ABSTRACT
Although prior evidence supports women’s mating behaviors and preferences being related to ovarian hormonal levels, there is conflicting evidence about exactly which hormones predict sexual function best, which specific psychosexual facets are affected and how between-individual and within-individual differences relate to this question. In this study levels of estradiol and progesterone were measured (once daily for 15 days for each participant) for 97 women, who attended two testing sessions, in times of the cycle varying in conception probability (based on the luteinizing hormone (LH) test result). Women completed surveys on their sexual desire, arousal, sexual activity frequency and initiation. There was a significant difference between peri-ovulatory and luteal values for all sexual function variables. Between-subject progesterone negatively predicted sexual activity frequency only. Within-subject estradiol positively and progesterone negatively predicted sexual desire. The findings provide support for hormonal underpinnings of sexual desire and sexual activity frequency fluctuations during the menstrual cycle. The findings did not yield support for hormonal influences on sexual arousal and initiation of sexual encounters. The main findings are consistent with the excitatory and inhibitory effects of estradiol and progesterone, respectively, on measures of women’s sexual motivation.
Introduction
Women’s mating behaviors and preferences are modulated, at least partly, by ovarian hormonal levels (Arslan et al., Citation2018; Jones et al., Citation2018; Marcinkowska et al., Citation2018; Roney & Simmons, Citation2013). Despite numerous studies associating facets of sexuality and hormones, there are some conflicting findings regarding which exact hormones predict sexual function best, which specific psychosexual facets are affected (Grebe et al., Citation2016; Roney & Simmons, Citation2016) and whether between-individual differences or within-individual changes are of significance (Havlicek et al., Citation2015; Marcinkowska et al., Citation2018; Roney & Simmons, Citation2013). Here, we tested whether facets of sexual function (sexual desire, arousal, and frequency and initiating of sexual activity) are related to both daily and average levels of two sex hormones: estradiol and progesterone.
Levels of sex hormones fluctuate significantly across the menstrual cycle. In a typically functioning menstrual cycle, on average two weeks after the menstrual bleeding onset, an egg is released from the follicle, allowing fertilization to occur. This process is called ovulation and the time near this event (between 5 days before through the day of ovulation; see Wilcox et al., Citation1998) is the only time in the cycle when conception is possible (late follicular or peri-ovulatory period). Toward the end of the follicular phase, the follicle secretes high doses of estradiol until, on average, one day before ovulation (Lipson & Ellison, Citation1996). After the release of an egg, levels of estradiol decrease, while levels of progesterone rise, preparing the lining of the uterus for possible implantation of the successfully fertilized egg. If fertilization and consequent implantation does not happen, the progesterone level drops and menstrual bleeding starts, marking the onset of the next menstrual cycle.
As studies have found links between hormones and various dimensions of individual differences (Luine, Citation2014), it has been suggested that the fluctuations of sex hormones during the menstrual cycle should predict corresponding changes in sexual behavior, desire and preferences (Thornhill & Gangestad, Citation2008). As conception is possible only during the narrow peri-ovulatory period (when estradiol levels peak), enhanced sexual activity and desire at that time could facilitate obtaining “good genes” for a woman’s offspring, if such desire is specifically focused on men with cues of higher genetic fitness (Thornhill & Gangestad, Citation2008). Recent sufficiently powered studies, however, have failed to support the idea that preferences for more masculine traits are higher when women are tested near ovulation (e.g., Jünger et al., Citation2018; Stern et al., Citation2020; for a review, see Jones et al., Citation2019), thereby calling this idea into question. Another motivational mechanism could be a general increase in sexual motivation during the peri-ovulatory period when the fitness benefits (conceiving a child) are the highest relative to the fitness costs of sexual behavior, such as risk of infection (Roney, Citation2018; Roney & Simmons, Citation2008, Citation2013, Citation2017). Alternatively, it has been suggested that within-woman changes in mating psychology are simply a by-product of adaptive expression of these behaviors between women varying in reproductive condition (the “Spandrel Hypothesis,” Havlicek et al., Citation2015). According to this hypothesis, sex hormones that are responsible for the between-women differences in sexual behavior will affect the within-women variation in the same manner.
