ABSTRACT
Although fear learning mechanisms are implicated in the development, maintenance, exacerbation, and reduction of genital pain, systematic research on how fear of genital pain emerges, spreads, persists, and reemerges after treatment is lacking. This paper provides an overview of the literature on pain-related fear, integrates the ideas on learning and sexual arousal responding, and specifies the pathways through which compromised learning may contribute to the development and persistence of genital pain. In order to refine theories of genital pain and optimize treatments, we need to adopt a biopsychosocial framework to pain-related fear learning and uncover potential moderators that shape individual trajectories. This involves examining the role of physiological processes, subjective experiences, as well as partner and relational cues in fear acquisition, excessive generalization and impaired safety learning, extinction of fear, counterconditioning, and return of fear. Recent methodological advances in fear conditioning and sex research are promising to enable more symptom-specific and ecologically valid experimental paradigms.
A Biopsychosocial Approach to Genital Pain: The Key Role of Fear of Pain
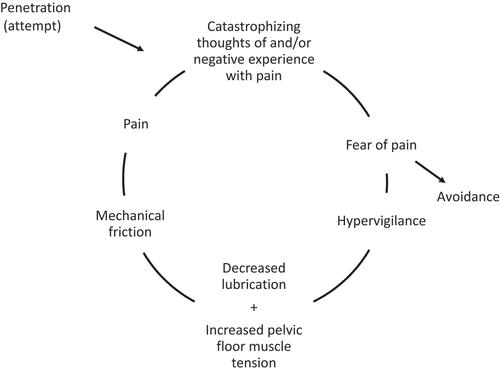
Genital pain is a disabling health problem in women, with a lifetime prevalence between 10–28%, causing substantial daily distress (Arnold et al., Citation2007; Reed et al., Citation2008). For a long time, genital pain has been approached from a biomedical perspective, studying physical markers such as inflammation, (vaginal) infections, hormonal triggers, central sensitization, pelvic floor dysfunction and genetic factors that may cause or put women at risk for genital pain (Bergeron et al., Citation2020; Dewitte, Citation2016). The biomedical approach, which is often still the first-line option, targets the periphery or central nervous system via pharmacological agents and surgery. The role of psychological and social factors, that are likely more involved in maintaining and exacerbating the pain, has been acknowledged only during the past few decades. Several psychosocial parameters have been identified, ranging from personality traits, (sexual) self-concept, sexual autonomy, depression and anxiety to partner responses and relationship variables, with the largest consensus being reached on the role of fear in the pathogenesis of genital pain (Bergeron et al., Citation2020; Dewitte et al., Citation2018). Fear of pain is a key factor in current models of genital pain because it prompts a cascade of cognitive-motivational and physiological processes that interact with each other and influence pain processing via multiple pathways (Dewitte et al., Citation2011; ter Kuile et al., Citation2010; ter Kuile & Weijenborg, Citation2006). Fear is known to induce hypervigilance to painful sexual stimuli and diminish attention for sexually exciting stimuli. The latter are negatively appraised, often in terms of bodily threat. Attentional distraction from sexual cues and negative, threatening appraisals in turn inhibit genital arousal, resulting in vaginal dryness, and increase pelvic floor muscle tension, consequently reducing genital hiatus. These physiological reactions, together with low sexual readiness, are thought to cause mechanical friction causing pain, which then interacts with partner responses and the relational context (See ; Spano & Lamont, Citation1975; ter Kuile & Weijenborg, Citation2006). When sexual activities, as well as intimacy- and partner-related cues, come to signal pain and bodily threat, sexual encounters will be increasingly avoided (Ekdahl et al., Citation2018). Amending the pain experience may require more than only understanding and tackling the fear, but its central impact on the psychophysiological mechanisms underlying pain does indicate that fear is the first obstacle to overcome when pursuing sexual pleasure.
Figure 1. Anxiety-pain circle by Spano and Lamont (Citation1975) and ter Kuile and Weijenborg (Citation2006).
The central role of pain-related fear is also reflected in current diagnostic classifications of genital pain. Although female genital pain is a heterogenous condition, yielding a diversity in response patterns that vary along pain location, onset, temporal dynamics, and situations that elicit pain, the DSM-5 now describes only one diagnostic category of genital pain, with fear of pain being a common criterion across conditions (American Psychiatric Association[APA], Citation2013; Bornstein et al., Citation2016). On the broadest level, a distinction can be made between primary or lifelong genital pain, i.e., present from the first sexual encounter, and secondary or acquired pain, i.e., acquired through experience. Although both types of pain are likely the result of distinct learning processes, pain-related fear is clearly involved in acquiring and generalizing the pain and turning it into a chronic pain condition. Pain-related fear and behavioral coping with pain are considered key factors in the transition from acute to chronic pain (Bergeron et al., Citation2011; ter Kuile et al., Citation2010; ter Kuile & Weijenborg, Citation2006). Accordingly, the most common treatment of genital pain, namely Cognitive Behavioral Therapy (CBT), targets fear and avoidance and aims at increasing sexual arousal (Bergeron et al., Citation2008, Citation2011; Goldstein et al., Citation2016; ter Kuile et al., Citation2009, Citation2010).
It is remarkable that the treatment of genital pain implements learning principles without drawing on a solid base of evidence to support the role of fear learning in genital pain. Furthermore, current treatment protocols are not well aligned with recent developments in fear learning research. Another important concern is the one-size-fits-all approach of genital pain treatment, which does not take into account the observed heterogeneity in clinical profiles showing a wide range of pain-related fear expression and behavioral coping strategies and therefore does not achieve maximal therapeutic benefit (Dewitte et al., Citation2018; De Kruiff et al., Citation2000; Lahaie et al., Citation2015). For example, while some women with genital pain display phobic avoidance of sex to prevent pain during penetration or seek pain relief, others persist with penetrative sex because they fear negative relational outcomes such as relational conflict or their partner leaving them (Brauer et al., Citation2014). Current treatments tend to ignore patients' idiosyncratic needs and disregard individual vulnerabilities and resiliencies.
To summarize, both on a conceptual and a clinical level, fear (learning) is implicated in the development, maintenance, exacerbation, as well as the reduction of genital pain (see also Both et al., Citation2008, Citation2017; Brom et al., Citation2014). Yet, systematic experimental research examining whether and how compromised fear learning (i.e., referring to a disbalance in threat-safety learning, see below) contributes to genital pain disability, and how “unlearning” (i.e., extinction) techniques can be clinically applied to reduce pain-related fear and avoidance is largely lacking (see also Both et al., Citation2008, Citation2017). In this paper, we argue that in order to refine our theories of genital pain and optimize/customize treatments, scientific attention should be directed to learning mechanisms underlying the development and maintenance of genital pain. In addition, potential moderators that shape individual trajectories in pain-related fear responding should be scrutinized. We start with describing how learning mechanisms are generally involved in shaping sexual responses and then describe the learning mechanisms underlying fear and apply this to genital pain.
A Learning Theory Perspective on Sexual Arousal and Pain-Related Fear
Learning to Become Sexually Aroused
Research on conditioning of sexual arousal has gained ground over the years. However, the majority of studies to date have focused on appetitive conditioning, i.e., learning about positive outcomes such as pleasure or sexual reward, while aversive conditioning, i.e., learning about negative outcomes such as pain or discomfort, has been largely neglected (Ågmo et al., Citation2004; Both et al., Citation2008; Brom et al., Citation2014; O’Donohue & Plaud, Citation1994). To better understand how learning mechanisms are involved in sexual dysfunctions, such as female genital pain, we start with explaining how sexual responses unfold as a cascade of cognitive, emotional, motivational, and behavioral processes (see ). According to incentive motivation models, the sexual system is triggered by stimuli that have acquired incentive value, as learned by previous experiences or by being innately sexually competent (i.e., “prepared” stimuli such as touching and kissing; Bindra, Citation1974; Both et al., Citation2007; Toates, Citation1998, Citation2009). These stimuli will pre-attentively capture attention and will automatically be appraised as sexually meaningful (or not) via automatic matching in memory. This appraisal process automatically evokes a genital arousal response, which then motivates people to maintain their attention to the sexual stimulus and cognitively elaborate (i.e., consciously appraise) it. When this results in a positive evaluation (reinforced by memories of positive sexual events) and the expectation of reward, a subjective sense of arousal is experienced, which further increases genital and subjective arousal, resulting in a full-blown sexual response. These ongoing sexual responses may then trigger the desire and motivation to actually engage in sexual activities (see also Bindra, Citation1974; Toates, Citation2009). Whenever in this process negative appraisal occurs, resulting from aversive learning experiences, sexual responses may be attenuated or inhibited. In the context of genital pain, it can thus be assumed that fear of pain will interfere with several of these phases, resulting in hypervigilance to painful sexual stimuli, distraction from sexually exciting stimuli, self-monitoring, threat appraisals, impaired genital and subjective sexual arousal, and avoidance motivation (Bergeron et al., Citation2011; Dewitte et al., Citation2011; ter Kuile et al., Citation2010).
Figure 2. Sexual arousal responding (Bindra, Citation1974; Dewitte, Citation2014; Janssen et al., Citation2000; Toates, Citation2009).
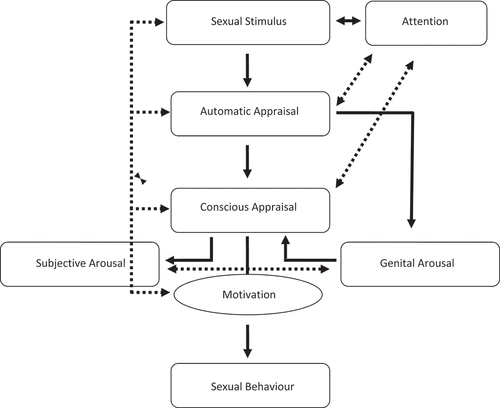
The incentive motivation model explains that sexual arousal consists of a subjective and a physical component and both processes may diverge in women (Chivers et al., Citation2010). Although the clinical relevance of sexual desynchrony has not been demonstrated (Meston & Stanton, Citation2018), it could be that the divergence between how sexually aroused women feel and how their body reacts to sexual stimuli relates to sexual problems that are characterized by low sexual arousability such as genital pain (Brotto et al., Citation2016). It is worth exploring whether genital and subjective sexual arousal are shaped by different learning principles or have different thresholds for modulation. Although learning processes are clearly implicated in the model of sexual responding, there is only limited research on how stimuli acquire sexually arousing or threat properties and activate subjective and autonomic responses associated with sexual arousal. Given its clinical relevance and cardinal role in treatment models, the role of aversive sex-related conditioning in particular requires further scrutiny.
Learning to Fear and Avoid Pain
Learning to predict pain is adaptive for survival as pain threatens bodily integrity and is a warning signal for damage (Meulders, Citation2020; Vlaeyen, Citation2015). Classical or Pavlovian conditioning, which is a type of S-S (stimulus-stimulus) or signal learning, is the principal mechanism by which pain-related fear learning can take place (Pavlov, Citation1927a, Citation1927b; Vlaeyen, Citation2015; for a review on conditioning, see also De Houwer, Citation2020a, Citation2020b; De Houwer & Hughes, Citation2020). In the context of genital pain, repeatedly pairing a sexual stimulus (CS), such as a picture of a couple engaging in penetration, with a pain-evoking stimulus (US), such as an electrical stimulus, will evoke fear responding (CR) when the sexual stimulus is presented alone because of its previous pairing with a pain-evoking stimulus. Learning to fear genital pain can also occur in non-sexual situations when tactile stimulation of the vulva or vaginal penetration by non-sexual objects (e.g., a tampon) is repeatedly associated with pain sensations.
Pain and pain-related fear will most often evoke protective behaviors, such as withdrawal and avoidance (Vlaeyen et al., Citation2016; Vlaeyen & Linton, Citation2000). Avoidance behavior is mainly directed at reducing fear, although accumulating evidence now suggests that avoidance is not merely a by-product of fear and might be affected by other factors than fear (Beckers et al., Citation2013; Volders et al., Citation2015). The relationship between fear and avoidance is most likely bidirectional (Pittig et al., Citation2020). Research has shown that letting participants believe they have the opportunity to avoid a painful stimulus paradoxically increases pain-related fear (van Vliet et al., Citation2021). By avoidance, the stimulus retains its threat value because the absence of the painful outcome is attributed to the avoidance response, which is referred to as protection from extinction (Lovibond et al., Citation2009; Volders et al., Citation2012). As such, dysfunctional threat beliefs cannot be disconfirmed, thereby maintaining pain-related fear (Engelhard et al., Citation2015). Avoidance learning is a type of operant conditioning, which refers to S-R (stimulus-response) learning (Fordyce, Citation1976; De Houwer & Hughes, Citation2020; Skinner, Citation1938). Research into operant conditioning describes how a response will increase or decrease in frequency based on its consequences, defined in terms of reinforcement or punishment. In the case of genital pain, avoidance of sexual penetration will prevent pain from occurring and/or reduce fear, which can be conceptualized as negative reinforcement. Although reinforcement of behaviors that maximize benefit and reduce loss or injury is crucial for survival, long-term avoidance will increase fear because every time the woman avoids fear-inducing thoughts, feelings, and activities, she is actually reinforcing them. Avoidance behavior may also lead to relief which is thought to be rewarding (Rizvi et al., Citation2021). Relief is a positive emotion that is triggered during unexpected omissions of a negative outcome, which induces prediction error (“pleasant surprise”) and reinforces the avoidance behavior in order to minimize or prevent future punishments (Deutsch et al., Citation2015; Vervliet et al., Citation2017). Accordingly, the avoidance behavior becomes associated with the positive feelings associated with safety, which motivates people to continue avoiding in similar situations (Vervliet et al., Citation2017). The ineffectiveness of long-term avoidance is indicated by the fact that the omission of a negative outcome is no longer considered as a “pleasant” surprise when the safety consequences are fully anticipated, thereby reducing prediction error and preventing new learning (Maia, Citation2010; Moutoussis et al., Citation2008).
