ABSTRACT
A new species of the genus Diachea (order Physarales, Myxomycetes, Amoebozoa) is described from Peru. Relevant details on spore germination, as well as morphological and phylogenetic data, are provided. At first glance, the new species shares some morphological similarities with both D. leucopodia, type of the genus, and D. koazei, but it strikingly differs from all other species of its genus by combining a short dark stalk, with a reticulate columella, and clustered spores. Moreover, it seems to be the only species of Diachea exclusively associated with Polylepis tropical forests at elevations above 3500 m. Apart from a comprehensive morphological study of 31 specimens, we here provide phylogenetic evidence to confirm the inclusion of this species in the genus Diachea. Specifically, our phylogenetic analyses of the nuclear 18S rDNA (18S), mitochondrial 17S rDNA (17S), and elongation factor-1 alpha (EF-1α) genes show that the new species is related to D. leucopodia and D. bulbillosa. The remarkably different morphological characters distinguishing the new Diachea from all other species of its genus, along with its particular ecological preferences and geographic distribution, indicate that it is a distinct entity deserving recognition as an independent species.
INTRODUCTION
Myxomycetes, also known as slime molds, is a monophyletic group of amoebozoans (Adl et al. Citation2019) characterized by producing spore-bearing fruiting bodies. More than 1000 species, organized in five orders and 64 genera, are currently recognized (Lado 2005–2022). They are found in virtually all terrestrial ecosystems and all biogeographic regions, including the Neotropics (Lado et al. Citation2018; Lado and Wrigley de Basanta Citation2008).
Several studies have been conducted in Peru, where up to 174 myxomycete species, associated with all types of decaying vegetable remains, have been cataloged (Treviño-Zevallos and Lado Citation2020b), including some recently described on the basis of morphological and molecular data (Citation2018; Wrigley de Basanta et al. Citation2015). Myxomycete species of Peru have been identified from the coastal deserts to the Amazonian rain forest, and from the Andes Mountains (Lado et al. Citation2016, Citation2019; Rojas et al. Citation2011b; Treviño-Zevallos and Lado Citation2020a, Citation2020b).
The Tropical Andes of Peru is a megadiverse region considered one of the most species-rich biodiversity hot spots on Earth (Hughes Citation2017; Myers et al. Citation2000), but, so far, it has been underexplored for myxomycetes. During intense field surveys, conducted by members of the Myxotropic Project (www.myxotropic.org) to this Andean region, special attention was focused on the myxobiota associated with species of the genus Polylepis Ruiz y Pav. (1794) (Rosaceae, Sanguisorbeae). This neotropical genus comprises several tree species, mostly 5 to 10 m high, distributed along the Andes Mountains, from northern Venezuela to northern Chile, and northeast and central Argentina (Kessler Citation2006; Kessler and Schmidt-Lebuhn Citation2006). The members of the genus Polylepis, normally growing on rocky slopes and ravines between 3500 and 4400 (–5000) m elevation, form the world’s highest forests and are among the most threatened ecosystems worldwide (Ames-Martínez et al. Citation2021; Kessler Citation2006). Despite the fact that the current distribution of these high-elevation forests, locally known as “queñuales” or “queñoales,” is highly reduced and fragmented due to anthropogenic influence (Zutta and Rundel Citation2017), they play an important role in the ecology of the Tropical Andes, being the preferential habitat of many plant and animal species (Kessler Citation2006). In addition, given that their tree roots stabilize the soil, Polylepis woodlands regulate runoff and control soil erosive processes (Fjeldså et al. Citation1996), and they provide a natural environment for a variety of myxomycete species (Lado et al. Citation2007; Treviño-Zevallos Citation2021; Wrigley de Basanta et al. Citation2015).
In these humid and protected woodlands, we found a species of the order Physarales, associated almost exclusively with the bark and wood of Polylepis spp. Based on morphology, it appeared to belong to a new species of Diachea Fr. (Fries Citation1825).
According to different authors, the genus Diachea currently comprises between 10 and 13 accepted species (Lado 2005–2022; Poulain et al. Citation2011), all of them originally described on the basis of morphological features (SUPPLEMENTARY TABLE 1), from the Northern Hemisphere (SUPPLEMENTARY TABLE 2). The members of this genus are characterized by their stalked sporocarps, rarely subsessile, with a single, limeless, iridescent peridium, and a limy columella (Martin and Alexopoulos Citation1969; Poulain et al. Citation2011). Some species, such as the type Diachea leucopodia (Bull.) Rostaf., have a worldwide distribution, with most occurrences being reported from Europe. Still, this species and other species of the genus, e.g., Diachea bulbillosa (Berk. & Broome) Lister and Diachea subsessilis Peck, have been found in several southern countries (SUPPLEMENTARY FIG. 1). Specifically, D. leucopodia has been reported from Peru by several authors, whereas congeneric species have been collected in surrounding countries (Lado et al. Citation2017; Lado and Wrigley de Basanta Citation2008). As occurs with other genera of the order Physarales (Lado and Wrigley de Basanta Citation2008), the genus Diachea is well represented in the Neotropics, with its species frequently colonizing bark, wood, leaves, and leaf litter in humid environments, but it does not seem to be very common in Peru.
In order to confirm the identity and phylogenetic placement of the species presented in this paper, we conducted a comprehensive micro- and macromorphological study, along with a phylogenetic analysis, based on three independent molecular regions.
MATERIALS AND METHODS
Sampling.—
Material was collected in the field in 11 different localities as part of a study of the myxobiota from high elevations of the Tropical Andes of Peru, in seven consecutive years (2012 to 2018). Samples of dead bark, branches, and litter were examined in the field or collected for culture from different Polylepis spp. (FIG. 2A), in an elevation gradient ranging from 3400 to 4150 m, in the Peruvian departments of Ancash, Ayacucho, Cusco, Huancavelica, Junín, and Moquegua (latitudinal gradient: 9.98°–16.99°S). All localities were geo-referenced using a Garmin eTrex Vista HCX Global Positioning System (GPS; datum WGS84; Olathe, KS, USA).
Dead litter and bark from most localities were used to prepare 30 moist chamber cultures in sterile 9-cm plastic Petri dishes in the manner described by Wrigley de Basanta et al. (Citation2009). The pH of each culture was determined with a portable pH meter after 24 h; then excess water was poured off from each dish. Cultures were maintained at room temperature (21–25 C) in diffuse daylight and examined at regular intervals with a dissecting microscope for up to 3 months.
