ABSTRACT
Dictyostelids are a monophyletic group of sorocarp-forming social amoebae in the major eukaryotic division Amoebozoa. Members of this taxon, which is made up of almost 200 described species, are common in terrestrial soils globally. Still, the alpha diversity is not well known in many areas, and new species are frequently recovered. The highest species richness is found in the tropics. Here, five new species are described from soil samples collected in Madagascar. These species—Cavenderia basinodulosa, C. canoespora, Heterostelium radiatum, H. versatile, and Raperostelium stabile—are described based on both morphological characteristics and molecular data, with sequence data from the rDNA small subunit (SSU). The five new species are morphologically disparate, ranging from relatively small, robust taxa such as R. stabile to taxa with variable morphologies such as the larger H. radiatum and H. versatile and the yellow-tinted and irregularly branched species C. canoespora and C. basinulosa. These new species, together with earlier work where 13 other species were described from the island, suggest that there is a range of genetically diverse and highly morphologically variable dictyostelid taxa occurring on Madagascar, suggesting biogeographic patterns even within these very small organisms.
INTRODUCTION
The island nation of Madagascar is known for species that are morphologically unique and often with high levels of evolutionary distance separating them from sister taxa found on the mainland of Africa. This owes to both the island’s isolated position in the Indian Ocean as well as the unique ecosystems that are found there (Myers et al. Citation2000). Although the flora and fauna are well documented on Madagascar, relatively little is known about the protist diversity there (Goodman and Benstead Citation2005).
Protists are diverse and abundant, yet only a small fraction of their total diversity is described (Mora et al. Citation2011). This taxonomic gap, also known as the Linnaean shortfall, can be explained by a number of factors, including their small size, the difficulty in cultivating them under laboratory conditions, and the paucity of taxonomic experts in the field, as well as limited sampling coupled with the potential for numerous geographically restricted taxa (Foissner Citation2009).This pattern holds true for the dictyostelids, which are widely distributed globally (Swanson et al. Citation1999) but require extensive laboratory cultivation to recover.
The dictyostelids are a monophyletic group of sorocarpic amoebae belonging to the supergroup Amoebozoa (Schaap et al. Citation2006; Fiore-Donno et al. Citation2010; Adl et al. Citation2012, Citation2019) and are composed of ca. 200 species. These terrestrial, bacterivorous amoebae are found in soil and organic litter habitats around the world (Raper Citation1984). The highest species diversity is known from tropical habitats, although they are not uncommon at higher latitudes and have even been recovered in low numbers from both the Arctic Circle and sub-Antarctic Macquarie Island (Cavender Citation1978; Stephenson et al. Citation1998; Swanson et al. Citation1999; Perrigo et al. Citation2013).
Recently, extended taxon sampling, along with whole-genome sequencing, has led to a better understanding of dictyostelid evolution (Singh et al. Citation2016; Sheikh et al. Citation2018; Schilde et al. Citation2019). Yet, even with whole-genome and extensive multisequence data (Schilde et al. Citation2019), some relationships remain unresolved. There are still myriad taxa that are not consistent with described species based on morphology, molecular data, or both. There are also a number of “species complexes” made up of morphologically similar but molecularly distinct taxa (Romeralo et al. Citation2007; Kalla et al. Citation2011; Perrigo Citation2013). To address this situation, broad sampling is needed, in addition to producing more complete data (multilocus or genome) from the known taxa. For this reason, finding new dictyostelid species and describing them based on both morphology and molecular data remains important. Geographically disconnected areas with high levels of endemicity, such as Madagascar, are hypothesized to have unique protist assemblages, mirroring the patterns seen in macroflora and -fauna. A study of the sister taxon to the dictyostelids—the myxomycetes—suggests this pattern in amoebae (Wrigley de Basanta et al. Citation2013).
To explore the protist diversity of Madagascar, soil samples were taken as part of the Global Biodiversity of Eumycetozoans project. In addition to documenting the variable diversity of myxomycetes in Madagascar, these samples were also assessed for their dictyostleid diversity, which recovered many morphologically and molecularly unique isolates. Thirteen species in the dictysotelid genus Heterostelium (formerly Polysphondylium) were described from Madagascar in 2016 (Cavender et al. Citation2016). Herein, we describe five additional new species, belonging to three genera, from the island. All five have dictyostelid-type morphology: a cellular stalk but no whirled side branches. The species described herein are Cavenderia basinodulosa, C. canoespora, Raperostelium stabile, Heterostelium radiatum, and H. versatile. These collective 18 new species, together with the 13 previously described species that have been found on Madagascar, account for ca. 15% of the known dictyostelid diversity. This suggests that Madagascar may be a biodiversity hot spot for dictyostelids, as it is for larger organisms, based on both the high levels of diversity as well as possible endemicity.
MATERIALS AND METHODS
Isolation, culture, and morphological assessment.—
A total of 54 soil samples were collected from study sites throughout southern, central, and northern Madagascar. Protocols for isolation and morphological identification follow those described in Cavender et al. (Citation2016) and are briefly summarized here.
Soil dilutions and culturing were carried out according to Raper (Citation1984). Soil samples were diluted individually in a 1:25 soil:water suspension and plated on Petri dishes with hay infusion agar and a heavy bacterial suspension. The Petri dishes were checked daily for up to 2 wk, and all dictyostelid colonies that appeared were subcultured on non-nutrient agar plates for identification.
The five isolates described herein were identified as potential new species based on unique combinations of morphological characteristics, which were assessed according to the methods in Cavender et al. (Citation2016). Morphological descriptions and terminology are based on Raper (Citation1984). In this paper, we use the terms “dictyostelid-type” and “polysphondylid-type” to describe a fruiting body with a multicellular stalk and a nonwhorled branching pattern (with or without side branches) and a fruiting body with a multicellular stalk and a whorled branching pattern, respectively. These terms are not independently informative in an evolutionary sense (Sheikh et al. Citation2018) but are still used as a primary morphological descriptor, and the branching type is often indicative of secondary morphological characteristics (Raper Citation1984; Schaap et al. Citation2006).
Isolates of the five new species are deposited at The Dicty Stock Center, Northwestern University, Chicago, Illinois, USA (Basu et al. Citation2012).
Phylogenetic analyses.—
DNA was extracted from all five isolates, following Romeralo et al. (Citation2011), and the ribosomal small subunit (rDNA SSU) was amplified using polymerase chain reaction (PCR), following the protocol described in Perrigo et al. (Citation2013). The rDNA SSU is a relatively long ribosomal region, where the section amplified from the primers described below is usually ca. 1800 nucleotide base pairs. It contains both functionally conserved regions with a slower rate of molecular evolution, as well as spacer regions that have highly variable sequences that are used to resolve relatively recent molecular divergence. This region has been used extensively for molecular delimitation of novel dictyostelid species (e.g., Cavender et al. Citation2016; Liu et al. Citation2019; Vadell et al. Citation2018). For sequence amplification, the primers 18S-FA (5′→3′: AACCTGGTTGATCCTGCCAG) and 18S-RB (5′→3′: TGATCCTTCTGCAGGTTCAC) were used, following Medlin et al. (Citation1988), and these plus the primers D542F (5′→3′: ACAATTGGAGGGCAAGTCTG) and D134R (5′→3′: TCGAGGTCTCGTCCGTTATC) were used for internal sequencing and improved read quality, as described in Schaap et al. (Citation2006). The thermocycler program used for the PCR was 95 C for 5 min, 30 cycles of 95 C for 30 s, 56 C for 1 min, and 72 C for 2 min, and 72 C for 10 min, following Medlin et al. (Citation1988). All new rDNA SSU sequences are deposited in National Center for Biotechnology Information (NCBI) GenBank (accession numbers: MN338953–MN338957).