The learning principles of classical and operant conditioning likely interact. The traditional two-factor theory describes how stimuli that have acquired sexually arousing versus threatening properties through classical conditioning can shape behavior, namely motivating approach or avoidance behavior toward these stimuli, which is then positively or negatively reinforced (Mowrer, Citation1947). In particular, when someone learns to fear a CS via Pavlovian pairings of the CS with a pain-US and this person is subsequently allowed to perform a response that prevents the CS, this response will be reinforced, which will increase the likelihood that they will respond in the same way during future encounters with the CS. The interaction between Pavlovian and instrumental learning is also evidenced by the so-called Pavlovian-to-Instrumental transfer (PIT; e.g., Estes, Citation1943; Holland, Citation2004). In support of this, it has been demonstrated that a cue predicting pain increases pain-related fear and avoidance tendencies, whereas a cue predicting reward decreases these tendencies (Claes, Crombez, Franssen et al., Citation2016; Claes, Crombez, & Vlaeyen Citation2016). Newer accounts of avoidance learning, however, challenge the necessity of fear (reduction) as an instrumental reinforcer of avoidance behavior (Lovibond et al., Citation2008). In line with emotion theories, it has been argued that avoidance-related action tendencies are an intrinsic part of fear emotions (Frijda, Citation2010; Lang, Citation1985). Accordingly, a CS eliciting fear after pairing with a pain-US will automatically elicit avoidance tendencies, which implies that avoidance can result from mere Pavlovian learning without the involvement of an instrumental component such as fear reduction (Krypotos et al., Citation2014).
Although both pain-related fear and avoidance behavior are central in theoretical and clinical models of genital pain, we limit this discussion to fear conditioning because fear of pain is an essential diagnostic criterion that is central to all genital pain conditions, despite the large variety in avoidance versus persistence behaviors coping (APA, Citation2013; Reissing et al., Citation2014). Furthermore, anomalies in fear learning have been observed in people with anxiety disorders as well as chronic pain and have been identified as a transdiagnostic pathological marker (Boddez et al., Citation2012; Lissek et al., Citation2010; Meulders, Citation2020; Van den Bergh et al., Citation2021). We focus mainly on learning mechanisms leading to genital pain in sexual situations. Yet, many individuals experience pain in response to any type of vulvar and/or vaginal stimulation such as tampon insertion or bicycling, which may point toward overgeneralization facilitated by hypersensitivity and central sensitization.
More concretely, we integrate the ideas on learning and sexual arousal responding, specify the pathways through which compromised learning may contribute to the development and persistence of genital pain, and approach compromised learning from a biopsychosocial framework. More specifically, we define the role of genital sensations and related physiological processes, subjective experiences, as well as partner and relational cues in fear acquisition (learning to associate vaginal sensations with pain); excessive generalization or “overgeneralization” and impaired safety learning (how fear generalizes to a range of “safe” stimuli that gradually build toward sexual penetration); extinction of fear (reduction of conditioned fear responding as a function of context and partner presence); counterconditioning (learning to associate vaginal sensations with pleasure instead of pain); and return of fear as a model of clinical relapse. Finally, we discuss some challenges and future directions to further our understanding of learning mechanisms underlying the development, persistence, and treatment of chronic genital pain.
Fear Acquisition: How Pain-Related Fear is Acquired in the Context of Genital Pain
Sexual responses such as sexual arousal and orgasm are intrinsically rewarding. Hence, any stimulus in the environment can get associated with sexual reward and become conditioned sexual incentive stimuli (Ågmo, Citation1999; O’Donohue & Plaud, Citation1994). It follows that when expected sexual reward and positive affect are absent, the same environmental stimuli can become associated with aversive experiences and elicit a decrease in sexual arousal and/or sexual approach behavior (Both et al., Citation2008, Citation2017; Brom et al., Citation2014). In the context of genital pain, it is relevant to study how an originally rewarding stimulus such as sexual touch and penetration gains aversive properties, thereby triggering protective behavior.
There is preliminary evidence showing that fear of pain can be acquired to initially arousing stimuli that are paired with pain, which then lowers sexual arousal responses. Using a differential fear conditioning paradigm, in which an erotic picture (CS+) was paired with a painful electrocutaneous stimulus delivered at the wrist and another erotic picture (the CS-) was never paired with pain, it was found that both women with genital pain and healthy controls showed weaker subjective sexual arousal to the CS+ as measured via self-reports (Both et al., Citation2017). However, the difference in affect and sexual arousal toward the stimulus paired with pain (CS+) and the stimulus not paired with pain (CS-) was less pronounced in women with genital pain compared to controls. In addition, genital arousal was measured using vaginal photoplethysmography, revealing that healthy control women displayed a lower genital response to the CS+ during acquisition than women with genital pain. These results suggest that women with genital pain show less differential conditioning and impaired safety learning, meaning that the learned aversiveness of the CS+ tends to generalize toward the CS-. Alternatively, these results could also indicate a lack of inhibitory learning to the CS- itself, meaning that the process of transferring the inhibitory properties of the CS- is attenuated or slowed down.
Although the previously described study provides the first important evidence on the potential role of fear learning in attenuating the sexual arousal response, more research is needed to replicate these findings and to address some methodological shortcomings. First, in most studies on human sex-related conditioning, only genital and subjective sexual arousal are considered as the primary CRs (Brom et al., Citation2014; Hoffmann, Citation2017). Given that the role of low genital arousal in genital pain has not yet received sufficient empirical support (Brauer et al., Citation2006), and that pelvic floor muscle tension is also an important component of defensive responding to pain (Reissing et al., Citation2005; Thibault-Gagnon & Morin, Citation2015), research on sex-related fear conditioning needs to include pelvic floor activity in addition to indicators of sexual arousal to understand the temporal dynamics of pain-related fear and genital pain responses. An electromyography (EMG) probe that allows simultaneous assessment of pelvic floor muscle activity and genital sexual arousal has been validated in previous research and may be useful to study conditioned responding to genital pain (Both et al., Citation2012). A recent study by Pawłowska et al. (Citation2020), examining differential disgust conditioning and extinction of female sexual arousal responses, did include pelvic floor EMG to mark a disgust defensive reflex, in addition to a VPA measure of genital arousal. This study showed that pelvic floor muscle activity was higher in response to the disgust-evoking versus neutral stimulus, which is an important finding given the potential role of disgust in the etiology and persistence of genital pain (de Jong et al., Citation2013).
Second, latent constructs such as fear need to be measured explicitly and throughout the conditioning procedure. Fear can be measured directly via fear-potentiated startle responses or verbal reports of fear learning after each (block of) CS presentations (e.g., ratings of US-expectancy, contingency, and risk as well as ratings of affect and fear; Beckers et al., Citation2013; Bradley & Lang, Citation2000). Note that Both and colleagues did include continuous reports of fear, affect, and US-expectancy in several of their studies on sexual conditioning (Brom et al., Citation2015a, Citation2015b; Pawłowska et al., Citation2020).
Third, the operationalization of the US, i.e., the pain induction, needs to align with the clinical presentation of genital pain and mimic what women actually experience during sexual penetration to ensure an ecologically valid design. Administering electrical pain stimulation to the wrist, as in the study of Both et al. (Citation2017), does not simulate penetration pain, especially because there is no solid evidence for an increased generalized pain response in women with genital pain (Dewitte et al., Citation2018; Hellman et al., Citation2015). Differences in fear learning will manifest more readily when using a disorder-specific US (Meulders, Citation2020; Pittig et al., Citation2018). This emphasizes the importance of using relevant CSs and USs and thinking about relevant control conditions and stimuli when developing experimental fear conditioning models. Dynamic sex stimuli such as videos may yield stronger conditioning effects than static sexual pictures (Dawson & Chivers, Citation2018). In addition, habituation of sexual stimuli occurs rapidly (Koukounas & Over, Citation1993; O’Donohue & Geer, Citation1985); therefore, the number of trials should be limited, and a rapid stimulus sequence is recommended.
A final concern regarding fear conditioning research in genital pain is that we cannot simply assume that exteroceptive aversive stimuli operate the same way as interoceptive stimuli in genital pain-related fear learning. Women with genital pain most often experience pain in the outer third part of the vagina, exactly where the largest pressure is experienced during penetration (Farmer et al., Citation2013). This means that vaginal sensations at initial penetration are experienced as most painful. A large range of aversive exteroceptive stimuli have been used in the context of fear conditioning, but interoceptive signals that stem from within the body are less commonly investigated (Meulders, Citation2020). Whereas most traditional fear conditioning research has relied on exteroceptive – often arbitrary – visual and auditory stimuli as CS and US, there is a growing body of research on interoceptive conditioning in which the CS, US, or both are endogenous events that reflect subjective changes in bodily signals (Meulders, Citation2020). There are indications that associative learning involving interoceptive stimuli operates differently than exteroceptive conditioning, yielding specific effects (e.g., rather unconscious, slower acquisition, more fixed, and resistant to extinction) on the physiological regulation of responses (Van Diest, Citation2019). Given that penetration pain is an interoceptive cue, research on fear of penetration pain may reveal unique insights that will further advance our knowledge on interoceptive fear conditioning and potentially reveal new targets for interoceptive exposure treatment.
Fear Generalization: How Pain-Related Fear Generalizes to Safe Stimuli
Fear generalization occurs when conditioned fear responding to a certain stimulus is transferred to novel stimuli that were never paired with the US (generalization stimuli or GSs) based on the perceptual or conceptual resemblance with the original CS+. This learning mechanism is highly adaptive as long as it allows discriminating between effective responding to novel stimuli – based on previous experiences with similar cues that predict aversive reactions such as pain – and refraining from responding to cues that do not predict pain (Dymond et al., Citation2015). In other words, there is a delicate balance between discrimination and generalization toward novel stimuli, with generalization becoming maladaptive when safe stimuli are needlessly feared (Lonsdorf et al., Citation2017). Fear generalization can occur based on the perceptual similarity with the original pain-associated CS, which is the most commonly investigated type of fear generalization. Pain-related fear can also spread to physically dissimilar stimuli that are semantically related or belong to the same category (i.e., via induction) or via derived relationships with pain such as conceptual equivalence between stimuli (i.e., via inferential reasoning; Bennett et al., Citation2015; Dunsmoor & Murphy, Citation2015). In category-based conditioning, which is probably most relevant to explain genital pain in sexual situations, people acquire fear to a class of (symbolically related) stimuli rather than to a specific CS. This type of generalization is enhanced when the CS is a typical member of a category rather than an atypical one (Dunsmoor & Murphy, Citation2005), which depends on one’s personal sexual script in the case of genital pain. Furthermore, an initial pain experience upon touching the vulva or inserting an object into the vagina (e.g., tampon) may spread across situations and generalize into fear for any type of stimulus that involves vulvar and/or vaginal touch. Category-based generalization may thus be one of the learning mechanisms involved in provoked vulvodynia, one of the most common types of genital pain (Bergeron et al., Citation2020). Generalization can be visualized via a graph marking how the strength of the elicited responses changes with similarity between the stimuli. The generalization gradient reflects the degree of generalization, with a steep gradient indicating narrow responses toward more similar stimuli and a flat gradient indicating substantial responding even to stimuli that are highly dissimilar to the CS+ (Dymond et al., Citation2015).
Excessive fear generalization is assumed to be a hallmark feature of anxiety disorders and may well be the central mechanism involved in genital pain (Craske et al., Citation2009; Dymond et al., Citation2015). That is, compromised fear learning in genital pain may not result from excessive conditioned responses to threat cues, but rather a failure to inhibit fear responding in the presence of safety cues. As described previously, there is evidence suggesting that women with genital pain may have difficulties in discriminating between safe and unsafe CSs. Using a differential learning paradigm with erotic pictures as CSs and a painful electrocutaneous stimulus as US, women with genital pain showed less differential responding (Both et al., Citation2017). Given that impaired safety learning may in turn cause overgeneralization, it is plausible to assume that excessive generalization of fear and pain expectancies is involved in the development and maintenance of genital pain.
Fear Extinction: How Pain-Related Fear is Reduced in the Context of Genital Pain
When assuming that fear acquisition and (particularly) fear generalization underlie the development and maintenance of chronic genital pain, it makes sense to rely on conditioning procedures to reduce the pain-related fear. Current treatments of genital pain are mainly based on Pavlovian fear extinction, which is a laboratory analogue for exposure and refers to the reduction of conditioned fear responding after presenting the CS repeatedly without the pain-US (Bergeron et al., Citation2008; Brom et al., Citation2014, Citation2015a, Citation2015b; ter Kuile et al., Citation2013). In other words, when the CS is no longer paired with the US (or its mental representation), the CS loses its predictive value and will stop eliciting conditioned responses.
Translated to clinical protocols of genital pain, most treatments introduce a “pain-stop” (i.e., cessation of any activity that causes genital pain) followed by systematic desensitization to gradually transform penetration into an arousing and pleasant experience (Bergeron et al., Citation2008, Citation2016). Women and their partners are exposed in a step-wise fashion to increasingly fear-inducing experiences, most often using sensate focus techniques that build up from non-genital touching to genital touching to gradually engaging in penetrative sexual activities (Masters & Johnson, Citation1970). These exercises are mostly combined with a penetration ban, allowing women to cultivate a non-demand, non-pressuring mind-set and learn to relax and refocus on pleasurable bodily sensations during sexual intimacy with their partner in order to decouple the associations between sex, fear, and pain (Dewitte et al., Citation2018; Goldstein et al., Citation2016; ter Kuile & Weijenborg, Citation2006). This treatment is based on traditional models of fear learning, assuming that, after repeated exposure to a fear-inducing stimulus, the fear will habituate, which then acts as a corrective experience. As a result, the fear-inducing stimulus will no longer evoke a fear response (Thompson, Citation2009). In addition, women are encouraged to explore new types of sexual stimulation so they learn to expect pleasure instead of pain when engaging in sexual activities, which can be viewed as a type of counterconditioning (Keller et al., Citation2020).