In addition, cultures were made on agar media using spores from mature sporocarps of the holotype (Lado 21834). Spores were sown on 0.75% water agar (WA) at pH 7.0. The sporocarps were crushed and spores released over the agar in each of four quadrants of the sterile 9-cm plastic Petri dishes. Germinated spores were transferred to weak malt-yeast agar (wMY) or 1.5% water agar. A sterile nutrient solution was made from 25 g substrate (Polylepis sp. remains) in 1 L distilled water to be added to germination and early growth plates. These cultures were kept in an incubator at 23 C, with an approximate 12-h light-dark regime. Further details of media and techniques used can be found in Wrigley de Basanta and Estrada-Torres (Citation2022).
For the description of the new species, we used a Leica M205C stereomicroscope (Wetzlar, Germany) and a Nikon Eclipse 80i microscope with differential interference contrast (DIC) (Tokyo, Japan). For light micrographs, a Leica DFC 550 and a Nikon DS-Fi1 digital camera were used. Material was mounted in Hoyer’s medium to examine spore characters under oil immersion. The critical-point drying technique was used for scanning electron microscopy (SEM) preparations, and the SEM analyses and photomicrographs of specimens were made by the Scanning Electron Microscopy Department of the Royal Botanic Garden of Madrid, by using a Jeol T 330 A scanning electron microscope (Tokyo, Japan), at 10–15 kV. Color notations of the descriptions, in parentheses, correspond to the Inter- Society Color Council–National Bureau of Standards (ISCC-NBS) Color-Name Charts (Kelly and Judd Citation1976).
All specimens of the new species analyzed in this study were deposited in the myxomycete collection of the MA-Fungi Herbarium (sub Lado), Real Jardín Botánico, CSIC (Spain), with duplicates in USM and TLXM.
DNA extraction, amplification, and sequencing.—
Six specimens of the new species (including the type specimen, Lado 21834, MA-Fungi 91212) and 16 samples of additional Diachea species and other Didymiaceae were selected for molecular studies (). Sample processing was made as described elsewhere (García-Martín et al. Citation2018). Partial sequences of two benchmark molecular regions, i.e., the nuclear small subunit ribosomal gene (also known as nuc 18S rDNA gene, 18S or SSU) and the elongation factor-1 alpha (EF-1α), were amplified by polymerase chain reaction (PCR). The primer pairs used to amplify such regions were S1/SR4Dark (Fiore-Donno et al. Citation2012, Citation2008) for 18S and KEF_F2/KEF_R3 and EF02F/EF02R (García-Martín et al. Citation2018) for EF-1α. A third molecular region, the mitochondrial small subunit ribosomal gene (hereafter 17S, also known as mtSSU), was also amplified using primers newly designed by the J.M.G.-M., i.e., the pair Kmit_F (5′-AGTGTTATTCGTGATGACTGG-3′)/Kmit_R (5′-CGAATTAAACCACATCTCCACC-3′) and an internal forward primer Kmit_Fi (5′-ATGACTGGGCGTAGGGTA-3′).
Table 1. Summarized data on the 22 collections, including six of the new species, used for DNA extraction and sequence generation.
PCRs were performed using MyTaq Red Mix DNA Polymerase (Bioline, London, United KIngdom) with the following cycling parameters: an initial denaturation step at 95 C for 2 min, followed by 30 cycles of denaturation at 94 C for 60s, annealing at 52 C for 90s, and polymerization at 72 C for 2 min, with a final extension step at 72 C for 10 min. All PCR products were purified using the method described in Dentinger et al. (Citation2010). Cleaned amplicons were sequenced in both directions by Macrogen (Madrid, Spain) with the same primers used for amplification.
Sequence assembly and alignment.—
The edition and assembly of raw sequences were performed in Geneious 7.1.9 (Kearse et al. Citation2012). GenBank was searched for all 18S, 17S, and EF-1α sequences from other Diachea species, and also for representatives of both families of the order Physarales, i.e., Didymiaceae and Physaraceae, with sequences available for the genes analyzed here. In total, a selection of 51 GenBank sequences, generated from 32 specimens, were also included in our phylogenetic analyses (SUPPLEMENTARY TABLE 3).
Three single-gene alignments were built using MAFFT 7.017 (Katoh et al. Citation2002) in Geneious, removing priming sites, gaps, and low-quality ends. Before conducting phylogenetic analyses, EF-1α introns were removed and the 17S alignment was trimmed to exclude a variable region (ca. 70 pb) located in the last third of the fragment amplified. The final combined data set comprised 52 specimens (six samples of the new species and 46 specimens that were either represented by GenBank sequences or newly sequenced in this study). All sequences newly obtained in this study were submitted to GenBank under accession numbers ON059420–ON059432, ON059548–ON059569, and ON081604–ON081615 (). The alignments are available as SUPPLEMENTARY MATERIAL hosted on Taylor & Francis figshare platform (https://tandf.figshare.com/umyc).
Phylogenetic analyses.—
Phylogenetic analyses were first done separately for each gene, using two common approaches, i.e., maximum likelihood (ML) and Bayesian inference (BI), as implemented in CIPRES Science Gateway 3.3 portal (Miller et al. Citation2010). After observing that no well-supported incongruences existed among different trees, we also estimated a three-gene phylogeny using both methods. All ML analyses were conducted with IQ-TREE 2.1.2 (Minh et al. Citation2020; Nguyen et al. Citation2015), using the option “complete bootstrap” and 1000 nonparametric replicates. For each data set, the best-fit model of nucleotide substitution (and, in the case of EF-1α and the concatenated data set, the optimal partitioning scheme) was determined by the integrated version of ModelFinder (Kalyaanamoorthy et al. Citation2017). Both 18S and 17S alignments were not partitioned, whereas the protein-coding gene EF-1α was originally partitioned by codon position (SUPPLEMENTARY TABLE 4), with each codon position allowed to have independent parameter values for the model of evolution.
