ABSTRACT
Sexual motivation (desire) requires the simultaneous presence of an active central motive state and a stimulus with sexual significance. Once activated, sexual motivation leads to visceral responses and approach behaviors directed toward the emitter of the sexual stimulus. In humans, such behaviors follow cognitive evaluation of the context, including predictions of the approached individual’s response. After successful approach and establishment of physical contact, manifest sexual activities may be initiated. Sexual interaction is associated with and followed by a state of positive affect in most animals, whereas aversive consequences may be experienced by humans. The affective reactions may become associated with stimuli present during sexual interaction, and these stimuli may thereby alter their incentive properties. Here we show how the incentive motivation model can be used to explain the origins and possible treatments of sexual dysfunctions, notably disorders of desire. We propose that associations formed between negative outcomes of sexual interaction and the salient stimuli, for example, the partner, underlies hypoactive desire disorder. Highly positive outcomes of sexual interaction enhance the incentive value of the stimuli present, and eventually lead to hyperactive sexual desire. Treatments aim to alter the impact of sexual incentives, mainly by modifying cognitive processes.
Female low sexual interest/arousal disorder has attracted much attention among sexologists and other clinicians concerned with sexual functions (Brotto, Citation2017; Kleinplatz, Citation2018). Early epidemiological studies revealed that a substantial proportion of women complained of less sexual desire than they would like, and many of them reported this low desire as a source of interpersonal problems and distress (e.g. Segraves & Segraves, Citation1991; West et al., Citation2008). In view of the commercial success obtained with treatments for erectile dysfunction, it was expected that efficient treatment for female hypoactive sexual desire disorder would be still more successful, simply due to its higher prevalence. An intense search for pharmacological treatments was initiated, but the results have so far been meager. Two drugs for the treatment of low sexual interest/arousal disorder have been registered in the USA, but none in Europe. Whether these drugs really improve sexual desire in women remains unclear. Recent independent reviews conclude that their effects are not superior to placebo (Anderson & Moffatt, Citation2018; Jaspers et al., Citation2016; Saadat et al., Citation2017; Spielmans, Citation2022), an assertion contested by representatives of the proprietors of the marketing rights for these drugs (e.g. Kingsberg et al., Citation2021; Pyke & Clayton, Citation2018).
Psychotherapeutic procedures are also used for treating sexual disorders, including low sexual interest/arousal disorder (reviewed in Banbury et al., Citation2021; Frühauf et al., Citation2013). The efficiency of these procedures may be superior to drug treatment (Brotto et al., Citation2021; Pyke & Clayton, Citation2018), but conclusive evidence is lacking. It is not strange, then, that there is no standard first line approach to the treatment of disorders of sexual desire (Clayton et al., Citation2018). Considering the importance of sexual well-being for the quality of life (Anderson, Citation2013; Mitchell et al., Citation2021), this is an unfortunate situation.
One of the most important reasons for the difficulties in finding efficient treatment of sexual desire disorders is a deficient analysis of the concept of sexual desire, or sexual motivation as would be a more appropriate scientific term. If we are to understand the lack of desire for partnered sex, we first need to understand the sequence of events leading up to a sexual encounter, and the motivational mechanisms behind these events. Focusing directly on the copulatory interaction, basically mechanical stimulation of the genitals, is not enough. It would also be helpful to profit from the enormous amount of experimental data obtained in studies of non-human animals when elaborating a motivational model. Much of the non-human data are directly relevant for understanding basic principles of sexual motivation, and ignoring these data would probably lead to an incomplete model and a substantial loss of explanatory power.
We will present a model of the events from the detection of stimuli with sexual relevance until the postcoital, affective responses. This model is applicable to most mammalian species, perhaps to most vertebrates. Since we will not focus on pharmacological approaches to the treatment of sexual problems, we will only provide superficial mention of the neurobiological bases. An extensive review of that issue can be found elsewhere (Ågmo & Laan, Citation2022). The model will be supported by data from humans and other animals. Whenever of importance, we will point out species differences. After introducing the incentive motivational model, including the fundamental concept of sexual central motive state, we will show how the model can be applied to explain the etiology of disorders of sexual motivation, i.e. hypoactive and hyperactive desire in men and women. Specifically, we propose that the accumulated affective consequences of sexual encounters determine the incentive value of sexually relevant stimuli, hence their impact on the sexual central motive state. Reduced impact of sexual incentives would lead to weak sexual motivation and hypoactive desire disorder, whereas heightened impact would lead to strong sexual motivation and eventually hypersexuality. Furthermore, we suggest that the incentive motivation model can be used for proposing effective psychotherapeutic approaches, hopefully eliminating the need for additional pursuit of pharmacological treatments. We end this review by suggesting that many of the mysteries of sexual behavior can be uncovered, and some of the disorders of desire can be efficiently managed, without any reference to neurobiological processes.
This is not a meta-analysis, and consequently there was no need to include all published reports on a particular subject. For example, when describing the incentive motivation model, we cited some of the most relevant papers found in our personal databases. No effort was made to cite all papers on sexual incentive motivation. Likewise, when discussing hypoactive sexual interest/arousal disorder, we mainly cite relevant papers filed in our own databases. These were established several decades ago and contain thousands of papers published in the field of human and non-human sexual functions and dysfunctions. These databases are continuously updated, using appropriate bibliographic tools, including the Web of Science and PubMed. In every case, we have made an effort to be fair, i.e. to present both supporting and conflicting observations, when such exist.
Overview of the Incentive Motivation Model of Sexual Motivation
Many incentive-based models of sexual motivation have been presented, even long before the term “incentive” became established in behavior theory. Frank Beach (Citation1956) made explicit that rodents are not sexually motivated unless exposed to a sexually relevant stimulus, and William McDougall considered that sexual motivation in humans was activated by an external stimulus. This is clearly expressed in the following quote: “ … .the perception by the eye of the human form is one, and the principal one, of several innately provided roads to excitement of the sex instinct” (McDougall, Citation1914, p. 74). Freud also acknowledged the importance of external stimuli for enhancing sexual excitement, through the erotogenic zones (Freud, Citation1905). Since visual stimuli may cause sexual excitation, the eye is an erotogenic zone. This is also the case for the ear, since sounds may be sexually arousing.
A different approach to the analysis of sexual interactions was outlined by Donn Byrne (Citation1977). He maintained that conditioned and unconditioned sexually relevant stimuli activated physiological sexual responses, expectations and cognitive evaluation, which eventually could lead to “preparatory sexual behavior,” i.e. approach to a potential partner, and finally to sexual behavior, followed by an outcome. This sequence is very similar to what will be described in the present communication, but Byrne completely ignored the motivational mechanisms underlying the sequence of acts.
More recently, Toates (Citation1989) presented an outline of an explicit incentive motivational model, which was elaborated later (Toates, Citation2020; Singer & Toates, ; Toates, Citation2009). There are several other accounts of the workings of sexual desire, or motivation, based on incentive motivational concepts (Both et al., Citation2007; Hayes, Citation2011; Janssen, Citation2011; Laan and Both, Citation2008). Somewhat related to the incentive motivational models is the dual control model (Bancroft, Citation1999; Bancroft et al., Citation2009; Janssen & Bancroft, Citation2006). It is based on the notion that sexual functions are controlled by the balance between an inhibitory and an excitatory system. All these models search to explain human sexual motivation and they are neither based on nor applicable to other animals. However, sexual motivation is not exclusive to humans, and there is no reason to believe that human sexual motivation is entirely different from that in other animals. A motivational model applicable to all mammals, and perhaps also other classes of non-human animals, may benefit from the substantial amount of experimental data obtained in these animals.
The model to be used here (see for a schematic outline) is based on ideas derived from studies of sexual motivation in non-human animals, particularly rodents. The origin is an adaptation of the Bindra framework (Bindra, Citation1969, Citation1974, Citation1976, Citation1978) for the specific case of sexual activities (Ågmo, Citation1999, Citation2007, Citation2011). Social conventions and cognitive factors shape a substantial portion of human sexual behaviors (see Ford & Beach, Citation1951; Gagnon & Simon, Citation2002; Marshall & Suggs, Citation1971, for arguments supporting this assertion), whereas non-human sexual behaviors are series of preprogrammed action patterns. Because of this fundamental difference, some additional notions are necessary when analyzing the intricacies of human sexual interactions. We will discuss each of the elements in the model in the following sections, in the order in which they will occur during a sexual encounter and in which they appear from left to right in .
Figure 1. Schematic representation of the incentive motivation model. Empty arrows show unidirectional relationships, whereas filled arrows illustrate reciprocal relationships. The curved arrows represents feedback systems. For further details, see text. +, excitation. -, inhibition.
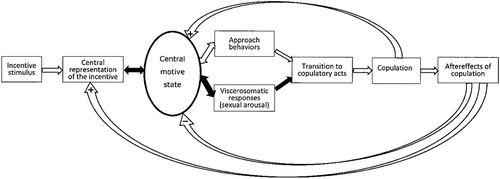
Before entering into specific issues, it is appropriate to explicitly define the subject on hand. We propose that sexual motivation should be understood as the mechanisms determining the probability of displaying sexual behavior when a mate is available or the intensity of that behavior when displayed. It can also refer to the intensity of approach to a potential sexual partner (Le Moëne & Ågmo, Citation2019, p. 2). Since sexual behavior often requires physical contact between at least two individuals, it is normally preceded by approach. It must be noted that sexual behavior, as it will be defined in the appropriate section, must involve the genitals. Penile-vaginal intercourse is one of many sexual behaviors.
The definition neither includes any mention of which the mental mechanisms might be nor any statement about the neurobiological bases. It refers exclusively to the behavioral manifestations of sexual motivation. This minimalist approach makes the definition applicable to species in which have little or no access to mental events, like rodents, as well as to species which have limited possibilities to establish the neurobiological bases of sexual motivation, like humans.
The Sexual Incentive Stimulus
The definition of a sexual incentive stimulus is straightforward: Any stimulus activating the sexual central motive state, a genital response and eventually sexual approach behavior in the receiving organism. The distinction between sexual approach and other approaches, for example to food or water when hungry or thirsty, is also straightforward: Sexual approach will potentially lead to sexual behavior but not to eating or drinking even when food and water are available. A similar argument is used for determining whether approach to a food stimulus is an expression of hunger, or of curiosity or of some other motive. It is an alimentary approach only if the approaching organism eventually will ingest the stimulus. Likewise, approach to water can only be considered an expression of thirst if the organism will drink when approach is complete. Whereas the motor patterns used for all these approaches can be identical, the motives activating them are entirely different. The specific motive activating approach can only be inferred from the kind of stimulus approached and the kind of commerce with the stimulus after successful approach.
In the case of the human, it must be added that external, sexually relevant stimuli are not necessary for the activation of sexual motivation and the ensuing behavior. Mental representations of such stimuli can be equally efficient. There are, for example, reports showing that women can activate genital responses and even attain solitary orgasm exclusively through sexual fantasies, without any concurrent stimulation of the genitals (Whipple et al., Citation1992). The physiological responses to imagery-induced orgasm and orgasm produced by clitoral stimulation are identical. Furthermore, the brain areas activated after both kinds of orgasms are similar (Wise et al., Citation2016). Men also show genital responses to mental representations of sexual stimuli (e.g. Heiman & Hatch, Citation1980), and there are anecdotal reports of fantasy-induced ejaculation and orgasm also in men (Klumbies & Kleinsorge, Citation1950). Nevertheless, it can be stated that the activation of sexual motivation usually requires sexually relevant, external stimuli.
Any individual, be it human or rodent, emits a large number of stimuli. Some of these act at a distance, namely olfactory, visual and auditory stimuli. Sexual approach behaviors must be activated by distant stimuli. Other modalities may be involved in further enhancement of sexual motivation, but only after that physical contact with another individual has been established. Studies in rats have shown that olfactory stimuli are necessary but not sufficient for activating sexual approach behaviors. It seems that an auditory or visual stimulus is necessary in addition to an olfactory stimulus (Ågmo & Snoeren, Citation2017). In humans, both visual and auditory stimuli can activate genital responses, and the combination of both is still more efficient (Gaither & Plaud, Citation1997; Julien & Over, Citation1988; McConaghy, Citation1974). The stimuli must have sexual content, and the illustration of explicit sexual acts or verbal descriptions of such acts are particularly efficient.
The Central Motive State
A specific stimulus can provoke widely different response intensities. A food pellet offered to a sated rat does not provoke any response whatsoever, whereas an identical pellet offered to a rat deprived of food for 24 h will immediately activate approach behavior and consumption, for example. To account for the varying responses to an invariant stimulus we need to assume that the nervous tissue involved in processing the stimulus, or in connecting the stimulus with motor output, or the motor output itself, can alter their responsivity to the stimulus. The ensemble of neural processes in charge of connecting the sensory input with organized motor output is labeled the central motive state. Even though this concept is of basic importance in Bindra’s theorizing, it stems from Morgan (Citation1942). It was originally proposed to determine “general activity, specific behavior, and the readiness to perceive and react to stimulus situations in particular ways” (Morgan, Citation1942, p. 461). Bindra (Citation1974) added specific characteristics to the central motive state, in the sense that once activated, it will generate a tendency to approach the incentive object as well as activating specific viscerosomatic reactions.
The operations of the sexual central motive state are unconscious, whereas most manifestations of these operations are conscious. This is the case for approach behavior (activity in skeletal muscles) as well as for the cognitive activities involved in the decision to approach or not to approach the emitter of the incentive stimulus. The viscerosomatic responses to sexual incentives, enhanced genital blood flow and release of some hormones (see section “Viscerosomatic responses”), do not involve consciousness (Laan & Everaerd, Citation1995). The viscerosomatic response of enhanced genital blood flow will manifest itself as erection of the penis and clitoris in men and women, respectively, and these responses have access to consciousness. Other visceral responses, such as hormone and transmitter release, will not be conscious.
In the case of sexual motivation, the central motive state is responsive to sexual incentives as long as it is exposed to gonadal hormones. This is certainly the case in rodents and other non-human mammals (reviewed in González-Flores et al., Citation2017; Hull & Rodríguez-Manzo, Citation2017; Wallen, Citation1990), and probably also in humans (Bagatell et al., Citation1994; Schmidt et al., Citation2009; Santi et al., Citation2018). In the absence of sexually relevant stimuli, this activity has no behavioral manifestation. As soon as a sexual incentive is perceived, excitation in the central motive state increases. When it passes a certain level, behavior will be activated.
The impact of a relevant stimulus is determined by the basic activity in the central motive state. When activity is low, the intensity of stimulation needs to be high. When activity is high, even modest stimulation may be enough for activating behavior. If the central motive state happens to be inactive, no stimulation will activate behavior, regardless of intensity. Likewise, in the absence of an incentive stimulus, no behavior will be activated, regardless of the intensity of the activity in the central motive state.
Sexual Approach Behavior
In humans, there is no automatic link between an activated sexual central motive state and approach behavior. Cognitive processes taking into account numerous factors, such as the place, time of day, social context and predictions about the responses of the subject emitting the incentive stimulus will determine whether approach behavior will occur or not. It is likely that an intensively active central motive state may override these cognitive factors, and impel the individual to manifest approach behavior even though the cognitive evaluation would advise against such a course of action. An additional feature of human sexual approach is that consent must be obtained from the approached individual.