Only a small number of studies have directly measured associations between within-women fluctuations in ovarian hormones and within-women changes in aspects of sexual desire and behavior. Two studies with repeated measurements spanning all phases of the cycle provided evidence that within-women shifts in self-reports of sexual desire were positively predicted by fluctuations in estradiol but negatively predicted by shifts in progesterone (Jones et al., Citation2018 [N = 375, >5 measurements per women]; Roney & Simmons, Citation2013 [N = 43, >14 measurements per women]). Roney and Simmons (Citation2013) also reported higher odds of sexual behavior on days when estradiol was higher, but found no between-women effects of average hormone concentrations on sexual desire or behavior. Neither study found significant associations between testosterone and any sexual variable examined. Positive effects of estradiol combined with the negative effects of progesterone on sexual desire could generate peri-ovulatory peaks in desire that have been reported in other studies (for a review, see Cappelletti & Wallen, Citation2016; Motta-Mena & Puts, Citation2017).
Despite the similar findings reported by Roney and Simmons (Citation2013) and Jones et al. (Citation2018), other studies have produced conflicting evidence regarding the relationship between ovarian hormones and women’s sexual motivation. Righetti et al. (Citation2019) targeted the middle 15 days of the cycle in a sample (N = 30), and did not reject the null hypothesis for associations between a daily measure of general sexual desire and fluctuations in estradiol, progesterone, or testosterone. Grebe et al. (Citation2016) collected two saliva samples one week apart (N = 33) and found that change in estradiol across the two samples was negatively correlated with change in general sexual desire, with no significant effects for change in progesterone. Likewise, Shirazi et al. (Citation2019) did not confirm relationships between change in estradiol or progesterone measured twice about two months apart (N = 87) and corresponding change in scores on the Sexual Desire Inventory (SDI-2, Spector et al., Citation1996). Rather than ovarian hormones predicting shifts in general sexual desire, both Grebe et al. (Citation2016) and Shirazi et al. (Citation2019) argued that the desire for uncommitted sex or extra-pair partners may be elevated when estradiol is higher (see also Marcinkowska, Citation2020), although some of the specific empirical patterns used to support those conclusions (i.e. multiple measurements of daily estradiol levels within cycle being related to solely extra-pair attraction) were not found in other studies (Jones et al., Citation2018; Roney & Simmons, Citation2016).
The conflicting findings described above underline the importance of gathering additional data assessing the relationship between ovarian hormones and measures of women’s sexual function. The current study analyzed hormonal predictors of general sexual function (in the sense that targets of desire were not specified), and included measures of desire, arousal, and sexual activity frequency and initiation. One limitation of some prior studies that have reported nonsignificant associations between hormones and general sexual motivation has been the imprecise control of time in the menstrual cycle. In the Grebe et al. (Citation2016) study, for instance, they reported that the majority of their participants appeared to collect both of their samples during the luteal phase, such that they were unable to consistently test hormonal predictors of desire over the entire range of hormone variability across the cycle.
We hypothesized a positive relationship between measurements of sexual desire and behavior and daily levels of estradiol, and a negative association between desire and daily levels of progesterone. We did not a priori hypothesize strong associations between facets of sexual function and between-individual differences in hormone values, in part due to the pronounced differences in hormonal levels between women (Marcinkowska, Citation2020).
Method
Participants
This study was part of a bigger project conducted in 2014–2019 (Marcinkowska et al., Citation2017). Participants were recruited from the Malopolska region of Poland (Mean age = 28.8, range = 21–37, SD = 4.56), and had: regular menstrual cycles (difference between consecutive cycles not larger than ±5 days), no medically diagnosed reproductive health problems and no diabetes. Additionally, only women who were not pregnant, breastfeeding, or taking hormonal contraception for at least 3 months prior were included in the study. All participants were Caucasian and of European descent. Out of 110 women recruited, 97 completed the study; 71 reported being in a romantic relationship, 4 identified as bisexual, and 4 as predominantly or entirely gay or lesbian. All participants provided their written, informed consent.
Measures
To measure multiple aspects of sexual function we asked participants questions about: sexual desire (two items capturing frequency and level of felt sexual desire), frequency of sexual activity (one item), initiation of sexual activity (one item) and sexual arousal (2 items capturing frequency and level of experiences of arousal) for the time period of “over the last 3 days.” To limit burden associated with participation, we used only selected items from well-established questionnaires, validated for use in Polish language and adjusted for frequent testing (FSFI; Rosen et al., Citation2000, SDI-2; Spector et al., Citation1996). For a full list of items, see ESM 1.
Procedure
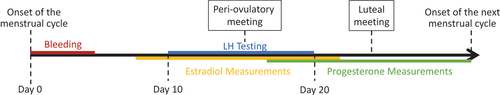
All participants completed questionnaires on 2 meetings during one menstrual cycle: once during the peri-ovulatory meeting and once during the luteal meeting. During both meetings, participants completed a survey related to sexual desire, behavior, preferences, and arousal. Even though participants attended 3 meetings in total, on the first meeting they did not answer the sexual activity questions. The first meeting was scheduled not more than 48 hours after obtaining a positive result from a urinary LH-based ovulatory test (peri-ovulatory meeting), and the second meeting took place approximately 7 days after the first one (luteal meeting). Participants were asked to report positive results of the LH test immediately, and if in doubt, they were asked to consult with the PI by sending a photo of the test result. Women who did not obtain a positive ovulation test result attended the first meeting around the 20th day of the cycle (see for a diagram of the procedure).