BI analyses were executed in MrBayes 3.2.7 (Ronquist et al. Citation2012) and consisted of four simultaneous runs of 10 million generations, each with four Monte Carlo Markov chains, sampling every 1000 trees, with the first 25% being discarded as burn-in. For EF-1α and the concatenated data set, the partitioning scheme previously obtained with IQ-TREE was used (SUPPLEMENTARY TABLE 4), unlinking model parameters across different partitions. In all cases, the reversible jumping model choice (lset nst = mixed) was used to estimate the best-fit substitution model(s), by sampling across the substitution model space in the Bayesian analysis itself (Huelsenbeck et al. Citation2004), allowing a gamma distributed rate heterogeneity across sites, with four rate categories, and a proportion of invariable sites. Mixing and convergence of the chains were assessed verifying that the standard deviation of split frequencies was <0.01. Convergence was further assessed by visualizing the log files in Tracer 1.7.2 (Rambaut et al. Citation2018), checking that all parameters reached stationarity and had an effective sampling size value >200. Both ML and BI phylogenetic trees were visualized in FigTree 1.4.4 (Rambaut Citation2018) and edited with Adobe Illustrator CS4 (San Jose, CA, USA). Members of the family Physaraceae were used to root the trees.
RESULTS
Molecular analyses.—
Forty-seven partial sequences (13 of 18S, 12 of EF-1α, and 22 of 17S), from 22 specimens representing 12 species of the family Didymiaceae and two of Physaraceae, have been generated for this study. Specifically, four almost identical 18S sequences (one variable position was found), six EF-1α sequences (nine variable positions), and six 17S sequences (three variable positions) have been generated from six specimens of the new species. The best substitution model(s) and partition schemes for the data sets used in the analyses are shown in SUPPLEMENTARY TABLE 4.
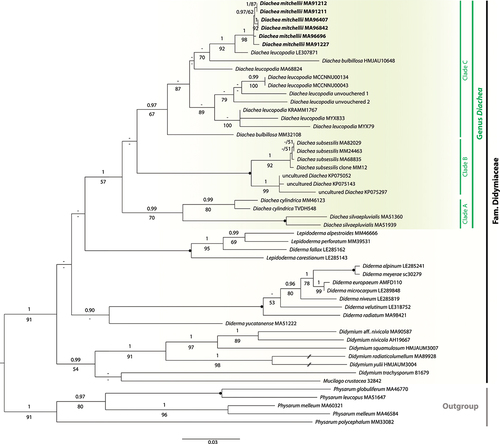
The topologies of the corresponding single-gene phylogenetic trees obtained by ML and BI analyses were similar to each other, so only the trees obtained by the ML approach are shown in the supplementary material (SUPPLEMENTARY FIGS. 2–4). In the 18S tree (SUPPLEMENTARY FIG. 2), a group containing all specimens of the genus Diachea, except for D. cylindrica MM46123 and D. silvaepluvialis MA-Fungi 51360 (which constitute a separate and well-supported clade; posterior probability [PP] = 1, bootstrap [BS] = 84%), received support only in the Bayesian analysis (PP = 0.98, BS = 45%). Within it, four specimens of the new species formed a well-supported clade (PP = 1, BS = 89%), sister to D. leucopodia LE307871 with strong support (PP = 1, BS = 84%). The species D. leucopodia is retrieved as para- or polyphyletic depending on whether the identity of the specimen HMJAU10648, from which the sequence MN722597 was obtained, is correct or not (PP = 1, BS = 90%) (unfortunately, we did not receive this specimen, nor D. leucopodia LE307871, on loan from the corresponding herbaria).
In the EF-1α tree (SUPPLEMENTARY FIG. 3), a clade constituted by all samples of Diachea included in the analysis is recovered with moderate support (PP = 0.98, BS = 74%). Within it, a clearly separated monophyletic group formed by six isolates of D. mitchellii is fully supported by both posterior probabilities and bootstrap values (PP = 1, BS = 100%). The closest relative to this clade seems to be the specimen D. leucopodia MA-Fungi 68824, although this relationship is not supported (PP = 0.58, BS = 43%). In the 17S tree (SUPPLEMENTARY FIG. 4), most relationships received low support, but the clade comprising the six individuals of the new species included in this analysis was strongly supported (PP = 1, BS = 89%), and sister to D. leucopodia MA-Fungi 68824 with high support (PP = 1, BS = 92%). For the sake of brevity, the remaining relationships in our individual phylogenies are not detailed, but, given that there were no major conflicts among them, all three regions were concatenated and analyzed together.
In the multigene phylogeny (), all 28 specimens representing the genus Diachea clustered together forming a moderately supported group (PP = 1, BS = 57%). Within it, although their relationships are unknown, three main highly supported monophyletic groups can be distinguished: clade A (formed by D. cylindrica and D. silvaepluvialis; PP = 0.99, BS = 70%), clade B (D. subsessilis and three uncultured Diachea; PP = 1, BS = 100%), and clade C (formed by D. bulbillosa, D. leucopodia, and D. mitchellii; PP = 0.97, BS = 67%). Within clade C, all six specimens of D. mitchellii formed a highly supported clade (PP = 1, BS = 98%), sister with strong support (PP = 1, BS = 92%) to D. leucopodia LE307871.
Figure 1. Maximum likelihood tree inferred from the concatenated sequences (1905 nucleotides) of three genes from 52 specimens, with members of the family Physaraceae as outgroup. Bayesian posterior probabilities (PPs) and maximum likelihood bootstrap values (BS) are shown above and below the branches, respectively (if PP ≥ 0.90 and BS ≥ 50%). Filled circles denote branches with PP = 1 and BS = 100%. Species names are followed by voucher number. The clade corresponding to the genus Diachea is highlighted by a dark-colored rectangle, and the new species, D. mitchellii, is in bold. The bar represents the number of substitutions per site. An interrupted branch (//) indicates its length has been reduced for representation purposes.
TAXONOMY
Diachea mitchellii Lado & Treviño, sp. nov. , 3, 4C–D, 5
Figure 2. A. Polylepis tree showing the red bark. B–G. Diachea mitchellii. B–D. Stipitate and subcylindrical to obovate sporocarps (holotype; Lado 21834, MA-Fungi 91212). E. Subglobose sporocarps with bluish shadows (Lado 25230, MA-Fungi 96415). F. Subglobose sporocarps with golden shadows (Lado 25684, MA-Fungi 96842). G. Iridescent sporocarps (Lado 26319, MA-Fungi 91227). Arrow shows the stalk and hypothallus. H–I. Diachea koazei (TNS-M R: 925). Closed and open sporocarps showing the iridescent peridium, and the limy hypothallus, stalk, and columella. Arrow shows the limy stalk and hypothallus. Bars: B–C, E–H = 0.5 mm; D, I = 0.2 mm.