An initial BLAST search was performed on each SSU sequence to identify similar sequences and give a tentative genus-level placement of the taxon, based on molecular data. This was cross-checked with the genus-level molecular signatures from the taxonomic treatment of Sheikh et al. (Citation2018). Based on both an initial BLAST search and the molecular signature taxonomy, the new taxa were preliminarily identified as two taxa in Cavenderia, one taxon in Raperostelium, and two taxa in Heterostelium.
Based on this genus-level placement, three molecular phylogenies were built using a selection of representative taxa from each of the respective genera. Sequences of closely related taxa were identified using a BLASTn search on NCBI GenBank. These results were cross-referenced with earlier phylogenies to ensure a broad and complete sampling of closely related taxa (Romeralo et al. Citation2011, Citation2012; Perrigo Citation2013; Cavender et al. Citation2016; Vadell et al. Citation2018). Additionally, three sequences from each sister genus, according to Sheikh et al. (Citation2018), were selected to serve as an outgroup for the Heterostelium and Raperostelium phylogenies, and three early-branching taxa were selected to root the Cavenderia phylogeny, based on Perrigo (Citation2013). All taxon names, strain designations, and GenBank accession numbers for the sequences used to build the phylogenies are listed in .
Table 1. Strain designations and NCBI GenBank accession numbers for all taxa used in the phylogenetic analyses
For each of the three phylogenies, the downloaded and new rDNA SSU sequences were aligned using AliView 1.19 (Larsson Citation2014). Phylogenies were then inferred using both maximum likelihood and Bayesian analyses, with the same parameters for each of the three data sets. Maximum likelihood analyses were inferred in RAxML 8 (Stamatakis Citation2014) using the raxmlGUI 1.31 (Silvestro and Michalak Citation2012) with the ML + thorough bootstrap setting, bootstrap branch lengths on, the GTR+gamma+i model of evolution, 100 runs, and 10 000Comp: “10 000”: Thin line space—but do not line break—between digits pls. replicates. Bayesian analyses were run using MrBayes 3.2.2 (Ronquist et al. Citation2012) through the CIPRES Science Gateway 3.3 (Miller et al. Citation2010) with the MC3 search algorithm and six independent sets of four chains. The analysis was run for 1 million generations, saving every 10 000thComp: “10 000th”: Thin line space—but do not line break—between digits pls. tree, removing the first 10% as burn-in. Each of the resulting phylogenies were rooted according to the outgroups described above, following Perrigo (Citation2013) and Sheikh et al. (Citation2018). The resulting phylogenies are shown in .
Figure 1. Phylogenetic positions of Cavenderia basinodulosa (sp. nov.) and C. canoespora (sp. nov.). Phylogeny of a subset of closely related species in the genus Cavenderia (Cavenderiaceae) indicating the phylogenetic position of the newly described species Cavenderia basinodulosa and C. canoespora, which are indicated in the phylogeny in bold. The strain indicator is listed to the right of the species names. Complete strain information and GenBank accession numbers for all taxa in this tree can be found in . The tree was derived by Bayesian analysis of the partial 18S ribosomal small subunit (rDNA SSU). Maximum likelihood bootstrap support values over 50% and Bayesian inference posterior probabilities over 0.70 are indicated on the branches, to the left and right of the slash, respectively. The phylogeny is rooted according to Sheikh et al. (Citation2018). C. = Cavenderia
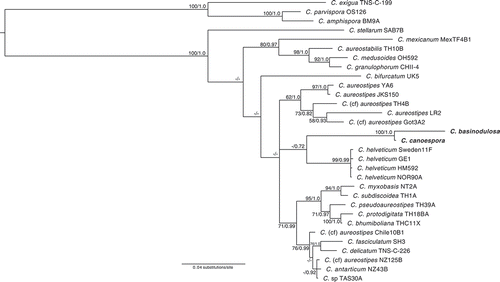
Figure 2. Phylogenetic position of Raperostelium stabile (sp. nov.). Phylogeny of a subset of closely related species in the genus Raperostelium (Raperosteliaceae) indicating the phylogenetic position of the newly described specie Raperostelium stabile, indicated in the phylogeny in bold. The strain indicator is listed to the right of the species names. Complete strain information and GenBank accession numbers for all taxa in this tree can be found in . The tree was derived by Bayesian analysis of the partial 18S ribosomal small subunit (rDNA SSU). Maximum likelihood bootstrap support values over 50% and Bayesian inference posterior probabilities over 0.70 are indicated on the branches, to the left and right of the slash, respectively. The phylogeny is rooted according to Sheikh et al. (Citation2018). R. = Raperostelium; Ha. = Hagiwaraea
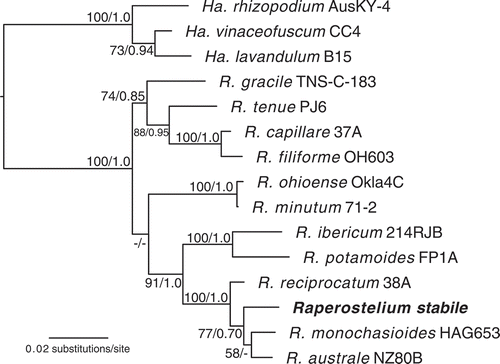
Figure 3. Phylogenetic positions of Heterostelium radiatum (sp. nov.) and H. versatile (sp. nov.). Phylogeny of a subset of closely related species in the genus Heterostelium (Acytosteliaceae) indicating the phylogenetic position of the newly described species Heterostelium radiatum and H. versatile, indicated in the phylogeny in bold. The strain indicator is listed to the right of the species names. Complete strain information and GenBank accession numbers for all taxa in this tree can be found in . All species that were originally described from Madagascar are indicated with an arrow. The tree was derived by Bayesian analysis of the partial 18S ribosomal small subunit (rDNA SSU). Maximum likelihood bootstrap support values over 50% and Bayesian inference posterior probabilities over 0.70 are indicated on the branches, to the left and right of the slash, respectively. The phylogeny is rooted according to Sheikh et al. (Citation2018). H. = Heterostelium; A. = Acytostelium
RESULTS
A total of 54 soil samples were collected from 18 study sites in Madagascar; two samples were not processed because they were very dry and consisted mostly of pebbles. Of the remaining 52 samples, isolation processes produced a total of 401 clones of dictyostelids. Nineteen of these samples were devoid of recoverable dictyostelids. Based upon the total amount of diluted soil samples examined in plate culture, the estimate of dictyostelid density in the sampled substrates is 193 clones/g fresh soil. Dictyostelids were observed in 15 of the general 18 study sites that were sampled.
All five of the isolates were subsequently determined to be unique and as new species based on both morphological and molecular data (). These criteria were considered to be fulfilled if the isolate was consistently morphologically distinguishable from the most closely related species according to the molecular phylogeny. Further, all new species described herein were morphologically distinct from any other known species, independent of the molecular affiliations. The new species are thus described herein as Cavenderia basinodulosa, C. canoespora, Raperostelium stabile, Heterostelium radiatum, and H. versatile.
