Abstract
Colloidal particles are distinguishable. Moreover, their thermodynamic properties are extensive. Statistical mechanics predicts such behaviour if one accepts that the configurational integral of a system of N colloids must be divided by N!. In many textbooks, it is argued that the factor N! corrects for the fact that identical particles (in the quantum mechanical sense) are indistinguishable. Clearly, this argument does not apply to colloids. This article explains why, nevertheless, all is well. The point has been made before, but has not yet sunk in. I also discuss the effect of polydispersity.
Keywords:
1. Background
There should be no need to write this article about the Gibbs paradox, but I am afraid that there is. The Gibbs paradox is based on the observation that when two systems of identical particles in the same thermodynamic state are brought into contact, the entropy of the combined system does not change. However, the entropy does increase when we allow mixing of two systems of ‘almost’ identical particles that we have somehow managed to separate.
In Gibbs's classical statistical mechanics, the entropy of a system with a fixed constant number of particles (N), volume (V) and energy (E) is related to the logarithm of Ω(N, V, E), the volume in phase space accessible to this system. In Boltzmann's (posthumous) notation,
(1) Gibbs (and, before him, Planck) realised that this expression only defines the entropy up to a constant that does not depend on V or E, but can depend on N. In fact, Boltzmann never wrote down Equation (Equation1
(1) ). Planck did [Citation1], acknowledging Boltzmann's influence. However, Planck wrote SN = k log W + const. Somehow, the constant got lost in translation. Probably because, by the time the famous text was written on Boltzmann's grave,Footnotequantum mechanics and the ‘quantum’ interpretation of Nernst thi
Law of ThermodynamicsFootnotewere known. Hence, for pure atomic or molecular systems, it is meaningful to speak about an absolute entropy. As we shall see below, this is not the case for colloidal systems.
In the final pages of his book on the Principles of Statistical Mechanics, Gibbs discusses the grand-canonical partition function. In this context he comments that there is no need to fix the constant as long as we do not consider exchange of particles between systems. However as soon as we do, the partition function should be divided by N!, because otherwise we do not arrive at extensive thermodynamic quantities. Gibbs writes [Citation2]:
… the principle that the entropy of any body has an arbitrary additive constant is subject to limitation, when different quantities of the same substance are concerned. In this case, the quantity being determined for one quantity of substance, is thereby determined for all quantities of the same substance.
Subsequently, with the advent of quantum mechanics, the existence of the factor 1/N! was related to the fact that the square of the quantum-mechanical wave function is invariant under permutation of identical particles, whereas classically, the permutation of identical particles results in a different configuration in phase space (be it with the same observable properties). The quantum mechanical indistinguishability of identical particles is now the standard ‘explanation’ of the Gibbs paradox in most textbooks on statistical mechanics. Some, such as Huang [Citation3], even go as far as stating that the factor N! only makes sense in the context of quantum mechanics: It is not possible to understand classically why we must divide by N! to obtain the correct counting of states. The reason is inherently quantum mechanical.
This is not true and several articles have been written that explain that there is no need to invoke quantum mechanics to arrive at the factor 1/N!. Particularly clear papers have been written on this subject by Jaynes [Citation4] and van Kampen [Citation5]. The focus of the papers by Jaynes and van Kampen has been on simple atomic or molecular systems where the concept of identical particles (in the quantum-mechanical sense), still is meaningful. Here I wish to consider the case of systems of particles that, although similar, are all different. This is the standard situation in colloid science: no two colloids are identical. Even if they would consist of the same number of atoms, their (amorphous) structure differs on a microscopic scale. I should state at the outset that the role of N! in that statistical mechanics of colloidal systems has been discussed by Swendsen [Citation6,Citation7] and Warren [Citation8]. Much of what I say echoes their comments.
The key point is that it is perfectly legitimate to apply statistical mechanics to colloidal systems – and all simulations of the phase behaviour of colloidal systems rely on this fact (see e.g. [Citation9]). It is worth analysing why this is the case.
2. Monodisperse colloids
Let us first consider a somewhat artificial situation where we have a solvent-free colloidal system in zero gravity. Moreover, we assume that the density of the system is so low that we can describe it as an ideal gas. In that case, we can use quantum mechanics to compute the partition function of a system of N ‘very similar’ colloidsFootnotein a volume V at temperature T. We will consider temperatures where the thermal de Broglie wavelength of the colloids is much smaller than the size of the particles. Then we can write the partition function of this system as
(2) where the subscript ‘QM’ indicates that this is a quantum partition function. As the colloids are ‘very similar’, I have assumed that they have the same thermal de Broglie wavelength and the same internal partition function
. If we take the logarithm of this purely quantum mechanical partition function, we obtain
(3) Clearly, ln Q is not extensiveFootnotebecause ln Q(2N, 2V, T) is not equal to 2ln Q(N, V, T), but
(4) Note that quantum indistinguishability cannot fix this non-extensivity because the particles are distinguishable in the quantum sense: permuting particles is not a symmetry operation on the wave function.