MycoBank MB843267
Typification: PERU. AYACUCHO: Lucanas, road PE-30A, km 103, 4 km E of Abra Condorsencca, 14°38ʹ28″S, 74°17ʹ57″W, 3947 m, bark of Polylepis sp., 27 Sep 2012, C. Lado, A. Estrada, A. Rollins, S.L. Stephenson & D. Wrigley de Basanta, Lado 21834 (holotype MA-Fungi 91212, isotype USM). GenBank: 18S = ON059421; 17S = ON059553; EF-1α = ON081607.
Diagnosis: Gregarious habit; hypothallus dark, not calcareous; stalk short, dark, noncalcareous; columella whitish, reticulate, packed with lime granules; spores in clusters, easily dissociating, blackish in mass, brown under LM, subglobose, (10–)11–12(–12.5) µm diam, densely and evenly warted.
Etymology: Named after Mr. David William Mitchell, dear friend, colleague, and renowned scientist, best known for his studies on corticolous myxomycetes, who sadly died at the end of 2019.
Description: Sporophores sporocarpic, grouped to gregarious, short-stalked to almost sessile, 0.8–1.5(–1.8) mm total height (). Hypothallus effuse, discoid or common to a group of sporocarps, solid to membranous, blackish, not calcareous (). Stalk short, 0.1–0.2 mm length, subcylindrical, erect () or prostrate as an extension of the hypothallus, not calcareous, blackish, dark brown (65. br. Black–61. gy. Br) under light microscope (LM). Sporotheca subglobose, turbinate, clavate or obovate, rarely subcylindrical (), 0.7–1.5 × 0.5–0.6 mm. Peridium single, membranous, iridescent, with golden, bluish, greenish, or purplish tints (), pale orange-yellow (73. p. OY–70. l. OY) under LM, partially persistent, smooth under LM and scanning electron microscope (SEM) (); dehiscence irregular (). Columella entirely calcareous, whitish, slightly yellowish toward the base, filled with calcareous granules (), reaching almost the top of the sporotheca, with many irregular projections and hollows arranged in a reticulate fashion (, 5B–C), some projections attached to the basal peridium and the stalk, merging into the capillitium (), more solid and with few projections in subglobose sporothecae. Capillitium abundant, filiform, 1–2.5 µm diam, branched and anastomosed, as a network (), emerging from the reticulate columella (), threads smooth, with dispersed small granules under SEM (), some nodes of the net slightly flattened (), threads brown (57. l. Br–58. m. Br) to grayish brown (62. d. gy. Br), sometimes with paler zones, almost hyaline toward the columella, peripheral free ends sharp and hyaline (), the basal capillitial net irregular, not filiform, and yellowish. Spores in clusters of (3–)5–10(–12) units (, 4C–D, 5F–H), but easily dissociating and so free, blackish in mass, brown (58. m. Br–60. l. gy. Br) under LM, with a slightly pale area, subglobose, (10–)11–12(–12.5) µm diam, warted (, 5F–H), the warts regularly distributed over the whole surface under LM (), densely covered with verrucae under SEM (). Plasmodium not observed. Germination of the spores by a V-shaped fissure in the spore wall with the spores still in clusters ().
Figure 3. Diachea mitchellii. A, D. Dehisced sporocarps showing the calcareous columella (holotype; Lado 21834, MA-Fungi 91212). B. Calcareous columella (Lado 25684, MA-Fungi 96842). C, F. Dehisced sporocarps showing the calcareous, reticulate columella (Treviño Myx 125, MA-Fungi 97929). E. Dehisced sporocarps (Lado 25230, MA-Fungi 96415). G–H. Detail of the calcareous, colorless columella, dark capillitium and spores (Lado 21858, MA-Fungi 94625). I–J. Clustered spores, some of them germinated (arrows) (holotype; Lado 21834, MA-Fungi 91212). K. Detail of the capillitium and clustered spores (Treviño Myx 125, MA-Fungi 97929). Bars: A–B, D–F = 0.2 mm; C = 0.5 mm; G–K = 20 µm.

Figure 4. Diachea koazei. A Clustered spores (TNS-M R: 925). B. Individual spores (TNS-M R: 926). C–D. Diachea mitchellii (holotype; Lado 21834, MA-Fungi 91212). C. Clustered spores. D. Individual spores. Bars: A–D = 10 µm.

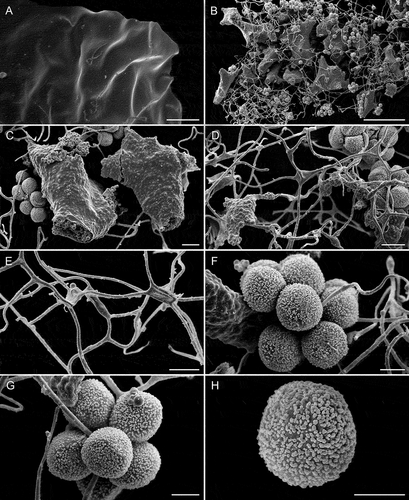
Figure 5. Scanning electron micrographs of Diachea mitchellii (Lado 23139, MA-Fungi 91227). A. Smooth peridium. B. Fragmented columella. C. Detail of calcareous columella showing internal lime nodes. D. Reticulate capillitium. E. Detail of the capillitial threads. F–G. Cluster of spores. H. Spore. Bars: A = 20 µm; B = 100 µm; C–E = 10 µm; F–H = 5 µm.
Habitat: On dead bark fallen on the ground, leaves, and wood remains of Polylepis spp., occasionally on grasses and other plants surrounding Polylepis trees.
Distribution: Tropical Andes of Peru, possibly present throughout the distribution range of the genus Polylepis.
