ABSTRACT
In Polyporales, the pore field immediately behind the basidiocarp margin may configure the hymenophore. Basidiocarp growth is not restricted to the margin, however. Here, the importance of the pore field was assessed from two years’ of observations on naturally occurring oak mazegill (Daedalea quercina, Polyporales) basidiocarps and tested by experimental perturbations in natural habitats. Oak mazegill was chosen because the formed hymenophore has a unique and stable combination of poroid and lamellate features. Whether the pore field is required for basidiocarp growth was tested in 10 basidiocarps in which one side was resected. New growth was observed in six basidiocarps, and it occurred equally from the cut hymenophore and the intact pore field. New formation of hymenophore and pileus even occurred in seven out of 10 basidiocarps that had the entire pore field resected. Whether the hymenophore is configured permanently was tested on 54 basidiocarps on 10 trunks that were turned upside down. A new hymenophore grew through the old pileus, often far from the pore field, and its hymenophore configuration was invariably poroid despite the old hymenophore had lamellate features. In 48 experimentally banded basidiocarps, new hymenophore grew in the insertion hole of the band despite this being far from the pore field. The banded basidiocarps grew at an average rate of 5 mm per year. In conclusion, the capacity to configure the hymenophore is not confined to the pore field and it could be broadly present in the basidiocarp, possibly due to ubiquitous hyphal totipotency.
GRAPHICAL ABSTRACT

KEYWORDS:
INTRODUCTION
Polypore basidiocarps can be considered to grow from the margin. That obstacles such as twigs can become imbedded in the basidiocarp rather than being pushed in front of it underscores the notion of growth from the margin. Basidiocarps not only grow broader and wider, however; they also increase in thickness, or vertical length. In the perennial polypore oak mazegill (Daedalea quercina), for example, this thickening can be up to approximately 8 cm and the thickening is most pronounced at the base and thus furthest from the margin (Lindner et al. Citation2011). It is unlikely, then, that growth is limited to the margin. Still, the margin may be the predominant site at which the various layers of the basidiocarp are patterned, and the in situ experiments reported here were designed to test this hypothesis.
Carpogenesis is monophasic when all contexts of the basidiocarp are formed continuously (Clémençon Citation2012). In monophasic polypores, such as the oak mazegill, a narrow and tube-free “pore field” immediately behind the margin may be important to pattern the configuration of the hymenophore (Clémençon Citation2012; Corner Citation1932). Corner (Citation1932) suggested that in the pore field “play[s] the lines of force which demarcate the pores” and it exhibits, in the rephrasing of Butler (Citation1992), a “localized development of different modes of hyphal growth” that results “in initiation of pore dissepiments.” Clémençon (Citation2012) perpetuated Corner’s concept and stated that the pore field is “supposed to ‘prepare’ the formation of the pores.” Although these authors do not clarify explicitly what properties are unique to the pore field, they did, at least in the reading of this author, assign a special status to this part of the basidiocarp.
Because a hymenophore can be grown in vitro seemingly from any part isolated from a basidiocarp, one might deduce that the pore field at best contributes an intermediate, or temporary, role in forming the hymenophore. In fact, hyphae in general likely have totipotent properties (Moore Citation2005). Such properties, however, should not necessarily imply that any type of hyphae can differentiate directly to any other type. Instead, basidiocarp hyphae can readily reverse into or give rise to a vegetative totipotent state and then differentiate again (Money Citation2002). By analogy to the human body, this could resemble the making of new somatic cells via stem cells derived from somatic cells. Whereas human stem cells are known to have distinct transcriptional attributes such as expression of NANOG and OCT4 (Wang et al. Citation2012), the equivalent profiling has not yet been found to ascertain totipotency of fungal hyphae or particular identities such as is presumed for the pore field. At this point, then, it is not possible to deduce from literature whether hymenophore formation in polypores requires a pore field or whether the hymenophore can be generated directly from a multitude of types of totipotent hyphae. The in vitro experiments of Butler (Citation1988, Citation1992) in the resupinate Phellinus contiguus, however, suggested that the appearance of hymenophoral dissepiments was preceded by the formation of a pore field-like structure. Additionally, in oak mazegill, the hymenophore has an essential constant configuration at any part of its depth (Ames Citation1913; Jensen et al. Citation2020), and this could suggest that the pore field patterns a fixed configuration.
