disgust type (sex-irrelevant disgust, sex-relevant disgust) and familiarity (parent, best friend, stranger) as within-subjects factors, and sex (male, female), age group (1 = nine to 11 years, 2 = 12 to 14 years, 3 = 15 to 17 years) and country (Study 1, Study 2), as between-subjects factors.
Results
Mauchly’s test indicated that the assumption of sphericity had been violated for the main effect of familiarity (χ2 (2) = 14.58, p = .001) and the interaction effect of disgust type and familiarity (χ2 (2) = 9.22, p = .010). Therefore, degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ε = .94 for the main effect of familiarity and ε = .96 for the interaction effect of disgust type and familiarity).
Consistent with the hypothesis that the variation in subjective disgust responses toward sex-relevant versus sex-irrelevant disgust across the three age groups would vary as a function of familiarity, a significant three-way interaction of disgust type, familiarity, and age group [F (3.83, 392.66) = 5.11, p < .001, ηp2 = .05; see ] was found. A visual representation of this three-way interaction can be found in .
Table 2. Mean Values of Disgust Type × Age Group in Parent, Best Friend, and Stranger Disgust
Figure 1. Three-way interaction of Disgust type × Familiarity × Age group. Upper graphic represents means of sex-irrelevant disgust with 95% confidence intervals. Lower graphic represents means of sex-relevant disgust with 95% confidence intervals.
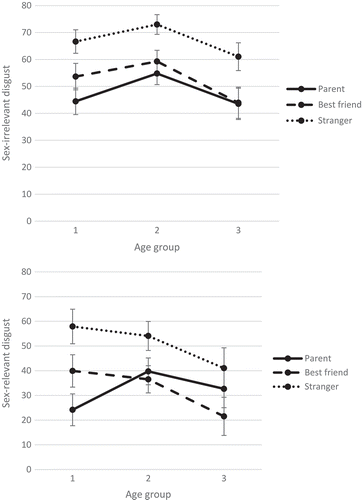
To decompose this three-way interaction, we carried out separate ANOVAs for parental, best friend, and stranger disgust. For parental disgust, the disgust type and age group interaction was significant, indicating that the differences in disgust responding across age groups varied between sex-irrelevant and sex-relevant disgust [F (2,218) = 4.15, p = .017; ηp2 = .037]. A subsequent one-way ANOVA with sex-irrelevant disgust as dependent variable showed a main effect of age group [F (2,220) = 8.76, p < .001]. Bonferroni post hoc comparisons indicated that ratings of sex-irrelevant disgust were significantly lower for age group 1 as compared to age group 2 (p < .001) and were significantly higher for age group 2 as compared to age group 3 (p = .001). There was no significant difference between age group 1 and age group 3 in sex-irrelevant disgust responses (p = .924). Also, for sex-relevant disgust, there was a significant difference across age groups [F (2,218) = 9.98, p < .001]. Bonferroni post hoc comparisons indicated that sex-relevant disgust ratings were also significantly lower for age group 1 as compared to age group 2 (p < .001) and significantly higher for age group 2 as compared to age group 3 (p = .031). Sex-relevant disgust was also significantly higher for age group 3 than for age group 1 (p = .028; see ).
For best friend as the source of disgust, the disgust ratings for sex-irrelevant and sex-relevant disgust did not differ significantly across the three age groups [F (2,217) = 2.81, p = .063; ηp2 = .025; see ]. Overall, disgust was not significantly lower in age group 1 as compared to age group 2 (p = .441). However, it was significantly higher in age group 2 as compared to age group 3 (p < .001); the disgust ratings of age group 3 were also significantly lower than those of age group 1 (p = .001).