TAXONOMY
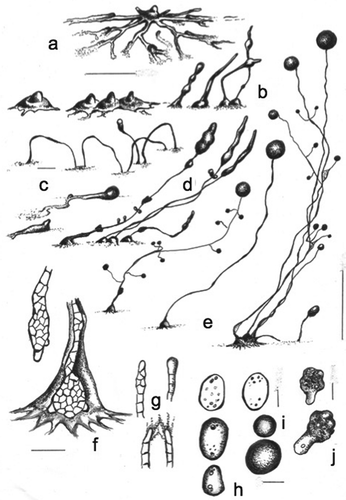
Cavenderia basinodulosa Cavender, J.C. Landolt, A. Perrigo, Vadell, Pu Liu & S.L. Stephenson, sp. nov. ,
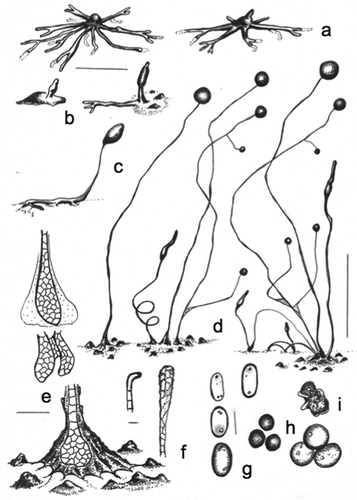
Figure 4. Morphology of Cavenderia basinodulosa, sp. nov. (strain MAD 5-1a). a. Ample radiate aggregations, one with the early sorogen emerging (right), and many streams leaving behind traces. b. Early sorogens rising up from the side: a large stream follows the development of an early sorogen already surrounded by clumps that characterize the species (right). c. Late prostrate lower-sorophore sorogen, with cell supporters. d. Three habits: solitary unbranched sorocarp (left), loosely clustered sorocarps, one is branched along with a helicoidal rising late sorogen; a tight clustered group of sorocarps and sorogens of different habits (right). The three habits surrounded by clumps or defined masses of steady pseudoplasmodia close to the bases. e. Bases. Below: a round base with its rugged sheath surrounded by the clumps. Center and above: two sketches show cellular union of two clavate bases (center) and the dense globular slime that covers a base (above). f. A curved, 1-celled tip (left) and a flexuous compound tip (right). g. Elliptical-oblong spores with consolidated polar-subpolar, and some unconsolidated, granules. h. Microcysts. i. A myxamoebae with a large vacuole (above) and a group of roundish myxamoebae. Bars: a, b, c = 300 µm; d = 0.5 mm; e = 25 µm; f = 10 µm; g, h, i = 6 μm
MycoBank MB836160
Typification: MADAGASCAR. Near Ambohimahasoa, 21.15°S, 47.25°E, elev 914 m, type isolate MAD 5-1A, Dicty Stock Center strain DBS0350767. GenBank: rDNA SSU = MN338955.
Etymology: The species name refers to the clumps of small pseudoplasmodia very close to the bases that give the appearance of plant nodes emerging to the surface (from Latin, basis = base; nodulum = plant node).
Sorocarps mostly solitary but some occurring in loose clusters that tend to become isolated, tight clusters when the culture ages, prone, decumbent to erect, mostly unbranched but sometimes 1–3-branched, slightly phototropic, golden yellow to faintly pigmented but fading with exposure to light, mostly 0.7–3 mm in length (commonly 1.5–2.7 mm), with a stoloniferous habit. Sorophores delicate, yellow, tapered, consisting mostly of one tier of cells except at the bases and tips, many times sigmoid to helicoidal, when branched the branches are of variable lengths (100–200 and 400–500 µm), a few branches (1–3) located in the upper portion of the sorophore when solitary (when sorophores occur in a dense cluster, the branches do not follow any pattern), delicate. Tip mostly compound capitate, progressively obtuse (10–30 µm diam), sometimes simple and consisting of one curved cell (when young), with individual myxamoebae and very small masses of pseudoplasmodia rising up the sorophores (some will eventually produce branches), with an abundant, dense, granulated slime matrix present on the sorophore and base, covered by a sheath, lower sorophores sometimes prostrate, with one to two lines of cellular small supporters sometimes present. Base mostly round (20–60 µm diam), covered by an abundant, granular, and very dense globoid matrix of slime, hyaline to yellowish, often with a well-developed apron-like sheath, then the base becomes very prominent, base typically surrounded by 4–12 median prominent clumps or small masses of nonmotile pseudoplasmodia and dense, mucilaginous matrix, irregular in shape and distribution (10–50 µm diam). Sori globose to globoid, yellow, always variable in diameter in a single culture (80–250 µm). Spores elliptical-oblong, regular, median to large, with prominent consolidated polar granules (PG+), not consistently polar, sometimes dispersed near the poles, with many small vacuoles, 4.5–6.5(–8.0) × 2.1–3.5 µm (mostly 6.0–7.5 × 2.5–3.0 µm), germinating immediately, even from the sori. Myxamoebae mostly active, 3.6–7.4 × 6.5–12 µm, with a dense granular cytoplasm, yellow, many come together and encyst (cysts 4.9–8.7 µm diam), many myxamoebae remain nonaggregated and dispersed. Microcysts often interspersed with amoebae around the edge of the colony. Aggregations mostly radiate, yellow to pale yellow, variable in size, small to median ones (200–600 µm) with well-defined ample streams that become condensed at the center of the aggregation and are more or less constant in section and with few ramifications. Larger aggregations commonly irregularly radiate at the edges, becoming massive, often with no disruption of flattened streams (0.8–2.0 mm, exceptionally 4 mm diam), stream terminus elevated, well defined, streams anastomosing massively at centers, aggregations developing quickly from relevant streams and traces except for the incoming masses that will surround the mature base, individual myxamoebae as well as small masses of pseudoplasmodia will continue creeping up the sorophores (mostly when the latter are clustered), commonly one to two early sorogens arise from the side (but sometimes the center) of each massive pseudoplasmodium, early sorogens (50–100 µm), early to late sorogens are variable and active, becoming very elongated, fusiform, sometimes with a thin terminus, 300–800 µm or more in length, many times helicoidal, sigmoid and 1–4 times stoloniferous, photodirected when settled.
Distinguishing features: This species will grow at temperatures from 20 to 27 C but shows optimal growth between 20 and 23 C. Loosely clustered sorocarps become isolated and tightly clustered, with the number of sorocarps developing continuously as the culture ages, while myxamoebae (many of which come together) remain on the substratum, where some will slowly approach mature isolated sorocarps and/or clusters while others will remain stationary. Characteristic clumps of stationary irregular masses of slime and cells are almost always surrounded by either individual sorocarps or clusters. Curved sorocaps that fall on the agar are observed mostly in old clusters. The combination of features that differentiate this species from C. aureostipes (formerly Dictyostelium aureo-stipes Cavender, Raper & Norberg and its variant D. aureostipes var. helvetium Cavender, Raper & Norberg), C. canoespora (isolate MAD 14-3C), and other yellow dictyostelid-type species are the production of prominent clumps around the base, sorocarps often clustered, a less intense yellow pigment, generally more regular spores with polar-subpolar consolidated granules, a much reduced production of branches, with both large massive aggregations and well-defined small to medium radiate ones, mostly with no disrupted streams, and the formation of isolated dense clusters when cultures age along with continuous developing sorogens. Pseudoplasmodia are golden yellow in fresh original cultures, as is also the case in Ha. lavandula (formerly D. lavandulum Raper & Fennell).