In non-human animals, there is a much simpler connection between activity in the sexual central motive state and approach behavior. For example, an intact, adult male rat will always approach a sexually receptive female. The exceptions would either be a male that has engaged in prolonged sexual activity (Spiteri & Ågmo, Citation2006), hence the activity of the central motive state is low or absent, or a male belonging to that rare group of males spontaneously lacking that state (Portillo & Paredes, Citation2004). Likewise, a female rat in the appropriate phase of the estrus cycle will always approach a sexually active male (Chu & Ågmo, Citation2014).
Viscerosomatic Responses
In humans, the most obvious of the visceral responses to a sexually relevant stimulus is enhanced genital blood flow, manifested as vaginal lubrication and engorgement of the external and internal aspects of the clitoris in women and penile erection in men. These responses seem to occur without any conscious evaluation of the incentive stimulus. There is an abundant, experimental literature concerning the genital responses (see Rosen & Beck, Citation1988, for a review of the fundamental issues). In fact, they may be regarded as the most exquisite measure of sexual motivation available for experiments and systematic study in humans. This assertion is not invalidated by the fact that both men and women may experience genital arousal and even orgasm during rape (Levin & van Berlo, Citation2004). It rather appears that the conscious cognitive process of giving consent to sexual interaction has little effect on the unconsciously controlled genital and orgasmic responses.
Endocrine and autonomic responses to sexual stimuli have also been reported in other mammals. In male rats, olfactory stimuli from a female in estrus activate erection (Sachs, Citation1997). In contrast to human males, male rodents and lagomorphs reliably respond to sexual stimuli with increased serum testosterone concentration (Macrides et al., Citation1974; Saginor & Horton, Citation1968). Increased release of some pituitary hormones has also been reported both in males and females (Beltramino & Taleisnik, Citation1983; Kamel et al., Citation1977; Macrides et al., Citation1974; Saginor & Horton, Citation1968). We have presented an extensive discussion of the endocrine response to sexual incentives elsewhere (Ågmo & Laan, Citation2022).
Sexual Behavior (Copulation)
Sexual behavior is “any action leading to sexual reward. Sexual reward is a state of positive affect activated by physical stimulation of the genitalia or mental representations of such stimulation” (Ågmo, Citation2007, p. 3). It is important to observe that this definition does not specify any motor actions, such as being penetrated or penetrating body orifices, nor the presence or absence of a partner or the sex of such a partner. Thereby the definition can include the stereotyped sexual activities of non-human animals as well as the enormous variety associated with human sexual interactions. Likewise, solitary sexual activities are not distinguished from those involving one or more partners. Finally, sexual fantasies are equaled to other kinds of sexual activities.
It may appear inappropriate to consider fantasizing leading to orgasm or causing non-orgasmic sexual pleasure as a sexual behavior. However, the overwhelming majority of sexual fantasies consists of imagined genital interactions with a mate (Leitenberg & Henning, Citation1995; Seehuus et al., Citation2019), i.e., about sexual behavior in a strict sense. The fact that the mate is imaginary rather than real may be considered irrelevant, as long as the consequences of the imagined sexual activity is similar to actual sexual activity. It may also be argued that humans may engage in sexual activities without obtaining or expecting to obtain sexual reward. In those cases a different reward, for example money, improved relationship or favors of all kinds, operates. Thus, motor patterns similar to those constituting sexual behavior become instrumental for obtaining non-sexual reward. The execution of these motor patterns may even be independent of activity in the central motive state controlling sexual activities. In fact, Meston and Buss (Citation2007) have listed more than 200 possible motives, most of them unrelated to sexuality, for engaging in motor patterns similar to intercourse. Here it may be appropriate to remember that Freud (Citation1915) considered that there is no obligatory relationship neither between the source of a motive and the object satisfying it nor between the actions performed and the aim of the motive. For example, the motive of greed (see Wang & Murnighan, Citation2011, for a discussion of this fascinating motive) may be satisfied by sexual behavior as long as that behavior leads to an outcome satisfying the motive. According to this reasoning, the execution of sexual motor patterns in the absence of an active sexual central motive state and with a purpose different from the obtention of sexual reward should not be considered sexual behavior. Examples of this could be someone coerced to perform sexual acts, or someone performing such acts solely for monetary reward, like a prostitute or an actor in a pornographic movie.
Motor Patterns
Humans display enormous variation in sexual acts (Kinsey et al., Citation1948, Citation1953). It appears that limits are imposed only by the extent of creativity and acrobatic proficiency of the participants.
Sexual behavior in non-human primates is far more stereotyped than in humans, yet less stereotyped than in other vertebrates (Dewsbury, Citation1972; Dewsbury & Pierce, Citation1989). In rodents, sexual behavior has been shown to be a series of tactile reflexes. Mechanical stimulation of the caudal ventral area, particularly the preputial region, in male rats is crucial for the three motor patterns constituting copulatory behavior, mount, intromission (vaginal penetration) and ejaculation (Contreras & Ågmo, Citation1993). All three motor patterns are extremely stereotyped, with a remarkably small interindividual variation (Moralí & Beyer, Citation1992; Moralí et al., Citation2003). In female rodents, the main element of copulatory behavior, lordosis, is a reflex dependent on tactile stimulation of the back and flanks (reviewed in Pfaff, Citation1980, Citation1999, Citation2017). In addition, female rats display a few additional, stereotyped motor patterns, such as ear-wiggling and running away from the male. Since these behaviors do not involve the genitals, they cannot be considered as part of copulation.
Aftereffects of Sexual Activity
In the Human
Sex is supposed to lead to a state of positive affect, or a rewarding state. There is no direct experimental evidence for this, probably because such evidence is not regarded as necessary. Even though experimental confirmation of the positive hedonic consequences of sexual activities is lacking, their existence can be presupposed.
In addition to the positive affect following sex, there is evidence showing that the act of intercourse and other intimate activities can cause negative affect. A considerable literature shows that sexual activities can give rise to the feelings of guilt or shame (reviewed in Emmers-Sommer et al., Citation2018) in addition to pleasure and satisfaction. Most studies of sexual guilt and shame have been limited to college students, but it appears that these feelings may also be present in adults as soon as transgression of social norms or expectations are associated with sexual activity (e.g. Cado & Leitenberg, Citation1990). Many other non-hedonic feelings or emotions have been reported after sexual activity, e.g. fear of pregnancy in women and postcoital dysphoria in men and women (Maczkowiack & Schweitzer, Citation2019; Schweitzer et al., Citation2015).
Non-human Animals
As was the case in the human, there is no experimental evidence for postcopulatory positive affect in other primates. The emotional aftereffects of sexual activity have been studied in a few non-primate species. The procedure that has been systematically used is a version of the conditioned place preference procedure, originally developed by H. D. Beach (Citation1957) and Rossi and Reid (Citation1976) for the study of affective consequences of drugs. It has also been used in thousands of studies of natural rewards (Tzschentke, Citation2007). Briefly, animals in a post-copulatory state are confined to an environment with distinctive characteristics. On alternate days, the animals are confined to a different environment in a neutral state. After a few sessions, the subjects are allowed to choose between the two environments. It is supposed that the subjects associate their affective state with environmental cues and therefore spend more time in the environment associated with positive affect than in the neutral environment (for a detailed description and analysis of the learning process, see Huston et al., Citation2013; Spiteri et al., Citation2000). In rats, there is experimental evidence for a reward state in females as well as in males after varying amounts of sexual interaction (reviewed in Paredes, Citation2009). The achievement of ejaculation is not necessary in males. A couple of non-ejaculatory vaginal penetrations is sufficient. In females, the receipt of the male ejaculation is unnecessary but several vaginal penetrations are required (Paredes & Alonso, Citation1997). Substantial amounts of artificial mechanical stimulation of the clitoris (60 one sec stimuli applied over a period of about 10 min) without vaginal stimulation is also sufficient for producing a reward state (Parada et al., Citation2010). In male mice, ejaculation (Popik et al., Citation2003) as well as a few non-ejaculatory vaginal penetrations have been reported to produce a reward state (Kudwa et al., Citation2005). There are no studies in female mice in which the emotional consequences of copulation have been evaluated.
This brief summary of the non-primate literature should have made it clear that there exist convincing experimental data supporting the notion of a postcopulatory reward state in rodents. Only in humans, there is evidence that sexual activity also can lead to negative affect.
Long-term Consequences of Postcoital Emotional Reactions
Humans
Orgasm may be associated with both positive and negative affect, as already mentioned. This affective state may become associated with sensory cues present during and shortly after orgasm through simple contiguity. Outstanding among the sensory cues is probably the partner, but inanimate objects present in the context may also become associated with the affect. In the case where orgasm gave rise to positive affect, the partner and other stimuli present may enhance their positive incentive properties, i.e. they may in the future activate more intense approach behavior. In cases where the affect was negative, the partner and other stimuli in the context may get their incentive value reduced or they may even acquire negative incentive properties, i.e. they may produce avoidance behavior on future occasions. It is reasonable to suppose that the association between stimuli present during and shortly after orgasm will become stronger with repeated exposure.
There is no direct experimental support for formation of associations between the affective consequences of orgasm and contextual cues. Nevertheless, it is generally assumed that this kind of association can explain some of the paraphilias, notably fetishism (reviewed in Bancroft, Citation2009). Studies in the quail have shown that these kind of associations are resistant to extinction, simply because the fetish continues to be associated with sexual satisfaction (Köksal et al., Citation2004, Citation2021). Unfortunately, similar experimental data from mammals, including the human, are not available.
Even though there are no systematic studies of how responses to sexual incentives may be altered by adverse postcoital emotions in humans, there are some approximations. In a Dutch study (Both et al., Citation2017), young women were repeatedly shown sexually arousing pictures. One of the pictures was followed by painful electric shock to the wrist, while another was followed by nothing. The vaginal response was recorded, and the women also rated their sexual arousal and affective reaction in response to the pictures. After a few pairings of picture and shock, both the genital response and self-reported sexual arousal were reduced in comparison to the picture not associated with shock. Likewise, the affective reaction to the picture predicting shock had become more negative. Thus, a single, ten trial conditioning session is enough for reducing the incentive value of a sexual stimulus. It must be noted that the non-paired picture continued to elicit an undiminished genital response in the study mentioned here. This means that the women in the Both et al. (Citation2017) study had acquired something similar to the situational type of low sexual interest/arousal. This issue will be discussed in the Low Sexual Desire in Women and Men section.
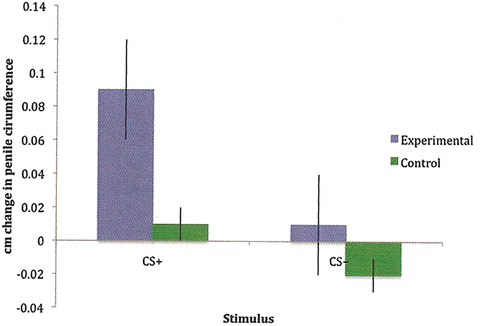
There are no experimental data concerning any kind of learning immediately after orgasm, neither in women nor in men. However, several studies have shown that preorgasmic genital stimulation produces learning in women (Both et al., Citation2011, Citation2008; Brom et al., Citation2016; Hoffmann et al., Citation2012). All these studies employed a classical conditioning procedure in which the presentation of a neutral stimulus immediately preceded tactile stimulation of the glans clitoris. The tactile stimulation is an unconditioned stimulus, activating the unconditioned response of enhanced genital blood flow and presumably also positive affect, which we may call sensory pleasure. After a few pairings, the neutral stimulus activated blood flow in the absence of tactile stimulation. It had acquired sexual incentive properties. In another study, couples were asked to expose themselves to a scent during sexual interaction in their home and another scent in other kinds of interactions. The men were later exposed to the scents in the laboratory while their penile response was recorded. As is illustrated in , only the sex-associated odor evoked a genital response (Hoffmann et al., Citation2012). This elegant experiment shows that learning can transform an arbitrary stimulus present during the execution of sexual acts into a sexual incentive. After learning, the stimulus will be able to activate the sexual central motive state. In the experimental setting, this activity caused enhanced genital blood flow, and it can be assumed that it should also provoke approach behavior in the appropriate context, pending the cognitive evaluation of the latter.
Figure 2. Changes in genital responding in young men having been exposed to an odor (CS+) during sexual interaction with their partner at home, and to another odor (CS-) during non-sexual interaction with the partner. A control group, exposed to the odors exclusively during non-sexual interactions, was also used. The penile response to the odors was assessed in the laboratory shortly after completing the conditioning procedure consisting of 3 sexual and 3 non-sexual interactions. Reproduced from Hoffmann et al. (Citation2012) with permission (Creative commons attribution noncommercial 3.0 unported license, CC BY-NC 3.0, https://creativecommons.org/licenses/by-nc/3.0/legalcode).
There have also been some efforts to associate a sexual incentive with aversive consequences. These studies were performed at a time when aversion treatment was used with the purpose of reducing responses to then socially unacceptable sexual incentives. In one of the classic studies, gay men were exposed to photographs of nude men combined with an electric shock to a finger while the penile response was monitored. Several pairings of the photograph with shock did indeed reduce the penile response (McConaghy, Citation1975). Likewise, men attracted to children were shown pictures of children of their preferred age followed by electric shock. The penile response was modestly reduced (Quinsey et al., Citation1976). There is no need to review this old literature. This has been done elsewhere (Quinsey & Marshall, Citation1983). The effects on genital responses of associating electric shock with a sexual incentive has also been studied in women. A pornographic picture was shown for 10s before shock was applied to the wrist. The vaginal response to the picture became gradually reduced (Both et al., Citation2017, Citation2008). It can be concluded that sexual incentives may be devalued when combined with aversive events in both men and women. The importance of this will become evident in a later section (Clinical applications of the model).
The arrows from aftereffects of sexual acts to the central representation of the incentive in illustrate the connection between the emotional consequences of sex and the future cognitive evaluation of sexual incentive stimuli. If the consequence were positive, then the stimuli present in the situation may acquire or enhance their incentive value. If it were negative, then the stimuli present in the situation may lose their incentive value or even be transformed into negative incentives.
Non-human Animals
We do not know if non-human animals experience anything similar to the human orgasm, but we know that sexual activity leads to positive affect both in males and females, and that the postcopulatory state can reinforce learning (Ågmo & Berenfeld, Citation1990; Meerts & Clark, Citation2007; Paredes & Vázquez, Citation1999; Tenk et al., Citation2009). This means that any stimulus present during the execution of sexual acts might become associated with that positive affect, and thereby acquire the property of conditioned incentive.
As is the case in humans, sexual activity and sexual incentives may become devaluated through association with aversive events. If ejaculation in male rats is followed by an injection of a nausea-producing compound, copulatory behavior will eventually become extinguished (Peters, Citation1983). Whether the treatment affected sexual approach behaviors or not was not evaluated. However, another study revealed that if copulation with a scented female became associated with aversive consequences, males would no longer approach similarly scented females. Since approach to the female was inhibited, there was logically no copulatory behavior. However, the males readily approached an unscented female, but they did not copulate with her (Ågmo, Citation2002). The conditioning procedure suppressed approach to a particular stimulus, whereas the execution of copulatory acts was generally suppressed.