Here, it is hypothesized that hymenophore patterning can occur outside the marginal pore field. This was tested in basidiocarps of oak mazegill by resecting the entire margin or parts thereof and by stimulating vertical growth by turning trunks with basidiocarps upside down. We have previously observed that basidiocarps that are flipped will grow a new hymenophore through the old pileus (Jensen et al. Citation2020). Although these tests are somewhat direct tests of the importance of the pore field, one limitation to the present study is that pore field may not only be a position but it may also be an identity to its constituent hyphae. Thus, although it is shown that hymenophore patterning can happen outside the first-established margin, it cannot be excluded that the experimentally induced patterning derives from hyphae with a secondarily derived identity akin to that of the hyphae of the undisturbed pore field.
MATERIALS AND METHODS
Localities.—
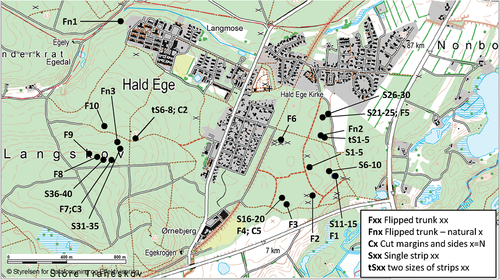
All monitored and sampled basidiocarps were found in the vicinity of the village Hald ege, Denmark, in forests in which most trees are oak (Quercus robur). The localities of the basidiocarps that were monitored are indicated in on a map from the Danish Agency for Data Supply and Efficiency (www.sdfekort.dk). All monitored basidiocarps were inspected approximately once every half year.
Growth in resected basidiocarps.—
To test the hypothesis that the patterning of the configuration of the oak mazegill hymenophore is not restricted to a marginally located pore field, we conducted two types of tests. In one type of tests, we removed entirely the basidiocarp margin (10 basidiocarps on three trunks). In the other type of tests, we removed approximately 20% of the basidiocarp from one lateral side (10 basidiocarps on three trunks).
Growth in flipped basidiocarps.—
We made observations on nine basidiocarps from three fallen trunks lying on the ground (Flipped trunk natural 1, N = 5; Flipped trunk natural 2, N = 3; Flipped trunk natural 3, N = 1). The basidiocarps showed various signs of having grown since having been flipped by natural occurrences. On the basis of these observations, we formulated two hypotheses that were tested by turning upside down 10 trunks of fallen oak such that the growing basidiocarps where oriented approximately 180 degrees to their original orientation, or gravity. On these 10 trunks, a total of 121 basidiocarps were monitored for two years, numerous of which were lost or responsive. To measure the progression of the formation of a new pileus and a new hymenophore, we measured the area of the new hymenophore relative to the old pileus through which it was growing and the area of the new pileus relative to the old hymenophore through which it was growing. Area measurements were made using the polygon tool in ImageJ (version 1.51a; National Institutes of Health, Bethesda, Maryland). The relative area of the new hymenophore was linearly correlated to the relative area of the new pileus, using a Pearson correlation test in Excel (version 16.16.27; Microsoft, Redmond, Washington).
Growth around obstacles.—
We found 53 basidiocarps of oak mazegill with incorporated twigs of various sizes and positions in the basidiocarp (for this we did not discriminate between dead basidiocarps, which are often dark and may be overgrown with moss, etc., and growing basidiocarps, which typically have a light brown pileus and an almost white hymenophore). From observations of these basidiocarps, we deduced how basidiocarps grow around obstacles and tested these deductions on basidiocarps in which we attached plastic strips (4.8 mm wide) on five basidiocarps of various sizes on each of eight trunks (total 40 basidiocarps, 30 in Nonbo forest and 10 in Langskov). To attach the strips, a small lesion was made on the pileus surface approximately halfway between the base and the margin, either with a screwdriver or a knife, and the strip was then forced through the basidiocarp and closed such that the strip made contact with the margin. We learned that the pileus is relatively brittle in abrupt boundaries between growth zones (these are revealed as a change in color and/or height of the pileus), and we preferentially inserted the strips through such boundaries. These basidiocarps were monitored for two years. In additional eight basidiocarps, we inserted a strip of medium size (4.8 mm wide, same width as above) and a strip of large size (7.6 mm wide) in each of eight basidiocarps situated on three different trunks, and these were also monitored for two years.