For stranger disgust, the disgust type and age group interaction was significant [F (2, 219) = 3.39, p = .035; ηp2 = .030; see ]. A subsequent one-way ANOVA with general sex-irrelevant disgust as a dependent variable showed a main effect of age group [F (2, 220) = 6.53, p = .002]. Bonferroni post hoc comparisons showed that, just as for parental disgust, disgust ratings for sex-irrelevant disgust were significantly lower for age group 1 as compared to age group 2 (p = .025) and were significantly higher in age group 2 as compared to age group 3 (p = .001). There was no difference between age group 1 and age group 3 (p = .166). Also for sex-relevant disgust, the subsequent one-way ANOVA showed a significant difference between age groups [F (2, 219) = 3.97, p = .020]. Bonferroni post hoc comparisons showed that there was no significant difference in age group 1 compared to age group 2 (p = .624) but that age group 2 reported significantly higher sex-relevant disgust compared to age group 3 (p = .017). The disgust ratings of age group 3 were significantly lower than those from age group 1 (p = .008).
In addition, our main analysis revealed a significant main effect of disgust type [F (1, 205) = 189.58, p < .001, ηp2 = .48], indicating higher disgust responses for sex-irrelevant disgust (M = 55.59, SE = 1.29) as compared to sex-relevant disgust (M = 38.62, SE = 1.56). Similarly, a significant main effect of familiarity was found [F (1.87, 383.54) = 128.61, p < .001, ηp2 = .39]. Specifically, disgust responses were lowest for parental disgust (M = 39.89, SE = 1.49), followed by best friend disgust (M = 42.47, SE = 1.51) and by stranger disgust (M = 58.94, SE = 1.47). Last, a significant main effect of age group was found [F (2, 205) = 7.48, p < .001, ηp2 = .07, suggesting that disgust responses were lowest in age group 3 (M = 40.61, SE = 2.59), followed by age group 1 (M = 47.79, SE = 2.19), and age group 2 (M = 52.89, SE = 1.85). However, these three main effects were qualified by the previously described three-way interaction. Furthermore, a significant main effect of sex [F (1, 205) = 6.17, p = .014, ηp2 = .03] was found. Overall, girls (M = 50.31, SE = 1.75) scored higher than boys (M = 43.89, SE = 1.90). Last, we found a significant main effect of the two studies [F (1, 205) = 28.17, p < .001, ηp2 = .12]. The children in Study 1 (M = 40.25, SE = 1.76) scored generally lower than the children in Study 2 (M = 53.95, SE = 1.89). The last two main effects were not qualified by an interaction.
Post hoc, we explored whether perhaps sex-relevant disgust was less connected to sex-irrelevant disgust in the higher age groups. The correlation between sex-relevant and sex-irrelevant disgust was, however, very similar for all age groups, indicating that the link between both types of disgust did not vary across the various age group (age group 1: r = .693; age group 2: r = .694; age group 3: r = .651).
Discussion
The main findings can be summarized as follows: First, there was a higher disgust response in early adolescence (age group 2: 12 to 14 years) compared to preadolescence (age group 1: 9 to 11 years), and disgust was again lower in middle (age group 3: 15 to 17 years) than in early adolescence. Second, in line with expectations and independent of age, disgust was generally more easily elicited by relatively unfamiliar than by familiar persons. Third, specifically when parents were the source of disgust, disgust for sex-relevant stimuli was higher in the groups of early and middle adolescents compared to the group of preadolescents. When strangers and best friends were the source of sex-relevant disgust, disgust showed a decline over the three age groups that was most prominent between the early adolescence to middle adolescence group. Last, for all sources of disgust (parent, best friend, stranger), disgust for sex-irrelevant stimuli was generally lower in preadolescents and middle adolescents compared to early adolescents.