Additional specimens examined: PERU. ANCASH: Bolognesi, Pachapaqui, San Judas Tadeo, road PE-3 N, km 35, 9°59ʹ23″S, 77°06ʹ40″W, 3828 m, bark and leaves of Polylepis sp., 23 Apr 2013, C. Lado, A. Estrada, A. Cano, J.M. García-Martín & D. Wrigley de Basanta, Lado 23139 (MA-Fungi 91227), Lado 23140 (MA-Fungi 95295, USM); Recuay, Catac, road AN-110, km 18, Querococha Lake, 9°43ʹ27″S, 77°20ʹ02″W, 4040 m, bark and wood of Polylepis sp., 24 May 2014, C. Lado, S. Castillo & D. Wrigley de Basanta, Lado 23848 (MA-Fungi 95501), branches of Polylepis sp., Lado 23862 (MA-Fungi 95512); Ticapampa, Catac, road AN-110, km 22, 9°43ʹ18″S, 77°19ʹ19″W, 4150 m, leaf litter and bark of Polylepis sp., 11 May 2018, C. Lado, A. Estrada, I. García-Cunchillos & I. Treviño, Lado 26319 (MA-Fungi 97117); AYACUCHO: Lucanas, road PE-30A, km 103, 4 km E of Abra Condorsencca, 14°38ʹ28″S, 74°17ʹ57″W, 3947 m, bark of Polylepis sp., 27 Sep 2012, C. Lado, A. Estrada, A. Rollins, S.L. Stephenson & D. Wrigley de Basanta, Lado 21829 (MA-Fungi 94601); wood of Polylepis sp., Lado 21833 (MA-Fungi 91211), Lado 21837 (MA-Fungi 94608, USM), grasses, Lado 21852 (MA-Fungi 94618) (moldy specimen), bark of Polylepis sp., Lado 21858 (MA-Fungi 94625, USM), Lado 21867 (MA-Fungi 94634); CUSCO: Canas, Kunturkanki, road from El Descanso to Sicuani, km 6, 14°29ʹ08″S, 71°17ʹ42″W, 4085 m, branch and bark of Polylepis sp., 22 Apr 2016, C. Lado, I. Treviño, A. Estrada & D. Wrigley de Basanta, Lado 24672 (MA-Fungi 95941, USM); HUANCAVELICA: Huancavelica, Acoria, road PE-26, km 39, Ccaccasiri, 12°38ʹ32″S, 74°55ʹ06″W, 3875 m, leaves, bark and branches of Polylepis racemosa, 25 Apr 2017, C. Lado, A. Estrada, I. García-Cunchillos & I. Treviño, Lado 25223 (MA-Fungi 96407, USM), Lado 25225 (MA-Fungi 96409, USM), Lado 25230 (MA-Fungi 96415, USM), Lado 25235 (MA-Fungi 96420, USM); Huaytara, Pilpichaca, KP 391, southern Peruvian gas pipeline, 13°26ʹ41″S, 74°55ʹ30″W, 3853 m, on bark of Polylepis sp., 31 Aug 2017, I. Treviño, Treviño Myx 125 (MA-Fungi 97929, HSP); JUNÍN: Concepción, Chambara, road PE-24, km 254, 3 km W from Roncha, 12°00ʹ32″S, 75°26ʹ50″W, 3428 m, bark and leaves of Polylepis racemosa, 28 Apr 2017, C. Lado, A. Estrada, I. García-Cunchillos & I. Treviño, Lado 25538 (MA-Fungi 96696, USM, HSP); Huancayo, Huancayo, road km 10, 8 km SW from Acopalca, 12°00ʹ18″S, 75°09ʹ38″W, 3505 m, bark and leaves of Polylepis racemosa and Eucalyptus globulus, 27 Apr 2017, C. Lado, A. Estrada, I. García-Cunchillos & I. Treviño, Lado 25451 (MA-Fungi 96604, USM); Yauli, Paccha, La Oroya, road PE-3 N, km 6, 6 km N of La Oroya, 11°29ʹ29″S, 75°56ʹ36″W, 3706 m, leaves and bark of Polylepis racemosa and grasses, 1 May 2017, C. Lado, A. Estrada, I. García-Cunchillos & I. Treviño, Lado 25663 (MA-Fungi 96821, USM), Lado 25666 (MA-Fungi 96824), Lado 25676 (MA-Fungi 96834), Lado 25677 (MA-Fungi 96835, USM), Lado 25678 (MA-Fungi 96836, USM), Lado 25682 (MA-Fungi 96840, USM), Lado 25684 (MA-Fungi 96842, USM), leaves and bark of Polylepis racemosa and leaves of Cortaderia jubata, Lado 25685 (MA-Fungi 96843), Lado 25686 (MA-Fungi 96844); MOQUEGUA: Mariscal Nieto, Torata, Pontón Cuellar, road PE-34D, km 69, 16°59ʹ54″S, 70°41ʹ54″W, 3815 m, bark of Polylepis rugulosa, 7 Oct 2012, C. Lado, A. Estrada, I. Treviño & D. Wrigley de Basanta, Lado 22176 (MA-Fungi 94731), Lado 22177 (MA-Fungi 94732).
Other specimens examined: Diachea koazei Y. Yamam. (as “Diachea syncarpa Koaze., nov. sp.” K 67, Sp. No. 2633). JAPAN. Mont. Takakuma, Osumi Peninsula, Aug 1935 [original text in Japanese], TNS-M R: 925. Diachea koazei Y. Yamam. (as “Diachea syncarpa Koaze., nov. sp.” K 13, Sp. No. 2733). JAPAN. Mont. Takakuma, Osumi Peninsula, Aug 1935 [original text in Japanese], TNS-M R: 926. Diachea cylindrica Bilgram. JAPAN. Imari-cho, Tanabe-shi, Wakayama Prefecture, 22 Jul 2012, Hikori Nishio, ex YY-33479 (MM46123). Diachea cylindrica Bilgram (as Paradiachea cylindrica (Bilgram) Hertel ex N. Neubert, Nowotny & K. Baumann). AUSTRALIA. Dorrigo National Park, Never Never area, −30.3583, 152.7793, forest litter, 27 Feb 2021, T. & J. van der Heul, TVDH548.