Estimation of growth rate.—
The increment in size of basidiocarps with attached plastic strips was used to deduce growth rates. Images of the basidiocarps were imported to ImageJ. Using the line tool, in each image the width of the plastic strip was measured for scale and then the outward growth was measured along a single line next to the perturbation of the margin created by the strip.
RESULTS
Growth despite resected pore field.—
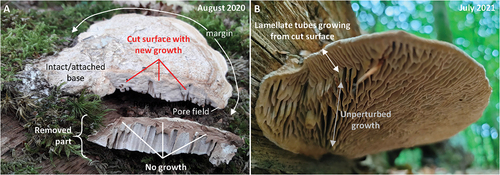
Of the 10 basidiocarps with a resected side, five exhibited pronounced growth from the cut surface already within three weeks after the resection (). This new growth occurred along the entire cut surface, and it was not restricted to the pore field nor was it preferentially originating from there (). The resected parts were left next to the cut basidiocarp, but they never exhibited observable growth or remodeling (). This suggests that the response to damage is mostly or fully dependent on nutrition from outside the basidiocarp and that the response could be considered new growth rather than reorganization. After one year, six out of 10 basidiocarps exhibited sustained growth from the cut surfaces, again showing substantial growth from the entire cut surface and no preferential growth from the pore field (). The configuration of the newly formed hymenophore was lamellate in some instances, showing that the poroid configuration is not a necessary configuration after perturbation (). The newly formed tubes were oriented perpendicular to the plane of the cut, i.e., following the direction of growth, suggesting that the lamellate configuration reflects growth rather than patterning.
Figure 2. Basidiocarps with resected sides. A. Basidiocarp growing on the same trunk as “S16-20” (see ). It exhibits new growth in the part that remains attached to the trunk, whereas the removed part does not show signs of new growth. The cut was made three weeks prior to the day the photos were taken (resection 26 July 2020, photography 16 August 2020). Notice that the growth response is on the entire cut surface, and it is therefore not restricted to the margin where the pore field is. B. Basidiocarp growing on the same trunk as “tS6-8” (see ), with a resected left side as the basidiocarp shown in A. It exhibits substantial growth from the cut surface. The new growth is not preferentially from the margin (and therefore pore field). In addition, the new hymenophore is predominantly lamellate, showing that a poroid configuration is not the necessary response to perturbed growth.
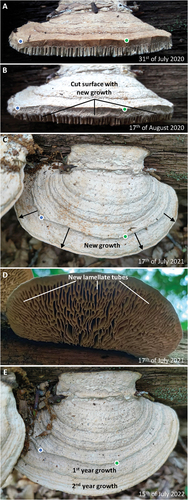
Ten other basidiocarps had their entire margin, including the pore field, resected. Five of these exhibited pronounced growth from cut surfaces already within three weeks after the resection (). Two years after the resection, seven basidiocarps exhibited substantial growth of both new pileus and new hymenophore from the cut surfaces (), which suggests that the pore field is not required for growth. The new hymenophore that had formed distal to the cut surface was lamellate in some cases (). These observations suggest that growth is not dependent on the presence of a pore field. The next experiments were conceived to further test the importance of the pore field by inducing the formation of a new hymenophore while the basidiocarp margin was left intact.
Figure 3. Growth despite resected margin and pore field. A. Resection of the margin of a basidiocarp from location “Flipped trunk 7.” Given that tubes are exposed on the cut surface, the pore field must have been resected. B. An initial growth response occurred already within three weeks. C. One year after the resection, substantial outward growth had taken place (notice the same positions in A–C and E are indicated with blue and green dots). D. The hymenophore that had formed after the resection had a predominantly lamellate configuration. This shows that new growth can occur even if the pore field is resected and that a poroid configuration is not the necessary response to perturbed growth. E. After two years, the basidiocarp still exhibits substantial growth.
