Molecular Physics, Vol. 106, No. 6 (2008), pp. 813–825
Ab initio potential energy curve for the neon atom pair and thermophysical properties for the dilute neon gas. II. Thermophysical properties for low-density neon
Eckard Bich, Robert Hellmann and Eckhard Vogel
The publishers mistakenly omitted the Appendices for the above paper. The complete version of this paper can now be found in this issue and this will represent its new citation information.
Taylor & Francis would like to unreservedly apologise for any inconvenience the missing appendices may have caused.
Research Article
Ab initio potential energy curve for the neon atom pair and thermophysical properties for the dilute neon gas. II. Thermophysical properties for low-density neon
Eckard Bich, Robert Hellmann and Eckhard Vogel Footnote*
Institut für Chemie, Universität Rostock, Rostock, Germany
A neon–neon interatomic potential energy curve determined from quantum-mechanical ab initio calculations and described with an analytical representation (R. Hellmann, E. Bich, and E. Vogel, Molec. Phys. 106, 133 (2008)) was used in the framework of the quantum-statistical mechanics and of the corresponding kinetic theory to calculate the most important thermophysical properties of neon governed by two-body and three-body interactions. The second and third pressure virial coefficients as well as the viscosity and thermal conductivity coefficients, the last two in the so-called limit of zero density, were calculated for natural Ne from 25 to 10,000 K. Comparison of the calculated viscosity and thermal conductivity with the most accurate experimental data at ambient temperature shows that these values are accurate enough to be applied as standard values for the complete temperature range of the calculations characterized by an uncertainty of about ±0.1% except at the lowest temperatures.
Keywords: neon pair potential; neon gas property standards; second and third pressure virial coefficients; viscosity; thermal conductivity
1. Introduction
Recently we calculated standard values for some thermophysical two-body and three-body properties of helium over the temperature range from 1 to 10,000 K with uncertainties that are superior to experimental data Citation1. Prerequisite for it was the determination of a state-of-the-art pair potential between two helium atoms Citation2. In order to establish a second standard for the calibration of high-precision measuring instruments at low density, we developed very recently a new interatomic pair potential for neon from high-level supermolecular ab initio calculations for a large number of internuclear separations R (paper I Citation3). The ab initio calculated interatomic potential energy values V(R), including core–core and core–valence correlation and relativistic corrections as well as coupled-cluster contributions up to CCSDT(Q), were listed in of paper I. They were used for the fit of an analytical potential function, which represents a modification of the potential function given by Tang and Toennies Citation4:
Table 1. Potential parameters.
A comparison in paper I with experimental rovibrational spectra Citation5 showed that the new potential function is superior to the ab initio potential by Cybulski and Toczylowski Citation6. This potential was given as an analytical function derived from ab initio values calculated for a large range of internuclear distances. Furthermore, the comparison made evident that our new potential is at least as good as the best semi-empirical potential by Aziz and Slaman Citation7 and also compares well with the potential of Wüest and Merkt Citation5 fitted directly to the rovibrational spectra under discussion. It is noteworthy that the rovibrational spectra are sensitive to the shape of the potential well. Hence it could be possible that the potential of Wüest and Merkt is not so effective with respect to other regions of the potential. Conversely, the transport properties are particularly sensitive to the repulsive part of the potential. Thus the potential of Aziz and Slaman could be expected to perform well in nearly all regions of the potential, since it was determined in a fit to high-energy beam data and to viscosity coefficients, considering calculated values of the C 6, C 8 and C 10 dispersion coefficients.
Table 2. Number of calculated phase shifts of neon for some reduced energies.
In this paper, we report standard values of the second and third pressure virial coefficients as well as of the viscosity and thermal conductivity coefficients in the limit of zero density for neon in its natural isotopic composition. Even though the quality of the new neon ab initio potential of paper I Citation3 is somewhat inferior compared with our recent ab initio potential for helium Citation2, the calculated thermophysical properties are expected to be as accurate as the best experimental measurements at room temperature and more accurate than available experimental data far above and far below room temperature. In order to assess as accurately as possible the quality of the potentials considered in this paper, some of the experimental viscosity data from the literature were recalibrated with reference values derived from the new interatomic potential for helium Citation1,Citation2.
2. Quantum-mechanical calculation of thermophysical properties
2.1. Evaluation of the phase shifts
A quantum-mechanical treatment of the elastic scattering is needed to obtain very accurate values for the thermophysical properties of neon. For this purpose the relative phase shifts δ
l
(k) have to be evaluated as asymptotic limiting values of the relative phases of the perturbed and unperturbed radial factor wave functions ψ
l
(R) and (the latter with V(R) = 0). Each of them corresponds to a particular state of the angular momentum of the system characterized by the quantum number l. To obtain the relative phase shifts δ
l
(k), the Schrödinger equation is to be solved by numerical integration for many values of the wave number k = (2μE)1/2/ℏ, where E is the energy of the incoming wave and μ = (m
1
m
2)/(m
1 + m
2) is the reduced mass.
In principle, neon can be considered as a mixture of the three isotopes 20Ne, 21Ne, and 22Ne with the relative atomic masses 19.9924356, 20.9938428, and 21.9913831, with the nuclear spins s of 0, 3/2, and 0, and with the natural abundances 90.48 atom%, 0.27 atom%, and 9.27 atom%. Hence there are six different interacting systems in naturally occurring neon with varying reduced masses and different statistics: 20Ne–20Ne and 22Ne−22Ne (both Bose–Einstein statistics), 21Ne−21Ne (Fermi–Dirac statistics), 20Ne–21Ne, 20Ne−22Ne, and 21Ne−22Ne (all Boltzmann statistics). As a consequence, the relative phase shifts have to be calculated for these six binary systems at a multiplicity of wave numbers k or of energies E for a substantial number of l values which requires plenty of computing time. In order to save time the semi-classical JWKB method was used as approximation. Problems and the procedure of the fully quantum-mechanical calculation as well as the JWKB method for the determination of the relative phase shifts were discussed in some detail in our paper on helium Citation1.