The capacity of learning to modify the sexual incentive properties of stimuli has not been systematically studied in species other than the rat (and the human). Nevertheless, it might be assumed that learning is of importance also in other species.
Clinical Applications of the Model
Low Sexual Desire in Women and Men
The best illustration of the usefulness of the incentive motivation model can probably be found in its application to the human dysfunction that was known as “hypoactive sexual desire disorder” in the DSM-IV TR (American Psychiatric Association, Citation2000). This category was divided in two in the latest edition of the DSM (DSM-5, (American Psychiatric Association, Citation2013): “Female sexual interest/arousal disorder” and “male hypoactive sexual desire disorder.” The diagnostic criteria for Female sexual interest/arousal disorder are absent or reduced interest in sexual activity, absent or reduced sexual or erotic thoughts or fantasies, no or reduced initiation of sexual activity, unresponsive to partner’s attempt to initiate sexual activity, and absent or reduced sexual interest/arousal in response to any internal or external cues (e.g., written, verbal, visual). The diagnostic criteria for male hypoactive sexual desire disorder are: persistently or recurrently deficient (or absent) sexual/erotic thoughts or fantasies and desire for sexual activity. A duration of at least six months is required for both diagnoses. An additional, and most important, diagnostic criterion is that the lack of sexual interest must cause clinically significant personal distress. This criterion excludes all those having low sexual desire without being concerned by it. Epidemiological data show that more than half of the women self-reporting low desire do not report any distress (Öberg et al., Citation2004; Shifren et al., Citation2008). Among men, a substantial proportion of those reporting low sexual interest do not report any distress, even when partnered (Hald et al., Citation2019; Træen et al., Citation2007). For obvious reasons, individuals classifying themselves as asexual do not find the lack of sexual interest problematic.
Both female sexual interest/arousal disorder and male hypoactive sexual desire disorder may either be acquired or lifelong, and they can be generalized or situation specific. In the latter case, the reduced or absent desire manifests itself only with a specific partner or a certain class of partners or with regard to specific sexual activities. This means that patients may have some sexual fantasies, masturbate or have sex with other partners. The situation specific form of low desire appears to be far more common than the generalized form. In fact, 72% of men and women seeking treatment for low desire reported that the problem was specific for their current partner (Kaplan, Citation1995). The many epidemiological studies of these conditions have systematically not specified whether the reduced desire was generalized or situational. Therefore, the figure given above cannot be confirmed. Likewise, the incidence of lifelong vs. acquired desire disorder is unknown.
The notion of situational sexual arousal disorder has been criticized on the grounds that lack of response to a particular individual or in specific contexts do not constitute signs of a disorder. Rather it is an adaptive response to stimuli having lost their sexual incentive properties for one reason or another (Laan et al., Citation2010). This rather obvious argument is based on certain assumptions concerning the etiology of the disorder (see below), and it has not been generally accepted.
The etiology of the dysfunctions remain unknown, although all kinds of hypotheses have been launched (reviewed in Ågmo et al., Citation2004; Clayton & Juarez, Citation2019). None has received general acceptance so far. Despite the unknown causes, pharmacological treatment has been suggested as a convenient cure. However, it has been argued that pharmacological treatment of sexual interest/arousal disorder may not be the most viable approach (Chanska & Grunt-Mejer, Citation2016; Charest & Kleinplatz, Citation2018; Laan & Both, Citation2011). Apart from severe hypogonadism in men (Gooren, Citation1987; Rastrelli et al., Citation2016) and very low serum testosterone concentration in women (Laan et al., Citation2019), there is no solid evidence for endocrine or neurochemical alterations in people diagnosed with hypoactive sexual interest/arousal disorder (Ågmo et al., Citation2004; Basson, Citation2021; Basson et al., Citation2010; Davis et al., Citation2005; DeRogatis et al., Citation2012; Wåhlin-Jacobsen et al., Citation2017).
Etiology According to the Incentive Motivation Model
In principle, low sexual desire could be a consequence of an intrinsically low activity in the sexual central motive state. This would lead to reduced responsivity to sexual stimuli and the resulting activity in the central motive state would be below the threshold needed for sexual approach and performance of sexual acts. In fact, low sexual desire may be an expression of a perfectly normal interindividual variation in activity in the sexual central motive state. It does not seem far-fetched to propose that there are large interindividual variations in the basic activity of that state, in the same way as there are large variations in the amount of sexual activity (Kinsey et al., Citation1948, Citation1953). Consequently, in some individuals the central motive state will be far less active than in others. It may even be possible that the activity is below the threshold required for responses to sexual stimuli. However, the fact that the genital response to sexual stimuli is unaltered both in asexuals and in women and men diagnosed with hypoactive sexual interest/arousal disorder (e.g. Heiman et al., Citation2011; Sarin et al., Citation2014, Citation2016) speaks against this.
The incentive motivation model outlined in this review can easily explain the origin of low sexual motivation without resorting to speculations about intrinsic variations in the central motive state. Excluding such variation there are only two likely explanations:
The cognitive evaluation of the incentive stimulus concludes that it is not predictive of sexual reward or that commerce with it might have aversive consequences. In that case, the stimulus would fail to activate the central motive state, and no sexual motivation or desire would be generated. No genital response would be present. Here is another example of how the genital responses sometimes recorded during rape could be used as an argument against the preceding proposal. However, even though sexual incentive stimuli fail to activate any response, mechanical stimulation of the genitals may do so, because of prewired, unconscious connections between genital mechanoreceptors and the central motive state. Even in involuntary sex, genital stimulation is a crucial element.
If the incentive is identified as predictive of sexual reward, and consequently activates the central motive state, a genital response will be displayed. However, the conscious decision to engage or not to engage in sexual approach behaviors and ultimately sexual activity will be negative. Likewise, no signs expressing consent to sexual activity will be displayed. Thus, the stimulus will not cause any behavioral response, with the possible exception of behavior indicating lack of consent. Visceral responses may, as mentioned, be unconsciously activated.
There may be many reasons why a stimulus normally functioning as a sexual incentive has lost its capacity to activate sexual approach responses. One reason may be that sex has been followed by dysphoria and other negative emotions (see section Aftereffects of sexual activity), and these may lead to the conscious or unconscious predictions that sexual approach and sexual acts will have aversive consequences. Furthermore, such consequences may have become associated with stimuli present during prior sexual acts, transforming them from positive to negative incentives.
The fact that women diagnosed with hypoactive sexual desire/interest disorder show undiminished genital responses to sexual incentives in laboratory studies may suggest that most of them suffer from the situational type of the disorder. Being exposed to different incentives in a context different from that in which sexual activities usually occur may reestablish the genital response to its normal level, just as the women in the Both et al. (Citation2017) study mentioned above showed a normal response to the stimulus not associated with painful shock.
A completely different situation is found in victims of childhood trauma. Such trauma has been reported to be a predictor of low sexual desire in adulthood (O’Loughlin et al., Citation2020). Unspecified sexual dysfunctions have also been reported among victims of childhood sexual abuse, with penetrative sex, particularly attempted or completed anal penetration, having more consequences than other forms of sexual behaviors (Najman et al., Citation2005). Trauma in adult individuals may also lead to sexual problems, and such problems are related to the kind of trauma suffered. Women having experienced completed rape are far more likely to experience sexual problems than women who were victims of other kinds of violence, for example (Letourneau et al., Citation1996). These authors suggested that the affected women had formed associations between sexual acts and strongly negative feelings, and that they therefore would be less prone to engage in future sexual activity. Since sexual acts rather than specific stimuli had become associated with negative feelings, these women may suffer from generalized low desire. We suggest that the basic activity of the central motive state has become reduced because of the traumatic experience. If this were the case, then these women should have a reduced genital response to sexual incentives. Unfortunately, there are no data available to confirm or reject this proposal. However, an MRI study of women that were victims of sexual abuse when children revealed reduced thickness of the genital representation field of the primary somatosensory cortex (Heim et al., Citation2013). This might constitute a structural basis for reduced genital responses.
There are also some observations suggesting that childhood sexual abuse may lead to hypersexuality (Meyer et al., Citation2017). A review of published studies suggests that any possible relationship is weak (Slavin et al., Citation2020), and the underlying mechanisms are most unclear (Aaron, Citation2012). Because of the divergent criteria used for describing abuse-related hypersexuality, meaningful speculations about the incentive motivational background are unfeasible at present.
Returning to the subject at hand, low sexual desire, we suggest that the generalized type is caused by low activity in the central motive state whereas the situational type is caused by reduced impact of specific sexual incentives. In the former case, genital responses would be reduced to all sexual incentives, whereas in the latter case they would be reduced to some stimuli but not to others. There is experimental evidence supporting the second part of this proposal only.
The preceding discussion of low sexual desire is based almost exclusively on data from women, simply because there are very few studies including men diagnosed with low sexual desire disorder. However, the association between a sexual incentive and an aversive event has also been reported to reduce sexual responses in men, as was mentioned in the section Aftereffects of sexual activity.
Absence of postcoital reward, i.e. lack of orgasm, or low intensity of the positive affect generated by preorgasmic activities, could also reduce the incentive properties of a sexual stimulus and predictions about the consequences of sexual activity. In support of this hypothesis, data show that couples in which one or both of the members suffer from low desire derive less pleasure from sexual activities and have a more limited repertoire of sex behaviors than healthy couples (Trudel et al., Citation1995, Citation1997). At the same time, there are reports showing that low probability of achieving orgasm is a predictor of low sexual desire (O’Loughlin et al., Citation2018; Segraves & Segraves, Citation1991). Likewise, painful intercourse, dyspareunia, is known to enhance the probability of developing low desire (e.g. Worsley et al., Citation2017). Insofar as absence of orgasm or pain during sex reduce the reward value of sex, this could devaluate sexual incentives and alter predictions about the consequence of sexual activity.
An intriguing study of responses to sexual incentives in healthy women and women satisfying the criteria for hypoactive sexual desire/interest disorder showed that the latter reported weaker positive associations than the healthy women (Brauer et al., Citation2012). There was no difference in attention to the stimuli between the groups. It was concluded that whereas the women with low desire attend to sexual stimuli, they do not experience the positive affect that healthy women do. It is even possible that sexual incentives are transformed into highly aversive stimuli. One of the pioneers in the field, Helen Kaplan, described how the simple mentioning of anything sexual may “bring tears to the eyes and outbursts of rage” in one of her patients with low sexual desire (Kaplan, Citation1979, p. 136).
Further support for the notion that some stimuli may lose sexual incentive properties or even become negative incentives comes from a most interesting study in which the vaginal response to sexual stimuli was evaluated in the laboratory as well as in the participants’ home (Bloemers et al., Citation2010). Two measures of the vaginal response were used: Vaginal plethysmography and clitoral blood volume. Women diagnosed with hypoactive sexual interest/arousal disorder and healthy women showed the same response to a pornographic movie in the laboratory. The vaginal and clitoral response to a similar movie at home was far larger than in the laboratory in healthy women, whereas there was no difference in vaginal response between home and laboratory in women with hypoactive sexual interest/arousal disorder. Furthermore, the home response in these women was far inferior to that of the healthy women. Interestingly, the clitoral response was smaller in the home setting than in the laboratory in women with hypoactive sexual interest/arousal disorder. Part of the results of this important experiment is illustrated in .
Figure 3. Genital responses in women exposed to a pornographic movie (hardcore) or asked to produce fantasies with sexual content (fantasy) either in the laboratory (institutional lab) or at home (ambulatory lab). Upper panel: Mean (and standard error) relative increases in VPA to erotic stimuli. Epochs 1 and 4 (E1 and E4; 1st and 4th 30-second epochs) in the fantasy and hardcore conditions are shown for both laboratory settings in both groups of women (healthy controls vs. HSDD). Lower panel: Mean (and standard error) relative increases in CBV to erotic stimuli. Epochs 1 and 4 (E1 and E4; 1st and 4th 30-second epochs) in the fantasy and hardcore conditions are shown for both laboratory settings in both groups of women (healthy controls vs. HSDD). VPA = vaginal pulse amplitude; CVB = clitoral blood volume; HSDD = hypoactive sexual desire disorder. Reproduced from Bloemers et al. (Citation2010) with permission.
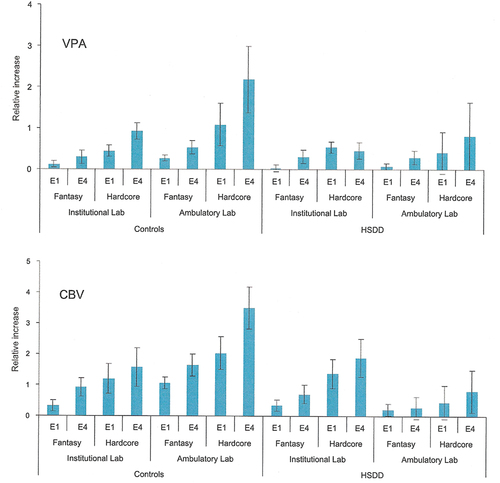
The different results obtained in the laboratory and in the participants’ home in the Bloemers et al. (Citation2010) study can easily be explained in terms of contextual conditioning. The healthy women may have associated pleasurable sexual experiences with cues in their homes, which can be assumed to be the place where most sexual activities occurred. Cues in the home may therefore enhance the response to the experimental sexual stimuli. Women with hypoactive sexual interest/arousal disorder may have associated home cues with absent sexual pleasure or even displeasure and these cues may reduce the response to sexual stimuli in the home environment. This was exactly what was observed. Considering that the experimental sexual stimuli were similar in both contexts, the altered impact of these stimuli must be due either to different cognitive evaluation of the stimuli in the laboratory and at home, or to the contribution of the cues in the home environment. Since the cues in the laboratory most likely are new, they cannot carry any sexual significance, and would therefore be unable to affect responding. At present, it is impossible to determine whether differences in cognitive evaluation of the sexual stimuli differed between home and lab, or if environmental home cues inhibited the genital response because of conditioning.
Regardless of whether the women diagnosed with hypoactive sexual interest/arousal disorder respond equally to or less than healthy women, they do respond genitally to sexual stimuli. This shows that the central motive state is sensitive to such stimuli. Since there is no relationship between the intensity of sexual desire (motivation) and the likelihood for initiating sexual activities, provided that motivation is above a threshold (see Le Moëne & Ågmo, Citation2019, for a discussion of this issue), another factor must cause the low sexual activity characteristic of individuals diagnosed with hypoactive sexual interest/arousal disorder. We propose that this other factor is the cognitive processes underlying the decision to sexually approach another individual, including predictions about the consequences of sex.
Cognitive Processes
The cognitive processes behind the decision to abstain from sexual activity are not known. It can be speculated that the consequences of sexual approach and eventually of the execution of sexual motor patterns have been negative on repeated occasions. Instead of or in addition to devaluating the sexual incentives preceding these motor patterns, the patterns themselves may have become associated with aversive consequences. Considering the temporal sequence of events from the detection of the sexual incentive to the approach and eventually the execution of copulatory acts, it is evident that the incentive is more distant in time from the emotional consequences of the copulatory acts than the event immediately preceding these consequences, which must be copulation itself. The association between consequences and copulatory acts should be stronger or more easily acquired than the association between the temporally more remote sexual incentives and the consequences of copulation. This, at least, would be the prediction from classical learning theory, and could explain why sexual stimuli may retain their incentive properties also in individuals with low desire and the resulting low or absent sexual activity.