Figure 1. Diagram of timing of the steroid testing, meetings, and LH testing across a menstrual cycle. Peri-ovulatory meeting took place after obtaining a positive LH test result or at the last day of the LH testing if no positive result appeared. Luteal meeting took place 7 days after the peri-ovulatory meeting.
To increase participants’ comfort, they completed the survey alone in an isolated space, they then put the surveys into a sealed envelope which was given to the principal investigator, and passed on to a research assistant who had never met participants and was not familiar with their names. Research assistants typed in the anonymized data, which were then used for statistical analyses.
Additionally, all participants collected daily saliva samples throughout the menstrual cycle. Participants were instructed to collect saliva samples upon waking up and not earlier than 30 min after eating, drinking, or smoking (Ellison et al., Citation1987). After the end of the menstrual cycle, all samples were transported in portable freezers from participants’ homes to the laboratory where hormonal assays were conducted. From each cycle, on average 15 samples were chosen for the hormonal analysis. As we were interested in hormonal changes throughout menstrual cycles, only cycle phases in which we expected to see pronounced variation were analyzed (14 days centered around ovulation for estradiol and 14 luteal phase days for progesterone). This allowed for increasing both the sample size and number of measurements per participant. Urine ovulation (LH) tests were conducted from the 10th day until obtaining a positive test result or until the 20th day of the cycle (following Blake et al., Citation2016).
Hormonal Data Collection
Levels of sex hormones were measured in saliva samples. Each sample was frozen in the participant’s home freezer immediately after collecting. Hormonal measurements were conducted in duplicates using commercially available hormonal assays of DRG International Inc. Elisa plates SLV4188 for 17-β-estradiol (E, sensitivity: 0.4 pg/ml, standard range: 1–100 pg/ml) and SLV3140 for 17-α-hydroxy-progesterone (P, sensitivity: 2.5 pg/ml, standard range:10–5000 pg/ml). To ensure high quality of the analyses, on each plate samples of known concentrations were added, i.e.: low control and high control. Inter- and intra-assay variability was 10.1% and 7.5% for E and 14.1% and 4.9% for P (for a detailed hormonal description of the sample, see Marcinkowska, Citation2020).
Data Analysis
All data is available in electronic supplementary material (ESM 2). Differences in hormonal and psychosexual variables between the two meetings were assessed using paired-samples t-tests.
To assess the contribution of both between-individual differences and within-individual changes in hormones to general sexual desire, arousal, frequency of activity, and activity initiation we estimated three groups of models. For a robustness test, all analyses described in the main text were run first using raw hormone values, and were then re-run using log-transformed hormone values. As log-transformed data may diminish the real variation between and within cycles (but see Gangestad et al., Citation2019; Roney, Citation2019), we chose to present analyses using the raw data analyses in the main text, and analyses using log-transformed data in ESM 3. Results were consistent across analyses using raw and log-transformed hormone values.
The first set of models included individual averages of estradiol and progesterone as predictors of sexuality variables of interest. The second set of models included within-person centered values of estradiol, progesterone, and their interaction to elucidate whether within-individual fluctuations in hormones predicted sexuality variables of interest. The third set of models was identical to the second, but additionally included terms for between-individual differences in average estradiol and progesterone levels.
We also estimated regressions using one- and two-day time lags for estradiol and progesterone levels, as previous work has suggested the strongest effects may be seen at these lags (Roney & Simmons, Citation2013). However, because these analyses were based on a smaller number of samples and were thus relatively underpowered, they should be considered exploratory. In addition, the temporal scope of the questions as “over the past 3 days” introduces ambiguity in the interpretation of these effects, and the analyses were thus moved to the online supplement (see ESM 3 for full results on lagged hormonal effects).
Results
Between-subject Effects
Between two testing sessions, 61.3% of women reported sexual activity, out of which 87.3% reported they were at least sometimes the initiator of the activity (see ESM 1 for the full list of possible answers and ESM 2 for raw data). There was a statistically significant difference between peri-ovulatory and luteal values for all examined outcome variables of interest, wherein levels of sexual desire, activity and arousal were higher in the peri-ovulatory phase relative to the luteal phase (). Levels of estradiol nor progesterone did not show a significant difference between meetings ().