To avoid uncertainties in the results of the calculated thermophysical properties, a very large number of phase shifts δ l (k) was determined for 500 values of the energy E from zero to about 135,000 K and for an increasing number of l values related to the energy. The calculations of the phase shifts were first performed fully quantum-mechanically and for comparison parallel to it according to the JWKB approximation. If the values resulting from both procedures became practically identical for certain values of the angular momentum quantum number l, the fully quantum-mechanical evaluation (QM) was finished and substituted by the semi-classical JWKB procedure at the higher l values. gives an overview about the number of phase shifts determined for some reduced energies E* = E/ϵ.
2.2. Calculation of the second and third pressure virial coefficients
In this paper two alternative ways were used to calculate the second virial coefficient of naturally occurring neon as a function of temperature T. In the first variant B(T) is determined like that of a mixture composed of the three neon isotopes:
In the second variant naturally occurring neon is considered as a pure gas consisting of atoms with the average relative atomic mass 20.1797. Then the second virial coefficient B(T) is derived as the sum of a classical contribution as well as of first-order, second-order, and third-order quantum corrections Citation9:
For the evaluation of the third pressure virial coefficient C(T), naturally occurring neon is again assumed to be a pure gas composed of atoms with the same mass. Furthermore, C(T) is calculated as a sum of three contributions Citation10,Citation11, one term for the pairwise additivity of the two-body interatomic potentials C add, an extra genuine term C non−add for the non-additivity ΔV 3(R 12, R 13, R 23) of the three-body interatomic interaction potential V 3(R 12, R 13, R 23), and a first-order correction term for the quantum effects C qm,1:
2.3. Calculation of the transport properties
Different alternative ways were used to determine the transport properties of naturally occurring neon as a function of temperature T. In the first variant η(T) and λ(T) are evaluated quantum-mechanically and approximately like that of a dilute-gas mixture in the limit of zero density composed of the three neon isotopes. In the first-order approximation of the kinetic theory the viscosity is formulated as:
In analogy to Equation (Equation16) an equation in which the elements H ij are replaced by elements L ij is applied for the thermal conductivity of a dilute gas mixture in its first-order approximation. The elements L ij are expressed as:
In principle, exact calculations require higher-order approximations of the kinetic theory. Therefore, we used fifth-order approximations in the case of the transport properties of helium Citation1, but the calculations for 3He and 4He concerned a pure gas each. Unfortunately, approximations of such a high order are not available for mixtures so that we were forced to choose any other reasonable way for the higher-order calculations of the transport properties of naturally occurring neon and tested thus two possibilities. On the one hand, the individual viscosity and thermal conductivity coefficients η ij and λ ij for the binary pairs with like and unlike interactions are calculated up to the fifth-order approximation according to:
Expressions for the quantum cross sections Q (m)(E) and for the quantum collision integrals Ω (m,s)(T) needed in the different approximations of the solution of the Boltzmann equation were derived by Meeks et al. Citation16. They were again collected in our paper for helium Citation1 for particles with spin s according to the Bose–Einstein (BE) or to the Fermi–Dirac (FD) statistics as well as for the Boltzmann statistics. The formulas for the different Q (m) are related to sums over the phase shifts δ l , either over only the odd l values or over only the even l values, but also over complete sums (Boltzmann statistics). The quantum collision integrals Ω(m,s)(T) result from the quantum cross sections Q (m)(E) according to:
In a second variant the viscosity of neon was determined classically for the first-order and the fifth-order approximations in order to examine whether a quantum-mechanical calculation is actually needed to achieve highly accurate values for the transport properties of neon in the zero-density limit. For this purpose the usual formulations for monatomics Citation17 were used, whereas neon was again considered to be a pure gas with the average relative atomic mass already given.
3. Comparison with experimental data and values for other interatomic potentials
3.1. Second and third pressure virial coefficients
The quantum-mechanical calculation of the second pressure virial coefficient requires the determination of the existing bound states. For this purpose the Level 7.7 program of LeRoy Citation18 was used. As already mentioned in Section 2.2, the bound states of the 20Ne–20Ne and 20Ne–22Ne dimers were listed in our paper I Citation3 in which they were compared with the experimental rovibrational spectra by Wüest and Merkt Citation5.
In the values calculated fully quantum-mechanically for the interatomic potential of the present paper are opposed to those resulting from quantum corrections of increasing order added to the classical contribution. The figure illustrates that the classical contribution alone is completely insufficient to describe adequately the second pressure virial coefficient. The agreement between both ways of calculation improves, with the quantum corrections included, what becomes obvious particularly at low temperatures. To obtain close agreement even at the lowest temperatures, the third-order quantum correction is needed.
Figure 1. Differences ΔB = B qm,full − [B cl + ∑λ i B qm,i] between the fully quantum-mechanically calculated values and the values resulting from the sum of a classical contribution and of different orders of quantum corrections to the second pressure virial coefficient for the new interatomic potential for Ne. Differences related to: ··· ··· ··· classical contribution B cl; – · – · – · sum of classical contribution and of first-order quantum correction B cl + λB qm,1; – – – – sum of classical contribution as well as of first-order and second-order quantum corrections B cl + λB qm,1 + λ2 B qm,2; ———– sum of classical contribution as well as of first-order, second-order, and third-order quantum corrections B cl + λB qm,1 + λ2 B qm,2 + λ3 B qm,3.