In the agar cultures of spores from the holotype, germination occurred in most quadrants in both germination plates by a small V-shaped fissure of the wall of spores that were still attached in the cluster (). Germination took 2–4 days producing a single myxamoeba from each spore. Not all spores in a cluster germinated. Germinating spores and amoebae were transferred onto a weak malt-yeast agar (wMY) or 1.5% water agar (WA), but they rapidly encysted. Attempts to stimulate amoebal growth using drops of a nutrient solution of Polylepis or sterile oat flour caused rapid overgrowth of bacteria and yeast from the spores and the cultures failed to progress further.
DISCUSSION
Knowledge of neotropical Myxomycetes is still fragmentary given that efforts have been focused on other regions (Treviño-Zevallos and Lado Citation2020b). This is especially true for Peru, where records of the genus Diachea are merely anecdotal. Here, we describe a new Diachea species, D. mitchellii, morphologically and ecologically distinct from other in its genus (SUPPLEMENTARY TABLES 1, 4). Diachea mitchellii was found abundantly and almost exclusively on the dead bark, branches, and litter of Polylepis spp. Specifically, it was found at 11 different locations of the Tropical Andes of Peru, across a large latitudinal gradient (6°–14°S), restricted to elevations ranging from 3400 to 4150 m, but always linked to the mentioned substrate. Interestingly enough, some neotropical myxomycetes, such as Didymium azorellae D. Wrigley de Basanta, Lado & Estrada (Wrigley de Basanta et al. Citation2018), Licea aurea D. Wrigley, Lado & Estrada (Wrigley de Basanta et al. Citation2019), and many others (Rojas et al. Citation2011a, Citation2011b), have been found in elevations above 3500 m, which seems to indicate that several species may have evolved to exploit the coldest areas of the Tropical Andes. In addition, due to the humidity, Polylepis forest soils are usually covered by large colonies of bryophytes forming a moist carpet that accumulates the pieces of fallen bark, leaves, and tree branches. This environment seems to serve as a natural habitat for myxomycetes, with more than 100 species identified so far (Treviño-Zevallos Citation2021).
None of the 30 moist chamber cultures of this substrate produced identifiable sporocarps of the new species. The cultures were generally less productive than cultures of other substrates, but the reasons for this are unknown. In all cultures, there was evidence of plasmodial tracks and/or phaneroplasmodia, possibly of the new species, but since in some cultures other myxomycete species developed mature identifiable sporocarps, it is not possible to be certain. It is possible that the rather acidic pH of the cultures (range 3.7 to 6.9; bark average pH 4.5; litter average pH 5.9) adversely affected the new species, or some other microhabitat condition or combination of conditions in the cultures were unfavorable for its life cycle to be completed.
The combination of characters that distinguishes the new species from others in the genus are its gregarious habit (); the dark, not calcareous hypothallus; the short, dark noncalcareous stalk (); the whitish reticulate columella packed with lime granules (); and its clustered spores easily dissociating (, 4C–D, 5F–G), densely and uniformly ornamented with warts, as evidenced under SEM (). The new species can be easily distinguished from Diachea koazei Y. Yamam., 1987 [basionym D. syncarpa Koaze, 1935, inval. name, and synonym D. synspora H.Z. Li, 1988 (Yamamoto Citation1998)], the only described species of its genus with clustered spores and an irregular columella. The differences are (i) the usually obovate, clavate, or turbinate sporotheca vs. the cylindrical sporotheca in D. koazei (); (ii) its blackish, noncalcareous hypothallus that is sometimes discoid and individual vs. the whitish, strongly calcareous hypothallus, common to several sporocarps in D. koazei (); (iii) the columella in D. koazei is solid, cylindrical, and has irregular projections (Yamamoto Citation1987) (), whereas it shows an irregular reticulate pattern in D. mitchellii (), and there is more abundant and dense capillitium; (iv) the new species has easily dissociated clusters of spores, with fewer spores by cluster (); and finally (v) the two species differ in the color, measurement, shape, and ornamentation of the spores (darker, 10–12 µm diam, subglobose, uniformely warted in D. mitchellii vs. paler, usually turbinate or pyriform, 8–10 × 8–10 in diam, and densely ornamented on the outside of the spore cluster, almost smooth on the inner side in D. koazei) () (see also Yamamoto (Citation1987) and SUPPLEMENTARY TABLE 1).
Because of the subcylindrical shape of some sporothecae, the new species could also be confused with depauperated forms of the common species D. leucopodia. However, D. mitchellii and D. leucopodia can be easily distinguished by (i) the arrangement of the spores (clustered vs. free), (ii) the length and color of the stalk (short, 0.1–0.2 mm, dark brown to blackish vs. long, 0.25–1 mm, whitish), and (iii) the shape of the columella (reticulate vs. cylindrical) (Martin and Alexopoulos Citation1969; Nannenga-Bremekamp Citation1991; SUPPLEMENTARY TABLE 1).
The calcareous and irregular columella of D. mitchellii resembles that of Physarum crateriforme Petch and stipitate forms of Ph. mutabile (Rostaf.) G. Lister, but in these two species the peridium is white due to the existence of lime deposits (Martin and Alexopoulos Citation1969), which are absent in the peridium of D. mitchellii.
Macroscopically, the new species is also similar to two species currently placed in the genus Paradiachea Hertel, i.e., P. caespitosa (Sturgis) Hertel ex H. Neubert, Nowotny & K. Baumann and P. cylindrica (Bilgram) Hertel ex H. Neubert, Nowotny & K. Baumann (≡ Diachea cylindrica Bilgram), that are considered, by some authors, to be belonging to the genera Comatricha or Diachea (Lister Citation1925; Lister and Lister Citation1907; Martin and Alexopoulos Citation1969; Sturgis Citation1893; Yamamoto Citation2021). These two species differ by their cylindrical, not reticulate columella, brown and limeless (Bilgram Citation1905; Lister Citation1925; Macbride Citation1922; Macbride and Martin Citation1934; Martin and Alexopoulos Citation1969; Sturgis Citation1893). However, some authors, such as Farr (Citation1979), Neubert et al. (Citation2000), and Yamamoto (Citation2021), describe and illustrate the columella of P. cylindrica as whitish and partially calcareous, as in the collections from Australia and Japan examined here. We consider P. cylindrica to be better placed in the genus Diachea, as it was originally described and as our analyses indicate (; SUPPLEMENTARY TABLES 2–4). In any event, in both species currently adscribed to Paradiachea, the spores are free and their ornamentation is different, subreticulated or incompletely verrucose-reticulate in P. cylindrica, irregularly spiny in P. caespitosa (Martin and Alexopoulos Citation1969; Neubert et al. Citation2000), in clusters of (3–)5–10(–12) units, and densely warted in D. mitchellii.