Figure 2. Values of sexual function variables at two meetings with SE (dark gray – peri ovulatory meeting, light gray – luteal meeting).
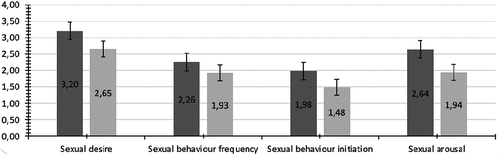
Table 1. Descriptive statistics of variables included in the model and significance tests of differences between two meetings.
In the model including terms for between-individual effects only, average estradiol did not significantly predict desire, arousal, or sexual activity frequency and initiation (). Average progesterone significantly negatively predicted only sexual activity frequency (β = −0.21, 95% CI = −0.30, −0.12, p = .019). This pattern of results was replicated when using log-transformed hormone values (ESM 3).
Table 2. Model coefficient estimates (and p-values) for between-subject effects of estradiol and progesterone on sexuality-related variables.
Within-subject Effects
Within-subject changes in estradiol positively (β = 0.29, 95% CI = 0.20, 0.38, p = .004), and progesterone negatively (β = −0.24, 95% CI = −0.34, −0.14, p = .015), predicted sexual desire (). The interaction between daily estradiol and progesterone level was not significant (β = 0.02, 95% CI = −0.08, 0.12, p = .805). The statistical significance and magnitude of these effects was virtually unchanged when substituting raw hormone values for log-transformed values with a single exception: the effect size of estradiol positively predicting sexual activity frequency was slightly larger for raw (β = 0.22, 95% CI = 0.13, 0.31, p = .029) than for log-transformed (β = 0.16, 95% CI = −0.01, 0.32, p = .052) values. No other effects were statistically significant ().
Table 3. Model coefficient estimates (and p-values) for within-subject effects of raw values of estradiol and progesterone on sexuality-related variables.
As a check of the robustness of the obtained results, and to account for the possible between-individual differences, we repeated these models based on daily hormonal measurements while including average estradiol and progesterone concentrations per participant. The inclusion of between-subject terms did not meaningfully change our findings. Namely, while including the between-subject differences, estradiol positively predicted (β = 0.32, 95% CI = 0.23, 0.41, p = .002) and progesterone negatively predicted (β = −0.35, 95% CI = −0.43, 0.27, p < .001) sexual desire, and the interaction between estradiol and progesterone was not significant (for full results, see ESM 4). Fluctuations in estradiol positively predicted sexual activity frequency when using both raw values (β = 0.28, 95% CI = 0.20, 0.36, p = .006) and log-transformed values (β = 0.24, 95% CI = 0.16, 0.32, p = .008). No other effects were statistically significant ().
A number of supplementary analyses and robustness checks are presented in the ESM files. Supplementary analyses included exploratory examinations of time-lagged effects of hormones (ESM 3) and the influence of estradiol-to-progesterone ratio on sexual variables (this variable did not have significant effects when added to models including estradiol and progesterone; see ESM 5). The hormone effects reported above were robust to exclusion of outliers and also to alternative decisions regarding the addition of random slope terms in the multilevel statistical models (see ESM 6 and ESM 7, respectively).
Discussion
All sexual function variables decreased from the peri-ovulatory to the luteal meeting. Women showed lower sexual arousal and desire, experienced fewer sexual encounters, and initiated them less often when conception probability was low. These results are consistent with the idea that mating psychology functions to facilitate increased sexual interest when conception probability is highest (Roney & Simmons, Citation2013). For sexual desire in particular, some researchers have argued that significant peri-ovulatory increases are absent when desire is measured in a target-general way (e.g., Gangestad et al., Citation2002; Haselton & Gangestad, Citation2006). The current demonstration of greater general desire near ovulation adds important evidence to this debate from a study with precise determination of ovulatory timing via LH tests.
An advantage of the current study was its use of LH tests to schedule testing sessions within the peri-ovulatory and luteal phases, thus ensuring that hormonal predictors were assessed across phases that vary in conception probability. Although sexuality measures were collected twice per cycle for each woman, multiple hormonal measurements occurred throughout the entire menstrual cycle. This allowed for precisely estimating, and thus analyzing the effects of, both between-individual differences and within-individual fluctuations in hormone levels.
Interestingly, levels of neither estradiol nor progesterone showed a significant difference between meetings. Some women attended the peri-ovulatory meeting around 48 hours after a positive result of the LH test; hence, it is possible that it was just after the estradiol peak. On the other hand, differences in progesterone between meetings were marginally significant in the expected direction. Lack of significant differences here could be caused by a pronounced inter-individual variation in average hormonal levels – presumably much greater than the cyclical within-individual changes (for an in-depth description of hormonal data for the current sample, see Marcinkowska, Citation2020).