Habitat: Found in primary tropical moist forest near Ambohimahasoa, and just outside of Ranomafana National Park on the eastern side of central Madagascar.
Cavenderia canoespora Cavender, J.C. Landolt, A. Perrigo, Vadell, Pu Liu & S.L. Stephenson, sp. nov. ,
Figure 5. Morphology of Cavenderia canoeospora, sp. nov. (strain MAD 14-3 c). a. Median aggregation with ample deltoid streams closer to the irregular central mound, which terminates in thin, dendroid open ends. b. Early rising sorogen (left); a cluster of two late sorogens (center); a solitary late sorogen (right). c. Stoloniferous habit of a late migrating pseudoplasmodium (above); solitary prone sorocarp with prostrate lower portion and with supporter cells (below). d. Three habits of mature sorocarps: unbranched solitary (left); solitary sorocarp with few branches (center); clustered sorocarps (right). e. Bases: few-celled, irregular base covered with a resistant sheath and many roundish cells (above); a one tier of cell sorocarp that ends in a roundish irregular base with some protruding cells. The sheath is wrinkled and gives the appearance of being immersed in the substratum (below). f. Simple (left) and compound (right) tips, both of them flexuous. g. Large canoe-shaped spores, with large regular consolidated polar granules. Their size and shape are similar to the shape of canoes, for which the species is named. h. Myxamoebae with at least one large vacuole (above); a circularized myxamoebae (below). Bars: a, b = 300 µm; c, d = 0.6 mm; e = 20 µm; f = 10 µm; g, h = 8 µm
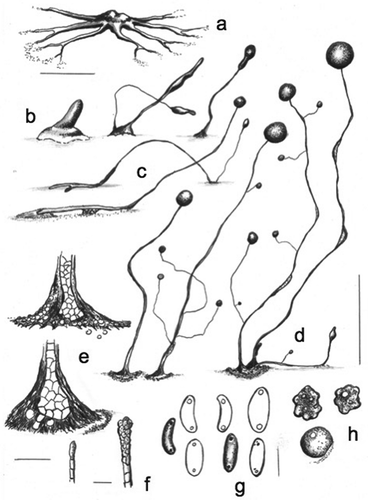
MycoBank MB836161
Typification: MADAGASCAR. Ambalavao, in southern-central Madagascar, 22.07°S, 46.53°E, elev 1632 m, type isolate MAD 14-3C, Dicty Stock Center strain DBS0350766. GenBank: rDNA SSU = MN338956.
Etymology: The species name refers to the appearance of the large elliptical to reniform spores that are similar to the shape of canoes: a light, narrow boat used by the aboriginal Caribbean people. Initially from Arawak, Carib, and other native American languages, then from Spanish, canoa; Latin, spora = spore and/or sporae (pl) = spores.
Sorocarps solitary to clustered, prone, decumbent, unbranched to branched, phototropic, intense yellow to faintly pigmented but fading with light, mostly 0.8–4 mm in length but occasionally longer (commonly 2–3 mm). Sorophores delicate, yellow, sections irregular in diameter, mostly consisting of one tier of cells except at the bases and in some sections, with constrictions, sometimes sigmoid to tortuous, changing direction abruptly, often with a few branches (when clustered: 4–6, rarely more), the basal ones larger (200–700 µm), those on the upper sorophore smaller and very delicate (50–150 µm); sorophores irregularly tapered from base to the simple, sometimes capitate tips (5–10 µm), although flexuous, with masses of pseudoplasmodia rising up the sorophore, or nonmotile (some produce branches), with abundant, dense, granulated yellow slime matrix at the angles, covered by a strong sheath; lower sorophore, when prostrate, has one to two lines of small cellular supporters. Base mostly clavate, sometimes acuminate or roundish, from 15 to 35 µm diam and with an apron of slime when aged (55 µm), the latter producing a concavity on the substratum with a surrounding halo, sometimes with adhering cells forming a disk. Base covered by an abundant, dark, granular, and very dense yellow matrix of slime, covered by a strong and rugged apron-like sheath. Sori globose, white to intensely yellow, sometimes citrine-yellow, 70–200 µm, lateral sori variable (20–90 µm diam). Spores elliptical-oblong and reniform, mostly large, with prominent consolidated polar granules (PG+), sometimes dispersed near the poles, with many vacuoles, not regular in shape, 7.5–11.0 × 2.5–4.0 µm (mostly 8.0–9.5 × 3.0 µm), most spores germinating immediately. Myxamoebae large, slow, 6.5–10.0 × 15.0 µm, with a large vacuole, yellow, many soon rounding up and producing cysts (8–10 µm) settling around beside the base, while others remain nonaggregated. Aggregations yellow to pale yellow, variable, small ones (100–200 µm) with few discrete, ample deltoid streams (sea-star-like in shape), larger ones irregularly radiate, becoming massive and similar to those of C. aureostipes by subdividing into secondary centers (500–1500 µm diam), streams soon anastomosing, they are plane and peripheral ones are short and may adopt a fiord-like shape, early-late sorogens 300–600 µm in length, late sorogens larger and developing rapidly into a mature sorocarp, sometimes with a stoloniferous habit.
Distinguishing features: The optimal temperature for growth is 20–23 C, whereas the range for germination and development varies between 18 and 24 C. The species becomes strongly citrine (old sorophores), and the sori become yellow to pale yellow with age. Under certain conditions, the pigment is more intense than that of the pseudoplasmodia of C. basinodulosa or the sorophores of C. aureostipes. The slime matrix is also yellow, and the pigment appears to be synthesized by the myxamoebae. Massive flat aggregations, some edges with fine streams are sometimes anastomosed or somewhat globoid or may adopt a “fiord-like” shape. Small aggregations are “sea-star”-shaped. Large curved sorocarps that fall on the agar and refruit (stoloniferous habit) may rebranch. The features that differentiate this species from C. aureostipes and its variant C. aureostipes var. helvetium are its large spores, a much lower number of branches that do not follow strictly the “aureostipes” pattern, a more intense yellow citrine pigment that becomes stronger with age, and the aggregations have variants not observed in other related yellow-pigmented species. Bases develop an apron of slime with age and are conspicuously attached to the substratum, forming a crateriform concavity on the latter. The sheath is rugged and opaque, sometimes forming a disk with cellular components. These basal cells are very adherent. The production of the yellow granulated, mucilaginous matrix is important for diagnosis.
Habitat: Found in fallow grassland overgrown with Asteraceae shrubs near Ambalavao, in southern-central Madagascar.