The underlying idea of this treatment approach, namely tackling the fear response via fear habituation, has been challenged in recent treatment models on anxiety disorders (Craske et al., Citation2014). Expectancy violation and inhibitory learning have now been forwarded as the core mechanisms of exposure, with stronger effects being achieved when the discrepancy between expected and actual outcomes (i.e., prediction error) becomes larger (Bouton, Citation2004; Craske et al., Citation2014; Delamater, Citation2004; Rescorla, Citation2001). Extinction does not erase the original CS-US association that is learned during acquisition, but that a new inhibitory CS-noUS association is formed inhibits the retrieval and behavioral expression of the acquisition memory (Bouton, Citation2002, Citation2004; Craske et al., Citation2014). When considering extinction as a form of new learning, it also involves the initial acquisition, consolidation, generalization, and later retrieval of the CS-noUS relationship. Extinction is a fragile process and does not always yield enduring behavioral effects because it is context-specific. Furthermore, it is difficult to consolidate and retain the new inhibitory learning and generalize it to novel stimuli (Bouton, Citation2002; Vervliet et al., Citation2013). Hence, inhibition can be released when leaving the extinction context again, favoring the retrieval of the original CS-US association. We will elaborate on this in the following section when explaining the return (i.e., post-extinction increase) of extinguished fear.
Impaired fear extinction learning might explain persistence of fear in the context of genital pain. Initial evidence, using the previously described differential learning paradigm with erotic pictures as CSs and a painful electrocutaneous stimulus as US, showed that conditioned genital responses and subjective affect to the CS+ did not diminish significantly during the extinction phase, suggesting resistance to extinction (Both et al., Citation2017). Interestingly, the lack of expressed extinction learning appeared in both women with and without genital pain. Note, however, that the extinction phase consisted of fewer unreinforced CS+ and CS- presentations compared to the acquisition phase (four presentations during extinction compared to 10 during acquisition), which makes it difficult to draw solid conclusions on extinction learning because it could be that the inhibitory CS-noUS association was not sufficiently trained. Typically, inhibitory (safety) learning takes longer than excitatory (threat) learning, so any procedure should present at least an equal number of extinction trials compared to acquisition trials to establish the role of (reduced) extinction in the context of genital pain. Other studies by Both and colleagues did establish successful extinction when using a more extensive series of extinction trials, though not in the context of genital pain (Brom et al., Citation2015a, Citation2015b).
Return of Fear: How Pain-Related Fear Reemerges after Successful Extinction
As discussed previously, extinction of fear is highly context-dependent and may easily return (Bouton, Citation2002). Hence, treatment protocols should take into account the role of learning context and anticipate that fear may return when the CS is encountered in a different context than the extinction context (Vervliet et al., Citation2013). Renewal of pain-related fear after extinction learning is common in women with genital pain who learn to insert their fingers or a progressive set of vaginal dilators in a clinical setting but fail to overcome their fear once they are at home in the presence of their partner. Post-extinction, both the acquisition and extinction memories co-exist. Which of these will be retrieved and expressed largely depends on the context (including physical characteristics, internal states, temporal factors, and social situations; Bouton, Citation2002; Vervliet et al., Citation2013). When a certain context reactivates features of the treatment context (e.g., a relaxed state after having performed abdominal breathing or pelvic floor exercises), the extinction memory will be retrieved. However, when certain characteristics of the context reactivate the original learning context (e.g., the bedroom), the acquisition memory will be triggered, resulting in conditioned responding and thus return of fear (Dymond et al., Citation2015; Vervliet et al., Citation2005).
Return of pain-related fear may also be triggered by any aversive stimulus or context following extinction learning, even when not clearly related to the fear-evoking stimulus (context; Hermans et al., Citation2005; Rescorla & Heth, Citation1975; Zbozinek et al., Citation2015). This reinstatement of conditioned fear may explain unexpected pain flare-ups in genital pain patients, for example, during periods of increased stress due to work or relational conflicts (den Hollander et al., Citation2015; Meulders et al., Citation2015; Sokol & Lovibond, Citation2012). The fragility of extinction is also indicated by the fact that fear of penetration pain can be rapidly reacquired after successful extinction when penetrative sexual acts become associated with pain again (Culver et al., Citation2015; Ricker & Bouton, Citation1996; Zbozinek & Craske, Citation2017a). For example, when a couple devotes extra time to engage in foreplay and moves toward penetration only when the woman is sufficiently sexually aroused to ensure a pain-free experience, but then after time they start to engage in unaroused intercourse again, the fear of pain may rapidly resurge. Finally, we need to consider that fear may spontaneously return after extinction training with the passage of time (Vervliet et al., Citation2013). It is therefore important to instruct the couple to regularly engage in penetrative sex following treatment completion to prevent spontaneous recovery (Craske & Mystkowski, Citation2006; Quirk, Citation2002; Vervliet et al., Citation2013).
The Way Forward: Future Research Directions
We have now reviewed the current theoretical and empirical knowledge on how fear conditioning mechanisms are implicated in genital pain. Given that research on (aversive) sex-related conditioning is still limited, a number of challenges and fascinating questions remain unaddressed, both on a theoretical and clinical level, which we outline below.
Unraveling Mechanisms of Fear Acquisition in Genital Pain
More research is needed to understand the pathways of fear learning in the context of genital pain. Fear acquisition can occur via different routes, namely learning contingencies on the basis of stimulus pairings (as in classical conditioning via direct experience) but also on the basis of instructions, observation, or inference (Debiec & Olsson, Citation2017; Mertens et al., Citation2018). The majority of studies on pain-related fear conditioning rely on the actual pairing of conditioned and painful stimuli. However, in the context of genital pain, it is plausible that pain-related fear can also be acquired without direct experience, being transmitted through verbal instructions or observation of others. Such a propositional perspective on learning (De Houwer, Citation2020a) is particularly relevant for understanding the development of primary genital pain. Fear of pain and its physical correlates often occur during the very first sexual encounter, thus without having actually experienced the pairing of penetration and pain before. Such observation suggests an important role of learning via instruction or inference (De Houwer, Citation2020a; Mertens et al., Citation2018). This has important implications for clinical practice in which little or no differentiation is made between primary and secondary genital pain (Dewitte et al., Citation2018; Pukall, Citation2016). Developing a line of research that examines the direct and indirect effects of instructions about stimulus properties on genital pain-related fear responding within an ecologically valid setting will crucially enhance insights on this pain condition.
Further elaborating on the distinction between primary and secondary genital pain, research on learning mechanisms may be particularly useful to understand the source of variation in the clinical profiles of women with lifelong versus acquired genital pain problems (Pukall, Citation2016). Differences in the strength of fear conditioning might occur as women with primary genital pain have never experienced a positive, non-painful sexual experience (with a partner) before. Prior experience might play a role in attenuating the defensive fear response in women who do have a history of sexually rewarding sensations during penetration, either with a partner or via masturbation. Previous pairings between stimuli that have been learned to evoke a sexually arousing response may limit the transfer of conditioned fear responding when one of the stimuli undergoes direct conditioning with the pain-US. That is, pre-exposure to a CS results in an initially learned CS-noUS or CS-posUS association, which exerts an inhibitory effect on the expression of the subsequently learned CS-US association (i.e., latent inhibition; Lubow, Citation1973; Meulders et al., Citation2012; Pavlov, Citation1927a, Citation1927b), which can occur both via informational transmission and via direct experience. This indicates the importance of taking a detailed sexual history assessment when deciding on a treatment for a woman with genital pain in order to get a clear picture of the learning history of that woman, including any instances of sexual reward versus pain.
In relation to this, it is important to consider that sexual stimuli are a priori rewarding rather than threatening and would thus be more prone to trigger appetitive responses rather than defensive responses (Ågmo, Citation1999; Hoffmann, Citation2017; O’Donohue & Plaud, Citation1994). It is known that the strength of conditioned fear responses is affected by the salience of the CS. As explained in preparedness theory (Ohman & Mineka, Citation2001; Seligman, Citation1970), fear-relevant CSs, which are evolutionarily relevant for survival, lead to faster fear learning and resistance to extinction learning. Considering that sexual stimuli show higher associability with sexual arousal, which is a strong reinforcing stimulus that would enhance appetitive conditioning effects, it is interesting to explore under which specific conditions pain will diminish the rewarding properties of sexual stimuli. For some people, for example those involved in Bondage and Discipline, Dominance and Submission (BDSM) practices, the association between sexual and pain stimuli does not evoke defensive responses but induces sexual pleasure (Dunkley et al., Citation2019). Although various mechanisms are likely involved to explain why painful sexual stimuli can be experienced as pleasurable, BDSM practitioners are an interesting comparison group because they offer the possibility to differentiate between the learning mechanisms underlying sex-pain versus sex-pleasure associations which may add to our understanding of genital pain.
The duration of the CS also plays a role in fear learning (Prenoveau et al., Citation2013). When presented with a longer conditioned stimulus, fear responding will usually be delayed until the final segment of the conditioned stimulus. That is, the US is often preceded by a chain of CSs and people learn to inhibit their fear response to the first CSs because they know the US is not occurring yet, a phenomenon referred to as inhibition of delay (Lubow, Citation1973; Pavlov, Citation1927a, Citation1927b; Vogel et al., Citation2003; but see Escobar et al., Citation2015). As described previously, women with genital pain will start to fear pain right from the beginning of a sexual episode. It is thus relevant to determine whether women with genital pain will show a decreased inhibition of delay as indicated by defensive activation in the first segment and a flatter peak response across the duration of the CS. To this aim, we need to apply longer CSs or chains of CSs and explore the temporal dynamics of fear acquisition as a function of genital pain status.
It is also relevant to explore how fear of vulvar and vaginal touch is acquired and spreads across sexual and non-sexual situations. Fear of pain-evoking touch is a hallmark feature of provoked vulvodynia (Bergeron et al., Citation2020). Tactile information can precede, co-occur or follow the onset of a pain experience and thus become part of the pain-related learning episode (Meulders, Citation2020), a mechanism that is likely involved in provoked vulvodynia and thus worth exploring in future research. Because fear of touch is a key symptom in several chronic pain conditions, researchers have started exploring the role of tactile acuity on the acquisition and generalization of fear of pain-evoking touch using a differential fear conditioning paradigm with vibrotactile stimulation (Biggs et al., Citation2017; Harvie et al., Citation2016).
As a final remark on fear acquisition in genital pain, it is important to consider the relational setting in which pain and fear learning takes place. Given that genital pain most often occurs in the context of sex with a partner, it is essential to specify the role of both the partner and the relational context when trying to understand genital pain responding (Dewitte et al., Citation2011, Citation2018). When experiencing pain during penetration, the partner can become part of the learning context and start to signal pain. When the pain-US is contingent on the presence of certain stimuli, e.g., only when the partner is present and when there is an opportunity or cue to initiate sex, the context can start to function as an occasion setter. The latter refers to a stimulus that is not directly related to the US but moderates the association between the CS and US. In other words, a certain context may set the occasion for the CS-US memory and its associated CRs to be activated (Fraser & Holland, Citation2019). For example, kissing the partner in a public setting may induce less fear because there is no threat of penetration pain compared to kissing the partner in an intimate setting in which the prospect of penetration, and hence, pain is more likely. In the absence of predictors, the context in itself may gain associative strength, which refers to contextual learning. A failure to accurately use contextual information to modulate responding to the CS+ allows fear to generalize to stimuli that are presented in a safe context (Andreatta et al., Citation2015; Lonsdorf et al., Citation2017). Various cues can become part of the pain experience, meaning that not only temporal associations between CS and US need to be considered but any other stimulus that precedes pain, including behavioral sequences (e.g., engaging in more or less foreplay) or internal states (e.g., being more relaxed or stressed during the day). The latter could induce a chronic anticipation of the pain-US which will continue to evoke high levels of anxiety (Meulders, Citation2020; Pittig et al., Citation2018).
When evaluating the role of partner and relational dynamics, it is relevant to approach genital pain from a motivational perspective (Dewitte et al., Citation2011). Previous research has suggested that learning about pain and defensive responses in anticipation of pain can be affected by the presence of other concurrent and competing goals (Claes et al., Citation2014; Van Damme et al., Citation2010). Pain control is not the only goal that drives the behavioral expression of fear learning. Overall, women do not necessarily consider sexual activity as a goal in itself but often engage in sex as a means to strive for emotional intimacy (Basson, Citation2000). This implies that the motivational determinants of genital pain may originate from or serve interpersonal dynamics that center around pleasuring the partner or avoiding anger and disappointment, keeping the ideal image of being a “normal” woman, and feelings of guilt and fear of losing the relationship (Elmerstig et al., Citation2008). Fear learning may thus not only be driven by the goal to control pain (by avoiding sex), but also by the goal to avert negative relational outcomes (Dewitte et al., Citation2011). It is interesting to examine whether the induction of relationship goals or relationship rewards interferes with fear conditioning effects or behavioral coping with fear. It is plausible that a focus on avoiding pain versus safeguarding the relationship (i.e., avoiding negative relational outcomes) will yield the same effects on fear learning but have different effects on defensive responding to anticipated pain, namely avoiding versus persisting with penetrative sex.