As a secondary finding, female participants generally reported more intense disgust compared to their male counterparts. This finding is convergent with adult literature and has been explained by the increased risk of contamination in women, by the need for women to protect their unborn offspring, and by specific phases in their menstrual cycle marked with lower immunity (e.g., Fessler et al., Citation2005). Finally, the findings indicated that participants in Study 2 systematically reported higher disgust scores than participants in Study 1. Participants of Study 1 lived in the urban area of Hamburg, whereas those of Study 2 were recruited from a small village in a rural area of Poland. Because it is known that people from rural areas tend to be more conservative and people from urban areas more liberal (Nelsen & Yokley, Citation1970), one could speculate that this difference in value orientation might underlie the difference in disgust propensity between both groups of participants. Such explanation would be consistent with previous research indicating that disgust responding is generally higher in individuals with relatively strong conservative and/or low liberal values (e.g., Inbar, Pizarro, & Bloom, Citation2009; Smith, Oxley, Hibbing, Alford, & Hibbing, Citation2011). However, one cannot exclude that other factors, such as financial status, might be influencing both the relationship between the environment and global value orientation, as well as the relationship between value orientation and disgust responding.
Variation in Patterns of Disgust Responding Subject to Age
As the intrinsic function of disgust is disease avoidance (Curtis et al., Citation2011; Oaten et al., Citation2009), the pattern of intensification in disgust responding from preadolescence to early adolescence can be explained as a compensatory mechanism to protect against the rising contamination risks that come part and parcel with increasing autonomy (Curtis, Citation2013). Thus, the finding that disgust responding was generally higher in early adolescence than in preadolescence is in line with the view that the disgust response will increase when the risk of contamination increases (Curtis, Citation2013). However, the finding that overall disgust responses declined from early to middle adolescence seems to challenge this stance.
One way to explain this reduction in disgust from early to middle adolescence could be related to the implications of this new developmental stage (and the adjustment to it). From the perspective that disgust serves the adaptive function to prevent contamination by pathogens that are omnipresent but cannot be detected by mere visual inspection, it has been argued that disgust responding typically follows a “better safe than sorry” heuristic (Bosman et al., Citation2016). Repeated safe experiences with “new” stimuli and behaviors during adolescence may have attenuated and corrected the initially overly conservative disease-avoidance mechanism, resulting in a decline of disgust response from early to middle adolescence.
The overall decline of sex-relevant disgust may be largely explained along the same lines. Early adolescence coincides with the phase in which youngsters generally start engaging in sexual activities. This initial sexual contact could be driven by the surge of sex hormones, which promotes the generation of sexual arousal and subsequent approach behavior (e.g., Halpern, Udry, & Suchindran, Citation1997). It has been shown that heightened sexual arousal can reduce feelings of disgust and disgust-induced avoidance (Borg & de Jong, Citation2012). Thus, from the start of puberty, the typical disgust-induced avoidance of sex stimuli might be reduced or counteracted by the hormone-based capacity of sex stimuli to elicit sexual arousal next to disgust, which in turn might lower the threshold for engaging in “disgusting” sexual behaviors (see de Jong, van Overveld, & Borg, Citation2013). Following repeated and prolonged contact, which goes hand in hand with sexual behavior, disgust will typically decline (Bosman et al., Citation2016; Rozin & Fallon, Citation1987). An alternative explanation for the motivation for initial sexual contact is related to the nature–nurture notion. For example, Pfaus et al. (Citation2012) emphasized the interaction between hormones and experience with sexual reward as key factors involved in sexual motivation. In other words, sex hormones support the initial approach and, in turn, the subsequent reward may further contribute to the following approach behaviors, thereby not only counterforcing the inhibitory quality of disgust but (probably) also reducing disgust by supporting repeated intense physical contact with initially disgusting stimuli.
Pattern of Disgust Responding Subject to Familiarity
In line with expectations, this study provided converging evidence that the intensity of disgust responding varied as a function of the familiarity of the source of disgust. In other words, unfamiliar individuals generally triggered more disgust, which can partially be explained by the increased risk of contamination that unfamiliarity brings with it, due to a new pool of pathogens (e.g., Curtis, Citation2013; Peng et al., Citation2013; Stevenson & Repacholi, Citation2005). Thus, the finding that disgust was generally more easily elicited by unfamiliar (e.g., a stranger) than by familiar persons (e.g., a parent) is consistent with the conceptualization of disgust as a disease-avoidance mechanism, because increased disgust protects the youth from involvement in situations that are relatively high risk for contamination.