Raperostelium stabile Cavender, J.C. Landolt, A. Perrigo, Vadell, Pu Liu & S.L. Stephenson, sp. nov. ,
Figure 6. Morphology of Raperostelium stabile, sp. nov. (strain M12A). a. Small, irregularly radiate aggregations with dendroid streams; one (or few) large stream connects each aggregation. Many small masses of pseudoplasmodia and myxamoebae remain on the substratum. b. Early sorogens rising up apart from each other: a large stream contacts another small aggregation (right). c. Late sorogens that keep the pseudoplasmodia bridge, and myxamoebae dispersed at the bases. d. Habits: tightly coremiform clustered lower sorocarps (left); four mature sorocarps (right). e. Two loosely clustered sorocarps (left); and one solitary decumbent sorocarp with a bridge to another aggregation (right). Sori are disproportionally large and of different sizes; lower sorophores in a tight coremiform cluster. f. Top: curved, clavate 3-celled base within a hyaline matrix of slime, surrounded by roundish cells. Below: four bases: 1-celled (left above), clavate 4-celled (left below), and two roundish bases (above and below right). g. Simple-celled tips: 1-celled (left), two pilliform tips (center), flexuous (right). h. Elliptical spores with polar-subpolar granules. Granules are consolidated of different shapes and sizes as well as unconsolidated dispersed. i. Free migrating globular myxamoebae with many black particles (above); joined myxamoebae (below). Bars: a, b, c = 100 µm; d, e = 0.7 mm (d left = out of scale); f, g = 10 µm; h = 5 µm; i = 7 µm
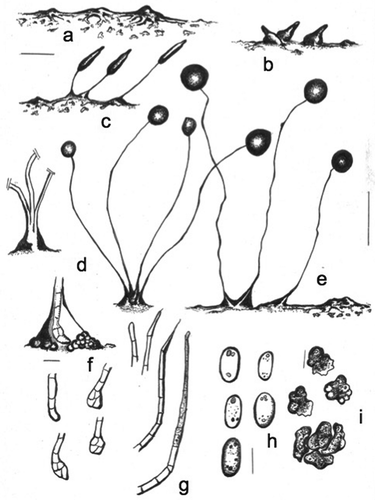
MycoBank MB836162
Typification: MADAGASCAR. Near Ambohimahasoa, 21.15°S, 47.25°E, elev 922 m, type isolate M12A, Dicty Stock Center strain DBS0350763. GenBank: rDNA SSU = MN338957.
Etymology: The species name refers to the capability and/or adaptation of the mature sorocarps to remain stable without major changes, when spores are cultured in a standard way, for longer periods of time, even under drastic changes of temperature (within the range 15–30 C). From Latin, stabilis or stabile = stable; constant, permanent; syn. in Latin = firmum.
Sorocarps prone to erect, solitary to clustered, small, 0.35–1.0 mm, unbranched, not phototropic. Sorophore consisting of one tier of cells except at the base, delicate, straight to somewhat curved to sigmoid, sometimes solitary, tightly clusteres to coremiform at base. Tip simple, 1-celled, often terminated in a piliform end, sometimes flexuous (1–4 µm). Base round to clavate, irregular, 1–4-celled (5–20 µm diam), immersed in a dense hyaline slime matrix and sometimes covered in part by roundish cells, sheath hyaline. Sori globose to globoid, large, pale white, 50–110 µm diam, not uniform in diameter in the same culture, commonly 70–100 µm diam. Spores elliptical-oblong, regular, 2.3–3.8 × 5.0–7.0 µm, with consolidated polar granules, occasionally granules are unconsolidated and subpolar, with small vacuoles visible as points, germinating immediately. Myxamoebae small, roundish, slow at first, with globoid protrusions, ranging from 3.0 to 8.5 µm diam, soon gathering together. Sorogens rising up from the center of each mound before the completion of the whole aggregation, when clustered, each cluster commonly producing 2–4 synchronous sorogens that develop apart from one central point. Aggregations irregularly radiate at first, 150–500 µm diam, streams not well defined and becoming intermixed with the closest aggregations, aggregation centers become irregular mounds, many larger aggregations keep connected with each other and produce elevated, mound-like pseudoplasmodia with one or few short streams, after full maturation, some minute masses of pseudoplasmodia and myxamoebae remain on the substratum, early and late sorogens are uniform, fusiform in shape and soon elongate, and immediately form a mature sori.
Distinguishing features: This species has a wide temperature tolerance, fruiting when grown at temperatures ranging from 15 to 30 C, but the optimal temperature for growth is 27–29 C. Culture remains alive for 3–4 wk without major changes, and sori remain hydrated and do not collapse for a relatively long period of time, then they slowly dry out. This species has affinities with other small species in the genus Raperostelium (formerly “Group 3 dictyostelids” sensu Romeralo et al. Citation2011) described from Central America by Cavender et al. (Citation2013). It has radiate streaming aggregations as well as mound-like aggregations.
Habitat: Found in primary tropical moist forest near Ambohimahasoa, and just outside of Ranomafana National Park on the eastern side of central Madagascar.
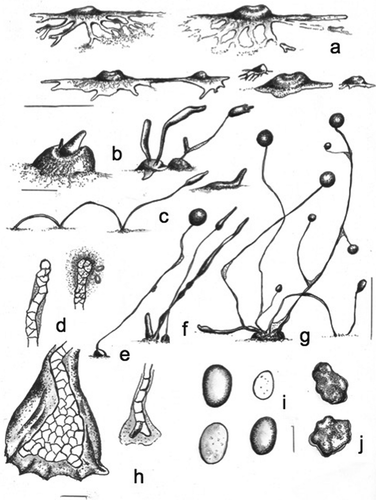
Heterostelium radiatum Cavender, J.C. Landolt, A. Perrigo, Vadell, Pu Liu & S.L. Stephenson, sp. nov. ,
Figure 7. Morphology of Heterostelium radiatum, sp. nov. (strain M26B). a. Radiate aggregations with incoming streams that follow previous streams. b. A series of early rising sorogens: solitary from the center (left), clustered (many times very tight) (center), and four early-late sorogens with bulbous basal slime (right). c. Stoloniferous migrative habit of a late sorogen (above); two free migrating pseudoplasmodia, one of them globose (below). d. Solitary late sorogen with pseudoplasmodia masses rising up (left); two coremiform late enlarged sorogens (center); a decumbent sorogen (right). e. Branched solitary mature sorocarp (left); solitary unbranched sorocarp (center); clustered sorocarps in a coremiform habit (right). f. Clavate base with protruding cells (above); roundish base with some enlarged protruding cells (below); the slime matrix is dense and the sheath pronate. g. Two one-terminal-cell tips (above); two simple tips united by slimy filaments (when sori are tangled) (below). h. Different spores mostly broadly elliptical to oblong, some misshapen and short. Spores display large consolidated polar-subpolar granules, some others have unconsolidated granules and many vacuoles. i. A typical microcyst (above); an encysted large myxamoebae (below). j. Small myxamoebae (above), and a larger one (below). Bars: a, b = 300 µm; c = 50 µm; d, e = 1 mm; f = 20; g = 10 µm; h = 6 µm; i = 5; j = 7 µm
MycoBank MB836163
Typification: MADAGASCAR. Near the Menarandra River in southern Madagascar, 24.15°S, 45.39°E, elev 509 m, type isolate M26B, Dicty Stock Center strain DBS0350765. GenBank: rDNA SSU = MN338953.
Etymology: The species name refers to the appearance of the regular radiate aggregation. From Latin, rad = ray, hence radiate = surrounded or bordered with rays or the like (e.g., radiata corona = radiate crown).