We have assumed that the decision to respond to sexual incentives with activity in skeletal muscles (i.e. approach behavior and sexual activity) is conscious. Likewise, the perception of consent from the partner or partners is conscious. This seems to be generally accepted, since humans are considered responsible for their sexual actions. In contrast, the decision not to respond is not necessarily conscious. Those seeking help for low sexual interest/arousal disorder probably do so because they cannot solve the problem themselves, i.e. they cannot voluntarily enhance the pleasure of sexual activities and their desire to engage in them. The associations formed between negative consequences of sexual approach and sexual activity may well, as so many other associations, remain unconscious.
In this section we have proposed some mechanisms by which sexual desire might become reduced. However, low or absent desire is not by itself sufficient for the diagnosis of hypoactive sexual interest/arousal disorder. The lack of desire must also cause distress. There is no automatic association between low desire and distress, since less than half of the women reporting low desire also report distress (Öberg et al., Citation2004; Shifren et al., Citation2008), and asexuality is not at all associated with distress. There are no systematic studies exploring the reasons why some women find low desire distressing while others do not. It has been proposed, however, that discrepant desire among the members of the couple is associated with low sexual satisfaction and poor relationship quality (Davies et al., Citation1999; Sutherland et al., Citation2015), factors often mentioned as associated with hypoactive sexual interest/arousal disorder. These and many other variables, such as feelings for partner, attraction, cognitive focus, attachment, self-esteem, and stress, have been proposed to be associated with sexual dysfunctions (Hayes et al., Citation2008; Mark & Lasslo, Citation2018). Most of these variables have been implicated in a host of other psychic and somatic disorders, and it is not evident that the pursuit of correlations will shed any light on the origin of low sexual desire and distress.
The previous discussion of the etiology of low sexual interest/arousal disorder is exclusively based on data from women. Although data from men diagnosed with this dysfunction are lacking, there are some studies suggesting that the many proposed determinants of sexual desire in men are similar to those proposed for women (Murray et al., Citation2017). This would suggest that the reasoning outlined above with regard to female hypoactive sexual desire disorder can also be applied to male hypoactive sexual desire disorder. It should illustrate how the incentive motivation model can explain these disorders. Whether the proposals made are true or not is far too early to determine, but at least they are reasonable. As will be evident in the following section, they nicely fit with the few established therapeutic approaches to sexual desire disorders.
Treatment
The scientific and financial effort invested in the search for psychotherapeutic treatment of hypoactive sexual interest/desire disorder is far inferior to that invested in the pursuit of drug treatment. The reason is probably that whereas efficient drug treatment could generate substantial financial benefits for the drug owner, little such benefit would be obtained by successful psychotherapy. Whereas the pharmaceutical industry provides generous funds for drug development, the improvement of psychotherapeutic treatments is almost exclusively financed by public funds. Because of lack of resources, only a handful of studies have evaluated the effects of psychotherapeutic treatment options for sexual dysfunctions. Nevertheless, recent reviews have concluded that low sexual interest/desire disorder can be efficiently treated by a combination of Masters’ and Johnson’s classical sex therapy (Masters & Johnson, Citation1970) and cognitive behavior therapy (reviewed in Frühauf et al., Citation2013; Schmidt et al., Citation2017). During the last couple of years, mindfulness meditation training has also been successfully used (Jaderek & Lew-Starowicz, Citation2019; Kingsberg et al., Citation2017). The long-term effects of these treatments have not been sufficiently evaluated, but some data suggest that the positive effect remains at least for 6 months (e.g. Brotto et al., Citation2012; Hurlbert, Citation1993; Hurlbert et al., Citation1993). Even though none of these therapeutic approaches have attained the status of routine treatment for hypoactive sexual interest/arousal disorder, measures of treatment outcome show larger effect sizes of these treatments than of the two drugs approved in the US for treating low sexual desire, provided the data are adjusted for the large placebo effect observed in drug studies (Pyke & Clayton, Citation2018).
Elements of Psychotherapeutic Treatment
There are a few common features of all the therapeutic approaches mentioned above. First, they all include one variant or another of Master’s and Johnson’s sensate focus training. The main purpose of this is to make the patient discover her or his own sensations, particularly genital sensations. Visual examination of one’s own or the partner’s genitals, and tactile stimulation produced by touching one’s own or the partner’s genitals are important elements. At this stage, the participants are encouraged to abstain from sexual activity. The sensate focus training is normally performed as homework after receiving instructions from the therapist. Second, at one stage or another during treatment, efforts are made to modify the patient’s cognitive appraisal of sexual activities, the partner, and the relationship with the partner, as well as the emotional responses to all these elements. Third and last, the patient is encouraged to engage in sexual activity only when fully sexually aroused and feeling strong desire to do so. Sometimes there is an insistence on sexual techniques maximizing the likelihood for achieving orgasm (e.g. Hurlbert, Citation1993; Hurlbert et al., Citation1993). It must be noted that penile-vaginal intercourse is not the most efficient way for women to achieve orgasm (Eschler, Citation2004), meaning that alternative means of genital stimulation need to be encouraged. Some programs require 15–20 sessions (e.g. LoPiccolo & Friedman, Citation1988) whereas the more recent mindfulness procedures often are limited to three sessions with a therapist plus an extensive amount of homework practice between sessions (e.g. Brotto et al., Citation2012). This is a much simplified description of the therapeutic process, but it should be sufficient for the present purpose.
Treatment Interpreted in Terms of the Incentive Motivation Model
All therapeutic interventions mentioned above can be interpreted in terms of the incentive motivation model outlined in this review. The first part of treatment, improving attention to and appreciation of the sensory experience provided by proximate sexual stimuli, would improve the incentive properties of these stimuli. By focusing on the pleasurable aspects of, for example genital stimulation, the cognitive evaluation of the stimulation could change from aversive to neutral or perhaps positive. The consequence is that these stimuli further enhance the activity in the central motive state. The cognitive part of the therapeutic program will change the patient’s evaluation of the situation and consequently the ensuing decision on how to proceed. The habitual decision to withdraw will be replaced by a decision to approach. Furthermore, instead of expressing lack of consent for further sexual activity, consent will be expressed. The insistence on maximizing the probability of attaining orgasm will assure that the sexual activity will be generating positive affect, and the cognitive part of therapy may eliminate or reduce potential negative emotional reactions following coitus. The experience of positive affect will then be undisturbed and it can become associated both with the performance of sexual acts and the salient stimuli present during these acts, and enhance the likelihood for future sexual activity.
The studies showing that a genital response to sexual stimuli is present in women suffering from hypoactive sexual interest/arousal disorder suggest that the unconscious response to such stimuli is not affected by the negative cognitive evaluation of them. Furthermore, successful psychotherapeutic treatment of the disorder does not alter the genital response. An interesting study of the effects of mindfulness therapy showed that most measures of sexual function improved in the treatment group, both compared to itself at pretreatment and when compared with a waiting list control at posttreatment, whereas there was no effect of treatment on the genital response, as evaluated with vaginal plethysmography (Brotto et al., Citation2012). One possible interpretation of this observation is that the sexual central motive state remained unaltered after treatment. If this were the case, then the treatment effect would be limited to changing the cognitive evaluation of the stimuli and the ensuing conscious decision to proceed with sexual approach and eventually sexual behavior. This change in cognitive evaluation of the stimuli due to therapy may result in women becoming more aware of, paying more attention to, being more “open” to, the genital response evoked by sexual stimuli, and thus feeling more excitement even though intensity of genital arousal is not greater. There is no experimental support in favor of this proposal, but at the same time there are no data contradicting it.
In women diagnosed with the general type of hypoactive sexual interest/arousal disorder it may be different. Here, successful therapy may have altered the basic activity of the sexual central motive state. This would manifest itself in an increased genital response to sexual incentives. Since there are no psychophysiological studies specifically including women with the generalized type of the disorder, this proposal is not more than speculation.
Hyperactive Sexual Desire
Some individuals engage in frequent sexual activities. When this frequency, or the time devoted to using pornographic materials, surpasses an arbitrary limit, it is customary to speak of hypersexuality or even compulsive sexual behavior or sexual addiction (e.g. Asiff et al., Citation2018). The forthcoming version of the International Classification of Diseases (ICD-11) even includes a category (code 6C72) “compulsive sexual behavior disorder” (https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1630268048, retrieved November 4, 2021). In brief, it is described as “persistent pattern of failure to control intense, repetitive sexual impulses or urges resulting in repetitive sexual behavior.” As always, the condition should last for more than 6 months and be associated with distress. A similar category was rejected for inclusion in the DSM-5 (Kafka, Citation2014). It has, perhaps with good reasons, been argued that frequent sexual behavior or intense pornography use are rather inoffensive leisure activities without any need to be pathologized (Williams et al., Citation2020).
Much effort has been invested in a search for objective criteria for defining hypersexuality. Kafka (Citation1997) suggested that a total sexual outlet (number of orgasms experienced) above seven per week should be defined as hypersexuality. In the Kinsey et al. (Citation1948) study, 7.6% of adult men reported a total sexual outlet higher than seven per week, and the sample mean was 2.14. Based on this, Kafka (Citation1997) established the criterion mentioned above. Most of the studies performed after Kinsey’s have used other quantifications of the amount of sexual behavior displayed, such as the number of penile – vaginal intercourses per unit time, or the number of masturbatory events per unit time. Unfortunately, these measures fail to take into account the varied ways in which humans may attain sexual satisfaction. There is one notable exception, though. A Canadian study with university students between 18 and 24 years reported not only the total sexual outlet, but it also included women, something most unusual in studies of hypersexuality (Levaque et al., Citation2016). The median total sexual outlet per week was 3.0 for women and 5.0 for men. The 10% with the highest sexual activity had a total sexual outlet of 20.0 and 28.3, for women and men, respectively. Had the Kafka (Citation1997) criterion been used, 22.6% of the women and 37.8% of the men would have been characterized as hypersexual. It was concluded that a numerical cutoff criterion is too simplistic.
There have been many other intents to establish criteria for defining hypersexuality, many including questionnaires of different types (Hook et al., Citation2010). So far, no agreement has been achieved (Montgomery-Graham, Citation2017). Likewise, many studies have tried to associate hypersexuality with paraphilia and other psychopathologies (e.g. Castellini et al., Citation2018; Raymond et al., Citation2003) without arriving at any undisputed conclusion.
Etiology
There are no known endocrine or neural differences between people characterized as hypersexual and those not characterized as such (reviewed in Bradford, Citation2001; Krueger & Kaplan, Citation2001). Recently, lower methylation of the corticotropin releasing hormone (CRH) gene have been found in men diagnosed with hypersexual disorder according to the proposed (and rejected) DSM-5 criteria when compared to healthy men (Jokinen et al., Citation2017). Low methylation is associated with high gene expression. Despite this, there was no difference in serum concentration of adrenocorticotropic hormone or cortisol between these two groups of men. Although an interesting observation, it is not entirely clear how altered CRH gene expression would affect sexual behavior. In the absence of any convincing explanation based on brain function or endocrine alterations, there has been launched a series of more or less extravagant hypotheses, most with no or extremely limited empirical support (reviewed in Walton et al., Citation2017).
Within the framework of the present model, it may be suggested that hypersexuality is a direct consequence of unusually high intrinsic activity in the sexual central motive state. Since that activity determines the responsivity to sexual incentives, it is to be expected that individuals with high activity will respond far easier or more intensely to sexual stimuli than most other individuals. It is also possible that the former will respond strongly to stimuli that are only weak sexual incentives in the latter. A bigger pool of stimuli with sexual significance combined with heightened response to these stimuli would probably lead to more frequent sexual activity or more intense approach to sexual stimuli than most individuals will show.
If sexual experiences are intensely rewarding, the stimuli associated with these acts should acquire strong sexual incentive properties through conditioning. Thus, even if the basic activity in the central motive state is not unusually high, the powerful incentive properties of sexual stimuli would enhance activity far more than in individuals not experiencing such intense sexual reward. Instead of assuming that high basic activity in the central motive state underlies hypersexuality, the high intensity of sexual reward would be the cause. Again, it may be supposed that there are large interindividual variations in sexual reward intensity, with a small proportion of individuals experiencing a much more intense reward than others. The causes for interindividual differences in the intensity of the experience of sexual reward remain entirely unknown.
In addition to these parsimonious hypotheses concerning the origin of unusually intense sexual motivation and behavior, it must be recognized that more complex factors may be involved. Among those are personality traits such as impulsivity (Reid et al., Citation2015) and sensation seeking (Kingston & Firestone, Citation2008). Recently, it has been suggested that the feeling of boredom may enhance interest in hedonic forms of sex (Moynihan et al., Citation2021). A convoluted motivational model, based on the questionable assumptions that dopaminergic systems are of great importance for sexual function and the existence of an inhibitory system, has also been proposed (Toates, Citation2022).
The hypotheses proposing enhanced intrinsic activity in the sexual central motive state or enhanced reward after sexual events as causes for hypersexuality use perfectly normal interindividual variation as the ultimate cause. The first assumes an inborn heightened activity in the sexual central motive state whereas the second assumes an inborn variation in the amount of pleasure derived from sexual acts. This pleasure would need to become associated with environmental stimuli, which in turn would lead to heightened response to these stimuli in the central motive state. The end result, intense activity in the sexual central motive state following exposure to sexual stimuli, would be identical in both cases.
There are indeed some data concerning the processing of sexual stimuli in individuals with unusually high sexual desire or motivation. A study of event related potentials in response to pictures with sexual content revealed that there was a correlation between the P300 component and sexual desire as evaluated by the Sexual Desire Inventory (Steele et al., Citation2013). Participants in the study were young men and women scoring high on questionnaires for sexual compulsivity, sometimes considered a manifestation of hypersexuality. The P300 is thought to represent the cognitive evaluation of the stimulus, and may be thought to precede its possible impact on motivational systems. This observation suggests that the level of sexual desire indeed affects the processing of sexual stimuli, potentially enhancing their impact on the central motive state. This hypothesis was strengthened in another study of P300 responses to pictures with sexual content. Instead of relating these responses to sexual desire, they were now related to the number of sexual partners during the preceding year. Those with many partners showed a higher response than those with few partners to pictures with weak sexual content, whereas there was no difference when the pictures were explicitly sexual (Prause et al., Citation2015). If we assume that the number of sexual partners is related to the level of sexual desire, it must be concluded that the higher the desire, the more responsive is the individual to weak sexual stimuli. Whether the enhanced responsiveness depends on the cognitive evaluation of the stimuli or on high intrinsic activity in the central motive state is impossible to determine because of the reciprocal relationship between the central motive state and the central representation of the sexual incentive. Both are necessary for the activation of sexual motivation but the relative contribution of each of them may not always be known.