In a between-women comparison, only sexual activity frequency was negatively related to average progesterone levels (no other between-women effects were statistically significant). In other words, although there was no within-cycle relationship between progesterone and sexual frequency, women with higher average progesterone across the cycle reported a lower frequency of sex across the two test session measurements. This pattern was not predicted and is difficult to interpret given the absence of a comparable effect within women.
Within cycle levels of estradiol positively predicted levels of general sexual desire and frequency of sexual activity. The desire effects are consistent with prior studies that have reported some evidence for positive within-cycle associations between estradiol and general sexual desire (Jones et al., Citation2018; Roney & Simmons, Citation2013) but inconsistent with studies that have reported non-significant (Righetti et al., Citation2019; Shirazi et al., Citation2019) or negative (Grebe et al., Citation2016) relationships. The sexual activity effect replicates a prior finding that women showed higher odds of sexual behavior on those days when their estradiol concentrations were higher (Roney & Simmons, Citation2013).
Within-women daily levels of progesterone were negatively related to general sexual desire between the two meetings. This result replicates the consistent, negative within-women associations between progesterone and desire reported in two prior studies (Jones et al., Citation2018; Roney & Simmons, Citation2013). Levels of progesterone rise during the luteal phase (in preparation for a possible pregnancy) when conception is not possible. Progesterone may inhibit sexual motivation to avoid the fitness costs of sex, other things equal, when conception is absent as a possible fitness benefit (Roney, Citation2018). Excitatory effects of estradiol in the peri-ovulatory period coupled with the inhibitory effects of progesterone in the luteal phase can combine to cause peri-ovulatory increases in general sexual desire, as demonstrated in the present study via differences in desire across the two testing sessions.
The overall results for sexual desire appear most consistent with motivational priorities theory (Roney, Citation2018). The theory posits that estradiol and progesterone shift motivation between sexuality and alternative priorities such as food intake via opposite effects on the different motivations. In many nonhuman mammals, estradiol promotes sexual receptivity but inhibits feeding, whereas progesterone has the opposite pattern of effects (reviewed in Schneider et al., Citation2013). Roney and Simmons (Citation2017) likewise provided preliminary evidence for these same patterns in women. Although food intake was not measured in the current study, the endocrine and cycle phase predictors for sexual desire were as predicted by motivational priorities theory, in which sexual motivation is elevated (and prioritized over alternative priorities) during the fecund peri-ovulatory phase relative to the non-fecund luteal phase. Because the specific targets of attraction were not measured in the current research, the findings do not provide strong evidence regarding the ovulatory shift hypothesis whereby women are predicted to show a selective increase in attraction to men with good genes indicators when tested in the periovulatory phase (Thornhill & Gangestad, Citation2008). Finally, regarding sexual desire, the current findings provide no support for the “Spandrels Hypothesis” (Havlicek et al., Citation2015) that posits that within-cycle psychological shifts are by-products of between-women hormone effects. Between-women correlations between hormones and desire were non-significant in the current study (see ) in spite of significant within-cycle correlations (see ).
In contrast to effects for desire, hormone effects were not significant for measures of initiation of sexual encounters and arousal in response to sexual activity, despite cycle phase effects for these variables. The reasons for these dissociations between cycle phase and hormone effects are unclear, although similar dissociations have been reported in other studies in humans (Stern et al., Citation2021). Likewise, in nonhuman primates, there is often variability in the relationships between hormones, cycle phase, and distinct behavioral and morphological outcomes (Higham, Citation2019). If the brain mechanisms regulating arousal and behavioral initiation are slightly different than those regulating desire, then the distinct mechanisms may differ in their sensitivity to hormone effects in ways that produce such dissociations (see Higham, Citation2019). In addition, other physiological signals that vary with cycle phase might help to explain the variability in the initiation and arousal variables. Identifying and measuring such physiological signals would be a valuable future direction of peri-ovulatory shifts studies.
It is also possible that the variables based on physical contact with the partner were determined by the partner’s current accessibility, rather than participants’ fertility (Arslan et al., Citation2018). Not all participants lived with their partners; hence, even in the presence of increased sexual desire, no increase in sexual activity with the partner, nor arousal during the activity would be reported (although as many as 61% of participants reported sexual activity between the two sessions). Previous studies also showed that women’s sexual function is related to a variety of factors, including the length of current relationship or sexual problems experienced by partners (McCabe & Goldhammer, Citation2012), type of attachment style and relational dimensions such as communication and compatibility with the partner (Peixoto & Lopes, Citation2022), as well as intimacy level and partner’s emotional responsiveness (van Lankveld et al., Citation2021). Both demographic and psychological factors can therefore account for significant variability in the dynamics of women’s sexual functioning.