Sex-Irrelevant Disgust
Overall, sex-irrelevant disgust responding was higher in early adolescence than in preadolescence, whereas disgust was again lower in middle adolescence than in early adolescence independent of familiarity. This finding suggests that the development of sex-irrelevant disgust seems to be independent of the degree of familiarity or the source of disgust. As previously elaborated, disgust serves disease-avoidance functions (Curtis et al., Citation2011; Oaten et al., Citation2009); the apparent rise in sex-irrelevant disgust from preadolescence to early adolescence is in line with Curtis’s (Citation2013) idea that it may act as a compensation process to protect against the heightened contamination risks that occur with increasing autonomy. The decline from early adolescence to middle adolescence may further be explained as a weakened response in disgust responding due to the repeated exposure as part of this developmental stage that generally comes with more autonomy. It needs to be noted, however, that this explanation cannot be supported by our current data, emphasizing the need for future research to investigate the underlying mechanisms of the differences in sex-irrelevant disgust across age groups.
Sex-Relevant Disgust
Interestingly, specifically for scenarios involving the parent (“familial” familiar person) sex-relevant disgust was higher in early adolescence than in preadolescence and was similarly high in the middle adolescence group. This finding is consistent with the view that disgust is not only involved in disease avoidance but also evolved to facilitate avoidance of sexual partners and behaviors that challenge long-term reproductive success (Tybur, Lieberman, & Griskevicius, Citation2009). One important factor that might jeopardize reproductive success is high genetic similarity. Accordingly, people typically respond with disgust when asked to imagine having sex with a close genetic relative (e.g., Lieberman, Tooby, & Cosmides, Citation2007). In these cases, disgust might help prevent having sex with a potential sex partner who is characterized by too high genetic similarity, which thus might interfere with producing healthy offspring. Thus, the current finding—that during the developmental stage of adolescence, youngsters reported a persistently heightened sex-relevant disgust toward familial persons—may be interpreted as reflecting a defensive mechanism that protects against inbreeding and its consequential high reproductive costs (Westermarck, Citation1891). On the other hand, the finding that sex-relevant disgust toward a parent was relatively low in age group 1 is in line with the notion that preadolescents are not concerned with reproductive success, as they usually are not sexually mature yet. Accordingly, they may interpret a kiss on the mouth of a parent differently (i.e., in a nonsexual way) as compared to adolescents, who are sexually mature. Further, the relative low (sex-relevant) disgust expressed when the parents were the source of disgust, as compared to when the source of disgust was a stranger or best friend, may be explained by the more common phenomenon that familiar sources typically tend to elicit less disgust than unfamiliar sources (Peng et al., Citation2013; Stevenson & Repacholi, Citation2005).
Complementing this pattern of disgust regarding scenarios that involved parents, sex-relevant disgust was lower for middle adolescence in scenarios involving unknown persons. The latter is hypothesized to allow for opportunities to engagement with potential sex mates, facilitating healthy and pleasurable sexual development.
Limitations
Some comments are in order with regard to the limitations of the current research. First, it needs to be mentioned that we used a cross-sectional approach and thus we cannot draw strong developmental inferences. It would require future longitudinal studies to examine whether the current findings represent an adequate representation of actual developmental trajectories.
Second, we grouped participants on the basis of their calendar age. Yet it is well known that there are large individual differences with regard to the age of onset of puberty. This variation might have rendered the three groups less consistent than ideal with regard to developmental stage or developmental labels. It would be important for future research to include objective measures to define participants’ developmental stages. Moreover, by including both an objective measure of puberty and a measure of interest in sex/initial sexual behaviors, it would allow testing whether indeed the influence of puberty is expressed via more interest in sex or if sex hormones promote approach and that only after some decline of disgust, interest in sex increases. Such approach would be helpful to arrive at more concrete analysis of the sex-relevant and sex-irrelevant disgust development.