![Figure 1. Differences ΔB = B qm,full − [B cl + ∑λ i B qm,i] between the fully quantum-mechanically calculated values and the values resulting from the sum of a classical contribution and of different orders of quantum corrections to the second pressure virial coefficient for the new interatomic potential for Ne. Differences related to: ··· ··· ··· classical contribution B cl; – · – · – · sum of classical contribution and of first-order quantum correction B cl + λB qm,1; – – – – sum of classical contribution as well as of first-order and second-order quantum corrections B cl + λB qm,1 + λ2 B qm,2; ———– sum of classical contribution as well as of first-order, second-order, and third-order quantum corrections B cl + λB qm,1 + λ2 B qm,2 + λ3 B qm,3.](/cms/asset/40a0fcef-0000-44e1-9371-9016be189d01/tmph_a_330433_o_f0001g.gif)
There exists only a limited number of experimental data for second and third pressure virial coefficients of neon in the literature compared with those of common gases like argon and nitrogen as well as with those of helium. Furthermore, one should point out that experimental data for the third pressure virial coefficient are not independent of the values for the second pressure virial coefficient derived from the same experiments. Hence third pressure virial coefficients combined with second ones are included in the comparison. Second and third pressure virial coefficients were determined by Holborn and Otto Citation19, Nicholson and Schneider Citation20, Michels et al. Citation21, and Gibbons Citation22 from isothermal measurements of volume (and density, respectively) and pressure. Vogl and Hall Citation23 used a Burnett apparatus to derive isothermal compression factors and to obtain finally second and third pressure virial coefficients. Unfortunately, in none of these papers an error propagation analysis or uncertainties of the second and third pressure virial coefficients adequately deduced from the experiments were reported.
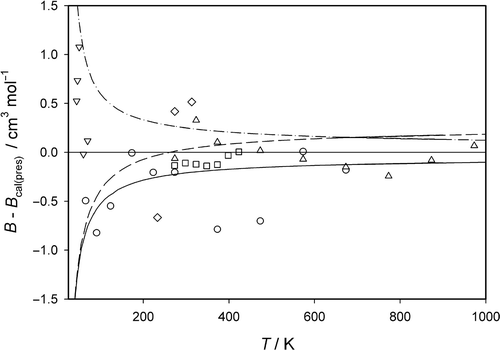
The experimental B data are compared with the values calculated fully quantum-mechanically for the neon–neon interatomic potential of the present paper in , in which the absolute deviations B exp − B cal(pres) are displayed. The figure demonstrates a very good agreement for the excellent data by Michels et al. Citation21 at medium temperatures. A good agreement is also found for the data by Nicholson and Schneider Citation20 up to high temperatures of 1000 K. Conversely, the very old data of Holborn and Otto Citation19 as well as the more recent but also already 35 years old data of Vogl and Hall Citation23 are characterized by comparably larger differences to the theoretically calculated values. The data of Gibbons Citation22 determined at low temperatures show partly large deviations, but agree partly very well. In our calculated values are additionally compared with the values calculated for the interatomic potentials by Aziz and Slaman Citation7, Cybulski and Toczylowski Citation6, and Wüest and Merkt Citation5. The differences B cal(lit) − B cal(pres) derived for the different interatomic potentials increase to low temperatures, where the values derived from the potentials by Aziz and Slaman Citation7 and Wüest and Merkt Citation5 are too small and the values resulting from the potential by Cybulski and Toczylowski Citation6 are too large. At medium and higher temperatures the B cal values for all four potentials do not differ much so that the second pressure virial coefficient is not suitable for distinguishing between the different interatomic potentials.
Figure 2. Differences (B − B cal(pres)) of experimental (B exp) and calculated (B cal(lit)) second pressure virial coefficients from values (B cal(pres)) calculated with the new interatomic potential for Ne. Experimental data: ○ Holborn and Otto Citation19; ▵ Nicholson and Schneider Citation20; □ Michels et al. Citation21; ▽ Gibbons Citation22; ⋄ Vogl and Hall Citation23. Calculated values: ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6; – – – – potential by Wüest and Merkt Citation5.
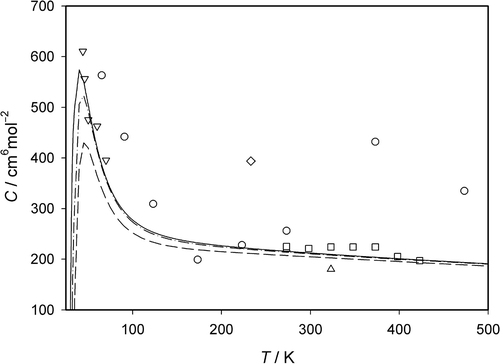
In a comparison between experimental data of the third pressure virial coefficient of neon and values calculated for the new interatomic potential is shown. The figure elucidates that good agreement of the experimental data by Michels et al. Citation21 and by Nicholson and Schneider Citation20 at medium temperatures and of the data by Gibbons Citation22 at low temperatures with the calculated values is only achieved in the case of the complete sum of the contributions for the pairwise additivity C add, for the non-additivity of the three-body interatomic interactions according to Axilrod and Teller C non-add, and for the first-order quantum-mechanical correction λC qm,1. The experimental data by Holborn and Otto Citation19 as well as Vogl and Hall Citation23 possess again larger differences to the calculated values. The comparison makes evident that the calculation procedure for the third pressure virial coefficient predicts very good values.
Figure 3. Comparison of experimental data and and of calculated values for the third pressure virial coefficient C derived from the new interatomic potential for Ne. Experimental data: ○ Holborn and Otto Citation19; ▵ Nicholson and Schneider Citation20; □ Michels et al. Citation21; ▽ Gibbons Citation22; ⋄ Vogl and Hall Citation23. Calculated values: – – – – classical contribution C add, – · – · – · classical and non-additivity contributions C add + C non-add, ——— sum of classical and non-additivity contributions and of the first-order quantum correction C add + C non-add + λC qm,1.