Sorocarps solitary to clustered, then coremiform when tall and aged, unbranched to branched, irregular, eventually may occasionally form one to rarely more small, irregular whorls (pseudowhorls) distributed irregularly, migrating, then becoming prostrate, prone to decumbent and yellow when aged, commonly 1–3 mm long but may become elongated up to 5 mm, very active with a resumption of early structures. Sorophores irregular, ranging from 1 tier of cells to 2–3, curved, sigmoid, sometimes cells discontinuous, with diameters from 10 to 30 µm, terminal segment delicate, consisting of one tier of cells. Tip generally consisting of a single short, ample cell with filaments and a dense matrix of slime attaching spores. Base round to clavate, generally with large cell protruding supporters, very irregular, 20–30 µm diam and covered with a dense, granulated apron-like sheath, sheath and slime matrix also in parts of the sorophores and joining sorophores and branches, clustered bases may share common cellular structures, when branched, branches are short (100 µm) to medium (300 µm) with or without a whorled formation and may soon resume or be reabsorbed, sorogens may rise up from spherical pseudoplasmodial clumps coming from late aggregations or from the tip of the pseudoplasmodial mass. Sori globose white (80–150 µm) at first, becoming large and yellow with age (300 µm), lateral sori small (20–30 µm), sori many times tangled. Spores elliptical-oblong, broad (mean: 6.8 × 4.0 µm), commonly 7.3 × 4.0 µm (range 6–8 × 3.5–5 µm), regular, with many vacuoles and dispersed granules, some consolidated, mostly at poles, spores germinating immediately. Microcysts varying in diameter from 4 to 6 µm. Myxamoebae generally small, globoid with protruding pseudopodia (12–7 × 6–4 µm), hyaline, with many blackish particles and small vacuoles, slow. Some larger myxamoebae (25–20 × 10 µm) present as uropods with particles, with a large vacuole, early sorogens may migrate extensively, forming a sphere at the terminus or migrating freely, late sorogens also migrate extensively or soon rise up, often with a globoid tip and sorophore formation proceeds with continuous growth. Aggregations with radial irregular streams that anastomose, becoming massive (1200 µm or more), streams flat at first, uniform in section, ending in a spatula-shaped globoid flat terminus, varying in extension, small aggregations (100 µm) reunite and from each arises a centrally located single sorogen, large aggregations produce clustered sorogens from the center, development may begin at 15 C but the optimum is 20–23 C, fruiting form up to 27 C. This species has a polysphondylium-type aggregation, still the formation of whorled branched depends on environmental conditions and time of cultivation.
Habitat: Found in very open, tropical dry forest near the Menarandra River in southern Madagascar.
Heterostelium versatile Cavender, J.C. Landolt, A. Perrigo, Vadell, Pu Liu & S.L. Stephenson, sp. nov.,
Figure 8. Morphology of Heterostelium versatile, sp. nov (strain MAD 52). a. Small to median aggregations that show ample flat and large streams (right above), two interconnected aggregations (center below), and two small aggregations at culmination surrounding a larger one (left below), most with the appearance of a fried egg. b. Early pseudoplasmodia from the center and side (left); early-late clustered and solitary sorogens (right). c. Stoloniferous habit of a late migrating pseudoplasmodium (left); solitary small shortly migrating pseudoplasmodium (right above). d. Simple single-celled tip (left) and a capitate tip with a dense sticky slime (right). e. Solitary sorocarp with small masses of pseudoplasmodia. f. Cluster of late rising sorogens. g. Tight cluster of branched and unbranched sorocarps, some decumbent and creeping. h. Massive roundish base, with some protruding cells (left); one tier of cell terminal sorophore with two bifurcate basal cells (right). i. Elliptical-oblong spores, some with many small vacuoles. j. Myxamoebae with many small vacuoles. Bars: a = 300 µm; b, c = 50 µm; d, h = 15 µm; e, f, g = 0.5 mm; i, j = 6 µm
MycoBank MB836164
Typification: MADAGASCAR. Near Ambohimahasoa, 21.17°S, 47.19°E, elev 1222 m, type isolate MAD 52, Dicty Stock Center strain DBS0351279. GenBank: rDNA SSU = MN338954;
Etymology: The species name refers to the highly variable morphology observed during the developmental stages as well as during the life span of mature sorocarps, under standard conditions of cultivation. From Latin, versatile (or versatilis) = of unpredictable, character, unstable (e.g., meaning: ingenio varius; syn.: levis, inconstants). By extension: “versatile, adj. = able to change easily from one activity to another”).
Sorocarps erect to prone, decumbent to prostrate, generally sinuose, solitary to clustered, unbranched to branched, curved near the base, 0.7–2.5 mm, exceptionally reaching 4.5 mm in height, not phototropic, sometimes coremiform, 1–4 times stoloniferous in habit under moist conditions, when cultivated with E. coli at 23–24 C on non-nutrient agar. Sorophores irregular, varying from one tier of cells to more than one in the same specimen, with small (100–150 µm) secondary branches when aged, terminal segment consisting of one tier of cells, delicate. Tip simple to compound, not uniform, 5–15 µm wide, or flexuose. Base inconspicuous, generally clavate to round, 2–4-celled, many times diverging, 10–25 µm diam or larger and with protruding cells, reaching 30–40 µm, covered by a dense granular covering of slime and with an apron-like sheath. Aggregations irregularly radiate, 500–1200 µm diam, when smaller (100–200 µm) having a few ample streams, many times with one stream connecting closer aggregations, then with each becoming a mound (“fried-egg-shaped” aggregation) surrounded by irregular flat, sometimes nodulose continuous or disrupted streams, late aggregations irregular in shape and dimensions, with condensed central mounds having a wrinkled surface, then producing a single conical uniform early sorogen that comes from a crateriform depression (50–100 µm), afterward irregular, small pseudoplasmodial masses rising up and becoming enlarged (250–800 µm in length). Sorocarps clustered with the appearance of closer secondary smaller sorogens (2–6) and/or when smaller late egg-shaped mounds condense together, early streams frequently disrupted. Sori globose to globoid, white-hyaline, white to cream, 50–170 µm diam, mostly 70–130 µm, with a dense covering of granulated slime that firmly attaches the spores. Spores elliptical-oval, without polar granules (PG−), 3.9–5.9 × 6.3–8.7 µm (mostly 6 × 3 µm), strongly adherent. Myxamoebae slow, mostly globoid, 6.5–12.0 × 4.3–8.0 µm, optimal growth observed at 20–23 C, although the species is sensitive to temperature, it may germinate and grow over a range of 15–24 C.
Distinguishing features: Larger aggregations are generally surrounded by smaller ones at the culmination stage, the latter sometimes with short flat, anastomosed irregular and inconspicuous streams. Streams in a single aggregation may differ in length (200–600 µm) and morphology, often terminating as spatula-shaped projections. In smaller aggregations, sorogens rise up immediately. Some early sorogens bend over and soon collapse. Early and late sorogens may migrate shortly or keep creeping on the agar surface. Late sorogens very thin and versatile: they generally form a crateriform depression from which a conical mass rises up as in the earlier rugged crateriform mound. Generally, myxamoebae do not leave a streak on the bacterial surface of the agar. Smaller oblong spores may show large dispersed vacuoles as well as some granulations and are difficult to separate from the covering slime matrix. The granulated slime-sheath also occurs at the bases of branches (when they are formed) and/or join sorophores. The stoloniferous (creeping, refruiting) behavior recalls that of Dictyostelium implicatum, where it is more substantial.
Habitat: Found in montane tropical wet forest in a steep river valley near Ambohimahasoa, and just outside of Ranomafana National Park on the eastern side of central Madagascar.
DISCUSSION
These five new species, together with the 13 polysphondylium-type dictyostelids described by Cavender et al. (Citation2016), bring the total number of species described from Madagascar to 18. This represents nearly 10% of the described dictyostelid diversity, although it is probably a much lower proportion of the actual worldwide diversity, as sampling efforts are not globally consistent. The ongoing rate of new species discovery, in Madagascar and globally, supports earlier estimates that only a small fraction of earth’s protist diversity is known (Mora et al. Citation2011).