3. N! recovered
Let us next consider two systems, one containing N1 particles in volume V1 and the other with N2 particles in volume V2. We prepare the systems at the same temperature and pressure. This means that (away from a phase transition) the systems have the same density and, hence N1/N2 = V1/V2. If we make a small opening in the wall dividing the two systems, mass exchange is possible. We should not expect a net flow of particles between two systems with the same temperature and pressure. However, if we write the total partition function of the combined system as
(5) then this product is not at a maximum for N1/N2 = V1/V2. The underlying problem is that Equation (Equation5
(5) ) is wrong. When we consider the total partition function of the combined systems 1 and 2, we must include all possible realisations of the system that result in the same macroscopic state. That means we must consider all possible ways in which we can distribute the N colloids, such that there are N1 in volume V1 and N2 in volume V2. That is
(6) This point has been made explicitly by Swendsen [Citation6,Citation7] and Warren [Citation8]. If we now differentiate
with respect to N1 at fixed N, we get
(7) Using dN2 = −dN1, it is clear that the condition for equilibrium is
(8) Equation (Equation8
(8) ) must express the equality of chemical potential between two phases that are in macroscopically identical states. That is
(9) In other words, we are forced to conclude that the Helmholtz free energy of a system of ‘very similar’ but distinguishable particles is given by
(10) although A(N, V, T) + αN, with α being an arbitrary constant independent of N and V Footnotewould work just as well. The factor N! is there, but it is not related to quantum-mechanical indistinguishability but to the fact that permuting very similar colloids does not change the observable properties of the macroscopic systems. What is, and what is not, an observable difference is, in the end, determined by our ability to detect differences. This point was eloquently made by Jaynes [Citation4] who discussed the entropy of a mixed system of hypothetical elements (Whifnium, Whafnium and Whoofnium) that are so similar, initially they were considered as one, then as two and finally as three. The entropy depends on what we know about the system or, more precisely, about what we care to know. Jaynes writes: … an illustration of the ‘anthropomorphic’ nature of entropy, would not be apparent to, and perhaps not believed by, someone who thought that entropy was, like energy, a physical property of the micro state. Of course, particles that are indistinguishable in the quantum sense, meaning that permuting them is a symmetry operation on the wave function can never be distinguished, no matter what we do, or do not care to know. However, if we were to label every individual colloid, then the whole concept of equilibrium under particle exchange becomes meaningless. If we gave every colloid a name, then we would consider the situation where the colloid named ‘Julius Caesar’ is in volume one distinct from the situation where this colloid had crossed the Rubicon into volume two: equilibrium is never possible because every permutation of particles creates a new situation. It is a bit like stamp collectors exchanging stamps that, to the uninitiated, look identical. The experts will consider every distribution of stamps over collectors as a distinct state.
Figure 1. The observable properties of two glasses of milk poured from the same bottle are the same. However, all the colloidal particles in the milk are different. Therefore the ‘quantum’ view (left) is that the two systems are not in the same state, whereas the ‘thermodynamic’ view (right) would be that they are in the same state. Disclaimer: neither Gibbs nor Nernst ever made the statements above.
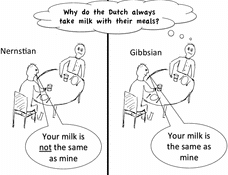
Although I have taken an ideal gas of colloids as an example, Equation (Equation10(10) ) also applies to systems of interacting colloids or, for that matter to any system consisting of large numbers of very similar particles. An immediate consequence of Equation (Equation10
(10) ) is that we can write for the entropy of our colloidal gas,
(11) which is not quite the same as what is written on Boltzmann's grave (but it is perfectly compatible with what Planck wrote in 1901).
Some readers may feel uncomfortable with the idea of dividing Ω by N!, only to ensure that ln (Ω/N!) of a system of very similar particles behaves like the entropy. However that is precisely the procedure that has to be followed in constructing statistical mechanics: we start with postulating a correspondence between a computable quantity (Ω) and a thermodynamic quantity that should be at a maximum in a closed system in equilibrium. In the present case, we find that S = kBln Ω does not do the job, but S = kBln [Ω(N, V, E)/N!] does.
4. Polydisperse colloids
Up to this point, we have assumed that the colloids are so similar that we cannot separate them. However, in practice, colloidal suspensions are usually polydisperse and we can separate colloids of different sizes by fractionation. The question is how polydispersity will affect the discussion above. As I will show, it makes the absolute value of the entropy meaningless, but the factor 1/N! remains. I note that the role of the factor N! in the entropy of polydisperse systems was discussed in a paper by Warren [Citation8] – hence, what I say here is again not very original.