It is to be stressed that the calculated values for the second and the third pressure virial coefficients are more reliable than the experimental data at low and high temperatures.
3.2. Viscosity
First, the results of the different alternative ways of the calculation of the transport properties of naturally occurring neon are compared. In this context it is sufficient to consider only the viscosity, since the effects are the same for the thermal conductivity. illustrates the relative differences between viscosity values derived for the different approximation procedures and the viscosity values obtained from the quantum-mechanical calculation up to the fifth-order approximation for the individual [η ij ]qm,5 within the first-order formulation of [ηmix]1 (see Section 2.3). The figure makes evident that the first-order approximation of the classical calculation leads to values which are nearly 1% too small in the complete temperature range except at the lowest temperatures. The agreement improves when the fifth-order approximation of the classical evaluation is applied. But even for this high-order approximation it becomes obvious that the classical evaluation is not appropriate with regard to highly accurate values. Thus the deviations from the results for the quantum-mechanical calculation of the same fifth-order approximation amount to −0.1% at room temperature increasing up to −1.1% at about 60 K. On the other hand, the first-order approximation of the quantum-mechanical calculation for a dilute-gas mixture composed of the three neon isotopes according to [ηmix]qm,1 is not adequate, too. The differences are approximately −0.7% at most temperatures and decrease to zero at the lowest temperatures. Further it is to note that there are only differences of <±0.0004% (not visible in ) between the results for the two ways to correct the first-order approximation [ηmix]qm,1 to an appropriate fifth-order approximation of the quantum-mechanical determination. In the following the comparisons with experimental data are performed with values resulting for the fifth-order approximation of the individual [η ij ]qm,5 and [λ ij ]qm,5 within the first-order formulations of [ηmix]1 and [λmix]1.
Figure 4. Relative deviations Δ = (η − ηqm,5)/ηqm,5 between viscosity values calculated for different approximation procedures and viscosity values resulting from quantum-mechanical calculations up to the fifth-order approximation for the individual [η ij ]qm,5 within the first-order formulation of [ηmix]1 for the new interatomic potential for Ne. Differences related to: ··· ··· ··· first-order classical calculation [η]cl,1; – ·· – ·· – ·· fifth-order classical calculation [η]cl,5; – · – · – · first-order quantum-mechanical calculation [ηmix]qm,1.
![Figure 4. Relative deviations Δ = (η − ηqm,5)/ηqm,5 between viscosity values calculated for different approximation procedures and viscosity values resulting from quantum-mechanical calculations up to the fifth-order approximation for the individual [η ij ]qm,5 within the first-order formulation of [ηmix]1 for the new interatomic potential for Ne. Differences related to: ··· ··· ··· first-order classical calculation [η]cl,1; – ·· – ·· – ·· fifth-order classical calculation [η]cl,5; – · – · – · first-order quantum-mechanical calculation [ηmix]qm,1.](/cms/asset/a235c3b6-0fbb-47db-9b83-0c0db2e1d7b2/tmph_a_330433_o_f0004g.gif)
With regard to the transport properties it should be considered that most measurements at low densities were performed at atmospheric pressure, whereas the theoretical calculations are valid for the limit of zero density. Hence the initial density dependence of the experimental data would have to be taken into account. However apart from the very low temperatures near to the normal boiling point of neon, the effect of the initial density dependence on the transport properties concerning the change in density from that at atmospheric pressure to zero density is comparably small (<0.1%) for all other temperatures. Furthermore, the experimental uncertainty is distinctly increased at low temperatures.
In our paper concerning the thermophysical standard values for low-density helium Citation1 we argued that it is difficult to perform genuine absolute measurements of the gas viscosity with an uncertainty <±0.1%, even at room temperature. The same complex of problems is illustrated in in which the best experimental data for neon near to ambient temperature are characterized by error bars for the uncertainties, given by the authors themselves, and are compared with the viscosity values calculated quantum-mechanically. For helium we demonstrated that the measurements with an oscillating-disc viscometer by Kestin and Leidenfrost Citation24, approved as one of the most accurate and additionally one of the few absolute measurements on gases, can only partly be considered as absolute ones, since they were finally adjusted to a value for the viscosity of air at 293.15 K and at atmospheric pressure determined by Bearden Citation31 in an absolute measurement with a rotating-cylinder viscometer. Thus the viscosity value of Kestin and Leidenfrost for neon at 20°C (uncertainty: ±0.05%) shown in corresponds as well to a relative measurement, whereas the genuine absolute measurement is that of Bearden in air. Measurements by Kestin and Nagashima Citation25, performed in a nearly analogous procedure, led to values which are 0.15–0.3% higher than those of Kestin and Leidenfrost Citation24, but also by the same percentage higher than further data obtained in relative measurements of the same research group by DiPippo et al. Citation26 as well as a best estimate reported by Kestin et al. Citation27 in 1972 as a result of their measurements in foregoing years. This shows that there were sometimes surprisingly large differences in the results of the measurements of this group. Nevertheless, the results of the most reliable measurements by Kestin and co-workers at ambient temperature are characterized by a tendency to values increased by +0.1% compared to the calculated values of the present paper. The same findings concerning the measurements by Kestin and co-workers were observed in the case of the values derived from our interatomic helium potential. As a consequence, the measurements on helium by Vogel Citation29 (uncertainty: ±0.15% at room temperature) performed with an all-quartz oscillating-disc viscometer in a relative manner using a viscometer constant derived from the best estimate by Kestin et al. should be affected by the same impact. Therefore, the viscometer of Vogel was recalibrated with the new helium standard for a rehandled evaluation of the measurements on helium Citation1 and on neon, too. The influence of the recalibration on the results of Vogel for neon is additionally demonstrated in . A value at 298.15 K resulting from the fitting function given by Vogel Citation29 deviates from the value calculated for the interatomic neon potential of the present paper by +0.18%. Conversely, the direct experimental data of the measurement series by Vogel at room temperature show only differences of +0.04% and +0.08% after the recalibration.