Establishing the Role of Fear Generalization in Genital Pain and Unraveling its Underlying Mechanisms
More research is needed to examine excessive generalization in response to sexual stimuli that gradually build up toward sexual penetration, which is the most fear-provoking and threatening stimulus for women with genital pain. Research and clinical reports suggest that women with genital pain tend to avoid intimate, non-penetrative sexual acts such as kissing and touching out of fear that these will progress to painful intercourse (Gordon et al., Citation2003; Sheppard et al., Citation2008). They feel that once involved in intimacy or once their male partner has an erection, they cannot refuse penetration, even when their body is not ready to engage in penetrative sex. This implies that fear might spread across the sexual script of the couple, i.e., a learned interaction sequence of sexual activities (Wiederman, Citation2015). Over time, every couple develops their own unique sexual script that endorses a progressive set of sexual actions that organize their sexual behavior. An example of such a sexual script may start with engaging in a sexual conversation, which then progresses into kissing, undressing, touching non-genital bodily parts, manually and/or orally stimulating the genitals, inserting a finger into the vagina, possibly inserting the penis into the vagina, ending with penile thrusting (for those with partners who have a penis). When vaginal penetration has repeatedly been paired with pain, any stimulus in the sexual script of the couple may start to evoke a fearful reaction and start to signal pain because the predictive value of painful penetration generalizes to every stimulus in the sexual script based on their conceptual relatedness. To test the role of fear generalization in an experimental lab design, two movie clips of distinctive actions (i.e., a couple having penetrative sex versus a couple engaging in a neutral, non-sexual interactive conversation) may serve as CS+ and CS- during fear acquisition training. In a subsequent generalization test phase, the generalization of fearful conditioned responding to intermediate actions in the sexual script, temporally occurring between the CS+ and CS-, may be investigated as the slopes of generalization gradients. The strength of the fear responses would gradually decrease as the GSs are temporally more distant from the CS+ (i.e., sexual penetration). Previous research with a similar approach has shown that anxiety and chronic pain patients display reduced differential learning and flatter generalization gradients (Lissek et al., Citation2010, Citation2014; Meulders et al., Citation2015). The shape of the gradient may thus vary as a function of clinical status but may also reflect the impact of different experimental manipulations such as verbal instructions. Along these lines, it is relevant to explore how different types of touch, both sexually and non-sexually related, may develop into a generalized fear of touch response.
As mentioned previously, it is also informative to consider the impact of prior experiences in women with genital pain in order to understand differentiation in the level and range of pain-related fear responding to sexual stimuli. When pre-exposed to stimuli that are not followed by defensive reactions prior to acquisition (e.g., latent inhibition), people will show less generalization to these stimuli in a subsequent generalization phase (Kaye et al., Citation1987; Sanjuan et al., Citation2006). In the case of genital pain, it is thus plausible that prior non-fearful and rewarding sexual experiences may protect against fear generalization and can thus be used as a buffering mechanism. Another process that may explain variation in genital pain responding refers to the unexpected spread of fear toward stimuli that are not perceptually or conceptually related to penetration pain. When pairing two neutral stimuli and, in a following phase, pairing one of the stimuli with a pain-US, the single presentation of the other stimulus will also elicit a fear response (Pittig et al., Citation2018). This type of sensory preconditioning might explain why some women start fearing and avoiding stimuli that are not directly related to the fearful sexual event.
Treatment Implications of Impaired Fear Extinction and Return of Fear in Genital Pain
Research has shown that people with chronic pain do not only show slower extinction rates, they also display deficient extinction of generalization learning (Dymond et al., Citation2015). For example, people with chronic pain continue to report stronger pain-related fear and US-expectancy to the unreinforced GSs compared to healthy controls, indicating that they fail to update their beliefs about the GSs not being followed by the US (Meulders et al., Citation2016). Such corrective learning occurs via explicit expectancy violation, which can be encouraged by maximizing the mismatch between fear-related expectancies and actual experiences (Craske et al., Citation2014; Rescorla & Wagner, Citation1972). Treatment protocols should endorse the explicit instruction not to engage in any sexual activities that may lead to pain and to pace sexual acts when progressing toward penetration in order to reduce the likelihood that sexual stimuli remain associated with pain, thereby facilitating disconfirmation of pain expectancies. Note, however, that this penetration ban might also interfere with fear extinction learning because women learn that their sexual acts are safe (i.e., no penetration, hence pain, will follow), thereby inducing protection from extinction.
Considering the importance of expectancy violation in extinction learning, the basic principle of graded exposure, in which individuals are progressively exposed to a hierarchy of increasingly more fear-inducing activities, has been challenged (Craske et al., Citation2014; Gatzounis et al., Citation2021; Pittig et al., Citation2016; Weisman & Rodebaugh, Citation2018). That is, moving forward in small steps allows patients to adjust their expectations based on positive experiences during the previous steps. This implies less prediction error and thus smaller violation of pain expectations, leaving less room for learning new inhibitory associations (Bouton, Citation2002; Weisman & Rodebaugh, Citation2018). Accordingly, it has been suggested that identifying the exact pain expectancies underlying different (sexual) activities and designing behavioral experiments to disconfirm these specific expectancies may be more effective than applying a fear hierarchy (e.g., den Hollander et al., Citation2016; Gatzounis et al., Citation2021).
Another issue regarding gradual exposure therapy is that it is often conducted with generalization stimuli that build up to the original pain-provoking stimulus, namely penetration. These GSs may have unique features that retain their excitatory association with the pain stimulus and are therefore resistant to inhibitory learning (Craske et al., Citation2018, Citation2014). In general, extinction with generalization stimuli might lead to more extensive return of fear, compared to extinction with the original CS (Vervliet et al., Citation2005). It is, however, sometimes difficult to retrieve the original CS, for example, in the case of trauma such as rape/violence or painful sexual experiences with a previous partner.
Extinction is often combined with counterconditioning as a means to further reduce pain-related fear by decoupling the association between sex and pain and learning a new association between sex and pleasure. During a counterconditioning procedure, the pain-associated CS+ is no longer followed by the pain-US, but paired with a US of the opposite valence (e.g., vibrotactile stimulation of the clitoris to increase sexual arousal). This should then increase prediction error, thereby reducing the predictive value, and even the affective valence, of the CS+ (Hermans et al., Citation2002; De Houwer et al., Citation2001; Scheveneels et al., Citation2016). In other words, counterconditioning maximizes the violation of pain expectancies because the presence of a positive outcome after a fear-inducing stimulus is even more unexpected than the mere absence of pain (Keller et al., Citation2020). Adding rewarding experiences may also keep patients motivated during therapy (den Hollander et al., Citation2017). Based on this principle, gradual exposure to penetrative sex, using for example, sensate focus exercises, is commonly complemented by sexual fantasy and/or masturbation training as well as the advice to explore additional and new sexual stimulation to increase sexual arousal levels during sexual activities (Bergeron et al., Citation2008; ter Kuile & Weijenborg, Citation2006). However, a direct experimental test of the effects of mere extinction learning in combination with counterconditioning on reducing fear of penetration pain is currently lacking. Note that the previously mentioned study on differential disgust conditioning did include both an extinction and a counterconditioning procedure, showing that sexual responses can be attenuated by learned sex-disgust associations and restored by extinction and counterconditioning procedures, though both procedures seemed equally effective (Pawłowska et al., Citation2020). Taking this one step further, it would also be interesting to examine the effect of conflicting outcomes (e.g., adding a sexual reward to a CS+ that is still followed by pain) on pain-related fear because, during the course of treatment, sexual arousal may coincide with pain and pain-related fear. Pairing the CS+ with sexual reward may also be useful to counteract the behavioral expression of deficient safety learning. It is plausible that not only adding specific sexual reward, but also increasing positive affect – which is the cornerstone of positive psychology interventions – might enhance inhibitory learning of pain-related fear (Hanssen et al., Citation2017; Zbozinek et al., Citation2015). It has been suggested that boosting positive affect during extinction learning will strengthen the encoding of the inhibitory memory, thereby reducing the return of pain-related fear (Pittig et al., Citation2016). Furthermore, positive affect may protect against the spreading of pain-related fear (Geschwind et al., Citation2015).
The main challenge of treatment is not only to extinguish pain-related fear, but to prevent and attenuate the return of extinguished fear. Most effective are techniques that maximize the discrepancy between expected pain when engaging in sexual activity and the actual outcome of this sexual activity. This implies that boosting new inhibitory learning by targeting memory consolidation and memory retrieval after treatment and maximizing prediction error will improve exposure treatment and its long-term effects (Craske et al., Citation2008; Gatzounis et al., Citation2021; Weisman & Rodebaugh, Citation2018). The encoding and retrieval of the inhibitory memory can be enhanced by inducing positive affect and adding positive outcomes via counterconditioning (Zbozinek & Craske, Citation2017a, Citation2017b; Zbozinek et al., Citation2015). In addition, trying to reduce the context-specificity of extinction learning by exposing patients to a wide range of sexual stimuli and in different contexts is an important target of extinction-based therapy (Gatzounis et al., Citation2021).
Individual Differences and Moderator Variables: Individual Trajectories of Pain-Related Fear Responding in Genital Pain
An important challenge of genital pain research is the question of how an acute pain experience during sexual activity develops into a chronic pain condition. Many women have memories about one or even multiple instances of pain during penetration, but only a subset of women get trapped into a vicious circle of pain-related fear responding. Unraveling the pathways by which pain-related fear is acquired, generalized, and extinguished is an important avenue for future research to understand the transition from acute to chronic pain. This also suggests including individual difference variables that result in stronger or more rapid fear acquisition, excessive generalization, and reduced extinction learning (Lonsdorf & Merz, Citation2017). We need to identify the specific moderators and mediators of sex-related fear conditioning so we can gain clearer insight into vulnerability factors that put individuals at risk for developing chronic genital pain (Lonsdorf & Merz, Citation2017; Meulders, Citation2020; Pittig et al., Citation2018). Here it is relevant to differentiate between modifiable versus fixed moderators because treatment will benefit more from targeting modifiable variables, such as patterns of dysfunctional thoughts, rather than focusing on non-malleable variables, such as previous aversive (e.g., traumatic) experiences.
Personality traits such as trait anxiety and neuroticism have been found to moderate fear conditioning effects (Jackson et al., Citation2006; Mineka & Zinbarg, Citation2006; Pittig et al., Citation2018). It is difficult to change these rigid patterns, but people can learn to cope with this vulnerability in order to prevent its behavioral expression. Although the evidence is not straightforward, there are indications that people with higher scores on these traits show stronger fear acquisition, less differential learning, broader generalization of conditioned fear responses, and impaired extinction learning as well as long-term extinction retention (Craske et al., Citation2009, Citation2018; Pittig et al., Citation2018; Van den Bergh et al., Citation2021). Women with genital pain have been found to display a personality profile that facilitates the processing of fear- and pain-related information. They report increased levels of neuroticism, pessimism, trait anxiety, perfectionism, harm avoidance, disgust propensity, and fear of negative evaluations (Dewitte et al., Citation2018). Although it is unclear whether these factors preceded pain or developed after the pain began, several of these personality traits will likely contribute to the chronicity of the pain. In relation to this, it has been argued that personality profiles, including neuroticism and negative affect, reflect a transdiagnostic vulnerability marker for psychopathology, yielding altered cognitive and affective processing such as compromised fear learning.
Recently, it has been proposed that these different personality characteristics reflect a common underlying “better safe than sorry” processing strategy (Van den Bergh et al., Citation2021). This refers to the tendency to process new information in a more superficial way, thus being low on sensory-perceptual detail, while prior, threatening, information remains highly detailed. Given that prior beliefs are used to predict the likelihood of future threat, failing to update these beliefs will stagnate the error reduction process and thus reduce the prediction error between actual and expected experiences because the highly detailed prior beliefs will have a stronger impact on the perception of actual threat. This processing strategy might be installed by repeated experiences with unpredictable and uncontrollable aversive life events, which means that a prior history of trauma can modulate (differential) fear learning (Dunsmoor et al., Citation2013; Pittig et al., Citation2018). As a result, people show less inhibitory fear learning and thus poor extinction, poor safety learning and overgeneralization, and they need more expectancy violations to shift a danger cue into a safety cue. Given that women with genital pain report a higher incidence of sexual abuse or an intense fear of any abuse in childhood (Khandkern et al., Citation2014), it is worth exploring whether this “better safe than sorry processing style” underlies the development and maintenance of chronic genital pain. Although past aversive experiences are fixed and cannot be erased, interventions can change the way people process and approach emotional experiences, for example, by learning to process aversive information thoroughly and with openness for sensory-perceptual threat-related details, thereby enabling prediction errors to modify previous threat information and facilitate error minimization toward more adaptive new information (Van den Bergh et al., Citation2021).