Treatment
Several treatments aiming at a reduction of inappropriate sexual activity have been used for many years. Men diagnosed with any of the paraphilias have been the most frequent target. Drugs reducing the serum concentration of testosterone to levels typical of hypogonadism, for examples medroxyprogesterone or analogues of luteinizing hormone releasing hormone, have been extensively used, usually with the intended effect (reviewed in Guay, Citation2009; Lewis et al., Citation2017). Androgen receptor antagonists have also been successfully used (Cooper et al., Citation1972), in the sense that they reduce deviant sexual behavior. In addition to compounds altering endocrine function, some psychopharmacological agents have been employed. Notable among these are antidepressant drugs belonging to the group of selective serotonin reuptake inhibitors, such as fluoxetine and sertraline. However, it is still a matter of debate whether these drugs are efficient or not (Grubin, Citation2018).
It is most likely that all the treatments mentioned in the preceding paragraph reduce responsivity in the sexual central motive state. This action will inevitably decrease appropriate sexual behaviors as much as inappropriate ones. Insofar as some of the paraphilias are criminal offenses in many countries, this undesirable effect has not received much attention compared to the desirable effect, reducing the recidivism rate of convicted sex offenders. However, questions have been raised concerning the ethical justification of these treatments (Ward et al., Citation2007), and they may be entirely inappropriate for non-paraphilic but problematic high sexual activity.
It might be possible for psychotherapeutic treatment to reduce undesired sexual behaviors, should these be paraphilic or belong to some of the ill-defined labels “sex addiction” or “compulsive sexual behavior,” without also affecting desirable sex. There are several reports of more or less successful therapeutical approaches, but according to a review of the subject (Hook et al., Citation2014), none of these reports includes appropriate controls, precluding any firm conclusion. More recent data suggest that a cognitive behavioral therapy program consisting of seven weekly sessions + homework efficiently reduced hypersexuality in a group of young men, whereas no change was observed in the waiting list control group (Hallberg et al., Citation2019). The effect lasted for at least six months. It appears that the main focus of the program was directed toward impulse control, cognitive restructuring, assertiveness training and conflict management. It can be assumed that the therapy mostly affected the cognitive evaluation of the interpersonal context in such a way that the conscious decision to seek consent for performing sexual acts or for proceeding to such acts became less likely. Since there are no studies on genital responses in people characterized as hypersexual, and no studies of the effects of therapy (pharmacological or psychological) on these responses, it is impossible to know whether the unconscious responses to sexual stimuli are altered in this condition and if they are affected by treatment.
As mentioned earlier, several old studies have shown that the association of pictures of inappropriate sexual incentives with aversive consequences reduces the response to them. Aversive conditioning has come into disrepute, and is no longer used as a therapeutic approach. Nevertheless, from a theoretical point of view it could certainly decrease the impact of sexual stimuli on the central motive state, and alter the cognitive evaluation of these stimuli. The likelihood for execution of sexual acts would consequently be reduced. However, if the results of the Hallberg et al. (Citation2019) study were confirmed and extended, it could be concluded that there are other means to alter the cognitive evaluation of sexual contexts.
In conclusion, the short discussion of hyperactive sexual desire or hypersexuality illustrates that the incentive motivation model can provide testable hypotheses as to its origin, and offer an interpretative framework for the effects of different kinds of treatment. An important element is that hypersexuality is a manifestation of a completely normal interindividual variation in sexual activity. It is not inherently problematic, but turns into a problem when the individual experiences distress because of that high activity. The present model can account for intense sexual activity, but cannot explain why some individuals find it distressing. This is probably something unrelated to sexuality per se. Among the factors that might be related to hypersexuality-induced distress is religiosity. However, a carefully designed study did not find any relationship between these variables (Reid et al., Citation2016).
Intermediary Conclusion
The preceding accounts of the possible origin of high or low interest/desire illustrate that the incentive motivational model can offer credible and coherent explanations and that these explanations are amenable to empirical evaluation. Likewise, it can offer a credible and coherent explanation of how treatment may work, regardless of the kind of treatment. Thus, the model is not only useful for understanding the workings of sexual motivation in normal conditions, but it can also help understanding pathologies related to sexual motivation or desire. A discussion of the role of desire in other sexual disorders, for example erectile dysfunction or premature ejaculation, is beyond the scope of this review, but there are undoubtedly motivational components also there.
The Incentive Motivation Model, Disorders of Desire and the Brain
The brain mechanisms that may be involved in the various elements of the model presented herein have scarcely been mentioned. What we have outlined here is a theoretical or conceptual model of sexual behavior, not a neurobiological model. It is anchored in psychological notions rather than in neurobiological facts. This is not unusual within psychology. In fact, most psychological theorizing or modeling is made without any reference to the central nervous system. Perhaps the most eloquent example is Skinner’s analyses of the effects of different schedules of reinforcement (Ferster & Skinner, Citation1957). The predictions made from these analyses have been confirmed in all species studied, from invertebrates to humans, and in thousands of experiments, yet Skinner did not make any reference to neurobiological mechanisms. Many other examples could be given, but they are probably not needed. In the same way, it is perfectly possible that sexual behavior can be completely understood without any reference to the brain substrate. This assertion may appear unfounded or exaggerated. Therefore, we will provide a concrete example.
Among the best known aspects of the biological bases of sexual behavior is its endocrine control. In rodents, particularly rats, it is well known that sexual approach behaviors and copulation are dependent on the simultaneous activation of androgen receptors and the estrogen receptor α in males (e.g. Attila et al., Citation2010; Sano et al., Citation2013). In females, only the estrogen receptor α is necessary for these behaviors (e.g. Ogawa et al., Citation1998; Spiteri et al., Citation2010). Furthermore, the medial preoptic area in males and the ventromedial nucleus of the hypothalamus in females are essential sites of action for the gonadal hormones (e.g. Paredes, Citation2003; Takahashi, Citation1990). The neural and endocrine control of the female rat’s copulatory posture, lordosis, have been described in almost every detail, including the entire afferent pathway from the sensory receptors in the skin to the ventromedial nucleus of the hypothalamus and the efferent pathway from this nucleus to the muscles responsible for the behavioral response (Pfaff, Citation1980, Citation1999). The molecular actions of the estrogens within the hypothalamic neurons are now being elucidated (Micevych & Meisel, Citation2017; Pfaff, Citation2017). Even though this knowledge of hormone action in the brain may be most important, it does not in the slightest help us to understand the intricacies of sexual interactions within a small group of rats. In contrast, a careful behavioral analysis of these interactions shed light on how they work (Chu & Ågmo, Citation2014, Citation2015). In view of these experiences from studies of non-human sexual behavior, we have not made any effort to anchor the concepts used in the present model in neurobiological language.
The rodent data mentioned above are based on a host of neurobiological studies employing all the procedures available in neuroscientific research on non-human animals, such as manipulations of gene expression, lesions of specific brain sites, optogenetics and chemogenetics as well as in vivo single cell recordings in neural tissue. None of these powerful techniques are available in humans. Consequently, knowledge about the neurobiology of human sexual behavior remains in a quite precarious state compared to that in non-human animals. If the latter contributes little to the understanding of sexual interactions, it is evident that the former contributes even less. In fact, we do not believe that neurobiology can offer any important contribution to our understanding of most facets of human sexual behavior at present.
This assertion does not mean that knowledge of the neural control of sexual functions is irrelevant. For example, the rather detailed knowledge of the physiology of erection (Andersson, Citation2011; Andersson & Wagner, Citation1995) has allowed for the development of excellent pharmacological treatments of erectile disorder. Likewise, the observations showing that male hypogonadism leads to generalized sexual dysfunction have opened the way for androgen replacement therapy in men suffering from this condition (Bhasin et al., Citation2018). However, these facts do not invalidate the proposal made in the preceding paragraph. They only confirm the rather obvious facts that appropriate penile erection is necessary for penetrative sex, and that androgens are necessary for adequate sexual function in men. Neither the enhanced availability of NO in the corpora cavernosa nor the altered transcription of some proteins in neurons expressing androgen receptors can be used to explain the interindividual variations in the intensity of sexual motivation, for example (see Ågmo & Laan, Citation2022, for an extensive discussion). Likewise, with the exception of the rare cases of severe hypogonadism, neither hypo- nor hyperactive sexual desire can be understood in terms of any known neurobiological alteration. This may change in the future, but for now neurobiology is a weak explanatory tool.
Conclusion
The incentive motivation model of sexual motivation is a powerful explanatory tool for understanding the intricacies of sexual behavior in humans and other animals. It is based on a series of generally accepted notions concerning the characteristics of motivational systems, but it also adds novel elements. The emphasis on cognitive evaluation of sexual stimuli and of the context in which these appear is an important element in the model. The decisive role of cognitive processes may be exclusive for human sexual interactions, but they are certainly not entirely absent in other animals. We may also point out that the model is equally applicable, and employs the same concepts and principles, for sexual interaction between pairs of different sex, of the same sex or between groups of individuals. Similarly, it is applicable to all kinds of activities, with the only criterion that the genitals somehow must be involved. At the same time, it excludes motor patterns identical to sexual motor patterns as soon as these are performed for any other purpose than sexual pleasure. None of the many kinds of transactional sex can be explained by this model, nor are they relevant for it.
We have described the sequence of events, from the detection of a sexually relevant stimulus to the postcoital affective reactions, constituting sexual interaction. This description can be used to disentangle the factors underlying the disorders of sexual desire. It also provides a powerful tool for understanding how psychotherapy works. Furthermore, we propose that the motivational model outlined here can contribute to the design of more efficient therapies for disorders of desire. Finally, the model shows that there is no need to assume the existence of an inhibitory system to explain the lack of sexual desire or behavior in some individuals, should they be mice or men.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Aaron, M. (2012). The pathways of problematic sexual behavior: A literature review of factors affecting adult sexual behavior in survivors of childhood sexual abuse. Sexual Addiction & Compulsivity-the Journal of Treatment and Prevention, 19(3), 199–218. https://doi.org/10.1080/10720162.2012.690678
- Ågmo, A. (1999). Sexual motivation. An inquiry into events determining the occurrence of sexual behavior. Behavioural Brain Research, 105(1), 129–150. https://doi.org/10.1016/S0166-4328(99)00088-1
- Ågmo, A. (2002). Copulation-contingent aversive conditioning and sexual incentive motivation in male rats: Evidence for a two-stage process of sexual behavior. Physiology and Behavior, 77(2–3), 425–435. https://doi.org/10.1016/S0031-9384(02)00874-0
- Ågmo, A. (2007). Functional and dysfunctional sexual behavior. A synthesis of neuroscience and comparative psychology. Academic Press. https://doi.org/10.1016/B978-0-12–370590-7.X5000-2
- Ågmo, A. (2011). On the intricate relationship between sexual motivation and arousal. Hormones and Behavior, 59(5), 681–688. https://doi.org/10.1016/j.yhbeh.2010.08.013
- Ågmo, A, & Berenfeld, R. (1990). Reinforcing properties of ejaculation in the male rat: Role of opioids and dopamine. Behavioral Neuroscience, 104(1), 177–182. https://doi.org/10.1037/0735-7044.104.1.177
- Ågmo, A., & Laan, E. (2022). Sexual incentive motivation, sexual behavior, and general arousal: Do rats and humans tell the same story? Neuroscience and Biobehavioral Reviews, 135, Article 104595. https://doi.org/10.1016/j.neubiorev.2022.104595
- Ågmo, A., & Snoeren, E. M. S. (2017). A cooperative function for multisensory stimuli in the induction of approach behavior of a potential mate. PloS One, 12(3), e0174339. https://doi.org/10.1371/journal.pone.0174339
- Ågmo, A., Turi, A. L., Ellingsen, E., & Kaspersen, H. (2004). Preclinical models of sexual desire: Conceptual and behavioral analyses. Pharmacology Biochemistry and Behavior, 78(3), 379–404. https://doi.org/10.1016/j.pbb.2004.04.013
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). https://doi.org/10.1176/appi.books.9780890420249.dsm-iv-tr
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. https://doi.org/10.1176/appi.books.9780890425596
- Anderson, R. M. (2013). Positive sexuality and its impact on overall well-being. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz, 56(2), 208–214. https://doi.org/10.1007/s00103-012-1607-z
- Anderson, R., & Moffatt, C. E. (2018). Ignorance is not bliss: If we don’t understand hypoactive sexual desire disorder, how can flibanserin treat it? Journal of Sexual Medicine, 15(3), 273–283. https://doi.org/10.1016/j.jsxm.2018.01.001
- Andersson, K. E. (2011). Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacological Reviews, 63(4), 811–859. https://doi.org/10.1124/pr.111.004515
- Andersson, K. E, & Wagner, G. (1995). Physiology of penile erection. Physiological Reviews, 75(1), 191–236. https://doi.org/10.1152/physrev.1995.75.1.191
- Asiff, M., Sidi, H., Masiran, R., Kumar, J., Das, S., Hatta, N. H., & Alfonso, C. (2018). Hypersexuality as a neuropsychiatric disorder: The neurobiology and treatment options. Current Drug Targets, 19(12), 1391–1401. https://doi.org/10.2174/1389450118666170321144931
- Attila, M., Oksala, R., & Ågmo, A. (2010). Sexual incentive motivation in male rats requires both androgens and estrogens. Hormones and Behavior, 58(2), 341–351. https://doi.org/10.1016/j.yhbeh.2009.08.011
- Bagatell, C. J., Heiman, J. R., Rivier, J. E., & Bremner, W. J. (1994). Effects of endogenous testosterone and estradiol on sexual behavior in normal young men. Journal of Clinical Endocrinology and Metabolism, 78(3), 711–716. https://doi.org/10.1210/jcem.78.3.8126146
- Banbury, S., Lusher, J., Snuggs, S., & Chandler, C. (2021). Mindfulness-based therapies for men and women with sexual dysfunction: A systematic review and meta-analysis. Sexual and Relationship Therapy, 1–22. https://doi.org/10.1080/14681994.2021.1883578
- Bancroft, J. (1999). Central inhibition of sexual response in the male: A theoretical perspective. Neuroscience and Biobehavioral Reviews, 23(6), 763–784. https://doi.org/10.1016/S0149-7634(99)00019-6
- Bancroft, J. (2009). Sexual variations. In J. Bancroft (Ed.), Human sexuality and its problems (3rd ed., pp. 280–288). Churchill Livingstone. https://doi.org/10.1016/B978-0-443-05161-6.00009-4
- Bancroft, J., Graham, C. A., Janssen, E., & Sanders, S. A. (2009). The dual control model: Current status and future directions. Journal of Sex Research, 46 (2–3), 121–142. Article Pii 909792907. https://doi.org/10.1080/00224490902747222.
- Basson, R. (2021). Sexual dysfunctions in women: Are androgens at fault? Endocrinology and Metabolism Clinics of North America, 50(1), 125–138. https://doi.org/10.1016/j.ecl.2020.12.001
- Basson, R., Brotto, L. A., Petkau, A. J., & Labrie, F. (2010). Role of androgens in women’s sexual dysfunction. Menopause, 17(5), 962–971. https://doi.org/10.1097/gme.0b013e3181d59765
- Beach, F. A. (1956). Characterstics of masculine sex drive. In M. R. Jones (Ed.), Nebraska symposium on motivation (pp. 1–32). University of Nebraska Press.