Figure 5. Relative deviations of experimental and calculated viscosity coefficients from values ηcal(pres) calculated quantum-mechanically with the new interatomic potential for Ne near to room temperature. Experimental data with uncertainties characterized by error bars: • Kestin and Leidenfrost Citation24; ○ Kestin and Nagashima Citation25; ▾ DiPippo et al. Citation26; ▽ Kestin et al. Citation27, best estimate; ▪ Flynn et al. Citation28; ▵ Vogel Citation29, fitted value; ▴ Vogel Citation29, experimental data corrected according to new helium standard; ♦ Evers et al. Citation30. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6.
![Figure 5. Relative deviations of experimental and calculated viscosity coefficients from values ηcal(pres) calculated quantum-mechanically with the new interatomic potential for Ne near to room temperature. Experimental data with uncertainties characterized by error bars: • Kestin and Leidenfrost Citation24; ○ Kestin and Nagashima Citation25; ▾ DiPippo et al. Citation26; ▽ Kestin et al. Citation27, best estimate; ▪ Flynn et al. Citation28; ▵ Vogel Citation29, fitted value; ▴ Vogel Citation29, experimental data corrected according to new helium standard; ♦ Evers et al. Citation30. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6.](/cms/asset/bfcf5391-5ef0-4a48-9577-d9738f18cfeb/tmph_a_330433_o_f0005g.gif)
Furthermore, the absolute measurements by Flynn et al. Citation28 performed with a capillary viscometer led to a datum at 293.15 K differing only by +0.01% from the theoretically calculated value (uncertainties: ±0.1%). Recently, Evers et al. Citation30 utilized a rotating-cylinder viscometer for absolute measurements (uncertainty: ±0.15%) on several gases at different temperatures and pressures. Their result for neon at 298.15 K deviates from our calculated value by −0.12%. In conclusion, the comparison makes evident that the best experimental data at room temperature are characterized by an uncertainty of ±(0.1 to 0.15)% and that they agree within this limit with the values calculated for the interatomic neon potential of the present paper.
The situation deteriorates to the disadvantage of the experiment, if the measurements were not performed at ambient temperature. In , experimental data at low and medium temperatures between 20 and 373 K are compared with the calculated values. Error bars for one or two (in the case that the uncertainty changes with temperature) values of each data set are additionally plotted. The figure demonstrates that excellent agreement within ±0.1% exists only for the absolute measurement by Evers et al. Citation30 at 348 K and that the results of the absolute measurements by Flynn et al. Citation28 are adequately consistent within ±0.3%. The other data were determined by relative measurements, which are not only affected by the usual measurement errors, but also by the values used for the calibration. Johnston and Grilly Citation32 and Rietveld et al. Citation34 (both using oscillating-disc viscometers) as well as Clarke and Smith Citation35 (capillary viscometer) based their measurements on reasonable values for air, helium, and nitrogen at ambient temperature and achieved results with deviations up to −2%, +4%, and +1%. These data are not suitable to judge the appropriateness of any interatomic neon potential. On the other hand, the measurements by Coremans et al. Citation33 carried out with an oscillating-disc viscometer, which was calibrated using a very old viscosity value for 4He at 20 K reported by Kamerlingh Onnes and Weber Citation36, yielded values characterized by positive deviations up to 6% from the quantum-mechanically calculated values. These results were improved for the purposes of this paper by a recalibration with a value for 4He at 20 K taken from our new helium standard Citation1. makes obvious that the corrected data advanced after this correction partly in close agreement.
Figure 6. Relative deviations of experimental and calculated viscosity coefficients from values ηcal(pres) calculated with the new interatomic potential for Ne at low and medium temperatures. Experimental data with uncertainties characterized by error bars: □ Johnston and Grilly Citation32; ○ Coremans et al. Citation33; • Coremans et al. Citation33, corrected according to new helium standard; ▵ Rietveld et al. Citation34; ▪ Flynn et al. Citation28; ⋄ Clarke and Smith Citation35; ♦ Evers et al. Citation30. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6; – – – – potential by Wüest and Merkt Citation5.
![Figure 6. Relative deviations of experimental and calculated viscosity coefficients from values ηcal(pres) calculated with the new interatomic potential for Ne at low and medium temperatures. Experimental data with uncertainties characterized by error bars: □ Johnston and Grilly Citation32; ○ Coremans et al. Citation33; • Coremans et al. Citation33, corrected according to new helium standard; ▵ Rietveld et al. Citation34; ▪ Flynn et al. Citation28; ⋄ Clarke and Smith Citation35; ♦ Evers et al. Citation30. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6; – – – – potential by Wüest and Merkt Citation5.](/cms/asset/ce4bdb7e-695b-406d-adef-c0111bdd50a6/tmph_a_330433_o_f0006g.gif)
illustrates the analogous comparison at higher temperatures. The figure reveals a surprisingly large scattering of about ±0.3% in the data from different papers by Kestin and his research group Citation27,Citation37,Citation38 (the same order of magnitude as the uncertainty) and additionally a systematic trend to higher values with increasing temperature combined with again decreasing values at the highest temperatures. In this connection it is to be noted that all measurements by Kestin and his co-workers with the oscillating-disc viscometer by Di Pippo et al. Citation41 are affected by a temperature measurement error with thermocouples explained by Vogel et al. Citation42. also makes evident that the data by Vogel Citation29, originally fitted to his experiments which were based on a calibration with the best estimate value at room temperature by Kestin et al. Citation27, deviate by about +0.2% from the quantum-mechanically calculated values of this paper. After a recalibration of the measurement series on neon by means of the new helium standard Citation1 at room temperature, the corrected experimental data do only deviate by less than +0.1% on average from the theoretical values for the new neon potential in the complete temperature range of the measurements. This demonstrates that the measurements by Vogel with his all-quartz oscillating-disc viscometer represent the best experiments in this temperature range. The comparison concerning the experimental data by Dawe and Smith Citation39 and by Guevara and Stensland Citation40, which result from relative measurements with capillary viscometers based on a reasonable calibration at room temperature, shows that these data should be influenced by systematic errors. Lastly it is concluded that the theoretical determination of viscosity values is to be preferred to experiments at these high temperatures.