The most investigated moderator variable in the context of chronic pain is pain catastrophizing (more recently defined as catastrophic worrying) which refers to the tendency to magnify the threat value of a pain stimulus, feel helpless in the context of pain, and being unable to inhibit pain-related thoughts in response to a painful encounter (Petrini & Arendt-Nielsen, Citation2020). High catastrophizers show altered cognitive and affective processing that lead to increased pain, and these cognitive-affective biases may also compromise fear learning (Lethem et al., Citation1983; Meulders, Citation2020). Although it is not entirely clear yet whether women with genital pain are characterized by a general versus more specific tendency to catastrophize about genital pain, exaggerated cognitive and affective responses to (anticipated) genital pain do play a role in predicting pain-related outcomes (Desrochers et al., Citation2008; Payne et al., Citation2005). Both et al. (Citation2017) included a measure of pain catastrophizing in their study on aversive conditioning in women with genital pain and showed that pain catastrophizing was not associated with stronger but with weaker differential aversive conditioning effects. This finding was unexpected, indicating the need for further research on the moderating role of catastrophic worrying in pain-related fear learning. In addition to including pain catastrophizing as a trait variable, we can also manipulate state levels of pain catastrophizing to examine its direct effect during actual or anticipated pain stimulation in a fear acquisition and generalization paradigm. Given the central role of pain catastrophizing in fear-avoidance models to explain pain persistence and pain recurrence after treatment and given that catastrophic worrying is modifiable, it is crucial to target the way women catastrophize about their (anticipated) pain experiences in order to disrupt the pain-fear circle (Vlaeyen & Linton, Citation2000).
In the context of fear learning, it is also relevant to study the specifics of genital pain compared to chronic pain in general. Given that genital pain can be induced by nonsexual situations (for example, inserting a tampon, cycling, or wearing tight clothes), is not associated with a specific disruption of the sexual response cycle (as is the case with other sexual dysfunctions), and shows somatosensory and psychosocial mechanisms similar to those of other chronic pain conditions, it has been argued that genital pain should be classified as a specific type of chronic pain (Binik, Citation2005). This is particularly relevant when considering that one of the strongest risk factors for someone progressing from acute to chronic pain is a previous history of chronic pain or chronic pain elsewhere in the body (Chapman & Vierck, Citation2017). It is thus worth exploring whether women with genital pain show compromised fear learning in response to nonspecific, non-sexual, pain-related stimuli or whether this is specific to sexual pain cues.
Another potential risk factor for compromised fear learning concerns the role of gender (Pittig et al., Citation2018). Given that genital pain is much less prevalent in men, it could be that women are more sensitive to aversive fear conditioning in response to sexual stimuli than men. In a study comparing men and women’s sexual arousal responses in a differential acquisition and extinction paradigm with erotic pictures as CS+ and an electrocutaneous pain stimulus as US, both men and women rated the CS+ as more negative as compared with the CS-, but this differential effect disappeared during extinction. Importantly, women showed lower genital arousal in response to the CS+ than to the CS- during the first extinction trials and they rated the CS+ as less sexually arousing (Brom et al., Citation2014). Although further research is needed to substantiate gender differences in fear learning, these results may suggest that pairing sexual stimuli with pain disturbs the sexual response more in women than in men. It is relevant to investigate whether such differential effects should be solely ascribed to gender, which is a non-modifiable factor, or whether it reflects the fact that women are more likely exposed to (early) painful sexual experiences, which does provide opportunities for change.
It is also relevant to explore fear responses in the partners of women with genital pain. Given that the partner is typically present during the pain and both triggers and witnesses the pain, (s)he becomes part of the pain experience, which might result in more rapid fear acquisition or excessive fear generalization in the partner as well (Dewitte et al., Citation2018). Previous research has shown that the observation of pain leads to excessive fear and worry in observing partners (Goubert et al., Citation2005, Citation2008; Hadjistavropoulos et al., Citation2011), and both clinical and research reports suggest that male partners of women with genital pain display higher levels of sexual dysfunction such as erectile dysfunction which is likely driven by fear-related concerns (Nylanderlundqvist & Bergdahl, Citation2003; Smith & Pukall, Citation2014). Aligning with the biopsychosocial view on genital pain, it is important to focus not only on fear learning in women with genital pain but also in their partners, and to explore how these fear responses may interact to shape genital pain responding (Dewitte, Citation2014; Dewitte et al., Citation2018).
Compared to research on vulnerability factors, far less attention has been paid to resilience factors such as optimism and positive affect and how these can modulate fear learning and pain-related outcomes (Finan & Garland, Citation2015; Hanssen et al., Citation2017). The most appealing aspect of studying protective factors is that resilience can be trained using targeted treatment interventions. As discussed previously, initial research has shown that increasing positive affect in healthy participants can protect against generalization of fear to safe stimuli and prevent return of fear after successful extinction (Zbozinek & Craske, Citation2017a, Citation2017b; Zbozinek et al., Citation2015). Positive psychology-based interventions such as boosting optimism using, for example, a “best possible self” intervention (Peters et al., Citation2010), reinforcing positive and sexually rewarding experiences with the partner, and offering experiences that align with valued life goals such as strengthening the emotional bond with the partner using Acceptance and Commitment Therapy or mindfulness-based approaches (Brotto et al., Citation2015, Citation2019), may all prove useful to correct pain expectancies and reduce pain-related fear.
Being involved in a satisfying relationship with the partner may also protect against the negative sequels of genital pain. Feeling satisfied with the relationship may act as a general safety cue that prevents the spreading of fear across sexually intimate encounters and helps to extinguish learned fear responses to sexual stimuli (Smith & Pukall, Citation2011). More research is needed to examine the exact pathways through which relationship satisfaction may yield a protective effect. It may be interesting to experimentally induce positive affect or positive relational schemas and examine its effect on fear acquisition, generalization, and fear extinction in response to pain-related sexual stimuli.
Another protective factor with high explanatory value is self-efficacy, which has proven to play an important role in reducing sexual impairment and distress (Davis et al., Citation2015; Desrochers et al., Citation2009). Not believing in their own capacity to manage the pain experience, and thereby lacking a sense of control, is likely to increase women’s anticipated pain intensity and unpleasantness, worsening both the pain and the associated sexual dysfunction. Hence, increasing women’s sense of self-efficacy and control may help to overcome (the expression of) compromised fear learning.
Conclusion: Toward a Biopsychosocial, Ecologically Valid Model of Fear Learning in Genital Pain
We believe that research on fear conditioning will greatly contribute to our understanding of genital pain disorders. Pain seems to diminish the rewarding properties of sexual activity and to adversely influence sexual motivation. Yet, we lack a clear understanding of the pathways by which (the anticipation of) pain interfere with sexual arousal responding. Developing sound theoretical models and empirically testing the learning mechanisms underlying genital pain, specifying the critical components of fear learning as main targets of treatment to increase its cost-effectiveness, and considering individual differences and (modifiable) moderators of conditioned fear responding will help us identify women at risk and optimize current prevention and treatment protocols of (chronic) genital pain that are directed toward reduced suffering and increased sexual pleasure instead of penetration success. This fits within a broader trend in current clinical research to personalize treatments along a what-fits-for-whom approach.
To achieve these aims, we need to invest more in developing symptom-specific paradigms using ecologically valid stimuli as CS (e.g., videos of couples engaging in sex-related versus sex-unrelated activities) and US (e.g., simulating genital pain using vaginal pressure or thermal heat stimuli on the labia) and including various CRs involved in genital pain responding, such as subjective sexual arousal, genital arousal, pelvic floor muscle tension, physiological indices of fear (e.g., startle responses or skin conductance) and verbal reports of fear and pain expectancies. When using more dynamic conditioned stimuli such as sexual videos, which are usually longer in duration, we can explore the temporal dynamics of fear acquisition and generalization as a function of genital pain status. Recent methodological developments now enable us to conduct ecologically valid fear conditioning studies in the context of genital pain. Ecological validity also suggests that fear conditioning paradigms should take into account the role of partner variables and relationship context. Exploring the moderating role of relationship satisfaction in fear learning, manipulating the presence versus absence of the partner, and examining conditioned fear responses in the partners of women with genital pain are examples of how to integrate the partner relationship into research on fear conditioning. The innovation of the proposed paradigmatic shift resides in the specific focus on genital sensations instead of general nociception, the use of disorder-specific stimuli, and the implementation of a biopsychosocial framework. In addition to fear learning, the role of avoidance learning needs further exploration because the latter precludes the opportunity to learn that sexual activities can be non-painful and induce sexual pleasure. Understanding individual differences in avoidance learning and exploring its motivational underpinnings will help us understand why some women avoid versus persist with painful penetration.
In conclusion, research on genital pain operates at the intersection of various biopsychosocial disciplines, which has made it an increasingly popular and intriguing, but also complex, research topic. Promoting dialogue and integration between disciplines, such as learning theoretical perspectives and clinical approaches to genital pain, is important to better understand the unique features of different genital pain conditions. This line of research will not only advance our knowledge on genital pain but holds promise to set up a translational program that will generate new ideas, methodologies, and insights on fear conditioning in a multi-determined pain condition.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Ågmo, A. (1999). Sexual motivation – An inquiry into events determining the occurrence of sexual behaviour. Behavioural Brain Research, 105(1), 129–150. https://doi.org/10.1016/s0166-4328(99)00088-1
- Ågmo, A., Turi, A. L., Ellingsen, E., & Kaspersen, H. (2004). Preclinical models of sexual desire: Conceptual and behavioral analyses. Pharmacology Biochemistry and Behavior, 78(3), 379–404. https://doi.org/10.1016/j.pbb.2004.04.013
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.).
- Andreatta, M., Leombruni, E., Glotzbach-Schoon, E., Pauli, P., & Mühlberger, A. (2015). Generalization of contextual fear in humans. Behavior Therapy, 46(5), 583–596. https://doi.org/10.1016/j.beth.2014.12.008
- Arnold, L. D., Bachmann, G. A., Rosen, R., & Rhoads, G. G. (2007). Assessment of vulvodynia symptoms in a sample of US women: A prevalence survey with a nested case control study. American Journal of Obstetrics and Gynecology, 196(2), 128.e1–128.e6. https://doi.org/10.1016/j.ajog.2006.07.047
- Basson, R. (2000). The female sexual response: A different model. Journal of Sex & Marital Therapy, 26(1), 51–65. https://doi.org/10.1080/009262300278641
- Beckers, T., Krypotos, A.-M., Boddez, Y., Effting, M., & Kindt, M. (2013). What’s wrong with fear conditioning? Biological Psychology, 92(1), 90–96. https://doi.org/10.1016/j.biopsycho.2011.12.015
- Bennett, M. P., Meulders, A., Baeyens, F., & Vlaeyen, J. W. (2015). Words putting pain in motion: The generalization of pain-related fear within an artificial stimulus category. Frontiers in Psychology, 6, 520. https://doi.org/10.3389/fpsyg.2015.00520
- Bergeron, S., Khalifé, S., Dupuis, M.-J., & McDuff, P. (2016). A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. Journal of Consulting and Clinical Psychology, 84(3), 259–268. https://doi.org/10.1037/ccp0000072
- Bergeron, S., Khalifé, S., Glazer, H. I., & Binik, Y. M. (2008). Surgical and behavioral treatments for vestibulodynia: Two-and-one-half year follow-up and predictors of outcome. Obstetrics and Gynecology, 111(6), 159–166. https://doi.org/10.1097/01.AOG.0000295864.76032.a7
- Bergeron, S., Reed, B. D., Wesselmann, U., & Bohm-Starke, N. (2020). Vulvodynia. Nature Reviews Disease Primers, 6(1), 36. https://doi.org/10.1038/s41572-020-0164-2
- Bergeron, S., Rosen, N. O., & Morin, M. (2011). Genital pain in women: Beyond interference with intercourse. Pain, 152(6), 1223–1225. https://doi.org/10.1037/ccp0000072
- Biggs, E. E., Meulders, A., Kaas, A. L., Goebel, R., & Vlaeyen, J. W. S. (2017). The acquisition and extinction of fear of painful touch: A novel tactile fear conditioning paradigm. Journal of Pain, 18(12), 1505–1516. https://doi.org/10.1016/j.jpain.2017.08.002
- Bindra, D. (1974). A motivational view of learning, performance, and behavior modification. Psychological Review, 81(3), 199–213. https://doi.org/10.1037/h0036330
- Binik, Y. M. (2005). Should dyspareunia be retained as a sexual dysfunction in DSM-V? A painful classification decision. Archives of Sexual Behavior, 34(1), 11–21. https://doi.org/10.1007/s10508-005-0998-4
- Boddez, Y., Vervliet, B., Baeyens, F., Lauwers, S., Hermans, D., & Beckers, T. (2012). Expectancy bias in a selective conditioning procedure: Trait anxiety increases the threat value of a blocked stimulus. Journal of Behavior Therapy and Experimental Psychiatry, 43(2), 832–837. https://doi.org/10.1016/j.jbtep.2011.11.005
- Bornstein, J., Goldstein, A. T., Stockdale, C. K., Bergeron, S., Pukall, C., Zolnoun, D., & Coady, D. (2016). 2015 ISSVD, ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. Obstetrics and Gynecology, 127(4), 745–751. https://doi.org/10.1016/j.jsxm.2016.02.167
- Both, S., Brauer, M., Weijenborg, P., & Laan, E. (2017). Effects of aversive classical conditioning on sexual response in women with dyspareunia and sexually functional controls. The Journal of Sexual Medicine, 14(5), 687–701. https://doi.org/10.1016/j.jsxm.2017.03.244
- Both, S., Everaerd, W., & Laan, E. (2007). Desire emerges from excitement: A psychophysiological perspective on sexual motivation. In E. Janssen (Ed.), The psychophysiology of sex (pp. 327–339). Indiana University Press.
- Both, S., Laan, E., Spiering, M., Nilsson, T., Oomens, S., & Everaerd, W. (2008). Appetitive and aversive classical conditioning of female sexual response. The Journal of Sexual Medicine, 5(6), 1386–1401. https://doi.org/10.1016/j.jsxm.2017.03.244
- Both, S., van Lunsen, R., Weijenborg, P. T. M., & Laan, E. (2012). A new device for simultaneous measurement of pelvic floor muscle activity and vaginal blood flow: A test in a nonclinical sample. The Journal of Sexual Medicine, 9(11), 2888–2902. https://doi.org/10.1111/j.1743-6109.2012.02910.x
- Bouton, M. E. (2002). Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry, 52(10), 976–986. https://doi.org/10.1016/s0006-3223(02)01546-9
- Bouton, M. E. (2004). Context and behavioral processes in extinction. Learning & Memory, 11(5), 485–494. https://doi.org/10.1101/lm.78804
- Bradley, M. M., & Lang, P. J. (2000). Measuring emotion: Behavior, feeling, and physiology. In R. D. Lane & L. Nadel (Eds.), Cognitive neuroscience of emotion (pp. 242–276). Oxford University Press.