- Beach, H. D. (1957). Morphine addiction in rats. Canadian Journal of Psychology, 11(2), 104–112. https://doi.org/10.1037/h0083703
- Beltramino, C., & Taleisnik, S. (1983). Release of LH in the female rat by olfactory stimuli. Effect of the removal of the vomeronasal organs or lesioning of the accessory olfactory bulbs. Neuroendocrinology, 36(1), 53–58. https://doi.org/10.1159/000123436
- Bhasin, S., Brito, J. P., Cunningham, G. R., Hayes, F. J., Hodis, H. N., Matsumoto, A. M., Snyder, P. J., Swerdloff, R. S., Wu, F. C., & Yialamas, M. A. (2018). Testosterone therapy in men with hypogonadism: An endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism, 103(5), 1715–1744. https://doi.org/10.1210/jc.2018-00229
- Bindra, D. (1969). The interrelated mechanisms of reinforcement and motivation, and the nature of their influence on response. In W. J. Arnold & D. Levine (Eds.), Nebraska symposium on motivation (pp. 1–33). University of Nebraska Press.
- Bindra, D. (1974). A motivational view of learning, performance, and behavior modification. Psychological Review, 81(3), 199–213. https://doi.org/10.1037/h0036330
- Bindra, D. (1976). A theory of intelligent behavior. Wiley.
- Bindra, D. (1978). How adaptive behaviour is produced: A perceptual-motivational alternative to response reinforcement. Behavioral and Brain Sciences, 1(1), 41–52. https://doi.org/10.1017/S0140525X00059380
- Bloemers, J., Gerritsen, J., Bults, R., Koppeschaar, H., Everaerd, W., Olivier, B., & Tuiten, A. (2010). Induction of sexual arousal in women under conditions of institutional and ambulatory laboratory circumstances: A comparative study. Journal of Sexual Medicine, 7(3), 1160–1176. https://doi.org/10.1111/j.1743-6109.2009.01660.x
- Both, S., Brauer, M., & Laan, E. (2011). Classical conditioning of sexual response in women: A replication study. Journal of Sexual Medicine, 8(11), 3116–3131. https://doi.org/10.1111/j.1743-6109.2011.02453.x
- Both, S., Brauer, M., Weijenborg, P., & Laan, E. (2017). Effects of aversive classical conditioning on sexual response in women with dyspareunia and sexually functional controls. Journal of Sexual Medicine, 14(5), 687–701. https://doi.org/10.1016/j.jsxm.2017.03.244
- Both, S., Everaerd, W., & Laan, E. (2007). Desire emerges from excitement: A psychophysiological perspective on sexual motivation. In E. Janssen (Ed.), The psychophysiology of sex (pp. 327–339). Indiana University Press.
- Both, S., Laan, E., Spiering, M., Nilsson, T., Oomens, S., & Everaerd, W. (2008). Appetitive and aversive classical conditioning of female sexual response. Journal of Sexual Medicine, 5(6), 1386–1401. https://doi.org/10.1111/j.1743-6109.2008.00815.x
- Bradford, J. M. W. (2001). The neurobiology, neuropharmacology, and pharmacological treatment of the paraphilias and compulsive sexual behavior. Canadian Journal of Psychiatry, 46(1), 26–34. https://doi.org/10.1177/070674370104600104
- Brauer, M., van Leeuwen, M., Janssen, E., Newhouse, S. K., Heiman, J. R., & Laan, E. (2012). Attentional and affective processing of sexual stimuli in women with hypoactive sexual desire disorder. Archives of Sexual Behavior, 41(4), 891–905. https://doi.org/10.1007/s10508-011-9820-7
- Brom, M., Laan, E., Everaerd, W., Spinhoven, P., Trimbos, B., & Both, S. (2016). The effect of a dopamine antagonist on conditioning of sexual arousal in women. Psychopharmacology, 233(7), 1179–1189. https://doi.org/10.1007/s00213-015-4201-x
- Brotto, L. A. (2017). Evidence-based treatments for low sexual desire in women. Frontiers in Neuroendocrinology, 45, 11–17. https://doi.org/10.1016/j.yfrne.2017.02.001
- Brotto, L. A., Erskine, Y., Carey, M., Ehlen, T., Finlayson, S., Heywood, M., Kwon, J., McAlpine, J., Stuart, G., Thomson, S., & Miller, D. (2012). A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecologic Oncology, 125(2), 320–325. https://doi.org/10.1016/j.ygyno.2012.01.035
- Brotto, L. A., Zdaniuk, B., Chivers, M. L., Jabs, F., Grabovac, A., Lalumière, M. L., Weinberg, J., Schonert-Reichl, K. A., & Basson, R. (2021). A randomized trial comparing group mindfulness-based cognitive therapy with group supportive sex education and therapy for the treatment of female sexual interest/arousal disorder. Journal of Consulting and Clinical Psychology, 89(7), 626–639. https://doi.org/10.1037/ccp0000661
- Byrne, D. (1977). Social psychology and the study of sexual behavior. Personality and Social Psychology Bulletin, 3(1), 3–30. https://doi.org/10.1177/014616727600300102
- Cado, S., & Leitenberg, H. (1990). Guilt reactions to sexual fantasies during intercourse. Archives of Sexual Behavior, 19(1), 49–63. https://doi.org/10.1007/BF01541825
- Castellini, G., Rellini, A. H., Appignanesi, C., Pinucci, I., Fattorini, M., Grano, E., Fisher, A. D., Cassioli, E., Lelli, L., Maggi, M., & Ricca, V. (2018). Deviance or normalcy? The relationship among paraphilic thoughts and behaviors, hypersexuality, and psychopathology in a sample of university students. Journal of Sexual Medicine, 15(9), 1322–1335. https://doi.org/10.1016/j.jsxm.2018.07.015
- Chanska, W., & Grunt-Mejer, K. (2016). The unethical use of ethical rhetoric: The case of flibanserin and pharmacologisation of female sexual desire. Journal of Medical Ethics, 42(11), 701–704. https://doi.org/10.1136/medethics-2016-103473
- Charest, M., & Kleinplatz, P. J. (2018). A review of recent innovations in the treatment of low sexual desire. Current Sexual Health Reports, 10(4), 281–286. https://doi.org/10.1007/s11930-018-0171-4
- Chu, X., & Ågmo, A. (2014). Sociosexual behaviours in cycling, intact female rats (Rattus norvegicus) housed in a seminatural environment. Behaviour, 151(8), 1143–1184. https://doi.org/10.1163/1568539X-00003177
- Chu, X., & Ågmo, A. (2015). Sociosexual behaviors of male rats (Rattus norvegicus) in a seminatural environment. Journal of Comparative Psychology, 129(2), 132–144. https://doi.org/10.1037/a0038722
- Clayton, A. H., Goldstein, I., Kim, N. N., Althof, S. E., Faubion, S. S., Faught, B. M., Parish, S. J., Simon, J. A., Vignozzi, L., Christiansen, K., Davis, S. R., Freedman, M. A., Kingsberg, S. A., Kirana, P. S., Larkin, L., McCabe, M., & Sadovsky, R. (2018). The International Society for the Study of Women's Sexual Health, process of care for management of hypoactive sexual desire disorder in women. Mayo Clinic Proceedings, 93(4), 467–487. https://doi.org/10.1016/j.mayocp.2017.11.002
- Clayton, A. H., & Juarez, E. M. V. (2019). Female sexual dysfunction. Medical Clinics of North America, 103(4), 681–698. https://doi.org/10.1016/j.mcna.2019.02.008
- Contreras, J. L., & Ågmo, A. (1993). Sensory control of the male rat’s copulatory thrusting patterns. Behavioral and Neural Biology, 60(3), 234–240. https://doi.org/10.1016/0163-1047(93)90447-P
- Cooper, A. J., Ismail, A. A. A., Phanjoo, A. L., & Love, D. L. (1972). Antiandrogen (cyproterone acetate) therapy in deviant hypersexuality. British Journal of Psychiatry, 120(554), 59–63. https://doi.org/10.1192/bjp.120.554.59
- Davies, S., Katz, J., & Jackson, J. L. (1999). Sexual desire discrepancies: Effects on sexual and relationship satisfaction in heterosexual dating couples. Archives of Sexual Behavior, 28(6), 553–567. https://doi.org/10.1023/A:1018721417683
- Davis, S. R., Davison, S. L., Donath, S., & Bell, R. J. (2005). Circulating androgen levels and self-reported sexual function in women. JAMA - Journal of the American Medical Association, 294(1), 91–96. https://doi.org/10.1001/jama.294.1.91
- DeRogatis, L., Rosen, R. C., Goldstein, I., Werneburg, B., Kempthorne-Rawson, J., & Sand, M. (2012). Characterization of hypoactive sexual desire disorder (HSDD) in men. Journal of Sexual Medicine, 9(3), 812–820. https://doi.org/10.1111/j.1743-6109.2011.02592.x
- Dewsbury, D. A. (1972). Patterns of copulatory behavior in male mammals. Quarterly Review of Biology, 47(1), 1–33. https://doi.org/10.1086/407097
- Dewsbury, D. A., & Pierce, J. D., Jr. (1989). Copulatory patterns of primates as viewed in broad mammalian perspective. American Journal of Primatology, 17(1), 51–72. https://doi.org/10.1002/ajp.1350170106
- Emmers-Sommer, T. M., Allen, M., Schoenbauer, K. V., & Burrell, N. (2018). Implications of sex guilt: A meta-analysis. Marriage and Family Review, 54(5), 417–437. https://doi.org/10.1080/01494929.2017.1359815
- Eschler, L. (2004). The physiology of the female orgasm as a proximate mechanism. Sexualities, Evolution & Gender, 6(2–3), 171–194. https://doi.org/10.1080/14616660412331330875
- Ferster, C. B., & Skinner, B. F. (1957). Schedules of reinforcement. Prentice Hall.
- Ford, C. S., & Beach, F. A. (1951). Patterns of sexual behavior. Harper & Row.
- Freud, S. (1905). Drei abhandlungen zur sexualtheorie. F. Deuticke.
- Freud, S. (1915). Triebe und Triebschicksale. Internationale Zeitschrift für ärztliche Psychoanalyse, 3(2), 84–100.
- Frühauf, S., Gerger, H., Schmidt, H. M., Munder, T., & Barth, J. (2013). Efficacy of psychological interventions for sexual dysfunction: A systematic review and meta-analysis. Archives of Sexual Behavior, 42(6), 915–933. https://doi.org/10.1007/s10508-012-0062-0
- Gagnon, J. H., & Simon, W. (2002). Sexual conduct: The social sources of human sexuality (second ed.), Aldine Transaction.
- Gaither, G. A., & Plaud, J. J. (1997). The effects of secondary stimulus characteristics on men’s sexual arousal. Journal of Sex Research, 34(3), 231–236. https://doi.org/10.1080/00224499709551890
- González-Flores, O., Hoffman, K. L., Delgadillo, J. A., Keller, M., & Paredes, R. G. (2017). Female sexual behavior in rodents, lagomorphs, and goats. In D. W. Pfaff & M. Joëls (Eds.), Hormones, brain and behavior (3rd ed., pp. 59–82). Academic Press. https://doi.org/10.1016/B978-0-12-803592-4.00002-X
- Gooren, L. J. G. (1987). Androgen levels and sex function in testosterone-treated hypogonadal men. Archives of Sexual Behavior, 16(6), 463–473. https://doi.org/10.1007/BF01541711
- Grubin, D. (2018). The pharmacological treatment of sex offenders. In A. J. Beech, R. E. Mann, & P. Rothstein (Eds.), The Wiley Blackwell handbook of forensic neuroscience (Vol. 2, pp. 703–723). John Wiley & Sons.
- Guay, D. R. P. (2009). Drug treatment of paraphilic and nonparaphilic sexual disorders. Clinical Therapeutics, 31(1), 1–31. https://doi.org/10.1016/j.clinthera.2009.01.009
- Hald, G. M., Graham, C., Štulhofer, A., Carvalheira, A., Janssen, E., & Træen, B. (2019). Prevalence of sexual problems and associated distress in aging men across 4 European countries. Journal of Sexual Medicine, 16(8), 1212–1225. https://doi.org/10.1016/j.jsxm.2019.04.017
- Hallberg, J., Kaldo, V., Arver, S., Dhejne, C., Jokinen, J., & Oberg, K. G. (2019). A randomized controlled study of group-administered cognitive behavioral therapy for hypersexual disorder in men. Journal of Sexual Medicine, 16(5), 733–745. https://doi.org/10.1016/j.jsxm.2019.03.005
- Hayes, R. D. (2011). Circular and linear modeling of female sexual desire and arousal. Journal of Sex Research, 48(2–3), 130–141. https://doi.org/10.1080/00224499.2010.548611
- Hayes, R. D., Dennerstein, L., Bennett, C. M., Sidat, M., Gurrin, L. C., & Fairley, C. K. (2008). Risk factors for female sexual dysfunction in the general population: Exploring factors associated with low sexual function and sexual distress. Journal of Sexual Medicine, 5(7), 1681–1693. https://doi.org/10.1111/j.1743-6109.2008.00838.x
- Heiman, J. R., & Hatch, J. P. (1980). Affective and physiological dimensions of male sexual response to erotica and fantasy. Basic and Applied Social Psychology, 1(4), 315–327. https://doi.org/10.1207/s15324834basp0104_3
- Heiman, J. R., Rupp, H., Janssen, E., Newhouse, S. K., Brauer, M., & Laan, E. (2011). Sexual desire, sexual arousal and hormonal differences in premenopausal US and Dutch women with and without low sexual desire. Hormones and Behavior, 59(5), 772–779. https://doi.org/10.1016/j.yhbeh.2011.03.013
- Heim, C. M., Mayberg, H. S., Mletzko, T., Nemeroff, C. B., & Pruessner, J. C. (2013). Decreased cortical representation of genital somatosensory field after childhood sexual abuse. American Journal of Psychiatry, 170(6), 616–623. https://doi.org/10.1176/appi.ajp.2013.12070950
- Hoffmann, H., Peterson, K., & Garner, H. (2012). Field conditioning of sexual arousal in humans. Socioaffective Neuroscience and Psychology, 2(1), Article number 17336. https://doi.org/10.3402/snp.v2i0.17336
- Hook, J. N., Hook, J. P., Davis, D. E., Worthington, E. L., & Penberthy, J. K. (2010). Measuring sexual addiction and compulsivity: A critical review of instruments. Journal of Sex and Marital Therapy, 36(3), 227–260. https://doi.org/10.1080/00926231003719673
- Hook, J. N., Reid, R. C., Penberthy, J. K., Davis, D. E., & Jennings, D. J. (2014). Methodological review of treatments for nonparaphilic hypersexual behavior. Journal of Sex and Marital Therapy, 40(4), 294–308. https://doi.org/10.1080/0092623X.2012.751075
- Hull, E. M., & Rodríguez-Manzo, G. (2017). Male sexual behavior. In D. W. Pfaff & M. Joëls (Eds.), Hormones, brain and behavior (3rd ed., pp. 1–57). Academic Press. https://doi.org/10.1016/B978-0-12-803592-4.00001-8
- Hurlbert, D. F. (1993). A comparative study using orgasm consistency training in the treatment of women reporting hypoactive sexual desire. Journal of Sex and Marital Therapy, 19(1), 41–55. https://doi.org/10.1080/00926239308404887
- Hurlbert, D. F., White, L. C., Powell, R. D., & Apt, C. (1993). Orgasm consistency training in the treatment of women reporting hypoactive sexual desire: An outcome comparison of women-only groups and couples-only groups. Journal of Behavior Therapy and Experimental Psychiatry, 24(1), 3–13. https://doi.org/10.1016/0005-7916(93)90003-F
- Huston, J. P., Silva, M. A. D., Topic, B., & Muller, C. P. (2013). What’s conditioned in conditioned place preference? Trends in Pharmacological Sciences, 34(3), 162–166. https://doi.org/10.1016/j.tips.2013.01.004
- Jaderek, I., & Lew-Starowicz, M. (2019). A systematic review on mindfulness meditation-based interventions for sexual dysfunctions. Journal of Sexual Medicine, 16(10), 1581–1596. https://doi.org/10.1016/j.jsxm.2019.07.019
- Janssen, E. (2011). Sexual arousal in men: A review and conceptual analysis. Hormones and Behavior, 59(5), 708–716. https://doi.org/10.1016/j.yhbeh.2011.03.004
- Janssen, E., & Bancroft, J. (2006). The dual control model: The role of sexual inhibition & excitation in sexual arousal and behavior. In E. Janssen (Ed.), The psychophysiology of sex (pp. 197–222). Indiana University Press.