Figure 7. Deviations of experimental and calculated viscosity coefficients from values ηcal(pres) calculated with the new interatomic potential for Ne at higher temperatures. Experimental data with uncertainties characterized by error bars: ▽ Kestin et al. Citation27, best estimate; ○ Hellemans et al. Citation37; ⊙ Kestin et al. Citation38; ⋄ Dawe and Smith Citation39; □ Guevara and Stensland Citation40; ▵ Vogel Citation29, fitted values; ▴ Vogel Citation29, experimental data corrected according to new helium standard. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6; – – – – potential by Wüest and Merkt Citation5.
![Figure 7. Deviations of experimental and calculated viscosity coefficients from values ηcal(pres) calculated with the new interatomic potential for Ne at higher temperatures. Experimental data with uncertainties characterized by error bars: ▽ Kestin et al. Citation27, best estimate; ○ Hellemans et al. Citation37; ⊙ Kestin et al. Citation38; ⋄ Dawe and Smith Citation39; □ Guevara and Stensland Citation40; ▵ Vogel Citation29, fitted values; ▴ Vogel Citation29, experimental data corrected according to new helium standard. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6; – – – – potential by Wüest and Merkt Citation5.](/cms/asset/db1e279b-dba6-47dc-b4e6-5f4808112166/tmph_a_330433_o_f0007g.gif)
Figures , , and include once again a comparison with the values derived classically using the fifth-order approximation. The results of the classical calculation deviate by about −0.1% from those of the quantum-mechanical computation at ambient and higher temperatures. At lower temperatures the deviations are distinctly increased. elucidates further that at room temperature the results of the quantum-mechanical calculations for the potentials by Aziz and Slaman Citation7 and by Cybulski and Toczylowski Citation6 (both >±0.2%) and particularly by Wüest and Merkt Citation5 (−0.7%, not observable in the figure) do not match the best experimental data as well as the calculated values for the potential of the present paper within ±0.1%. Figures and demonstrate that the best experimental data allow one to distinguish between the different potentials proposed for neon. The values resulting from the potentials by Aziz and Slaman Citation7 and by Cybulski and Toczylowski Citation6 and particularly by Wüest and Merkt Citation5 are characterized by differences from the transport data that are distinctly larger than the experimental uncertainties. Here one should point to the differences for the values determined with the potential proposed by Wüest and Merkt Citation5. They arise with increasing temperature due to the fact that the rovibrational spectra used by Wüest and Merkt are sensitive to the shape of the potential well, but not to the repulsive part of the potential to which the transport properties are particularly sensitive.
3.3. Thermal conductivity
The uncertainty of measurements of the thermal conductivity is inferior to that of viscosity measurements due to different experimental difficulties, whereas the most accurate data can be obtained with the transient hot-wire technique, but essentially restricted to ambient temperature. This is demonstrated in , in which experimental data for neon at low and medium temperatures are compared with the values calculated quantum-mechanically. Here the experimental data are again, when available, characterized by error bars according to the uncertainties given by the experimenters themselves. The data by Kestin et al. Citation44 and Assael et al. Citation45, each gained with the transient hot-wire technique at room temperature, deviate from the calculated values by <−0.1% and <+0.2%. These differences are lower than the experimental uncertainties (±0.3% and ±0.2%). Although the data by Haarman Citation43 are characterized by larger deviations (−0.3% to −0.4%), the temperature function of these transient hot-wire data between 328 and 468 K corresponds closely to that of the calculated values. Conversely, the temperature function of the data by Millat et al. Citation46 shows an awkward behaviour so that these data are not useful with regard to the assessment of the values calculated for the different interatomic potentials of neon. But makes also evident that the deviations of the experimental data of Hemminger Citation47, derived from measurements with a guarded parallel-plate apparatus and carefully corrected for impurities caused by desorbed air, are within −0.35% and −0.6%; this means their temperature function and that of the calculated values are pretty much consistent from room temperature up to 470 K.
Figure 8. Deviations of experimental and calculated thermal conductivity coefficients from values λcal(pres) calculated with the new interatomic potential for Ne at low and medium temperatures. Experimental data with uncertainties characterized by error bars: • Haarman Citation43; ▴ Kestin et al. Citation44; ▪ Assael et al. Citation45; ▾ Millat et al. Citation46; ♦ Hemminger Citation47; ○ Weber Citation48; ▵ Kannuluik and Carman Citation49; □ Keyes Citation50; ▽ Sengers et al. Citation51; ⋄ Nesterov and Sudnik Citation52, smoothed values. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6; – – – – potential by Wüest and Merkt Citation5.