A related issue that could be enhanced in future research is using more items to measure each variable of interest. Measuring variables of interest based on only one or two items may lead to broad standard errors and impact the reliability of the measurement (Arslan et al., Citation2018). It is possible that multi-item measurements of sexual behavior, desire, and arousal would better track variation in women’s sexuality.
Another possible future direction would be more detailed measurements of daily hormonal levels and daily completion of the surveys asking about sexual activity restricted to a single day instead of over the past three days as in the current study. More frequent measurements would allow for running sufficiently powered models on the lagged effects. As measuring lagged effects was not of primary concern in this study, only some data were available for such analysis here and models based on lagged effects were underpowered.
To decrease hormonal measurement errors mass spectrometry could be used instead of immunoassays (Schultheiss et al., Citation2018). Mass spectrometry measurements are based on stable physical properties of molecules, while immunoassays are based on biochemical reactions, that can possibly be affected by changes in external factors, such as room temperature, enzyme purity, and enzyme activity (Selby, Citation1999). Using mass spectrometry, however, is very costly and implementing both this measuring method and frequent hormonal measurements was not possible for this study.
Additionally, in this study all women started their participation from the beginning of menses, meaning that the order of meetings (peri-ovulatory and luteal) was always the same. This could potentially lead to the exposure effect (Zajonc, Citation1968), where participants’ responses change merely due to previous exposure to a certain stimulus (in this case the particular set of questions). To account for this possible bias (stemming from lack of randomization of meetings), daily hormonal measurements of two full menstrual cycles would have had to be conducted, which in this study was not feasible.
Conclusions
In summary, the results of this study obtained from robust statistical analyses, including logged and raw data, and exploratory models of the effects of hormonal levels, provide support for hormonal underpinnings of general sexual desire and sexual activity frequency fluctuations during the menstrual cycle. We did not find support for hormonal influences on sexual arousal and initiation of sexual encounters. The main findings are consistent with the excitatory and inhibitory effects of estradiol and progesterone, respectively, on measures of women’s sexual motivation, the combined effects of which may increase sexual desire and behavior during the periovulatory region relative to the luteal phase.
Author Contribution
Conceptualization: UMM, MM. Data curation: UMM, MM. Formal analysis: TS. Funding acquisition: UMM. Methodology: UMM, MM. Statistical Analysis: TS. Writing and editing of the manuscript: UMM, TS, JR, MM.
Supplemental Material
Download Zip (259.2 KB)Disclosure Statement
No potential conflict of interest was reported by the authors.
Data Availability Statement
All raw data are available in the Electronic Supplementary Materials 2.
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00224499.2022.2110558
Additional information
Funding
References
- Arslan, R. C., Schilling, K. M., Gerlach, T. M., & Penke, L. (2018). Using 26,000 diary entries to show ovulatory changes in sexual desire and behavior. Journal of Personality and Social Psychology, 121(12), 140–431. https://doi.org/10.1037/pspp0000208
- Blake, K. R., Dixson, B. J. W., O’Dean, S. M., & Denson, T. F. (2016). Standardized protocols for characterizing women’s fertility: A data-driven approach. Hormones and Behavior, 81, 74–83. https://doi.org/10.1016/j.yhbeh.2016.03.004
- Cappelletti, M., & Wallen, K. (2016). Increasing women’s sexual desire: The comparative effectiveness of estrogens and androgens. Hormones and Behavior, 78, 178–193. https://doi.org/10.1016/j.yhbeh.2015.11.003
- Ellison, P. T., Lager, C., & Calfee, J. (1987). Low profiles of salivary progesterone among college undergraduate women. Journal of Adolescent Health, 8(2), 204–207. https://doi.org/10.1016/0197-0070(87)90266-x
- Gangestad, S. W., Dinh, T., Grebe, N. M., Del Giudice, M., & Thompson, M. E. (2019). Psychological cycle shifts redux, once again: Response to Stern et al., Roney, Jones et al., and Higham. Evolution and Human Behavior, 40(6), 537–542. https://doi.org/10.1016/j.evolhumbehav.2019.08.008