- Brauer, M., Laan, E., & ter Kuile, M. M. (2006). Sexual arousal in women with superficial dyspareunia. Archives of Sexual Behavior, 35(2), 187–196. https://doi.org/10.1007/s10508-005-9001-7
- Brauer, M., Lakeman, M., van Lunsen, R., & Laan, E. (2014). Predictors of task-persistent and fear-avoiding behaviors in women with sexual pain disorders. The Journal of Sexual Medicine, 11(12), 3051–3063. https://doi.org/10.1111/jsm.12697
- Brom, M., Both, S., Laan, E., Everaerd, W., & Spinhoven, P. (2014). The role of conditioning, learning and dopamine in sexual behavior: A narrative review of animal and human studies. Neuroscience and Biobehavioral Reviews, 38, 38–59. https://doi.org/10.1016/j.neubiorev.2013.10.014
- Brom, M., Laan, E., Everaerd, W., Spinhoven, P., & Both, S. (2015a). Extinction of aversive classically conditioned human sexual response. The Journal of Sexual Medicine, 12(4), 916–935. https://doi.org/10.1111/jsm.12800
- Brom, M., Laan, E., Everaerd, W., Spinhoven, P., Trimbos, B., & Both, S. (2015b). d- Cycloserine reduces context specificity of sexual extinction learning. Neurobiology of Learning and Memory, 125, 202–210. https://doi.org/10.1016/j.nlm.2015.09.010
- Brotto, L. A., Basson, R., Smith, K. B., Driscoll, M., & Sadownik, L. A. (2015). Mindfulness-based group therapy for women with provoked vestibulodynia. Mindfulness, 6(3), 417–432. https://doi.org/10.1007/s12671-013-0273-z
- Brotto, L. A., Bergeron, S., Zdaniuk, B., Driscoll, M., Grabovac, A., Sadownik, L. A., Smith, K. B., & Basson, R. A. (2019). A comparison of mindfulness-based cognitive therapy vs cognitive behavioral therapy for the treatment of provoked vestibulodynia in a hospital clinic setting. The Journal of Sexual Medicine, 16(6), 909–923. https://doi.org/10.1016/j.jsxm.2019.04.002
- Brotto, L. A., Chivers, M. L., Millman, R. D., & Albert, A. (2016). Mindfulness-based sex therapy improves genital-subjective arousal concordance in women with sexual desire/arousal difficulties. Archives of Sexual Behavior, 45(8), 1907–1921. https://doi.org/10.1007/s10508-015-0689-8
- Chapman, C. R., & Vierck, C. J. (2017). The transition of acute postoperative pain to chronic pain: An integrative overview of research on mechanisms. Journal of Pain, 18(4), 359.e1–359.e38. https://doi.org/10.1016/j.jpain.2016.11.004
- Chivers, M. L., Seto, M. C., Lalumière, M. L., Laan, E., & Grimbos, T. (2010). Agreement of self-reported and genital measures of sexual arousal in men and women: A meta-analysis. Archives of Sexual Behavior, 39(1), 5–56. https://doi.org/10.1007/s10508-009-9556-9
- Claes, N., Crombez, G., Franssen, M., & Vlaeyen, J. W. S. (2016). The impact of Pavlovian cues on pain avoidance: A behavioral study. Learning and Motivation, 56, 73–83. https://doi.org/10.1016/j.lmot.2016.10.001
- Claes, N., Crombez, G., & Vlaeyen, J. W. S. (2016). Pain in context: Cues predicting a reward decrease fear of movement related pain and avoidance behavior. Behaviour Research and Therapy, 84, 35–44. https://doi.org/10.1016/j.brat.2016.07.004
- Claes, N., Karos, K., Meulders, A., Crombez, G., & Vlaeyen, J. W. S. (2014). Competing goals attenuate avoidance behavior in the context of pain. Journal of Pain, 15(11), 1120–1129. https://doi.org/10.1016/j.jpain.2014.08.003
- Craske, M. G., Hermans, D., & Vervliet, B. (2018). State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philosophical Transactions of the Royal Society B Biological Sciences, 373(1742), 20180432. https://doi.org/10.1098/rstb.2017.0025
- Craske, M. G., Kircanski, K., Zelikowsky, M., Mystkowski, J., Chowdhury, N., & Baker, A. (2008). Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy, 46(1), 5–27. https://doi.org/10.1016/j.brat.2007.10.003
- Craske, M. G., & Mystkowski, J. L. (2006). Exposure therapy and extinction: Clinical studies. In M. G. Craske, D. Hermans, & D. Vansteenwegen (Eds.), Fear and learning: From basic processes to clinical implications (pp. 217–233). American Psychological Association. https://doi.org/10.1037/11474-011
- Craske, M. G., Rauch, S. L., Ursano, R., Prenoveau, J., Pine, D. S., & Zinbargh, R. E. (2009). What is an anxiety disorder? Depression and Anxiety, 26(12), 1066–1085. https://doi.org/10.1002/da.20633
- Craske, M. G., Treanor, M., Conway, C. C., Zbozinek, T., & Vervliet, B. (2014). Maximizing exposure therapy: An inhibitory learning approach. Behaviour Research and Therapy, 58, 10–23. https://doi.org/10.1016/j.brat.2014.04.006
- Culver, N. C., Vervliet, B., & Craske, M. G. (2015). Compound extinction: Using the analysis and brief review. Using the Rescorla-Wagner model to maximize exposure therapy effects for anxiety disorders. Clinical Psychological Science, 3(3), 335–34. https://doi.org/10.1177/2167702614542103
- Davis, S. N. P., Bergeron, S., Bois, K., Sadikaj, G., Binik, Y. M., & Steben, M. (2015). A prospective 2-year examination of cognitive and behavioral correlates of provoked vestibulodynia outcomes. Clinical Journal of Pain, 31(4), 333–341. https://doi.org/10.1097/AJP.0000000000000128
- Dawson, S. J., & Chivers, M. L. (2018). The effect of static versus dynamic stimuli on visual processing of sexual cues in androphilic women and gynephilic men. Royal Society Open Science, 5(6), 172286. https://doi.org/10.1098/rsos.172286
- Debiec, J., & Olsson, A. (2017). Social fear learning: From animal models to human function. Trends in Cognitive Science, 21(7), 546–555. https://doi.org/10.1016/j.tics.2017.04.010
- De Houwer, J. (2020a). Revisiting classical conditioning as a model for anxiety disorders: A conceptual analysis and brief review. Behaviour Research and Therapy, 127, 103558. https://doi.org/10.1016/j.brat.2020.103558
- De Houwer, J. (2020b). Conditioning is more than association formation: On the different ways in which conditioning research is valuable for clinical psychology. Collabra: Psychology, 6(1), 96–99. https://doi.org/10.1525/collabra.239
- De Houwer, J., & Hughes, S. (2020). The psychology of learning: An introduction from a functional-cognitive perspective. The MIT Press.
- De Houwer, J., Thomas, S., & Baeyens, F. (2001). Association learning of likes and dislikes: A review of 25 years of research on human evaluative conditioning. Psychological Bulletin, 127(6), 853. https://doi.org/10.1037/0033-2909.127.6.853
- de Jong, P. J., van Overveld, M., & Borg, C. (2013). Giving in to arousal or staying stuck in disgust? Disgust-based mechanisms in sex and sexual dysfunction. The Journal of Sex Research, 50(3–4), 247–262. https://doi.org/10.1080/00224499.2012.746280
- De Kruiff, M. E., ter Kuile, M. M., Weijenborg, P. T., & van Lankveld, J. J. (2000). Vaginismus and dyspareunia: Is there a difference in clinical presentation? Journal of Psychosomatic Obstetrics and Gynecology, 21(3), 149–155. https://doi.org/10.3109/01674820009075622
- Delamater, A. R. (2004). Experimental extinction in Pavlovian conditioning: Behavioural and neuroscience perspectives. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology, 57(2), 97–132. https://doi.org/10.1080/02724990344000097
- den Hollander, M., Goossens, M., de Jong, J., Ruijgrok, J., Oosterhof, J., Onghena, P., Smeets, R., & Vlaeyen, J. W. S. (2016). Expose or protect? A randomized controlled trial of exposure in vivo vs pain-contingent treatment as usual in patients with complex regional pain. Pain, 157(10), 2318–2329. https://doi.org/10.1097/j.pain.0000000000000651
- den Hollander, M., Goossens, M., de Jong, J., Ruijgrok, J., Oosterhof, J., Onghena, P., Smeets, R., & Vlaeyen, J. W. S. (2016). Expose or protect? A randomized controlled trial of exposure in vivo vs pain-contingent treatment as usual in patients with complex regional pain syndrome type 1. Pain, 157(10), 2318–2329. https://doi.org/10.1097/j.pain.0000000000000651
- den Hollander, M., Meulders, A., Jakobs, M., & Vlaeyen, J. W. (2015). The effect of threat information on acquisition, extinction, and reinstatement of experimentally conditioned fear of movement-related pain. Pain Medicine, 16(12), 2302–2315. https://doi.org/10.1111/pme.12836
- Desrochers, G., Bergeron, S., Khalifé, S., Dupuis, M. J., & Jodoin, M. (2009). Fear avoidance and self-efficacy in relation to pain and sexual impairment in women with provoked vestibulodynia. Clinical Journal of Pain, 25(6), 520–527. https://doi.org/10.1097/AJP.0b013e31819976e3
- Desrochers, G., Bergeron, S., Landry, T., & Jodoin, M. (2008). Do psychosexual factors play a role in the etiology of provoked vestibulodynia? A critical review. Journal of Sex and Marital Therapy, 34(3), 1998–226. https://doi.org/10.1080/00926230701866083
- Deutsch, R., Smith, K. J., Kordts-Freudinger, R., & Reichardt, R. (2015). How absent negativity relates to affect and motivation: An integrative relief model. Frontiers in Psychology, 6, e125. https://doi.org/10.3389/fpsyg.2015.00152
- Dewitte, M. (2014). On the interpersonal dynamics in sexuality. Journal of Sex and Marital Therapy, 40(3), 209–232. https://doi.org/10.1080/0092623X.2012.710181
- Dewitte, M. (2016). Gender differences in implicit processes of sexual stimuli. European Journal of Personality, 30(2), 107–124. https://doi.org/10.1007/s10508-014-0419-7
- Dewitte, M., Borg, C., & Lowenstein, L. (2018). A psychosocial approach to female genital pain. Nature Reviews Urology, 15(1), 25–41. https://doi.org/10.1038/nrurol.2017.187
- Dewitte, M., Van Lankveld, J., & Crombez, G. (2011). Understanding women suffering from sexual pain: A cognitive-motivational account. Pain, 152(2), 251–253. https://doi.org/10.1016/j.pain.2010.10.051
- Dunkley, C. R., Henshaw, C. D., Henshaw, S. K., & Brotto, L. A. (2019). Physical pain as pleasure: A theoretical perspective. The Journal of Sex Research, 57(4), 421–437. https://doi.org/10.1080/00224499.2019.1605328
- Dunsmoor, J. E., Kragel, P. A., Martin, A., & LaBar, K. S. (2013). Aversive learning modulates cortical representations of object categories. Cerebral Cortex, 24(11), 2859–2872. https://doi.org/10.1093/cercor/bht138
- Dunsmoor, J. E., & Murphy, G. L. (2015). Categories, concepts, and conditioning: How humans generalize fear. Trends in Cognitive Science, 19(2), 73–77. https://doi.org/10.1016/j.tics.2014.12.003
- Dymond, S., Dunsmoor, J. E., Vervliet, B., Roche, B., & Hermans, D. (2015). Fear generalization in humans: Systematic review and implications for anxiety disorder research. Behavior Therapy, 46(5), 561–582. https://doi.org/10.1016/j.beth.2014.10.001
- Ekdahl, J., Flink, I., Engman, L., & Linton, S. J. (2018). Vulvovaginal pain from a fear-avoidance perspective: A prospective study among female university students in Sweden. International Journal of Sexual Health, 30(1), 49–59. https://doi.org/10.1080/19317611.2017.1404543
- Elmerstig, E., Wijma, B., & Berterö, R. N. T. (2008). Why do young women continue to have sexual intercourse despite pain? Journal of Adolescent Health, 43(4), 357–363. https://doi.org/10.1016/j.jadohealth.2008.02.011
- Engelhard, I. M., van Uijen, S. L., van Seters, N., & Velu, N. (2015). The effects of safety behaviour directed towards a safety cue on perceptions of threat. Behavior Therapy, 46(5), 604–610. https://doi.org/10.1016/j.beth.2014.12.006
- Escobar, M., Suits, W. T., Rahn, E. J., & Arcediano, F. (2015). Do long delay conditioned stimuli develop inhibitory properties? Frontiers in Psychology, 6, 1606. https://doi.org/10.3389/fpsyg.2015.01606
- Estes, W. K. (1943). Discriminative conditioning. I. A discriminative property of conditioned anticipation. Journal of Experimental Psychology, 32(2), 150–155. https://doi.org/10.1037/h0058316
- Farmer, M. A., Maykut, C. A., Huberman, J. S., Huang, L., Khalifé, S., Binik, Y. M., Apkarian, A. V., & Schweinhardt, P. (2013). Psychophysical properties of female genital sensation. Pain, 154(11), 2277–2286. https://doi.org/10.1016/j.pain.2013.05.028
- Finan, P. H., & Garland, E. L. (2015). The role of positive affect in pain and its treatment. The Clinical Journal of Pain, 31(2), 177–187. https://doi.org/10.1097/AJP.0000000000000092
- Fordyce, W. E. (1976). Behavioral methods for chronic pain and illness. Mosby.