Third, the current research exclusively relied on subjective disgust ratings. It would be important for future research to complement self-report measures with behavioral and physiological indices of disgust to verify the robustness of the current pattern of findings. In the same vein, it should be acknowledged that the current research relied on disgust responding in the context of imagined instead of real-time confrontation with potential disgust elicitors. It would be important to examine in future research whether similar results will appear in the context of actual confrontations. In addition, with the current data, we are not able to compare with other studies or to infer whether we can set cut points. We hope that future work will develop these leads to give more meaning to the pattern of disgust responding we observed across these three age groups.
In addition, it should be acknowledged that whereas the items for the sex-irrelevant disgust items showed excellent reliability (Cronbach’s alpha was .98), internal consistency for the sex-relevant disgust items was just acceptable (Cronbach’s alpha was .69). The relatively low index of internal consistency for the sex-relevant disgust items may reflect that responses to various sexual behaviors are more variable than those to more general and perhaps less sensitive (sex-irrelevant) disgust elicitors (e.g., rotten food). In addition, the number of items was (much) lower for the sex-relevant pathogen disgust items, which may also be partly responsible for the apparent difference in internal consistency in responding between sex-relevant and sex-irrelevant items.
Finally, although the current pattern of disgust over the age groups is consistent with an adjustment explanation, it should be acknowledged that the current design does not contain indices to empirically corroborate such explanation. It would require future longitudinal research with repeated assessments of both disgust response and concrete behaviors to arrive at more final conclusions in explaining the global decline of (sex-relevant and sex-irrelevant) disgust from early to middle adolescence. For instance, future research can study the androgen patterns in a longitudinal design and the initial sexual engagements and/or behaviors to arrive at a more concrete analysis of disgust development.
Conclusion
In these two studies, the variation of disgust responding provided no evidence to suggest that early adolescence would be characterized by lowered disgust response as a means to support engagement in sexual behaviors. Instead, overall disgust ratings were higher in children ages 12 to 14 compared to children ages nine to 11, which might reflect a defense mechanism that protects against health risks involved in sexual behaviors and other “new” behaviors that are related to increased autonomy in adolescence. Importantly, disgust generally decreased from early to middle adolescence. The decline of (sex-relevant) disgust elicited by a stranger or best friend from early to middle adolescence might reflect a reduction of disgust responding due to repeated “safe” experiences and might contribute to healthy sexual functioning. The heightened sex-relevant disgust when parents were the source of disgust might reflect a functional adjustment to help avoid potential sex mates close genetic similarity.
Acknowledgments
The authors are grateful to numerous people who assisted in these two studies. Florian Zsok (†, January 16, 1993–December 28, 2017) assisted actively in Study 1; he contributed in the brainstorming, design, and data collection, and in the preliminary analysis of the German data set. Dr. E. Kuipers was indispensable in sharing her expertise on how to best recruit participants, and Professor R. Banse assisted in the ethical application for this project. Authors are grateful also to Karolina Grenda and Aleksandra Kustra for assisting in the Polish translation of the questionnaires and the Bachelor of Science students who helped with the data input (Amanda Daniel, Arron Mannix, Kira Brauns, Ronja Eike, and Selina Kexel) under the supervision of Jessica Hinzmann (second author of this article). Finally, we are grateful for the German school (Schülerschule) and the Polish schools (Szkoła Podstawowa w Purdzie (primary school] and Gimnazjum w Purdzie [secondary school]) and for all the participating children and their parents who made this study possible.