- Gangestad, S. W., Thornhill, R., & Garver, C. E. (2002). Changes in women’s sexual interests and their partners’ mate-retention tactics across the menstrual cycle: Evidence for shifting conflicts of interest. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269(1494), 975–982. https://doi.org/10.1098/rspb.2001.1952
- Grebe, N. M., Emery Thompson, M., & Gangestad, S. W. (2016). Hormonal predictors of women’s extra-pair vs. in-pair sexual attraction in natural cycles: Implications for extended sexuality. Hormones and Behavior, 78, 211–219. https://doi.org/10.1016/j.yhbeh.2015.11.008
- Haselton, M. G., & Gangestad, S. W. (2006). Conditional expression of women’s desires and men’s mate guarding across the ovulatory cycle. Hormones and Behavior, 49(4), 509–518. https://doi.org/10.1016/j.yhbeh.2005.10.006
- Havlicek, J., Cobey, K. D., Barrett, L., Klapilova, K., & Roberts, S. C. (2015). The spandrels of Santa Barbara? A new perspective on the peri-ovulation paradigm. Behavioral Ecology, 26(5), 1249–1260. https://doi.org/10.1093/beheco/arv064
- Higham, J. P. (2019). A comparative perspective on measures of cycle phase, and how they relate to cues, signals, and mating behavior: A commentary on Gangestad, Dinh, Grebe, Del Giudice, and Emery Thompson (2019). Evolution and Human Behavior, 40(6), 533–536. https://doi.org/10.1016/j.evolhumbehav.2019.08.007
- Jones, B. C., Hahn, A. C., & DeBruine, L. M. (2019). Ovulation, sex hormones, and women’s mating psychology. Trends in Cognitive Sciences, 23(1), 51–62. https://doi.org/10.1016/j.tics.2018.10.008
- Jones, B. C., Hahn, A. C., Fisher, C. I., Wang, H., Kandrik, M., & DeBruine, L. M. (2018). General sexual desire, but not desire for uncommitted sexual relationships, tracks changes in women’s hormonal status. Psychoneuroendocrinology, 88, 153–157. https://doi.org/10.1016/j.psyneuen.2017.12.015
- Jünger, J., Kordsmeyer, T. L., Gerlach, T. M., & Penke, L. (2018). Fertile women evaluate male bodies as more attractive, regardless of masculinity. Evolution and Human Behavior, 39(4), 412–423. https://doi.org/10.1016/j.evolhumbehav.2018.03.007
- Lipson, S. F., & Ellison, P. T. (1996). Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Human Reproduction, 11(10), 2090–2096. https://doi.org/10.1093/oxfordjournals.humrep.a019055
- Luine, V. N. (2014). Estradiol and cognitive function: Past, present and future. Hormones and Behavior, 66(4), 602–618. https://doi.org/10.1016/j.yhbeh.2014.08.011
- Marcinkowska, U. M. (2020). Importance of daily sex hormone measurements within the menstrual cycle for fertility estimates in cyclical shifts studies. Evolutionary Psychology, 18(1), 1474704919897913. https://doi.org/10.1177/1474704919897913
- Marcinkowska, U. M., Galbarczyk, A., & Jasienska, G. (2017). La donna e mobile? Lack of cyclical shifts in facial symmetry, and facial and body masculinity preferences-A hormone based study. Psychoneuroendocrinology, 88, 47–53. https://doi.org/10.1016/j.psyneuen.2017.11.007
- Marcinkowska, U. M., Kaminski, G., Little, A. C., & Jasienska, G. (2018). Average ovarian hormone levels, rather than daily values and their fluctuations, are related to facial preferences among women. Hormones and Behavior, 102, 114–119. https://doi.org/10.1016/j.yhbeh.2018.05.013
- McCabe, M. P., & Goldhammer, D. L. (2012). Demographic and psychological factors related to sexual desire among heterosexual women in a relationship. The Journal of Sex Research, 49(1), 78–87. https://doi.org/10.1080/00224499.2011.569975
- Motta-Mena, N. V., & Puts, D. A. (2017). Endocrinology of human female sexuality, mating, and reproductive behavior. Hormones and Behavior, 91(Suppl. C), 19–35. https://doi.org/10.1016/j.yhbeh.2016.11.012
- Peixoto, M. M., & Lopes, P. (2022). Solitary and dyadic sexual desire and sexual satisfaction in women with and without sexual concerns. Journal of Sex & Marital Therapy, 1–11. https://doi.org/10.1080/0092623X.2022.2077271
- Righetti, F., Tybur, J., Van Lange, P., Echelmeyer, L., Van Esveld, S., Kroese, J., Van Brecht, J., & Gangestad, S. (2019). How reproductive hormonal changes affect relationship dynamics for women and men: A 15-day diary study. Biological Psychology, 149, 107784. https://doi.org/10.1016/j.biopsycho.2019.107784
- Roney, J. R. (2018). Functional roles of gonadal hormones in human pair bonding and sexuality. In O. C. Schultheiss & P. H. Mehta (Eds.), Routledge international handbook of social neuroendocrinology (pp. 239–255). Routledge.