In our molecular analyses, the phylogenetic relationships within the genus Diachea are, in many cases, weakly supported. This could be probably due to (i) undersampling (there was not sufficient material for DNA extraction from other Diachea species or, if available, no sequences were obtained); (ii) the proportion of missing data; and (iii) the partial nature of the sequences used here. Consequently, a more comprehensive molecular study, including more taxa and, ideally, longer sequences of additional genes is needed to ultimately reveal the relationships among different species of Diachea.
Still, our molecular phylogenetic analyses have revealed support for the monophyly of the genus, and a close relationship between D. mitchellii, D. bulbillosa, and D. leucopodia (). It is noteworthy that all specimens of D. mitchellii molecularly characterized constitute a strongly supported monophyletic group, regardless of the gene analyzed (SUPPLEMENTARY FIGS. 2–4), nested within the clade C, which is also highly supported and comprises all accessions of D. leucopodia analyzed and two isolates of D. bulbillosa (). These two specimens of D. bulbillosa (MM32108, whose identity could be verified, and HMJAU10648, which could not be studied) did not cluster together in our three-gene phylogeny (), and their positions remain uncertain. If it was confirmed that they are not phylogenetically related, it could be assumed that HMJAU10648 most probably corresponds to D. leucopodia, instead of D. bulbillosa. Because the life cycle was not completed under controlled laboratory conditions, we could not determine whether the morphological traits of D. mitchellii are maintained in specimens grown from spore to spore. However, numerous field specimens were collected from 11 different localities over seven consecutive years, and all sequenced samples of D. mitchellii clustered together in a strongly supported clade. Moreover, this species does not share ecological requirements nor distribution with the species that seems to be the most closely related to it (D. mitchellii is only known from high-elevation Polylepis forests of Peru, whereas D. leucopodia is a cosmopolitan species that can be found on different substrates at lower elevations (Tran et al. Citation2014)). Thus, our consistent morphological, geographic, and ecological data, coupled with the fact that all specimens analyzed formed a monophyletic group, provide strong support for considering D. mitchellii as a new species.
Supplemental Material
Download Zip (3.1 MB)ACKNOWLEDGMENTS
We thank Carlos de Mier (RJB) for his help with the light micrographs, Yolanda Ruiz (RJB) for technical assistance with SEM, Diana Ramos for technical assistance in the laboratory, and all members of the Myxotropic project, especially Dr. Arturo Estrada Torres and Dr. Iván García-Cunchillos, who participated in field sampling trips. We are grateful to Dr. Zamora-Señoret and Dr. Tsuyoshi Hosoya who sent us some samples deposited at the Herbarium of the Conservatoire et Jardin botaniques de la Ville de Genève (CJB) and the National Museum of Natural History (TNS) from Tsukuba (Japan), respectively, and to Mrs. T. van der Heul for providing us with some specimens from Australia. Peruvian field work and sample collection were authorized by collecting and/or exportation permit no. 118-2016-SERFOR-DGGSPFFS from the Dirección General de Gestión Sostenible del Patrimonio Forestal y de Fauna Silvestre, and the Dirección General del Servicio Forestal (Peru).
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed on the publisher’s Web site.
Additional information
Funding
LITERATURE CITED
- Adl SM, Bass D, Lane CE, Lukeš J, Schoch CL, Smirnov AV, Agatha S, Berney C, Brown MW, Burki F. 2019. Revisions to the classification, nomenclature, and diversity of Eukaryotes. J Eukaryotic Microbiol. 66:4–119.
- Ames-Martínez FN, Quispe-Melgar HR, Renison D. 2021. Conservation status assessment of the highest forests in the world: polylepis flavipila forests as a case study. Neotrop Biodivers. 7:160–69.
- Bilgram H 1905. Diachea cylindrica, a new species of Mycetozoa. Proceedings of the Academy of Natural Sciences of Philadelphia. 57:524.
- Dentinger BT, Margaritescu S, Moncalvo JM. 2010. Rapid and reliable high-throughput methods of DNA extraction for use in barcoding and molecular systematics of mushrooms. Mol Ecol Resour. 10:628–33.
- Farr M. 1979. Notes on Myxomycetes II: new taxa and records. Nova Hedwigia. 31:103–18.
- Fiore-Donno AM, Meyer M, Baldauf SL, Pawlowski J. 2008. Evolution of dark-spored Myxomycetes (slime-molds): molecules versus morphology. Mol Phylogenet Evol. 46(3):878–89.
- Fiore-Donno AM, Kamono A, Meyer M, Schnittler M, Fukui M, Cavalier-Smith T, Salemi M. 2012. 18S rDNA phylogeny of Lamproderma and allied genera (Stemonitales, Myxomycetes, Amoebozoa). PLOS ONE. 7(4):e35359.
- Fjeldså J, Kessler M, Engblom G, Driesch P. 1996. Conserving the biological diversity of Polylepis woodlands of the highland of Peru and Bolivia: a contribution to sustainable natural resource management in the Andes. Copenhagen (Denmark): Nordeco.
- Fries EM. 1825. Systema Orbis Vegetabilis. Pars I. Plantae Homonemeae. Lund (Sweden): Typographia Academica.
- García-Martín JM, Mosquera J, Lado C. 2018. Morphological and molecular characterization of a new succulenticolous Physarum (Myxomycetes, Amoebozoa) with unique polygonal spores linked in chains. Eur J Protistol. 63:13–25.
- Huelsenbeck JP, Larget B, Alfaro ME. 2004. Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Mol Biol Evol. 21(6):1123–33.
- Hughes CE 2017. Are there many different routes to becoming a global biodiversity hotspot? Proceedings of the National Academy of Sciences. 114:4275–77.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–89.
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30(14):3059–66.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–49.
- Kelly KL, Judd DB. 1976. Color: universal language and dictionary of names. Washington (USA): Department of Commerce, National Bureau of Standards.
- Kessler M. 2006. Bosques de Polylepis. In: Moraes RM, Øllgaard B, Kvist LP, eds. Botánica Económica de los Andes Centrales. La Paz (Bolivia): Universidad Mayor de San Andrés. p. 110–20.