When discussing polydisperse mixtures, the ‘anthropomorphic’ nature of entropy is even more obvious than before. Basically, we have to specify what particles we can separate. The value of the entropy will depend on this choice, but of course, the macroscopic equilibrium will not. A clear discussion of this issue can be found in Jaynes paper on Information Theory and Statistical Mechanics [Citation10].
Let us consider an example where we have a colloidal mixture of spherical particles with different sizes. We characterise the size of colloid by its hard-core diameter σ, and the probability to find a colloid with a size between σ and σ+dσ is given by P(σ)dσ. The first step is to ‘bin’ the particle size distribution into fractions that can be separated. Suppose that there are m such fractions and that the fraction of all particle sizes in bin i that includes all particles with diameters between σi and σi +1 is denoted by Xi,
(12) As we cannot separate particles within one bin, permutations of such particles leave the macroscopic state of the system unaffected. As before, the total number of particles in the combined system (1 + 2) is denoted by N, with N1 particles in system 1 and N2 in system 2. Then
, the number of ways in which particles can be permuted between systems 1 and 2 is given by
(13) where N1, 2(i) = N1, 2Xi. As before, we require that, in equilibrium, the total partition function of the combined system must be at a maximum, and that its derivative with respect to all N1(i) = Ni − N2(i) must vanish. The immediate consequence is that the expression for the Helmholtz free energy of a system with N particles in volume V at temperature T must be of the form,
(14) where N(i) = NXi. We can now use the Stirling approximation to write
(15) Let us define an ‘entropy of mixing’ as
(16) then the expression for the Helmholtz free energy becomes
(17) Importantly,
depends linearly on N: it only changes the reference point for the chemical potential as long as the composition of the mixture is kept constant. However, in the case of coexistence between two polydisperse phases with a different composition, the mixing term does become important. It is useful to consider the limit where the number of bins goes to infinity and the width of the individual bin tends to zero. In that case, we could writeFootnote
(18) The first term on the right diverges in the limit dσ → 0. However, it is a term that does not depend on composition and is hence immaterial for phase coexistence. We can ignore it. The physically meaningful part of
is
(19) which is well defined.Footnote
5. Conclusions
Of course, the expression S = kBln Ω is valid for a system consisting of indistinguishable quantum particles: Ω(E, V, N) counts the number of eigenstates with energy E and permutations of particles will map a given eigenstate onto itself (possibly with a minus sign). However, as soon as we deal with distinguishable colloids, we need to divide the partition function by N! in order to obtain an extensive Helmholtz free energy. In that case, S = kBln (Ω/N!) = kBln Ω + const., the very expression that Planck wrote down in 1901.
Acknowledgements
I gratefully acknowledge heated discussions with Fabien Paillusson and Daniel Asenjo. I thank Patrick Warren for sending me Ref. [Citation8]. I thank Erika Eiser for carefully reading the manuscript.
Additional information
Funding
Notes
1. Apparently the text was proposed by Max Planck, around 1930.
2. Based on very limited statistics (2), it seems that three laws do not have the same generality as first or second laws. This holds not only for the Laws of Thermodynamics, but also for Newton's Laws.
3. I use the word ‘very similar’ to indicate that all the observable properties of the colloidal particles – e.g. mass, size, shape – are the same, but the microscopic structures of the individual colloids are different.
4. Free energies thus defined would not be extensive, but would be additive.
5. α can depend linearly on temperature.
6. Of course, the mathematics here are very sloppy. The way to ‘read’ this equation is to consider that ∫dσ(...) stands for ∑δσ(...) with δσ very small, but sufficiently large that we can apply Stirling's approximation to ln (Nδ)!. After that, we can take the thermodynamic limit...
7. Almost: if we change the integration variable from σ to a monotonic function of σ, a Jacobian will enter into Equation (Equation19(19) ), but not in any of the entropy differences that are important for phase equilibria.
References
- M. Planck, Ann. Phys. (Leipzig) 309, 553 (1901).
- J.W. Gibbs, Elementary Principles of Statistical Mechanics (Charles Scribner's Sons, New York, 1902).
- K. Huang, Statistical Mechanics (Wiley, New York, 1963).
- E.T. Jaynes, in Maximum Entropy and Bayesian Methods, edited by C.R. Smith, G.J. Ericksen, and P.O. Neudorfer (Kluwer, Dordrecht, 1992), pp. 1–22.
- N.G. van Kampen, in The Gibbs Paradox, edited by W.E. Parry(Pergamon Press, Oxford, 1984), pp. 303–312.
- R.H. Swendsen, Am. J. Phys. 74, 187 (2006).
- R.H. Swendsen, Entropy 10, 15 (2008).
- P.B. Warren, Phys. Rev. Lett. 80, 1369 (1998).
- P.G. Bolhuis and D. Frenkel, J. Chem. Phys. 106, 666 (1997).
- E.T. Jaynes, Phys. Rev. 106, 620 (1957).