Hymenophore formation in flipped basidiocarps.—
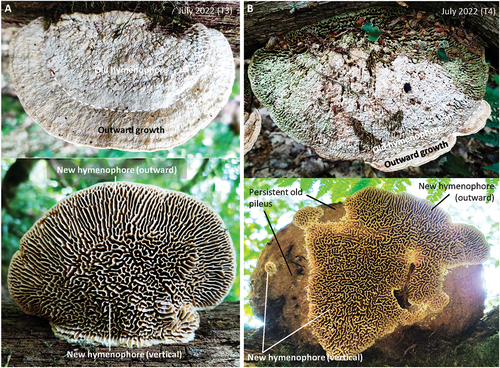
Nine naturally occurring basidiocarps were found with clear signs of having grown in one vertical direction and then later grown in the opposite vertical direction (), i.e., they had been flipped by presumably natural events. These grew on three trunks from different localities (, Flipped trunk natural 1–3). The signs of having been flipped were principally a partially covered hymenophore pattern on the present sky-facing pileus together with neighboring and darkly colored, or old, basidiocarps with sky-facing hymenophores. In the flipped basidiocarps, the new hymenophore that was vertically below the old hymenophore was characterized by a very low prevalence of lamellate tubes (). Lamellate tubes, however, were abundant in the part of the new hymenophore that appeared to have grown outward, since the basidiocarp was flipped (). Basidiocarps from the same trunk and which appeared to have started growing after the trunk was flipped showed the typical daedalean configuration with many lamellate tubes near the base (), as did basidiocarps from different trunks from the same location ().
Figure 4. Effect of growth direction on hymenophore configuration. A. A basidiocarp flipped by natural events in which the old hymenophore is partly obscured by a new pileus and there has been substantial outward growth since being flipped. The new (vertical) hymenophore configuration is almost hydnoid, and lamellate tubes with radial orientation are confined to the new part formed by outward growth. B. Basidiocarp from the same trunk as A with no obvious signs of having been flipped and in which the hymenophore configuration at the base has numerous lamellate tubes. C. Basidiocarp from the same location as A–B, but from a different trunk, in which the hymenophore configuration at the base has numerous lamellate tubes.

For the experimentally induced formation of a new hymenophore, we identified 10 trunks of oak lying on the ground and which had 121 basidiocarps growing on them. The hymenophore configuration of all basidiocarps was daedalean. The trunks were rotated approximately 180 degrees such that the basidiocarps were turned upside down. Already three weeks after being flipped (i.e., by mid-August 2020), some basidiocarps showed clear signs of responding to the new orientation. Most noticeable was the thickening of the tubes that were now facing the sky, and we found this to be occurring in 40 out of 121 basidiocarps. In the remaining 81 basidiocarps, there were no signs of response. In a few of the responding basidiocarps, the thickening was so advanced that pores were sealed (), whereas in others the thickening was in its earliest stage and was barely detectable. We also observed in three out of 121 basidiocarps the formation of a new hymenophore on the old pileus that was now facing the ground (). In these basidiocarps, the configuration of the old tubes was predominately lamellate, whereas the new hymenophore was poroid (). Most noticeably, the new hymenophores were closer to the base than to the margin and the pore field ().
Figure 5. Short-term changes to experimentally flipped basidiocarp. A–B. Basidiocarp from “Flipped trunk 6” where substantial thickening of the tubes has occurred in 22 days. C. New hymenophore in poroid configuration has started to develop on the pileus that faces the ground.

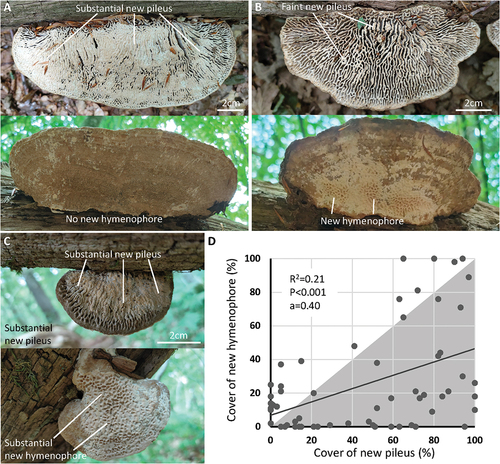
The same 121 flipped basidiocarps were reassessed after one year, and 66 exhibited a response as forming either a new pileus and/or a new hymenophore (). In 44 of the responders, a new hymenophore was forming, and in 10 a new hymenophore covered the entire new underside. No tubes were ever configured as lamellae, in contrast to what could be expected if the hymenophore configuration is fixed. Based on the analysis of 59 responding basidiocarps, we found that the formation of a new pileus had on average proceeded further than the formation of a new hymenophore () (seven out of 66 responding basidiocarps were excluded from this analysis because of 100% coverage of the new pileus and new hymenophore). When analyzed as a linear regression, there was a 0.4% increase in new hymenophore coverage for every 1% increase in new pileus coverage (P < 0.001, alpha = 0.40, 95% confidence interval: 0.19–0.60).