Cavenderia basinodulosa and C. canoespora are most closely related to one another, but no sister taxon to these two species is strongly supported based on the rDNA SSU phylogeny (). The two species share some morphological characteristics, including yellow sorocarp pigmentation, thin and delicate sorophores with few branches, spores with large consolidated polar-subpolar granules, a large variation in sorus diameter, and the formation of tight isolated clusters in old cultures (). However, they are still morphologically distinct. They differ mainly in spore size (larger in C. canoespora), intensity of color pigmentation (stronger in C. canoespora), and the sea-star aggregation of C. basinodulosa that is distinguishable from the radiated aggregation with sheath-slime traces of its club-shaped-ended streams. C. basinodulosa produces small clumps of nonmotile pseudoplasmodia surrounding the base of the sorophore and minute ascending masses of pseudoplasmodia, whereas those of C. canoespora are larger and without basal clumps. C. canoespora has larger, canoe-shaped spores, a heavy, rugged sheath, and a dense granulose slime at the base. Its aggregation is similar in shape to a sea star, and its sorocarps are slightly taller. Neither of these species strictly follows the aureostipe-type pattern of branching, and both have a dissimilar distribution of microcyst and generally develop as tight clusters.
The morphological and molecular differences between C. basinodulosa and C. canoespora compared with other Cavenderia species suggest that these two species may have been isolated from other closely related species for some time. The two new species are sister taxa, but they are morphologically different from one another. This is evident in the spores, which are larger (8.0–9.5 × 3.0 µm) and often reniform in C. canoespora, whereas they are elliptical to oblong and not as narrow (6.0–7.5 × 2.5–3.0 µm) in C. basinodulosa. C. canoespora also has a much smaller base, usually about 15–35 µm at its widest point, whereas the bases of C. basinodulosa are generally 20–60 µm at the widest point (, ). There is no clear support for the next closest taxon to C. basinodulosa and C. canoespora based on the molecular phylogeny (). This lack of sister-taxon support may be due to long-branch attraction and could further represent a sampling artifact due to the relatively low number of taxa known from southern African and other tropical regions where closely related taxa could be expected to occur.
Raperostelium stabile is a minute species distinguishable for its endurance in cultivation and tolerance to a large temperature span (, ). The rDNA SSU phylogeny suggests that Raperostelium stabile is most closely related to R. monochasioides and R. austral and together with these two species has a sister-group relationship to R. reciprocatum (). These three species are described from different localities around the world: R. monochasioides was described from the tropical Australasian island of New Guinea (Hagiwara Citation1973) and has since been found in multiple localities, including Japan, Mexico, and Ukraine (Cavender et al. Citation2012; Liu and Li Citation2010). R. australe was described from the far-south latitudes of New Zealand (Cavender et al. Citation2002), whereas R. reciprocatum was first recovered in Central America (Cavender et al. Citation2013). All four of these species are relatively small, usually with elliptical spores and prominent polar granules (although R. australe is a partial exception, as it often displays polar granules, but also alongside subpolar, consolidated or irregular granules). They all from mound-shaped aggregations, with varying levels of subdivision along the streams. However, R. stabile is the smallest among these four species, with sorphores rarely taller than 1.0 mm, whereas sorophores regularly reach 3.0, 1.7, and 3.5 mm in R. monochasioides, R. australe, and R. reciprocatum, respectively. R. stabile does not form lateral branches, a common feature in both R. monochasioides and R. australe.
The two new species of Heterostelium—H. radiatum and H. versatile—are molecularly distinct from one another, as well as from the 12 other Heterostelium taxa from Madagascar that have been sequenced (Cavender et al. Citation2016; ). The 14 species of Heterostelium that were first described from Madagascar are marked with arrows in . This suggests that members of this genus may have morphological, physiological, or other characteristics that allow them to survive and potentially diversify on the island. The species of Heterostelium from Madagascar do not form a monophyletic group, indicating that these species or their ancestors dispersed to and/or from the island many times. This is feasible, as dictyostelids are known to be transported by fruit flies, invertebrates, ground vertebrates, migratory songbirds, and even humans (Suthers Citation1985; Huss Citation1989; Stephenson and Landolt Citation1992; Perrigo et al. Citation2012; Smith et al. Citation2014). They have also been shown to be dispersed in fresh water over short distances (O’Dell Citation1979). However, there is presently no evidence that we are aware of for wind dispersal in dictyostelids or long-term persistence in salt water conditions.
Heterostelium radiatum is closely related to H. violaceotypum, with a weakly supported sister-taxon relationship, but is nonetheless morphologically distinct. Most noticeably, H. violaceotypum has many whorls, commonly 3-4 sets per sorophore but often more, whereas H. radiatum rarely has more than a single whorl per sorophore, although it produces ample nonwhorled lateral branches. In early stages, H. violaceotypum produces many tightly clustered pseudoplasmodia that later separate, a habit not observed in H. radiatum.
H. versatile is weakly supported as the sister taxon to a clade including three Australia species: H. granulosum, H. rotatum, and H. flexuosum (; Landolt et al. Citation2008). All four of these species are relatively small, with sorophores ca. 0.4–2.5 mm in height. They all have conspicuous slime sheaths covering the base as well, often with support cells around the base. However, H. versatile can be differentiated from the other three species by the strongly stoloniferous habit, as well as the larger spore, 6.3–8.7 × 3.9–5.9 µm, compared with an upper range of 5–7 × 4 µm in the other three species.
Efforts to describe the diversity and richness of dictyostelids in natural habitats must continue in order to collect sufficient data to understand protist distributions and patterns. The description of these five species, along with the other 13 species previously described from Madagascar, suggests that the island is home to a unique and diverse assemblage of dictyostelids and that biodiversity hot spots may be key localities for dictyostelid diversity and diversification, as they are for plants and animals.
Supplemental Material
Download Zip (14.7 KB)ACKNOWLEDGMENTS
Field work in Madagascar in 2009 was possible with the assistance provided by the staff at the Madagascar Institut pour la Conservation des Ecosystemes Tropicaux (MICET).
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
LITERATURE CITED
- Adl SM, Bass D, Lane CE, Lukeš J, Schoch CL, Smirnov A, Agatha S, Berney C, Brown MW, Burki F, Cárdenas P, Čepička I, Chistyakova L, del Campo J, Dunthorn M, Edvardsen B, Eglit Y, Guillou L, Hampl V, Heiss AA, Hoppenrath M, James TY, Karnkowska A, Karpov S, Kim E, Kolisko M, Kudryavtsev A, Lahr DJG, Lara E, Le Gall L, Lynn DH, Mann DG, Massana R, Mitchell EAD, Morrow C, Park JS, Pawlowski JW, Powell MJ, Richter DJ, Rueckert S, Shadwick L, Shimano S, Spiegel FW, Torruella G, Youssef N, Zlatogursky V, Zhang Q. 2019. Revisions to the classification, nomenclature, and diversity of eukaryotes. Journal of Eukaryotic Microbiology 66:4–119.