References
- Antfolk, J., Lieberman, D., & Santtila, P. (2012). Fitness costs predict inbreeding aversion irrespective of self-involvement: Support for hypotheses derived from evolutionary theory. PLOS ONE, 7, 1–8. doi:10.1371/journal.pone.0050613
- Ariely, D., & Loewenstein, G. (2006). The heat of the moment: The effect of sexual arousal on sexual decision making. Journal of Behavioral Decision Making, 19, 87–98. doi:10.1002/bdm.501
- Bittles, A. H. (1983). The intensity of human inbreeding. Behavioral and Brain Sciences, 6, 103–104. doi:10.1017/S0140525X00014874
- Borg, C., & de Jong, P. (2012). Feelings of disgust and disgust-induced avoidance weaken following induced sexual arousal in women. PLOS ONE, 7, 1–8. doi:10.1371/journal.pone.0044111
- Bosman, R. C., Borg, C., & de Jong, P. J. (2016). Optimising extinction of conditioned disgust. PLOS ONE, 11, 1–18. doi:10.1371/journal.pone.0148626
- Brislin, R. W. (1970). Back-translation for cross-cultural research. Journal of Cross-Cultural Psychology, 1, 185–216. doi:10.1177/135910457000100301
- Curtis, V. (2013). Don’t look, don’t touch: The science behind revulsion. Oxford, United Kingdom: Oxford University Press.
- Curtis, V., de Barra, M., & Aunger, R. (2011). Disgust as an adaptive system for disease avoidance behaviour. Philosophical Transactions of the Royal Society B: Biological Sciences, 366, 389–401. doi:10.1098/rstb.2010.0117
- de Graaf, H., & Rademakers, J. (2011). The psychological measurement of childhood sexual development in western societies: Methodological challenges. Journal of Sex Research, 48, 118–129. doi: 10.1080/00224499.2011.555929
- de Jong, P. J., & Peters, M. L. (2009). Sex and the sexual dysfunctions: The role of disgust and contamination sensitivity. In B. O. Olatunji & D. McKay (Eds.), Disgust and its disorders: Theory, assessment, and treatment implications (pp. 253–270). Washington, DC: American Psychological Association.
- de Jong, P. J., van Overveld, M., & Borg, C. (2013). Giving in to arousal or staying stuck in disgust? Disgust-based mechanisms in sex and sexual dysfunction. Journal of Sex Research, 50, 247–262. doi:10.1080/00224499.2012.746280
- Erdfelder, E., Faul, F., & Buchner, A. (1996). GPOWER: A general power analysis program. Behavior Research Methods, Instruments, and Computers, 28, 1–11. doi:10.3758/BF03203630
- Ersche, K. D., Hagan, C. C., Smith, D. G., Abbott, S., Jones, P. S., Apergis-Schoute, A. M., & Döfflinger, R. (2014). Aberrant disgust responses and immune reactivity in cocaine-dependent men. Biological Psychiatry, 75, 140–147. doi:10.1016/j.biopsych.2013.08004
- Fessler, D. M. T., Eng, S. J., & Navarrete, C. D. (2005). Elevated disgust sensitivity in the first trimester of pregnancy: Evidence supporting the compensatory prophylaxis hypothesis. Evolution and Human Behavior, 26, 344–351. doi:10.1016/j.evolhumbehav.2004.12.001
- Fleischman, D. S., & Fessler, D. M. T. (2011). Progesterone’s effect on the psychology of disease avoidance: Support for the compensatory behavioral prophylaxis hypothesis. Hormones and Behavior, 59, 271–275. doi:10.1016/j.yhbeh.2010.11.014
- Fleischman, D. S., Hamilton, L. D., Fessler, D. M. T., & Meston, C. M. (2015). Disgust versus lust: Exploring the interactions of disgust and fear with sexual arousal in women. PLOS ONE, 10, 1–22. doi:10.1371/journal.pone.0118151
- Fortenberry, J. D. (2013). Puberty and adolescent sexuality. Hormones and Behavior, 64, 280–287. doi:10.1016/j.yhbeh.2013.03.007
- Halpern, C. T., Udry, J. R., & Suchindran, C. (1997). Testosterone predicts initiation of coitus in adolescent females. Psychosomatic Medicine, 59, 161–171. doi:10.1097/00006842-199703000-00008
- Inbar, Y., Pizarro, D. A., & Bloom, P. (2009). Conservatives are more easily disgusted than liberals. Cognitions and Emotions, 23, 714–725. doi:10.1080/02699930802110007
- Lieberman, D., Tooby, J., & Cosmides, L. (2007). The architecture of human kindetection. Nature, 445, 727–731. doi:10.1038/nature05510
- May, R. (1979). When to be incestuous. Nature, 279, 192–194. doi:10.1038/279192a0
- Nelsen, H. M., & Yokley, R. L. (1970). Civil rights attitudes of rural and urban Presbyterians. Rural Sociology, 35, 161–174.