![Figure 8. Deviations of experimental and calculated thermal conductivity coefficients from values λcal(pres) calculated with the new interatomic potential for Ne at low and medium temperatures. Experimental data with uncertainties characterized by error bars: • Haarman Citation43; ▴ Kestin et al. Citation44; ▪ Assael et al. Citation45; ▾ Millat et al. Citation46; ♦ Hemminger Citation47; ○ Weber Citation48; ▵ Kannuluik and Carman Citation49; □ Keyes Citation50; ▽ Sengers et al. Citation51; ⋄ Nesterov and Sudnik Citation52, smoothed values. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6; – – – – potential by Wüest and Merkt Citation5.](/cms/asset/bbb5cb8d-3731-42d4-892e-b12d7d2c0c07/tmph_a_330433_o_f0008g.gif)
Experimental data determined with the common steady-state hot-wire technique often affected by convection are checked against the quantum-mechanically calculated values in , too. Differences of only < ±0.4% are found for the very old experimental datum by Weber Citation48 at 273 K and also for a value by Kannuluik and Carman Citation49 at the same temperature. But for the complete temperature range of the measurements of Kannuluik and Carman between 90 and 580 K the deviations increase up to −3%. On the other hand, the smoothed experimental values by Nesterov and Sudnik Citation52 between 90 K and ambient temperature created with the same technique are characterized by comparably small differences between −0.1% and −0.7%, with the best agreement at low temperatures. Further it becomes evident from this figure that the experimental data by Keyes Citation50 determined with the concentric-cylinder method (differences between −1% and −1.5%) and those of Sengers et al. Citation51 obtained with a parallel-plate apparatus (differences between −0.5% and −0.85%) are not suitable for a reasonable comparison with the theoretical values.
illustrates the comparison at higher temperatures. Neither the experimental data by Tufeu et al. Citation54 (concentric-cylinder method) nor the experimental values by Saxena and Saxena Citation53 (common hot-wire technique) enable one to verify the performance of the different potentials under discussion due to the differences exceeding −1%. Conversely, there occur surprisingly only very small deviations of −0.45% to + 0.05% for the experimental data by Springer and Wingeier Citation55 between 1000 and 1500 K using the concentric-cylinder method. In principle, this would support the new interatomic potential of this work. In addition, the values recommended by Ziebland Citation56 on the basis of different experimental data show deviations larger than +1% according to their estimated uncertainties. It is to note that thermal conductivity values at very high temperatures between 1500 K and at most 6000 K were derived from shock-tube measurements by Collins and Menard Citation57 and by Maštovský Citation58. Their data not shown in have deviations of −5.5% up to −11.5% and −2.5% up to −6.8%. At such high temperatures calculated values are to be preferred in any case.
Figure 9. Deviations of experimental and calculated thermal conductivity coefficients from values λcal(pres) calculated with the new interatomic potential for Ne at higher temperatures. Experimental data with uncertainties characterized by error bars: ○ Saxena and Saxena Citation53, smoothed values; ▵ Tufeu et al. Citation54; □ Springer and Wingeier Citation55; ▽ Ziebland Citation56, recommended values. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6; – – – – potential by Wüest and Merkt Citation5.
![Figure 9. Deviations of experimental and calculated thermal conductivity coefficients from values λcal(pres) calculated with the new interatomic potential for Ne at higher temperatures. Experimental data with uncertainties characterized by error bars: ○ Saxena and Saxena Citation53, smoothed values; ▵ Tufeu et al. Citation54; □ Springer and Wingeier Citation55; ▽ Ziebland Citation56, recommended values. Calculated values: ··· ··· ··· fifth-order classical calculation [η]cl,5; ———– potential by Aziz and Slaman Citation7; – · – · – · potential by Cybulski and Toczylowski Citation6; – – – – potential by Wüest and Merkt Citation5.](/cms/asset/7cc8409e-66b1-4946-a947-0939fb05ea8f/tmph_a_330433_o_f0009g.gif)
Both figures make evident that the interatomic potential by Wüest and Merkt Citation5 is not qualified to describe adequately the best experimental thermal conductivity data. On the other hand, there exist only a few experimental data to distinguish between the appropriateness of the other potentials. But if the best experimental transient hot-wire-data at room temperature are selected for the comparison, then there exists a stringent test of the new potential and of the correct application of the kinetic theory including the quantum-mechanical effects.
4. Summary and conclusions
A new interatomic potential for neon derived from quantum-mechanical ab initio computations Citation3 was utilized to calculate the second and third pressure virial, the viscosity, and the thermal conductivity coefficients for dilute neon gas in its natural isotopic composition in the temperature range from 25 to 10,000 K. For the second virial coefficient and for the transport properties fully quantum-mechanical calculations were performed with neon treated as an isotopic mixture, whereas for the third virial coefficient a classical mechanical evaluation with a quantum correction using the average mass of the isotopic mixture was applied. The comparison with available experimental data makes evident that the calculated thermophysical properties are as accurate as the best experimental data at room temperature and more accurate at temperatures above and below room temperature. The deviations between the results from the different potentials for all calculated properties increase at the lowest temperatures.
The viscosity values around ambient temperature derived theoretically with the interatomic potential of this paper are characterized by deviations smaller than ±0.1% compared to the best experimental data, whereas the results obtained from the potential energy curves by Cybulski and Toczylowski, by Aziz and Slaman Citation7, and by Wüest and Merkt Citation5 show larger deviations. We estimate summarily the uncertainties of the calculated transport properties resulting from our new potential to be about ±0.1% except at the lowest temperatures. It is to be stressed that this uncertainty is much below the experimental uncertainties at low as well as at high temperatures. All calculated data (see in Appendix 1) can be applied as standards values for the complete temperature range.
Notes
References
- Bich , E , Hellmann , R and Vogel , E . 2007 . Molec. Phys. , 105 : 3035
- Hellmann , R , Bich , E and Vogel , E . 2007 . Molec. Phys. , 105 : 3013
- Hellmann , R , Bich , E and Vogel , E . 2008 . Molec. Phys. , 106 : 133
- Tang , KT and Toennies , JP . 1984 . J. Chem. Phys. , 80 : 3726
- Wüest , A and Merkt , F . 2003 . J. Chem. Phys. , 118 : 8807
- Cybulski , SM and Toczylowski , RR . 1999 . J. Chem. Phys. , 111 : 10520
- Aziz , RA and Slaman , MJ . 1989 . Chem. Phys. , 130 : 187
- Boyd , ME , Larsen , SY and Kilpatrick , JE . 1969 . J. Chem. Phys. , 50 : 4034
- Mason , EA and Spurling , TH . 1969 . The Virial Equation of State , Oxford : Pergamon .