- Adl SM, Simpson AGB, Lane CE, Lukeš J, Bass D, Bowser S S, Brown MW, Burki F, Dunthorn M, Hampl V, Heiss A, Hoppenrath M, Lara E, le Gall L, Lynn DH, McManus H, Mitchell EAD, Mozley-Stanridge SE, Parfrey LW, Pawlowski J, Rueckert S, Shadwick L, Schoch CL, Smirnov A, Spiegel FW. 2012. The revised classification of eukaryotes. Journal of Eukaryotic Microbiology 59:429–514.
- Basu S, Fey P, Pandit Y, Dodson R, Kibbe WA, Chisholm RL. 2012. dictyBase 2013: integrating multiple Dictyostelid species. Nucleic Acids Research 41:D676–D683.
- Cavender J, Landolt JC, Suthers HB, Stephenson SL. 2012. Dictyostelid cellular slime molds of Mexico. Mycosphere 3:336–351.
- Cavender JC. 1978. Cellular slime molds in tundra and forest soils of Alaska including a new species, Dictyostelium septentrionalis. Canadian Journal of Botany 56:1326–1332.
- Cavender JC, Landolt JC, Romeralo M, Perrigo A, Vadell EM, Stephenson SL. 2016. New species of Polysphondylium from Madagascar. Mycologia 108:80–109.
- Cavender JC, Stephenson SL, Landolt JC, Vadell EM. 2002. Dictyostelid cellular slime moulds in the forests of New Zealand. New Zealand Journal of Botany 40:235–264.
- Cavender JC, Vadell EM, Landolt JC, Winsett KE, Stephenson SL, Rollins AW, Romeralo M. 2013. New small dictyostelids from seasonal rainforests of Central America. Mycologia 105:610–635.
- Fiore-Donno AM, Nikolaev SI, Nelson M, Pawlowski J, Cavalier-Smith T, Baldauf SL. 2010. Deep phylogeny and evolution of slime moulds (Mycetozoa). Protist 161:55–70.
- Foissner W. 2009. Protist diversity and distribution: some basic considerations. In: Foissner W, Hawksworth DL. eds. Protist diversity and geographical distribution. Dordrecht, The Netherlands: Springer. p. 1–8.
- Goodman SM, Benstead JP. 2005. Updated estimates of biotic diversity and endemism for Madagascar. Oryx 39:73–77.
- Hagiwara H. 1973. Enumeration of the Dictyosteliaceae. Mycological reports from New Guinea and the Solomon Islands. Bulletin of the National Science Museum Tokyo 16:493–497.
- Huss MJ. 1989. Dispersal of cellular slime molds by two soil invertebrates. Mycologia 81:677–682.
- Kalla SE, Queller DC, Lasagni A, Strassmann JE. 2011. Kin discrimination and possible cryptic species in the social amoeba Polysphondylium violaceum. BMC Evolutionary Biology 11:31.
- Landolt JC, Cavender JC, Stephenson SL, Vadell EM. 2008. New species of dictyostelid cellular slime moulds from Australia. Australian Systematic Botany 21:50–66.
- Larsson A. 2014. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30:3276–3278.
- Liu P, Li Y. 2010. Dictyostelids from Ukraine 1: two new records of Dictyostelium. Mycotaxon 111:275–278.
- Liu P, Zou Y, Li W, Li Y, Li X, Che S, Stephenson SL. 2019. Dictyostelid cellular slime molds from Christmas Island, Indian Ocean. mSphere 4:e00133–19.
- Medlin L, Elwood HJ, Stickel S, Sogin ML. 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71:491–499.
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, 14 Nov. 2010. pp 1–8.
- Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. 2011. How many species are there on earth and in the ocean? PLoS Biology, 9:e1001127.
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403:853–858.
- O’Dell W. 1979. Isolation, enumeration and identification of amebae from a Nebraska lake. Journal of Protozoology 26:265–269.
- Perrigo AL. 2013. Diversity underfoot: systematics and biogeography of the dictyostelid social amoebae [PhD dissertation]. Uppsala, Sweden: Uppsala University. 56 p.
- Perrigo AL, Baldauf SL, Romeralo M. 2013. Diversity of dictyostelid social amoebae in high latitude habitats of Northern Sweden. Fungal Diversity 58:185–198.
- Perrigo AL, Romeralo M, Baldauf SL. 2012. What’s on your boots: an investigation into the role we play in protist dispersal. Journal of Biogeography 39:998–1003.
- Raper KB. 1984. The dictyostelids. Princeton, New Jersey: Princeton University Press. p. 453.
- Romeralo M, Cavender JC, Landolt JC, Stephenson SL, Baldauf SL. 2011. An expanded phylogeny of social amoebas (Dictyostelia) shows increasing diversity and new morphological patterns. BMC Evolutionary Biology 11:84.
- Romeralo M, Escalante R, Baldauf SL 2012. Evolution and diversity of dictyostelid social amoebae. Protist 163:327–343.
- Romeralo M, Fiz-Palacios O, Lado C, Cavender JC. 2007. A new concept for Dictyostelium sphaerocephalum based on morphology and phylogenetic analysis of nuclear ribosomal internal transcribed spacer region sequences. Canadian Journal of Botany 85:104–110.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542.
- Schaap P, Winckler T, Nelson M, Alvarez-Curto E, Elgie B, Hagiwara H, Cavender J, Milano-Curto A, Rozen DE, Dingermann T, Mutzel R, Baldauf SL. 2006. Molecular phylogeny and evolution of morphology in the social amoebas. Science 314:661–663.
- Schilde C, Lawal HM, Kin K, Shibano-Hayakawa I, Inouye K, Schaap P. 2019. A well supported multi gene phylogeny of 52 Dictyostelia. Molecular Phylogenetics and Evolution 134:66–73.
- Sheikh S, Thulin M, Cavender JC, Escalante R, Kawakami S, Lado C, Landolt JC, Nanjundiah V, Queller DC, Strassmann JE, Spiegel FW, Stephenson SL, Vadell EM, Baldauf SL. 2018. A new classification of the dictyostelids. Protist 169:1–28.
- Silvestro D, Michalak I. 2012. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution 12:335–337.
- Singh R, Schilde C, Schaap P. 2016. A core phylogeny of Dictyostelia inferred from genomes representative of the eight major and minor taxonomic divisions of the group. BMC Evolutionary Biology 16:251.
- Smith J, Queller DC, Strassmann JE. 2014. Fruiting bodies of the social amoeba Dictyostelium discoideum increase spore transport by Drosophila. BMC Evolutionary Biology 14:105.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313.
- Stephenson SL, Landolt JC. 1992. Vertebrates as vectors of cellular slime moulds in temperate forests. Mycological Research 96:670–672.
- Stephenson SL, Laursen GA, Landolt JC, Seppelt RD. 1998. Dictyostelium mucoroides from subantarctic Macquarie Island. Mycologia 90:368–371.
- Suthers HB. 1985. Ground-feeding migratory songbirds as cellular slime mold distribution vectors. Oecologia 65:526–530.
- Swanson AR, Vadell EM, Cavender JC. 1999. Global distribution of forest soil dictyostelids. Journal of Biogeography 26:133–148.
- Vadell E, Cavender JC, Landolt JC, Perrigo AL, Liu P, Stephenson SL. 2018. Five new species of dictyostelid social amoebae (Amoebozoa) from Thailand. BMC Evolutionary Biology 18:198.
- Wrigley de Basanta D, Lado C, Estrada-Torres A, Stephenson SL. 2013. Biodiversity studies of myxomycetes in Madagascar. Fungal Diversity 59:55–83.