- Jaspers, L., Feys, F., Bramer, W. M., Franco, O. H., Leusink, P., & Laan, E. T. M. (2016). Efficacy and safety of flibanserin for the treatment of hypoactive sexual desire disorder in women: A systematic review and meta-analysis. JAMA Internal Medicine, 176(4), 453–462. https://doi.org/10.1001/jamainternmed.2015.8565
- Jokinen, J., Boström, A. E., Chatzittofis, A., Ciuculete, D. M., Öberg, K. G., Flanagan, J. N., Arver, S., & Schiöth, H. B. (2017). Methylation of HPA axis related genes in men with hypersexual disorder. Psychoneuroendocrinology, 80, 67–73. https://doi.org/10.1016/j.psyneuen.2017.03.007
- Julien, E., & Over, R. (1988). Male sexual arousal across 5 modes of erotic stimulation. Archives of Sexual Behavior, 17(2), 131–143. https://doi.org/10.1007/BF01542663
- Kafka, M. P. (1997). Hypersexual desire in males: An operational definition and clinical implications for males with paraphilias and paraphilia-related disorders. Archives of Sexual Behavior, 26(5), 505–526. https://doi.org/10.1023/A:1024507922470
- Kafka, M. P. (2014). What happened to hypersexual disorder? Archives of Sexual Behavior, 43(7), 1259–1261. https://doi.org/10.1007/s10508-014-0326-y
- Kamel, F., Wright, W. W., Mock, E. J., & Frankel, A. I. (1977). The influence of mating and related stimuli on plasma levels of luteinizing hormone, follicle stimulating hormone, prolactin and testosterone in the male rat. Endocrinology, 101(2), 421–429. https://doi.org/10.1210/endo-101-2-421
- Kaplan, H. S. (1979). Disorders of sexual desire and other new concepts and techniques in sex therapy. Simon and Schuster.
- Kaplan, H. S. (1995). The sexual desire disorders. Dysfunctional regulation of sexual motivation. Brunner/Mazel.
- Kingsberg, S. A., Althof, S., Simon, J. A., Bradford, A., Bitzer, J., Carvalho, J., Flynn, K. E., Nappi, R. E., Reese, J. B., Rezaee, R. L., Schover, L., & Shifrin, J. L. (2017). Female sexual dysfunction-medical and psychological treatments, committee 14. Journal of Sexual Medicine, 14(12), 1463–1491. https://doi.org/10.1016/j.jsxm.2017.05.018
- Kingsberg, S. A., Clayton, A. H., Portman, D., Krop, J., Jordan, R., Lucas, J., & Simon, J. A. (2021). Failure of a meta-analysis: A commentary on Glen Spielmans’s “Re-analyzing phase III bremelanotide trials for ‘hypoactive sexual desire disorder in women’. Journal of Sex Research, 58(9), 1106–1107. https://doi.org/10.1080/00224499.2021.1902926
- Kingston, D. A., & Firestone, P. (2008). Problematic hypersexuality: A review of conceptualization and diagnosis. Sexual Addiction & Compulsivity, 15(4), 284–310. https://doi.org/10.1080/10720160802289249
- Kinsey, A. C., Pomeroy, W. B., & Martin, C. E. (1948). Sexual behavior in the human male. Saunders.
- Kinsey, A. C., Pomeroy, W. B., Martin, C. E., & Gebhard, P. H. (1953). Sexual behavior in the human female. Saunders.
- Kleinplatz, P. J. (2018). History of the treatment of female sexual dysfunction (s). Annual Review of Clinical Psychology, 14(1), 29–54. https://doi.org/10.1146/annurev-clinpsy-050817-084802
- Klumbies, G., & Kleinsorge, H. (1950). Das herz in orgasmus. Medizinische Klinik, 45(31), 952–958.
- Köksal, F., Domjan, M., Kurt, A., Sertel, Ö., Örüng, S., Bowers, B., & Kumru, G. (2004). An animal model of fetishism. Behaviour Research and Therapy, 42(12), 1421–1434. https://doi.org/10.1016/j.brat.2003.10.001
- Köksal, F., Kumru, G., Anarat, C., Domjan, M., & Yeniceri, N. (2021). Compulsive conditioned sexual responding of male Japanese quail in extinction. Archives of Sexual Behavior, 50(3), 1207–1216. https://doi.org/10.1007/s10508-020-01906-5
- Krueger, R. B., & Kaplan, M. S. (2001). The paraphilic and hypersexual disorders: An overview. Journal of Psychiatric Practice, 7(6), 391–403. https://doi.org/10.1097/00131746-200111000-00005
- Kudwa, A. E., Dominguez-Salazar, E., Cabrera, D. M., Sibley, D. R., & Rissman, E. F. (2005). Dopamine D5 receptor modulates male and female sexual behavior in mice . Psychopharmacology, 180(2), 206–214. https://doi.org/10.1007/s00213-005-2150-5
- Laan, E., & Both, S. (2008). What makes women experience desire?. Feminism & Psychology, 18(4), 505–514. https://doi.org/10.1177/0959353508095533
- Laan, E., & Both, S. (2011). Sexual desire and arousal disorders in women. Advances in Psychosomatic Medicine, 31, 16–34. https://doi.org/10.1159/000328806
- Laan, E., Brauer, M., & van Lunsen, R. H. W. (2010). New classification of women’s sexual problems in DSM-V: Support for merging FSAD and HSDD. Journal of Sexual Medicine, 7(6), 2002–2004. https://doi.org/10.1111/j.1743-6109.2010.01865.x
- Laan, E., & Everaerd, W. (1995). Determinants of female sexual arousal: Psychophysiological theory and data. Annual Review of Sex Research, 6(1), 32–76.
- Laan, E. T. M., Prins, J. M., van Lunsen, R. H. W., Nieuwkerk, P. T., & Nievaard-Boon, M. A. F. (2019). Testosterone insufficiency in human immunodeficiency virus-infected women: A cross-sectional study. Sexual Medicine, 7(1), 72–79. https://doi.org/10.1016/j.esxm.2018.10.002
- Leitenberg, H., & Henning, K. (1995). Sexual fantasy. Psychological Bulletin, 117(3), 469–496. https://doi.org/10.1037/0033-2909.117.3.469
- Le Moëne, O., & Ågmo, A. (2019). Modeling human sexual motivation in rodents: Some caveats. Frontiers in Behavioral Neuroscience, 13, Article 187. https://doi.org/10.3389/fnbeh.2019.00187
- Letourneau, E. J., Resnick, H. S., Kilpatrick, D. G., Saunders, B. E., & Best, C. L. (1996). Comorbidity of sexual problems and posttraumatic stress disorder in female crime victims. Behavior Therapy, 27(3), 321–336. https://doi.org/10.1016/S0005-7894(96)80020-7
- Levaque, E., Sawatsky, M. L., & Lalumiere, M. L. (2016). Hypersexualité chez les étudiants universitaires hétérosexuels. Canadian Journal of Behavioural Science-Revue Canadienne Des Sciences Du Comportement, 48(3), 182–192. https://doi.org/10.1037/cbs0000042
- Levin, R. J., & van Berlo, W. (2004). Sexual arousal and orgasm in subjects who experience forced or non-consensual sexual stimulation – A review. Journal of Clinical Forensic Medicine, 11(2), 82–88. https://doi.org/10.1016/j.jcfm.2003.10.008
- Lewis, A., Grubin, D., Ross, C. C., & Das, M. (2017). Gonadotrophin-releasing hormone agonist treatment for sexual offenders: A systematic review. Journal of Psychopharmacology, 31(10), 1281–1293. https://doi.org/10.1177/0269881117714048
- LoPiccolo, J., & Friedman, J. M. (1988). Broad-spectrum treatment of low sexual desire: Integration of cognitive, behavioral, and systemic therapy. In S. R. Leiblum & R. C. Rosen (Eds.), Sexual desire disorders (pp. 107–144). Guilford Press.
- Macrides, F., Bartke, A., Fernandez, F., & D’Angelo, W. (1974). Effects of exposure to vaginal odor and receptive females on plasma testosterone in the male hamster. Neuroendocrinology, 15(6), 355–364. https://doi.org/10.1159/000122326
- Maczkowiack, J., & Schweitzer, R. D. (2019). Postcoital dysphoria: Prevalence and correlates among males. Journal of Sex and Marital Therapy, 45(2), 128–140. https://doi.org/10.1080/0092623X.2018.1488326
- Mark, K. P., & Lasslo, J. A. (2018). Maintaining sexual desire in long-term relationships: A systematic review and conceptual model. Journal of Sex Research, 55(4–5), 563–581. https://doi.org/10.1080/00224499.2018.1437592
- Marshall, D. S., & Suggs, R. C. (1971). Human sexual behavior: Variations in the ethnographic spectrum. Basic Books.
- Masters, W. H., & Johnson, V. E. (1970). Human sexual inadequacy. Little, Brown & Co.
- McConaghy, N. (1974). Penile volume responses to moving and still pictures of male and female nudes. Archives of Sexual Behavior, 3(6), 565–570. https://doi.org/10.1007/BF01541138
- McConaghy, N. (1975). Aversive and positive conditioning treatments of homosexuality. Behaviour Research and Therapy, 13(4), 309–319. https://doi.org/10.1016/0005-7967(75)90036-4
- McDougall, W. (1914). The definition of the sexual instinct. Proceedings of the Royal Society of Medicine, 7(Section Psychology), 65–88. https://doi.org/10.1177/003591571400701815
- Meerts, S. H., & Clark, A. S. (2007). Female rats exhibit a conditioned place preference for nonpaced mating. Hormones and Behavior, 51(1), 89–94. https://doi.org/10.1016/j.yhbeh.2006.08.007
- Meston, C. M., & Buss, D. M. (2007). Why humans have sex. Archives of Sexual Behavior, 36(4), 477–507. https://doi.org/10.1007/s10508-007-9175-2
- Meyer, D., Cohn, A., Robinson, B., Muse, F., & Hughes, R. (2017). Persistent complications of child sexual abuse: Sexually compulsive behaviors, attachment, and emotions. Journal of Child Sexual Abuse, 26(2), 140–157. https://doi.org/10.1080/10538712.2016.1269144
- Micevych, P. E., & Meisel, R. L. (2017). Integrating neural circuits controlling female sexual behavior. Frontiers in Systems Neuroscience, 11, Article number 42. https://doi.org/10.3389/fnsys.2017.00042
- Mitchell, K. R., Lewis, R., O’Sullivan, L. F., & Fortenberry, J. D. (2021). What is sexual wellbeing and why does it matter for public health? Lancet Public Health, 6(8), E608–E613. https://doi.org/10.1016/S2468-2667(21)00099-2
- Montgomery-Graham, S. (2017). Conceptualization and assessment of hypersexual disorder: A systematic review of the literature. Sexual Medicine Reviews, 5(2), 146–162. https://doi.org/10.1016/j.sxmr.2016.11.001
- Moralí, G., & Beyer, C. (1992). Motor aspects of masculine sexual behavior in rats and rabbits. Advances in the Study of Behavior, 21, 201–238. https://doi.org/10.1016/S0065-3454(08)60145-X
- Moralí, G., Soto, M. A. P., Contreras, J. L., Arteaga, M., González-Vidal, M. D., & Beyer, C. (2003). Detailed analysis of the male copulatory motor pattern in mammals: Hormonal bases. Scandinavian Journal of Psychology, 44(3), 279–288. https://doi.org/10.1111/1467-9450.00346
- Morgan, C. T. (1942). Physiological psychology. McGraw-Hill.
- Moynihan, A. B., Igou, E. R., & van Tilburg, W. A. P. (2021). Bored stiff: The relationship between meaninglessness, sexual sensation seeking, and promiscuous attitudes via boredom susceptibility. Personality and Individual Differences, 168, Article number 110295. https://doi.org/10.1016/j.paid.2020.110295
- Murray, S. H., Milhausen, R. R., Graham, C. A., & Kuczynski, L. (2017). A qualitative exploration of factors that affect sexual desire among men aged 30 to 65 in long-term relationships. Journal of Sex Research, 54(3), 319–330. https://doi.org/10.1080/00224499.2016.1168352
- Najman, J. M., Dunne, M. P., Purdie, D. M., Boyle, F. M., & Coxeter, P. D. (2005). Sexual abuse in childhood and sexual dysfunction in adulthood: An Australian population-based study. Archives of Sexual Behavior, 34(5), 517–526. https://doi.org/10.1007/s10508-005-6277-6
- Öberg, K., Fugl-Meyer, A. R., & Fugl-Meyer, K. S. (2004). On categorization and quantification of women’s sexual dysfunctions: An epidemiological approach. International Journal of Impotence Research, 16(3), 261–269. https://doi.org/10.1038/sj.ijir.3901151
- Ogawa, S., Eng, V., Taylor, J., Lubahn, D. B., Korach, K. S., & Pfaff, D. W. (1998). Roles of estrogen receptor-a gene expression in reproduction-related behaviors in female mice. Endocrinology, 139(12), 5070–5081. https://doi.org/10.1210/endo.139.12.6357
- O’Loughlin, J. I., Basson, R., & Brotto, L. A. (2018). Women with hypoactive sexual desire disorder versus sexual interest/arousal disorder: An empirical test of raising the bar. Journal of Sex Research, 55(6), 734–746. https://doi.org/10.1080/00224499.2017.1386764
- O’Loughlin, J. I., Rellini, A. H., & Brotto, L. A. (2020). How does childhood trauma impact women’s sexual desire? Role of depression, stress, and cortisol. Journal of Sex Research, 57(7), 836–847. https://doi.org/10.1080/00224499.2019.1693490
- Parada, M., Chamas, L., Censi, S., Coria-Avila, G., & Pfaus, J. G. (2010). Clitoral stimulation induces conditioned place preference and Fos activation in the rat. Hormones and Behavior, 57(2), 112–118. https://doi.org/10.1016/j.yhbeh.2009.05.008
- Paredes, R. G. (2003). Medial preoptic area/anterior hypothalamus and sexual motivation. Scandinavian Journal of Psychology, 44(3), 203–212. https://doi.org/10.1111/1467-9450.00337
- Paredes, R. G. (2009). Evaluating the neurobiology of sexual reward. Ilar Journal, 50(1), 15–27. https://doi.org/10.1093/ilar.50.1.15
- Paredes, R. G., & Alonso, A. (1997). Sexual behavior regulated (paced) by the female induces conditioned place preference. Behavioral Neuroscience, 111(1), 123–128. https://doi.org/10.1037/0735-7044.111.1.123
- Paredes, R. G., & Vázquez, B. (1999). What do female rats like about sex? Paced mating. Behavioural Brain Research, 105(1), 117–127. https://doi.org/10.1016/S0166-4328(99)00087-X
- Peters, R. H. (1983). Learned aversions to copulatory behaviors in male rats. Behavioral Neuroscience, 97(1), 140–145. https://doi.org/10.1037/0735-7044.97.1.140
- Pfaff, D. W. (1980). Estrogens and brain function: Neural analysis of a hormone-controlled mammalian reproductive behavior. Springer.