- Oaten, M., Stevenson, R. J., & Case, T. I. (2009). Disgust as a disease-avoidance mechanism. Psychological Bulletin, 135, 303–321. doi:10.1098/rstb.2010.0117
- Peng, M., Chang, L., & Zhou, R. (2013). Physiological and behavioral responses to strangers compared to friend as a source of disgust. Evolution and Human Behavior, 34, 94–98. doi:10.1016/j.evolhumbehav.2012.10.002
- Pfaus, J. G., Kippin, T. E., Coria-Avila, G. A., Gelez, H., Afonso, V. M., Ismail, N., & Parada, M. (2012). Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Archives of Sexual Behavior, 41, 31–62. doi:10.1007/s10508-012-9935-5
- Rozin, P., & Fallon, A. E. (1987). A perspective on disgust. Psychological Review, 94, 23–41. doi:10.1037/0033-295X.94.1.23
- Rozin, P., Nemeroff, C., Horowitz, M., Gordon, B., & Voet, W. (1995). The borders of the self: Contamination sensitivity and potency of the body apertures and other body parts. Journal of Research in Personality, 29, 318–340. doi:10.1006/jrpe.1995.1019
- Shor, E., & Simchai, D. (2009). Incest avoidance, the incest taboo, and social cohesion: Revisiting Westermarck and the case of the Israeli kibbutzim. American Journal of Sociology, 114, 1803–1842. doi:10.1086/597178
- Smith, K. B., Oxley, D., Hibbing, M. V., Alford, J. R., & Hibbing, J. R. (2011). Disgust sensitivity and the neurophysiology of left–right political orientations. PLOS ONE, 6, 1–9. doi:10.1371/journal.pone.0025552
- Stevenson, R. J., Case, T. I., & Oaten, M. J. (2011). Effect of self-reported sexual arousal on responses to sex-related disgust cues. Archives of Sexual Behavior, 40, 79–85. doi:10.1007/s10508-009-9529-z
- Stevenson, R. J., & Repacholi, B. M. (2005). Does the source of an interpersonal odour affect disgust? A disease risk model and its alternatives. European Journal of Social Psychology, 35, 375–401. doi:10.1002/ejsp.263
- Tybur, J. M., Lieberman, D., & Griskevicius, V. (2009). Microbes, mating, and morality: Individual differences in three functional domains of disgust. Journal of Personality and Social Psychology, 97, 103–122. doi:10.1037/a0015474
- Westermarck, E. (1891). The history of human marriage (2nd ed.). Oxford, United Kingdom: Macmillan.
Appendix A
Procedure for Age Group 0 (i.e., Six to Eight Years of Age)
For Study 1, children of six and seven years of age were tested individually due to their limited reading and reasoning skills (Graaf & Rademakers, Citation2011). Each child was taken out of the classroom to an interview room that the school allocated for the study. The researcher then completed the questionnaire together with the child in an interview-like manner. To make children feel comfortable and secure, two children were taken out of the classroom together. Both researchers performed the interview at the same time with one child each. The researchers made sure the children could not see each other and used different versions of the questionnaire.