- Kim , S and Henderson , D . 1966 . Proc. Nat. Acad. Sci. Wash. , 55 : 705
- Lucas , K . 1986 . Angewandte Statistische Thermodynamik , Berlin : Springer .
- Axilrod , BM and Teller , E . 1943 . J. Chem. Phys. , 11 : 299
- Axilrod , BM . 1951 . J. Chem. Phys. , 19 : 719
- Kumar , A and Meath , WJ . 1985 . Molec. Phys. , 54 : 823
- Viehland , LA , Janzen , AR and Aziz , RA . 1995 . J. Chem. Phys. , 102 : 5444
- Meeks , FR , Cleland , TJ Hutchinson , KE . 1994 . J. Chem. Phys. , 100 : 3813
- Maitland , GC , Rigby , M Smith , EB . 1987 . Intermolecular Forces. Their Origin and Determination , Oxford : Clarendon Press .
- LeRoy , RJ . 2005 . LEVEL 7.7. A Computer Program for Solving the Radial Schrodinger Equation for Bound and Quasibound Levels , Waterloo, Ontario, , Canada : University of Waterloo, Chemical Physics Research Report CP–661 .
- Holborn , L and Otto , J . Z. Phys. , 33 1 (1925) 38, 359 (1926)
- Nicholson , GA and Schneider , WG . 1955 . Can. J. Chem. , 33 : 589
- Michels , A , Wassenaar , T and Louwerse , P . 1960 . Physica , 26 : 539
- Gibbons , RM . 1969 . Cryogenics , 9 : 251
- Vogl , WF and Hall , KR . 1972 . Physica , 59 : 529
- Kestin , J and Leidenfrost , W . 1959 . Physica , 25 : 1033
- Kestin , J and Nagashima , A . 1964 . J. Chem. Phys. , 40 : 3648
- DiPippo , R , Kestin , J and Oguchi , K . 1967 . J. Chem. Phys. , 46 : 4758
- Kestin , J , Ro , ST and Wakeham , WA . 1972 . J. Chem. Phys. , 56 : 4119
- Flynn , GP , Hanks , RV Lemaire , NA . 1963 . J. Chem. Phys. , 38 : 154
- Vogel , E . 1984 . Ber. Bunsenges. Phys. Chem. , 88 : 997
- Evers , C , Lösch , HW and Wagner , W . 2002 . Int. J. Thermophys. , 23 : 1411
- Bearden , JA . 1939 . Phys. Rev. , 56 : 1023
- Johnston , HL and Grilly , ER . 1942 . J. Phys. Chem. , 46 : 948
- Coremans , JMJ , van Itterbeek , A Beenakker , JJM . 1958 . Physica , 24 : 557
- Rietveld , AO , van Itterbeek , A and Velds , CA . 1959 . Physica , 25 : 205
- Clarke , AG and Smith , EB . 1969 . J. Chem. Phys. , 51 : 4156
- Kamerlingh Onnes , H and Weber , S . 1913 . Vers. Kon. Acad. Wetenschapen Amsterdam , 21 : 1385
- Hellemans , JM , Kestin , J and Ro , ST . 1974 . Physica , 71 : 1
- Kestin , J , Khalifa , HE and Wakeham , WA . 1978 . Physica A , 90 : 215
- Dawe , RA and Smith , EB . 1970 . J. Chem. Phys. , 52 : 693
- Guevara , FA and Stensland , G . 1971 . Phys. Fluids , 14 : 746
- DiPippo , R , Kestin , J and Whitelaw , JH . 1966 . Physica , 32 : 2064
- Vogel , E , Küchenmeister , C Bich , E . 1998 . J. Phys. Chem. Ref. Data , 27 : 947
- Haarman , JW . 1973 . Am. Inst. Phys. Conf. Proc. , 11 : 193
- Kestin , J , Paul , R Clifford , AA . 1980 . Physica A , 100 : 349
- Assael , MJ , Dix , M Lucas , A . 1981 . J. Chem. Soc. Faraday Trans. I , 77 : 439
- Millat , J , Ross , M Wakeham , WA . 1988 . Physica A , 148 : 124
- Hemminger , W . 1987 . Int. J. Thermophys , 8 : 317
- Weber , S . 1927 . Ann. Phys. , 82 : 479
- Kannuluik , WG and Carman , EH . 1952 . Proc. Phys. Soc. London B , 65 : 701
- Keyes , FG . 1954 . Trans. ASME , 76 : 809
- Sengers , JV , Bolk , WT and Stigter , CJ . 1964 . Physica , 30 : 1018
- Nesterov , NA and Sudnik , VM . 1976 . Inzh.-fiz. Zh. , 30 : 863
- Saxena , VK and Saxena , SC . 1968 . J. Chem. Phys. , 48 : 5662
- Tufeu , R , LeNeindre , B and Bury , P . 1970 . C. R. Acad. Sci. Paris, B , 271 : 589
- Springer , GS and Wingeier , EW . 1973 . J. Chem. Phys. , 59 : 2747
- Ziebland , H . 1981 . Pure Appl. Chem. , 53 : 1863
- Collins , DJ and Menard , WA . 1966 . Trans. ASME , 88 : 52
- Maštovský , J . 1987 . Report Z-1026/87 , Prague : ČSAV, Ústav Termomechaniky .
Appendix 1. Thermophysical properties of neon calculated in this work
The thermophysical properties of naturally occurring neon are given in