- Kessler M, Schmidt-Lebuhn AN. 2006. Taxonomical and distributional notes on Polylepis (Rosaceae). Org Diversity and Evol. 6(1):67–70.
- Lado C, Estrada-Torres A, Stephenson SL. 2007. Myxomycetes collected in the first phase of a north-south transect of Chile. Fungal Divers. 25:81–101.
- Lado C, Wrigley de Basanta D. 2008. A review of Neotropical Myxomycetes (1828–2008). Anales del Jardín Botánico de Madrid. 65(2):211–54.
- Lado C, Wrigley de Basanta D, Estrada-Torres A, Stephenson SL. 2016. Myxomycete diversity in the coastal desert of Peru with emphasis on the lomas formations. Anales del Jardín Botánico de Madrid. 73(1):1–27.
- Lado C, Estrada-Torres A, Wrigley de Basanta D, Schnittler M, Stephenson SL. 2017. A rapid biodiversity assessment of myxomycetes from a primary tropical moist forest of the Amazon basin in Ecuador. Nova Hedwigia. 104:293–321.
- Lado C, Estrada-Torres A, Rojas C. 2018. New records of genera and species of Myxomycetes (Amoebozoa) from the Neotropics. Check List. 14(3):509–18.
- Lado C, Wrigley de Basanta D, Estrada-Torres A, Stephenson SL, Treviño-Zevallos I. 2019. Diversity of Myxomycetes in arid zones of Peru part II: the cactus belt and transition zones. Anales del Jardín Botánico de Madrid. 76:e083.
- Lister A, Lister G. 1907. Synopsis of the orders, genera, and species of Mycetozoa. J Bot. 45:176–97.
- Lister A. 1925. A monograph of the Mycetozoa: a descriptive catalogue of the species in the Herbarium of the British Museum. (3 ed., revised by G. Lister). London (UK): Printed by order of the Trustees.
- Macbride TH 1922. The North American slime-moulds: a descriptive list of all species of Myxomycetes hitherto reported from the continent of North America, with notes on some extralimital species. New York (USA): The Macmillan Company.
- Macbride TH, Martin GW. 1934. The Myxomycetes. New York (USA): Macmillan.
- Martin GW, Alexopoulos CJ. 1969. The Myxomycetes. Iowa City (USA): University of Iowa Press.
- Miller MA, Pfeiffer W, Schwartz T 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop (GCE). New Orleans, USA: IEEE. p. 1–8.
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, Von Haeseler A, Lanfear R, Teeling E. 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 37(5):1530–34.
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature. 403(6772):853–58.
- Nannenga-Bremekamp NE. 1991. A guide to temperate Myxomycetes. Bristol (UK): Biopress.
- Neubert H, Nowotny W, Baumann K. 2000. Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besonderer Berücksichtigung Österreichs: stemonitales band 3. Stemonitales. Gomaringen (Germany): Karlheinz Baumann Verlag.
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–74.
- Poulain M, Meyer M, Bozonnet J. 2011. Les Myxomycètes. Sévrier (France): Fédération mycologique et botanique Dauphiné-Savoie.
- Rambaut A. 2018. FigTree v 1.4.4. Available from: https://github.com/rambaut/figtree/releases/tag/v1.4.4
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67(5):901–04.
- Rojas C, Stephenson SL, Huxel GR. 2011a. Macroecology of high-elevation myxomycete assemblages in the northern Neotropics. Mycol Prog. 10(4):423–37.
- Rojas C, Stephenson SL, Pavlich M. 2011b. New additions to the myxobiota of Peru. Mycosphere. 2(5):583–92.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–42.
- Sturgis WC. 1893. On two new or imperfectly known Myxomycetyes. Bot Gaz (Crawfordsville). 18(5):186–87.
- Tran DQ, Nguyen H, Tran HT, Stephenson SL. 2014. Myxomycetes recorded from three lowland tropical forests in Vietnam. Mycosphere. 5(5):662–72.
- Treviño-Zevallos IF, Lado C. 2020a. Myxomycete diversity in a humid montane forest on the eastern slopes of the Peruvian Andes. Plant Ecol Evol. 153(3):390–98.
- Treviño-Zevallos IF, Lado C. 2020b. New records of Myxomycetes from Peru. Check List. 16(2):253–64.
- Treviño-Zevallos IF. 2021. Estudio biosistemático de Myxomycetes altoandinos. Madrid (Spain): Autonomous University of Madrid.
- Wrigley de Basanta D, Lado C, Estrada-Torres A, Stephenson SL. 2009. Description and life cycle of a new Didymium (Myxomycetes) from arid areas of Argentina and Chile. Mycologia. 101(5):707–16.
- Wrigley de Basanta D, Lado C, García-Martín JM, Estrada-Torres A. 2015. Didymium xerophilum, a new myxomycete from the tropical Andes. Mycologia. 107(1):157–68.
- Wrigley de Basanta D, Estrada-Torres A, García-Cunchillos I, Cano Echevarría A, Lado C. 2018. Didymium azorellae, a new myxomycete from cushion plants of cold arid areas of South America. Mycologia. 109(6):993–1002.
- Wrigley de Basanta D, Estrada-Torres A, Lado C. 2019. Licea aurea a new Myxomycete from the Peruvian Andes. Phytotaxa. 391(3):218–24.
- Wrigley de Basanta D, Estrada-Torres A. 2022. Techniques for recording and isolating Myxomycetes: updated. In: Rojas C, Stephenson SL, editors. Myxomycetes: biology, systematics, biogeography, and ecology. Second ed. Cambridge (USA): Academic Press; p. 417–51.
- Yamamoto Y. 1987. A new species of Diachea with clustered spores. J Jpn Bot. 62:346–48.
- Yamamoto Y. 1998. The Myxomycetes biota of Japan. Tokyo (Japan): Toyo Shorin Publishing; 700. Japanese.
- Yamamoto Y. 2021. Biota of Japanese Myxomycetes. Committee for the publication of Biota of Japanese Myxomycetes. Ibaraki (Japan). p. 1136.
- Zutta BR, Rundel PW. 2017. Modeled shifts in Polylepis species ranges in the Andes from the Last Glacial Maximum to the present. Forests. 8(7):232.