- Fraser, K. M., & Holland, P. C. (2019). Occasion setting. Behavioral Neuroscience, 133(2), 145–175. https://doi.org/10.1037/bne0000306
- Frijda, N. H. (2010). Impulsive action and motivation. Biological Psychology, 84(3), 570–579. https://doi.org/10.1016/j.biopsycho.2010.01.005
- Gatzounis, R., den Hollander, M., & Meulders, A. (2021). Optimizing long-term outcomes of exposure for chronic primary pain from the lens of learning theory. Journal of Pain, 22(11), 1315–1327. https://doi.org/10.1016/j.jpain.2021.04.012
- Geschwind, N., Meulders, M., Peters, M. L., Vlaeyen, J. W. S., & Meulders, A. (2015). Can experimentally induced positive affect attenuate generalization of fear of movement-related pain? Journal of Pain, 16(3), 258–269. https://doi.org/10.1016/j.biopsycho.2010.01.005
- Goldstein, A. T., Pukall, C. F., Brown, C., Bergeron, S., Stein, A., & Kellogg-Spadt, S. (2016). Vulvodynia: Assessment and treatment. The Journal of Sexual Medicine, 13(4), 572–590. https://doi.org/10.1016/j.jsxm.2016.01.020
- Gordon, A. S., Panahian-Jand, M., Mccomb, F., Melegari, C., & Sharp, S. (2003). Characteristics of women with vulvar pain disorders: Responses to a web-based survey. Journal of Sex and Marital Therapy, 29(sup1), 45–58. https://doi.org/10.1080/713847126
- Goubert, L., Craig, K. D., Vervoort, T., Morley, S., Sullivan, M. J. L., Williams, C. A. C., Cano, A., & Crombez, G. (2005). Facing others in pain: The effects of empathy. Pain, 118(3), 285–288. https://doi.org/10.1016/j.pain.2005.10.025
- Goubert, L., Vervoort, T., Sullivan, M. J. L., Verhoeven, K., & Crombez, G. (2008). Parental emotional responses to their child’s pain: The role of dispositional empathy and catastrophizing about their child’s pain. The Journal of Pain, 9(3), 272–279. https://doi.org/10.1016/j.jpain.2007.11.006
- Hadjistavropoulos, T., Craig, K. D., Duck, S., Cano, A., Goubert, L., Jackson, P. L., Mogil, J. S., Rainville, P., Sullivan, M. J. L., Williams, A. C. C., Vervoort, T., & Fitzgerald, T. D. (2011). A biopsychosocial formulation of pain communication. Psychological Bulletin, 137(6), 910–939. https://doi.org/10.1037/a0023876
- Hanssen, M. M., Peters, M. L., Boselie, J. J., & Meulders, A. (2017). Can positive affect attenuate (persistent) pain? State of the art and clinical implications. Current Rheumatology Reports, 19(12), 80. https://doi.org/10.1007/s11926-017-0703-3
- Harvie, D. S., Meulders, A., Reid, E., Camfferman, D., Brinkworth, R. S., & Moseley, G. L. (2016). Selectivity of conditioned fear of touch is modulated by somatosensory precision. Psychophysiology, 53(6), 921–929. https://doi.org/10.1111/psyp.12631
- Hellman, K. M., Patanwala, I. Y., Pozolo, K. E., & Tu, F. F. (2015). Multimodal nociceptive mechanisms underlying chronic pelvic pain. American Journal of Obstetrics and Gynecology, 213(6), 827.e1–827.e9. https://doi.org/10.1016/j.ajog.2015.08.038
- Hermans, D., Dirikx, T., Vansteenwegen, D., Baeyens, F., Van Den Bergh, O., & Eelen, P. (2005). Reinstatement of fear responses in human aversive conditioning. Behaviour Research and Therapy, 43(4), 533–551. https://doi.org/10.1016/j.brat.2004.03.013
- Hermans, D., Vansteenwegen, D., Crombez, G., Baeyens, F., & Eelen, P. (2002). Expectancy learning and evaluative learning in human classical conditioning: Affective priming as an indirect and unobtrusive measure of conditioned stimulus valence. Behaviour Research and Therapy, 40(3), 217–234. https://doi.org/10.1016/s0005-7967(01)00006-7
- Hoffmann, H. (2017). Situating human sexual conditioning. Archives of Sexual Behavior, 46(8), 2213–2229. https://doi.org/10.1007/s10508-017-1030-5
- Holland, P. C. (2004). Relations between Pavlovian- instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology: Animal Behavior Processes, 30(2), 104–117. https://doi.org/10.1037/0097-7403.30.2.104
- Jackson, E. D., Payne, J. D., Nadel, L., & Jacobs, W. J. (2006). Stress differentially modulates fear conditioning in healthy men and women. Biological Psychiatry, 59(6), 516–522. https://doi.org/10.1016/j.biopsych.2005.08.002
- Janssen, E., Everaerd, W., Spiering, M., & Janssen, J. (2000). Automatic processes and the appraisal of sexual stimuli: Toward an information processing model of sexual arousal. The Journal of Sex Research, 37(1), 8–23. https://doi.org/10.1080/00224490009552016
- Kaye, H., Preston, G. C., Szabo, L., Druiff, H., & Mackintosh, N. J. (1987). Context specificity of conditioning and latent inhibition: Evidence for a dissociation of latent inhibition and associative interference. The Quarterly Journal of Experimental Psychology Section B, 39(2), 127–145. https://doi.org/10.1080/14640748708402258
- Keller, N. E., Hennings, A. C., & Dunsmoor, J. E. (2020). Behavioral and neural processes in counterconditioning: Past and future directions. Behaviour Research and Therapy, 125, 103532. https://doi.org/10.1016/j.brat.2019.103532
- Khandkern, M., Brady, S. S., Stewart, E. G., & Harlow, B. L. (2014). Is chronic stress during childhood associated with adult-onset vulvodynia? Journal of Women’s Health, 23(8), 649–656. https://doi.org/10.1089/jwh.2013.4484
- Koukounas, E., & Over, R. (1993). Habituation and dishabituation of male sexual arousal. Behaviour Research and Therapy, 31(6), 575–585. https://doi.org/10.1016/0005-7967(93)90109-8
- Krypotos, A.-M., Effting, M., Arnaudova, I., Kindt, M., & Beckers, T. (2014). Avoided by association: Acquisition, extinction, and renewal of avoidance tendencies toward conditioned fear stimuli. Clinical Psychological Science, 2(3), 336–343. https://doi.org/10.1177/2167702613503139
- Lahaie, M. A., Amsel, R., Khalife´, S., Boyer, S., Faaborg-Andersen, M., & Binik, Y. M. (2015). Can fear, pain, and muscle tension discriminate vaginismus from dyspareunia/provoked vestibulodynia? Implications for the new DSM-5 diagnosis of genito-pelvic pain/penetration disorder. Archives of Sexual Behavior, 44(6), 1537–1550. https://doi.org/10.1007/s10508-014-0430-z
- Lang, P. J. (1985). The cognitive psychophysiology of emotion: Fear and anxiety. In A. H. Tuma & J. Maser (Eds.), Anxiety and the anxiety disorders (pp. 131–170). Erlbaum.
- Lethem, J., Slade, P. D., Troup, J. D., & Bentley, G. (1983). Outline of a fear-avoidance model of exaggerated pain perception. Behaviour Research And Therapy, 21(4), 401–408. https://doi.org/10.1016/0005-7967(83)90009-8
- Lissek, S., Kaczkurkin, A. N., Rabin, S., Geraci, M., Pine, D. S., & Grillon, C. (2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75(11), 909–9156. https://doi.org/10.1016/j.biopsych.2013.07.025
- Lissek, S., Rabin, S., Heller, R. E., Lukenbaugh, D., Geraci, M., Pine, D. S., & Grillon, C. (2010). Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry, 167(1), 47–55. https://doi.org/10.1176/appi.ajp.2009.09030410
- Lonsdorf, T. B., Menz, M. M., Andreatta, M., Fullana, M. A., Golkar, A., Haaker, J., Heitland, I., Hermann, A., Kuhn, M., Kruse, O., Meir Drexler, S., Meulders, A., Nees, F., Pittig, A., Richter, J., Römer, S., Shiban, Y., Schmitz, A., Straube, B., … Merz, C. J. (2017). Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience and Biobehavioral Reviews, 77, 247–285. https://doi.org/10.1016/j.neubiorev.2017.02.026
- Lonsdorf, T. B., & Merz, C. J. (2017). More than just noise: Inter-individual differences in fear acquisition, extinction and return of fear in humans - biological, experiential, temperamental factors, and methodological pitfalls. Neuroscience and Biobehavioral Reviews, 80, 703–728. https://doi.org/10.1016/j.neubiorev.2017.07.007
- Lovibond, P. F., Mitchell, C. J., Minard, E., Brady, A., & Menzies, R. G. (2009). Safety behaviours preserve threat beliefs: Protection from extinction of human fear conditioning by an avoidance response. Behaviour Research and Therapy, 47(8), 716–720. https://doi.org/10.1016/j.brat.2009.04.013
- Lovibond, P. F., Saunders, J. C., Weidemann, G., & Mitchell, C. J. (2008). Evidence for expectancy as a mediator of avoidance and anxiety in a laboratory model of human avoidance learning. Quarterly Journal of Experimental Psychology, 61(8), 199–216. https://doi.org/10.1080/17470210701503229
- Lubow, R. E. (1973). Latent inhibition. Psychological Bulletin, 79(6), 398–407. https://doi.org/10.1037/h0034425. PMID: 4575029.
- Maia, T. V. (2010). Two-factor theory, the actor-critic model, and conditioned avoidance. Learning & Behavior, 38(1), 50e67. https://doi.org/10.3758/LB.38.1.50
- Masters, W. H., & Johnson, V. E. (1970). Human sexual inadequacy. Bantam Books.
- Mertens, G., Boddez, Y., Sevenster, D., Engelhard, I. M., & De Houwer, J. (2018). A review on the effects of verbal instructions in human fear conditioning: Empirical findings, theoretical considerations, and future directions. Biological Psychology, 137, 49–64. https://doi.org/10.1016/j.biopsycho.2018.07.002
- Meston, C. M., & Stanton, A. M. (2018). Desynchrony between subjective and genital sexual arousal in women: theoretically interesting but clinically irrelevant. Current Sexual Health Reports, 10(3), 73–75. https://doi.org/10.1007/s11930-018-0155-4
- Meulders, A. (2020). Fear in the context of pain: Lessons learned from 100 years of fear conditioning research. Behaviour Research and Therapy, 131, 103635. https://doi.org/10.1016/j.brat.2020.103635
- Meulders, A., Jans, A., & Vlaeyen, J. W. S. (2015). Differences in pain-related fear acquisition and generalization: An experimental study comparing patients with fibromyalgia and healthy controls. Pain, 156(1), 108–122. https://doi.org/10.1016/j.pain.0000000000000016
- Meulders, A., Meulders, M., Stouten, I., De Bie, J., & Vlaeyen, J. W. S. (2016). Extinction of fear generalization: A comparison between fibromyalgia patients and healthy control participants. The Journal of Pain, 18(1), 79–95. https://doi.org/10.1016/j.jpain.2016.10.004
- Meulders, A., Rousseau, A., & Vlaeyen, J. W. S. (2015b). Motor intention as a trigger for fear of movement-related pain: An experimental cross-US reinstatement study. Journal of Experimental Psychopathology, 6(3), 206–228. https://doi.org/10.5127/jep.043614
- Meulders, A., Vervliet, B., Fonteyne, R., Baeyens, F., Hermans, D., & Vansteenwegen, D. (2012). Preexposure to (un)predictable shock modulates discriminative fear learning between cue and context: An investigation of the interaction between fear and anxiety. International Journal of Psychophysiology, 84(2), 180–187. https://doi.org/10.1016/j.ijpsycho.2012.02.004
- Mineka, S., & Zinbarg, R. E. (2006). A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. American Psychologist, 61(1), 10–26. https://doi.org/10.1037/0003-066X.61.1.10
- Moutoussis, M., Bentall, R. P., Williams, J., & Dayan, P. (2008). A temporal difference account of avoidance learning. Network: Computation in Neural Systems, 19(2), 137e160. https://doi.org/10.1080/09548980802192784
- Mowrer, O. H. (1947). On the dual nature of learning-a reinterpretation of conditioning and problem solving. Harvard Educational Review, 17, 102–148.
- Nylanderlundqvist, E., & Bergdahl, J. (2003). Vulvar vestibulitis: Evidence of depression and state anxiety in patients and partners. Acta Dermato Venereologica, 83(5), 369–373. https://doi.org/10.1080/00015550310003764
- O’Donohue, W. T., & Geer, J. H. (1985). The habituation of sexual arousal. Archives of Sexual Behavior, 14(3), 233–246. https://doi.org/10.1007/BF01542106
- O’Donohue, W. T., & Plaud, J. J. (1994). The conditioning of human sexual arousal. Archives of Sexual Behavior, 23(3), 321–344. https://doi.org/10.1007/BF01541567
- Ohman, A., & Mineka, S. (2001). Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review, 108(3), 483–522. https://doi.org/10.1037/0033-295x.108.3.483
- Pavlov, I. P. (1927a). Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Oxford University Press.