- Pfaff, D. W. (1999). Drive. Neurobiological and molecular mechanisms of sexual motivation. MIT Press.
- Pfaff, D. (2017). How the vertebrate brain regulates behavior. Direct from the lab. Harvard University Press.
- Popik, P., Wrobel, M., Rygula, R., Bisaga, A., & Bespalov, A. Y. (2003). Effects of memantine, an NMDA receptor antagonist, on place preference conditioned with drug and nondrug reinforcers in mice. Behavioural Pharmacology, 14(3), 237–244. https://doi.org/10.1097/00008877-200305000-00008
- Portillo, W., & Paredes, R. G. (2004). Sexual incentive motivation, olfactory preference, and activation of the vomeronasal projection pathway by sexually relevant cues in non-copulating and naive male rats. Hormones and Behavior, 46(3), 330–340. https://doi.org/10.1016/j.yhbeh.2004.03.001
- Prause, N., Steele, V. R., Staley, C., & Sabatinelli, D. (2015). Late positive potential to explicit sexual images associated with the number of sexual intercourse partners. Social Cognitive and Affective Neuroscience, 10(1), 93–100. https://doi.org/10.1093/scan/nsu024
- Pyke, R. E., & Clayton, A. H. (2018). Effect size in efficacy trials of women with decreased sexual desire. Sexual Medicine Reviews, 6(3), 358–366. https://doi.org/10.1016/j.sxmr.2018.01.003
- Quinsey, V. L., Bergersen, S. G., & Steinman, C. M. (1976). Changes in physiological and verbal responses of child molesters during aversion therapy. Canadian Journal of Behavioural Science-Revue Canadienne Des Sciences Du Comportement, 8(2), 202–212. https://doi.org/10.1037/h0081948
- Quinsey, V. L., & Marshall, W. L. (1983). Procedures for reducing inappropriate sexual arousal: An evaluation review. In J. G. Greer & I. R. Stuart (Eds.), The sexual aggressor. Current perspectives on treatment (pp. 267–289). Van Nostrand Reinhold.
- Rastrelli, G., Corona, G., Tarocchi, M., Mannucci, E., & Maggi, M. (2016). How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. Journal of Endocrinological Investigation, 39(4), 473–484. https://doi.org/10.1007/s40618-015-0425-1
- Raymond, N. C., Coleman, E., & Miner, M. H. (2003). Psychiatric comorbidity and compulsive/impulsive traits in compulsive sexual behavior. Comprehensive Psychiatry, 44(5), 370–380. https://doi.org/10.1016/S0010-440X(03)00110-X
- Reid, R. C., Berlin, H. A., & Kingston, D. A. (2015). Sexual impulsivity in hypersexual men. Current Behavioral Neuroscience Reports, 2(1), 1–8. https://doi.org/10.1007/s40473-015-0034–5
- Reid, R. C., Carpenter, B. N., & Hook, J. N. (2016). Investigating correlates of hypersexual behavior in religious patients. Sexual Addiction & Compulsivity-the Journal of Treatment and Prevention, 23(2–3), 296–312. https://doi.org/10.1080/10720162.2015.1130002
- Rosen, R. C., & Beck, J. G. (1988). Patterns of sexual arousal: Psychophysiological processes and clinical applications. Guilford Press.
- Rossi, N. A., & Reid, L. D. (1976). Affective states associated with morphine injections. Physiological Psychology, 4(3), 269–274. https://doi.org/10.3758/BF03332869
- Saadat, S. H., Kabir, A., Rahmani, K., Panahi, Y., Hosseinialhashemi, M., & Sahebkar, A. (2017). Systematic review and meta-analysis of flibanserin’s effects and adverse events in women with hypoactive sexual desire disorder. Current Drug Metabolism, 18(1), 78–85. https://doi.org/10.2174/1389200217666161026090333
- Sachs, B. D. (1997). Erection evoked in male rats by airborne scent from estrous females. Physiology and Behavior, 62(4), 921–924. https://doi.org/10.1016/S0031-9384(97)00307-7
- Saginor, M., & Horton, R. (1968). Reflex release of gonadotropin and increased plasma testosterone concentration in male rabbits during copulation. Endocrinology, 82(3), 627–630. https://doi.org/10.1210/endo-82–3-627
- Sano, K., Tsuda, M. C., Musatov, S., Sakamoto, T., & Ogawa, S. (2013). Differential effects of site-specific knockdown of estrogen receptor alpha in the medial amygdala, medial preoptic area, and ventromedial nucleus of the hypothalamus on sexual and aggressive behavior of male mice. European Journal of Neuroscience, 37(8), 1308–1319. https://doi.org/10.1111/ejn.12131
- Santi, D., Spaggiari, G., Gilioli, L., Poti, F., Simoni, M., & Casarini, L. (2018). Molecular basis of androgen action on human sexual desire. Molecular and Cellular Endocrinology, 467, 31–41. https://doi.org/10.1016/j.mce.2017.09.007
- Sarin, S., Amsel, R., & Binik, Y. M. (2014). How hot is he? A psychophysiological and psychosocial examination of the arousal patterns of sexually functional and dysfunctional men. Journal of Sexual Medicine, 11(7), 1725–1740. https://doi.org/10.1111/jsm.12562
- Sarin, S., Amsel, R., & Binik, Y. M. (2016). A streetcar named “derousal”? A psychophysiological examination of the desire-arousal distinction in sexually functional and dysfunctional women. Journal of Sex Research, 53(6), 711–729. https://doi.org/10.1080/00224499.2015.1052360
- Schmidt, H. M., Hohn, C., Widmeier, E., & Berner, M. M. (2017). Psychosoziale Interventionen für sexuelle Funktionsstörungen bei Frauen. Zeitschrift für Sexualforschung, 30(3), 213–247. https://doi.org/10.1055/s-0043-117321
- Schmidt, P. J., Steinberg, E. M., Negro, P. P., Haq, N., Gibson, C., & Rubinow, D. R. (2009). Pharmacologically induced hypogonadism and sexual function in healthy young women and men. Neuropsychopharmacology, 34(3), 565–576. https://doi.org/10.1038/npp.2008.24
- Schweitzer, R. D., O’Brien, J., & Burri, A. (2015). Postcoital dysphoria: Prevalence and psychological correlates. Sexual Medicine, 3(4), 235–243. https://doi.org/10.1002/sm2.74
- Seehuus, M., Stanton, A. M., & Handy, A. B. (2019). On the content of ”real-world” sexual fantasy: Results from an analysis of 250,000+ anonymous text-based erotic fantasies. Archives of Sexual Behavior, 48(3), 725–737. https://doi.org/10.1007/s10508-018-1334-0
- Segraves, K. B., & Segraves, R. T. (1991). Hypoactive sexual desire disorder: Prevalence and comorbidity in 906 subjects. Journal of Sex and Marital Therapy, 17(1), 55–58. https://doi.org/10.1080/00926239108405469
- Shifren, J. L., Monz, B. U., Russo, P. A., Segreti, A., & Johannes, C. B. (2008). Sexual problems and distress in United States women: Prevalence and correlates. Obstetrics and Gynecology, 112(5), 970–978. https://doi.org/10.1097/AOG.0b013e3181898cdb
- Singer, B., & Toates, F. M. (1987). Sexual motivation. Journal of Sex Research, 23(4), 481–501. https://doi.org/10.1080/00224498709551386
- Slavin, M. N., Scoglio, A. A. J., Blycker, G. R., Potenza, M. N., & Kraus, S. W. (2020). Child sexual abuse and compulsive sexual behavior: A systematic literature review. Current Addiction Reports, 7(1), 76–88. https://doi.org/10.1007/s40429-020-00298-9
- Spielmans, G. I. (2022). Re-analyzing phase III bremelanotide trials for “hypoactive sexual desire disorder” in women. Journal of Sex Research, 58(9), 1085–1105. https://doi.org/10.1080/00224499.2021.1885601
- Spiteri, T., & Ågmo, A. (2006). Modèles precliniques du désir sexuel. Sexologies, 15(4), 241–249. https://doi.org/10.1016/j.sexol.2006.05.001
- Spiteri, T., Le Pape, G., & Ågmo, A. (2000). What is learned during place preference conditioning? A comparison of food- and morphine-induced reward. Psychobiology, 28(3), 367–382. https://doi.org/10.3758/BF03331994
- Spiteri, T., Musatov, S., Ogawa, S., Ribeiro, A., Pfaff, D. W., & Ågmo, A. (2010). Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor a in the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology, 91(2), 142–154. https://doi.org/10.1159/000255766
- Steele, V. R., Staley, C., Fong, T., & Prause, N. (2013). Sexual desire, not hypersexuality, is related to neurophysiological responses elicited by sexual images. Socioaffective Neuroscience & Psychology, 3(1), 20770. https://doi.org/10.3402/snp.v3i0.20770
- Sutherland, S. E., Rehman, U. S., Fallis, E. E., & Goodnight, J. A. (2015). Understanding the phenomenon of sexual desire discrepancy in couples. Canadian Journal of Human Sexuality, 24(2), 141–150. https://doi.org/10.3138/cjhs.242.A3
- Takahashi, L. K. (1990). Hormonal regulation of sociosexual behavior in female mammals. Neuroscience and Biobehavioral Reviews, 14(4), 403–413. https://doi.org/10.1016/S0149-7634(05)80062-4
- Tenk, C. M., Wilson, H., Zhang, Q., Pitchers, K. K., & Coolen, L. M. (2009). Sexual reward in male rats: Effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Hormones and Behavior, 55(1), 93–97. https://doi.org/10.1016/j.yhbeh.2008.08.012
- Toates, F. (1989). Motivational systems. Cambridge University Press.
- Toates, F. (2009). An integrative theoretical framework for understanding sexual motivation, arousal, and behavior. Journal of Sex Research, 46(2–3), 168–193. https://doi.org/10.1080/00224490902747768
- Toates, F. M. (2020). The enigmatic urge: How sexual desire works. In J. H. Barkow, L. Workman, & W. Reader (Eds.), The Cambridge handbook of evolutionary perspectives on human behavior (pp. 330–341). Cambridge University Press. https://doi.org/10.1017/9781108131797.028
- Toates, F. (2022). A motivation model of sex addiction – Relevance to the controversy over the concept. Neuroscience and Biobehavioral Reviews, 142, Article number 104872. https://doi.org/10.1016/j.neubiorev.2022.104872
- Træen, B., Martinussen, M., Öberg, K., & Kavli, H. (2007). Reduced sexual desire in a random sample of Norwegian couples. Sexual and Relationship Therapy, 22(3), 303–322. https://doi.org/10.1080/14681990701381203
- Trudel, G., Aubin, S., & Matte, B. (1995). Sexual behaviors and pleasure in couples with hypoactive sexual desire. Journal of Sex Education and Therapy, 21(3), 210–216. https://doi.org/10.1080/01614576.1995.11074153
- Trudel, G., Fortin, C., & Matte, B. (1997). Sexual interaction and communication in couples with hypoactive sexual desire. Scandinavian Journal of Behaviour Therapy, 26(2), 49–53. https://doi.org/10.1080/16506079708412037
- Tzschentke, T. M. (2007). Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addiction Biology, 12(3–4), 227–462. https://doi.org/10.1111/j.1369-1600.2007.00070.x
- Wåhlin-Jacobsen, S., Kristensen, E., Pedersen, A. T., Laessøe, N. C., Cohen, A. S., Hougaard, D. M., Lundqvist, M., & Giraldi, A. (2017). Androgens and psychosocial factors related to sexual dysfunctions in premenopausal women. Journal of Sexual Medicine, 14(3), 366–379. https://doi.org/10.1016/j.jsxm.2016.12.237
- Wallen, K. (1990). Desire and ability: Hormones and the regulation of female sexual behavior. Neuroscience and Biobehavioral Reviews, 14(2), 233–241. https://doi.org/10.1016/S0149-7634(05)80223–4
- Walton, M. T., Cantor, J. M., Bhullar, N., & Lykins, A. (2017). Hypersexuality: A critical review and introduction to the “sexhavior cycle.” Archives of Sexual Behavior, 46(8), 2231–2251. https://doi.org/10.1007/s10508-017-0991-8
- Wang, L., & Murnighan, J. K. (2011). On greed. Academy of Management Annals, 5(1), 279–316. https://doi.org/10.5465/19416520.2011.588822
- Ward, T., Gannon, T. A., & Birgden, A. (2007). Human rights and the treatment of sex offenders. Sexual Abuse-a Journal of Research and Treatment, 19(3), 195–216. https://doi.org/10.1177/107906320701900302
- West, S. L., D’Aloisio, A. A., Agans, R. P., Kalsbeek, W. D., Borisov, N. N., & Thorp, J. M. (2008). Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Archives of Internal Medicine, 168(13), 1441–1449. https://doi.org/10.1001/archinte.168.13.1441
- Whipple, B., Ogden, G., & Komisaruk, B. R. (1992). Physiological correlates of imagery-induced orgasm in women. Archives of Sexual Behavior, 21(2), 121–133. https://doi.org/10.1007/BF01542589
- Williams, D. J., Thomas, J. N., & Prior, E. E. (2020). Are sex and pornography addiction valid disorders? Adding a leisure science perspective to the sexological critique. Leisure Sciences 41(3–4), 306–321. https://doi.org/10.1080/01490400.2020.1712284
- Wise, N. J., Frangos, E., & Komisaruk, B. R. (2016). Activation of sensory cortex by imagined genital stimulation: An fMRI analysis. Socioaffective Neuroscience & Psychology, 6 (1). Article number 31481. https://doi.org/10.3402/snp.v6.31481.
- Worsley, R., Bell, R. J., Gartoulla, P., & Davis, S. R. (2017). Prevalence and predictors of low sexual desire, sexually related personal distress, and hypoactive sexual desire dysfunction in a community-based sample of midlife women. Journal of Sexual Medicine, 14(5), 675–686. https://doi.org/10.1016/j.jsxm.2017.03.254