After a short introduction, the rating scale was explained to the child on an extra printed and laminated paper. The children were able to express the intensity of their feelings by indicating their score with their fingers on the paper. To make sure they understood the rating system, the pre–base state feeling questions were asked (e.g., disgust, good feeling; see Method). Together with all scenarios the children were (also) shown a printed and laminated picture (8.27 in. × 5.91 in. [21 cm × 15 cm]) containing disgusting content corresponding with the actual scenario. To prevent biases, the researchers used the same explanations throughout the study.
After completing all questions the researcher asked the children to think back to a happy moment that recently occurred to them. When they were not able to remember a happy moment, they were asked to remember their last favorite birthday present. This was done to create a positive ending and to reduce possible negative effects that the scenarios or pictures could have caused. After the session, the researcher accompanied each child back to the classroom.
Demographics of Age Group 0
Age group 0 of Study 1 consisted of N = 24 children with an age range from six to eight years, including seven boys (30%) and 17 girls. Mean disgust response for sex-irrelevant disgust was 55.23 (SD = 17.26), and for sex-relevant disgust 31.93 (SD = 18.07). provides an overview of the disgust response toward sex-irrelevant disgust and sex-relevant disgust items dependent on the level of familiarity as the source of disgust.
Table A1. Study 1, Age Group 0: Mean Values of Disgust Type × Level of Familiarity
Overall Analysis of the Main Variables Including Age Group 0
The disgust ratings (dependent variable) were subjected to a mixed-factor analysis of variance (ANOVA) with disgust type (sex-irrelevant disgust, sex-relevant disgust), and familiarity (parent, best friend, stranger) as within-subjects factors, and sex (male, female), age group (0 = six to eight years, 1 = nine to 11 years, 2 = 12 to 14 years, 3 = 15 to 17 years) and country (Study 1, Study 2) as between-subjects factors. The main findings of the analysis were unchanged in comparison with the analysis (of age groups 1, 2, and 3; see Results) in which age group 0 was excluded. The results are given in , , and .
Table A2. Study 1, Age Group 0: Main Effects of the Mixed-Factor Analysis
Table A3. Study 1, Age Group 0: Relevant Interaction Effects of the Mixed-Factor Analysis
Table A4. Study 1, Age Group 0: Follow-Up One-Way ANOVAs of Disgust Type × Age Group
Appendix B
Scenario-Based Questions of the Disgust Development Scenario-Based Assessment (DD-SBA)
Atypical appearance disgust
Imagine (person) has a strange/deformed hand. How would you feel shaking hands with (person)?
Imagine (person) spent some time with people living in an undeveloped country with limited hygiene facilities. How would you feel about spending time with (person) now, after he returns from there?
Lesion disgust
Imagine (person) has bumps/pimples that are filled with fluid and pus on his/her face. How would you feel hugging (person) with touching his/her face?
Imagine (person) has open wounds on his/her hands and you see him/her using your towel. How would you feel while using the towel again?
Hygiene disgust
Imagine (person) used the toilet before you and he/she left poop in the toilet base. How would you feel now that you need to use the toilet and sitting on the rim?
Imagine you forgot to take your toothbrush on holidays and you realize just before going to bed. How would you feel having to use the toothbrush of (person) for the first evening, before there is time to buy a new one the next day?
Food and animal disgust
Imagine you got your favorite fruit from (person) and you took a first bite from it. After that you turn it in your hand and you realize it is rotten. How would you feel about that?
Imagine the dog of (person) is running toward you while you are walking in a park. When he reaches you he starts licking your hand. How would you feel about that?
Vomit disgust
Imagine (person) was eating from your lunch, but there are still some leftovers. How would you feel finishing the lunch with the same fork?
Imagine (person) is changing the nappy of a baby and does not wash his/her hands afterward. How would you feel if he/she is now preparing your lunch?
Sex disgust (in the article, this is referred to as sex-relevant disgust)
Imagine (person) gives you a kiss on your mouth. How would you feel about that?
Imagine you are in a busy family changing room at the swimming pool and (person) sits down next to you wearing only a towel and touches your naked arm with his naked arm accidentally. How would you feel about that?