- Pavlov, I. P. (1927b). Conditioned reflexes. Oxford University Press.
- Pawłowska, A., Borg, C., de Jong, P. J., & Both, S. (2020). The effect of differential disgust conditioning and subsequent extinction versus counterconditioning procedures on women’s sexual responses to erotic stimuli. Behaviour Research and Therapy, 134, 103714. https://doi.org/10.1016/j.brat.2020.103714
- Payne, K. A., Binik, Y. M., Amsel, R., & Khalifé, S. (2005). When sex hurts, anxiety and fear orient attention towards pain. European Journal of Pain, 9(4), 427–436. https://doi.org/10.1016/j.ejpain.2004.10.003
- Peters, M. L., Flink, I. K., Boersma, K., & Linton, S. J. (2010). Manipulating optimism: Can imagining a best possible self be used to increase positive future expectancies? The Journal of Positive Psychology, 5(3), 204–211. https://doi.org/10.1080/17439761003790963
- Petrini, L., & Arendt-Nielsen, L. (2020). Understanding pain catastrophizing: Putting pieces together. Frontiers in Psychology, 11, 603420. https://doi.org/10.3389/fpsyg.2020.603420
- Pittig, A., Treanorc, M., LeBeauc, R. T., & Craske, M. G. (2018). The role of associative fear and avoidance learning in anxiety disorders: Gaps and directions for future research. Neuroscience and Biobehavioral Reviews, 88, 117–140. https://doi.org/10.1016/j.neubiorev.2018.03.015
- Pittig, A., van den Berg, L., & Vervliet, B. (2016). The key role of extinction learning in anxiety disorders: Behavioral strategies to enhance exposure-based treatments. Current Opinion in Psychiatry, 29(1), 39–47. https://doi.org/10.1097/YCO.0000000000000220
- Pittig, A., Wonga, A. H. K., Glücka, V. M., & Boscheta, J. M. (2020). Avoidance and its bi-directional relationship with conditioned fear: Mechanisms, moderators, and clinical implications. Behaviour Research and Therapy, 126, 103550. https://doi.org/10.1016/j.brat.2020.103550
- Prenoveau, J. M., Craske, M. G., Liao, B., & Ornitzc, E. M. (2013). Human fear conditioning and extinction: Timing is everything … or is it? Biological Psychology, 92(1), 59–68. https://doi.org/10.1016/j.biopsycho.2012.02.005
- Pukall, C. F. (2016). Primary and secondary provoked vestibulodynia: A review of overlapping and distinct factors. Sexual Medicine Reviews, 4(1), 36–41. https://doi.org/10.1016/j.sxmr.2015.10.012
- Quirk, G. J. (2002). Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learning and Memory, 9(6), 402–407. https://doi.org/10.1101/lm.49602
- Reed, B. D., Haefner, H. K., Sen, A., & Gorenflo, D. W. (2008). Vulvodynia incidence and remission rates among adult women: A 2-year follow-up study. Obstetrics and Gynecology, 206, 112–131. https://doi.org/10.1097/AOG.0b013e318180965b
- Reissing, E. D., Borg, C., Spoelstra, S. K., ter Kuile, M. M., Both, S., de Jong, P. J., van Lankveld, J. J. D. M., Melles, R. J., Weijenborg, P. T. M., & Weijmar Schultz, W. C. M. (2014). “Throwing the baby out with the bathwater”: The demise of vaginismus in favor of genito-pelvic pain/penetration disorder. Archives of Sexual Behavior, 43(7), 1209–1213. https://doi.org/10.1007/s10508-014-0322-2
- Reissing, E. D., Brown, C., Lord, M. J., Binik, Y. M., & Khalifé, S. (2005). Pelvic floor muscle functioning in women with vulvar vestibulitis syndrome. Journal of Psychosomatic Obstetrics and Gynecology, 26(2), 107–113. https://doi.org/10.1080/01443610400023106
- Rescorla, R. A. (2001). Retraining of extinguished Pavlovian stimuli. Journal of Experimental Psychology: Animal Behavior Processes, 27(2), 115–124.
- Rescorla, R. A., & Heth, C. D. (1975). Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes, 104, 88–96.
- Rescorla, R. A., & Wagner, A. R. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In H. Black & W. F. Prokasy (Eds.), Classical conditioning II: Current research and theory (pp. 64–99). Appleton Century Crofts.
- Ricker, S. T., & Bouton, M. E. (1996). Reacquisition following extinction in appetitive conditioning. Animal Learned Behavior, 24(4), 423–436. https://doi.org/10.3758/BF03199014
- Rizvi, S. J., Gandhi, W., & Salomons, T. (2021). Reward processing as a common diathesis for chronic pain and depression. Neuroscience and Biobehavioral Reviews, 127, 749–760. https://doi.org/10.1016/j.neubiorev.2021.04.033
- Sanjuan, M. C., Alonso, G., & Nelson, J. B. (2006). The contribution of latent inhibition to reduced generalization after pre-exposure to the test stimulus. Behavioural Processes, 71(1), 21–28. https://doi.org/10.1016/j.beproc.2005.09.002
- Scheveneels, S., Boddez, Y., Vervliet, B., & Hermans, D. (2016). The validity of laboratory-based treatment research: Bridging the gap between fear extinction and exposure treatment. Behaviour Research and Therapy, 86, 87–94. https://doi.org/10.1016/j.brat.2016.08.015
- Seligman, M. E. P. (1970). On the generality of the laws of learning. Psychological Review, 77(5), 406–418. https://doi.org/10.1037/h0029790
- Sheppard, C., Hallam-Jones, R., & Wylie, K. (2008). Why have you both come? Emotional, relationship, sexual and social issues raised by heterosexual couples seeking sexual therapy (in women referred to a sexual difficulties clinic with a history of vulval pain). Sexual and Relationship Therapy, 23(3), 217–226. https://doi.org/10.1080/14681990802227974
- Skinner, B. F. (1938). The behavior of organisms: An experimental analysis. Appleton-Century.
- Smith, K. B., & Pukall, C. A. (2011). Systematic review of relationship adjustment and sexual satisfaction among women with provoked vestibulodynia. The Journal of Sex Research, 48(2), 166–191. https://doi.org/10.1080/00224499.2011
- Smith, K. B., & Pukall, C. F. (2014). Sexual function, relationship adjustment, and the relational impact of pain in male partners of women with provoked vulvar pain. The Journal of Sexual Medicine, 11(5), 283–293. https://doi.org/10.1111/jsm.12484
- Sokol, N., & Lovibond, P. F. (2012). Cross-US reinstatement of human conditioned fear: Return of old fears or emergence of new ones? Behaviour Research and Therapy, 50(5), 313–322. https://doi.org/10.1016/j.brat.2012.02.005
- Spano, L., & Lamont, J. A. (1975). Dyspareunia: A symptom of female sexual dysfunction. Cancer Nurse, 71(8), 22–25.
- ter Kuile, M. M., Both, S., & van Lankveld, J. (2010). Cognitive behavioral therapy for sexual dysfunctions in women. Psychiatric Clinics of North America, 33(3), 595–610. https://doi.org/10.1016/j.psc.2010.04.010
- ter Kuile, M. M., Bulté, I., Weijenborg, P. T., Beekman, A., Melles, R., & Onghena, P. (2009). Therapist-aided exposure for women with lifelong vaginismus: A replicated single-case design. Journal of Consulting and Clinical Psychology, 77(1), 149–159. https://doi.org/10.1016/j.psc.2010.04.010
- ter Kuile, M. M., Melles, R., de Groot, H. E., Tuijnman-Raasveld, C. C., & van Lankveld, J. J. D. M. (2013). Therapist-aided exposure for women with lifelong vaginismus: A randomized waiting-list control trial of efficacy. Journal of Consulting and Clinical Psychology, 81(6), 1127–1136. https://doi.org/10.1037/a0034292
- ter Kuile, M. M., & Weijenborg, P. T. (2006). A cognitive-behavioral group program for women with vulvar vestibulitis syndrome (VVS): Factors associated with treatment success. Journal of Sex and Marital Therapy, 32(3), 199–213. https://doi.org/10.1080/00926230600575306
- Thibault-Gagnon, S., & Morin, M. (2015). Active and passive components of pelvic floor muscle tone in women with provoked vestibulodynia: A perspective based on a review of the literature. The Journal of Sexual Medicine, 12(11), 2178–2189. https://doi.org/10.1111/jsm.13028
- Thompson, R. F. (2009). Habituation: A history. Neurobiology of Learning and Memory, 92(2), 127–134. https://doi.org/10.1016/j.nlm.2008.07.011
- Toates, F. (1998). The interaction of cognitive and stimulus-response processes in the control of behavior. Neuroscience and Biobehavioral Reviews, 22(1), 59–83. https://doi.org/10.1016/s0149-7634(97)00022-5
- Toates, F. (2009). An integrative theoretical framework for understanding sexual motivation, arousal, and behavior. The Journal of Sex Research, 46(2–3), 168–193. https://doi.org/10.1080/00224490902747768
- Van Damme, S., Legrain, V., Vogt, J., & Crombez, G. (2010). Keeping pain in mind: A motivational account of attention to pain. Neuroscience and Biobehavioral Reviews, 34(2), 204–213. https://doi.org/10.1016/j.neubiorev.2009.01.005
- Van den Bergh, O., Brosschot, J., Critchley, H., Thayer, J. F., & Ottaviani, C. (2021). Better safe than sorry: A common signature of general vulnerability for psychopathology. Perspectives in Psychological Science, 16(2), 225–246. https://doi.org/10.1177/1745691620950690
- Van Diest, I. (2019). Interoception, conditioning, and fear: The panic threesome. Psychophysiology, 56(8), e13421. https://doi.org/10.1111/psyp.13421
- van Vliet, C., Meulders, A., Vancleef, L., & Vlaeyen, J. W. (2021). The perceived opportunity to avoid pain paradoxically increases pain-related fear through increased threat appraisals. Annals of Behavioral Medicine, 55(3), 216–227. https://doi.org/10.1093/abm/kaaa045
- Vervliet, B., Craske, M. G., & Hermans, D. (2013). Fear extinction and relapse: State of the art. Annual Review of Clinical Psychology, 9(1), 215–248. https://doi.org/10.1146/annurev-clinpsy-050212-185542
- Vervliet, B., Lange, I., & Milad, M. R. (2017). Temporal dynamics of relief in avoidance conditioning and fear extinction: Experimental validation and clinical relevance. Behaviour Research and Therapy, 96, 66–78. https://doi.org/10.1016/j.brat.2017.04.011
- Vervliet, B., Vansteenwegen, D., Baeyens, F., Hermans, D., & Eelen, P. (2005). Return of fear in a human differential conditioning paradigm caused by a stimulus change after extinction. Behaviour Research and Therapy, 43(3), 357–371. https://doi.org/10.1016/j.brat.2004.02.005
- Vlaeyen, J. W. S. (2015). Learning to predict and control harmful events: Chronic pain and conditioning. Pain, 156(Suppl. 1), S86–S93. https://doi.org/10.1097/j.pain.0000000000000107
- Vlaeyen, J., Crombez, G., & Linton, S. (2016). The fear-avoidance model of pain. Pain, 157(8), 1588–1589. https://doi.org/10.1097/j.pain.0000000000000574
- Vlaeyen, J. W. S., & Linton, S. (2000). Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain, 85(3), 317–332. https://doi.org/10.1016/S0304-3959(99)00242-0
- Vogel, E. H., Brandon, S. E., & Wagner, A. R. (2003). Stimulus representation in SOP: II. An application to inhibition of delay. Behavioural Processes, 62(1–3), 27–48. https://doi.org/10.1016/s0376-6357(03)00050-0
- Volders, S., Boddez, Y., De Peuter, S., Meulders, A., & Vlaeyen, J. W. (2015). Avoidance behavior in chronic pain research: A cold case revisited. Behaviour Research and Therapy, 64, 31–37. https://doi.org/10.1016/j.brat.2014.11.003
- Volders, S., Meulders, A., De Peuter, S., Vervliet, B., & Vlaeyen, J. W. S. (2012). Safety behavior can hamper the extinction of fear of movement-related pain: An experimental investigation in healthy participants. Behaviour Research and Therapy, 50(11), 735–746. https://doi.org/10.1016/j.brat.2012.06.004
- Weisman, J. S., & Rodebaugh, T. L. (2018). Exposure therapy augmentation: A review and extension of techniques informed by an inhibitory learning approach. Clinical Psychology Review, 59, 41–51. https://doi.org/10.1016/j.cpr.2017.10.010
- Wiederman, M. (2015). Sexual script theory: Past, present, and future. In J. DeLamater & R. Plante (Eds.), Handbook of the sociology of sexualities (pp. 7–22). Springer International Publishing.
- Zbozinek, T. D., & Craske, M. G. (2017a). Positive affect predicts less reacquisition of fear: Relevance for long-term outcomes of exposure therapy. Cognition and Emotion, 31(94), 712–725. https://doi.org/10.1080/02699931.2016.1142428
- Zbozinek, T. D., & Craske, M. G. (2017b). The role of positive affect in enhancing extinction learning and exposure therapy for anxiety disorders. Journal of Experimental Psychopathology, 8(1), 13–39. https://doi.org/10.5127/jep.052615
- Zbozinek, T. D., Holmes, E. A., & Craske, M. G. (2015). The effect of positive mood induction on reducing reinstatement fear: Relevance for long term outcomes of exposure therapy. Behaviour Research and Therapy, 71, 65–75. https://doi.org/10.1016/j.brat.2015.05.016