- Roney, J. R. (2019). On the use of log transformations when testing hormonal predictors of cycle phase shifts: Commentary on Gangestad, Dinh, Grebe, Del Giudice, and Emery Thompson (2019). Evolution and Human Behavior, 40(6), 526–530. https://doi.org/10.1016/j.evolhumbehav.2019.08.006
- Roney, J. R., & Simmons, Z. L. (2008). Women’s estradiol predicts preference for facial cues of men’s testosterone. Hormones and Behavior, 53(1), 14–19. https://doi.org/10.1016/j.yhbeh.2007.09.008
- Roney, J. R., & Simmons, Z. L. (2013). Hormonal predictors of sexual motivation in natural menstrual cycles. Hormones and Behavior, 63(4), 636–645. https://doi.org/10.1016/j.yhbeh.2013.02.013
- Roney, J. R., & Simmons, Z. L. (2016). Within-cycle fluctuations in progesterone negatively predict changes in both in-pair and extra-pair desire among partnered women. Hormones and Behavior, 81, 45–52. https://doi.org/10.1016/j.yhbeh.2016.03.008
- Roney, J. R., & Simmons, Z. L. (2017). Ovarian hormone fluctuations predict within-cycle shifts in women’s food intake. Hormones and Behavior, 90, 8–14. https://doi.org/10.1016/j.yhbeh.2017.01.009
- Rosen, R. C., Brown, C., Heiman, J., Leiblum, S. R., Meston, C., Shabsigh, R., Ferguson, D., & D’Agostino, R. J. R. (2000). The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. Journal of Sex & Marital Therapy, 26(2), 191–208. https://doi.org/10.1080/009262300278597
- Schneider, J. E., Wise, J. D., Benton, N. A., Brozek, J. M., & Keen-Rhinehart, E. (2013). When do we eat? Ingestive behavior, survival, and reproductive success. Hormones and Behavior, 64(4), 702–728. https://doi.org/10.1016/j.yhbeh.2013.07.005
- Schultheiss, O. C., Dlugash, G., & Mehta, P. (2018). Hormone measurement in social neuroendocrinology: A comparison of immunoassay and mass spectroscopy methods. In O. C. Schultheiss & P. H. Mehta (Eds.), Routledge international handbook of social neuroendocrinology (pp. 81–96). Routledge.
- Selby, C. (1999). Interference in immunoassay. Annals of Clinical Biochemistry: International Journal of Laboratory Medicine, 36(6), 704–721. https://doi.org/10.1177/000456329903600603
- Shirazi, T. N., Self, H., Dawood, K., Rosenfield, K. A., Penke, L., Carre, J. M., Ortiz, T., & Puts, D. A. (2019). Hormonal predictors of women’s sexual motivation. Evolution and Human Behavior, 40(3), 336–344. https://doi.org/10.1016/j.evolhumbehav.2019.02.002
- Spector, I. P., Carey, M. P., & Steinberg, L. (1996). The Sexual Desire Inventory: Development, factor structure, and evidence of reliability. Journal of Sex & Marital Therapy, 22(3), 175–190. https://doi.org/10.1080/00926239608414655
- Stern, J., Gerlach, T. M., & Penke, L. (2020). Probing ovulatory-cycle shifts in women’s preferences for men’s behaviors. Psychological Science, 31(4), 424–436. https://doi.org/10.1177/0956797619882022
- Stern, J., Kordsmeyer, T. L., & Penke, L. (2021). A longitudinal evaluation of ovulatory cycle shifts in women’s mate attraction and preferences. Hormones and Behavior, 128, 104916. https://doi.org/10.1016/j.yhbeh.2020.104916
- Thornhill, R., & Gangestad, S. W. (2008). The evolutionary biology of human female sexuality. Oxford University Press.
- van Lankveld, J. J. D. M., Dewitte, M., Verboon, P., & van Hooren, S. A. H. (2021). Associations of intimacy, partner responsiveness, and attachment-related emotional needs with sexual desire. Frontiers in Psychology, 12, 665967. https://doi.org/10.3389/fpsyg.2021.665967
- Wilcox, A. J., Weinberg, C. R., & Baird, D. D. (1998). Post-ovulatory ageing of the human oocyte and embryo failure. Human Reproduction, 13(2), 394–397. https://doi.org/10.1093/humrep/13.2.394
- Zajonc, R. B. (1968). Attitudinal effects of mere exposure. Journal of Personality and Social Psychology, 9(2, Pt.2), 1–&. https://doi.org/10.1037/h0025848