Figure 6. One-year changes to experimentally flipped basidiocarps. A. Example of basidiocarp that has grown a substantial new pileus, whereas there has been no growth of a new hymenophore. B. Example of basidiocarp with faint growth of a new pileus, whereas the growth of a new hymenophore is more pronounced. C. Example of basidiocarp with substantial growth of a new pileus and a new hymenophore. D. Pearson’s linear regression analysis (P < 0.001) of experimentally flipped basidiocarps with growth response (N = 59), showing that most basidiocarps had a more extensive new pileus than new hymenophore (gray triangular area).
That the new hymenophores had a poroid configuration appeared to be transitory, however. By 25 July 2022, basidiocarps exhibited a kind of “broken” poroid configuration (). This configuration approximated the one observed in the naturally flipped basidiocarps; notice, for example, how similar the basidiocarps of are. In both naturally and experimentally flipped basidiocarps, the “broken” poroid configuration was confined to the vertically grown hymenophore, whereas the new hymenophore that had grown outward was predominantly lamellate.
Figure 7. Configuration of new hymenophore two years since being experimentally flipped. A. Example of basidiocarp from Flipped trunk 3 in which the old hymenophore is entirely covered by a new pileus, the old pileus is entirely covered by a new hymenophore, and there has been outward growth. There is a broken poroid configuration to the new hymenophore that has grown vertically, whereas the outward-grown hymenophore is lamellate. B. Example of basidiocarp from Flipped trunk 4 in which the old hymenophore is not fully covered by a new pileus, the old pileus is not fully covered by a new hymenophore, and there has been outward growth. There is a broken poroid configuration to the new hymenophore that has grown vertically, whereas the outward-grown hymenophore is lamellate.
We noticed that a large fraction of the studied basidiocarps were not responsive and that among the responsive basidiocarps there was much variation in the extent of new growth. A final series of experiments were made to try to reproduce a common perturbation to normal growth, namely, growing around obstacles, which would give insight into whether a large fraction of unresponsive basidiocarps is to be expected and at the same time allow for the measurement of growth rates.
Natural growth around obstacles and growth around bands.—
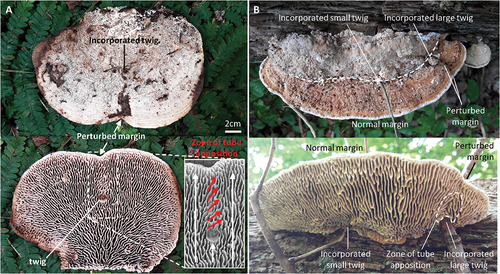
Encountering obstacles such as twigs constitutes a common perturbation to the normal growth of basidiocarps (). Perturbations to normal growth can be revealed as indentations to the margin and altered configuration to the hymenophore (). Greater obstacles tend to associate with greater perturbations (). Based on the observations of 53 basidiocarps in various stages of growing around twigs of various sizes, it was deduced that the encounter of obstacles will entail growth around the obstacle rather than through it. The distance around the obstacle, or perimeter, is greater than the distance through it. Consequently, if all parts of the margin grow approximately at the same rate, the part of the margin that grows around the twig will expand around the twig and less so distally. This results in the indentation of the margin. A zone of tube apposition is formed when margins merge, and this can persist over a number of growth periods. A very similar zone of apposition can be found where two basidiocarps merge (SUPPLEMENTARY FIG. 1). If growth is sustained since the incorporation, the margin may become normal. In this scenario, all parts of the margin are growing outward at a similar rate, and such growth in effect resembles fluid flow around a cylinder (e.g., Mo et al. Citation2013).
Figure 8. Observations on incorporation of naturally occurring obstacles. A. Basidiocarp with a single incorporated twig associating with a single indentation of the margin and a skewed orientation of the lamellate tubes between the twig and the margin. B. Basidiocarp with a small and a large twig incorporated in the same growth zone (dashed line). Only in association with the large twig is the margin (and pileus) perturbed, suggesting that a greater obstacle is associated with greater perturbation.
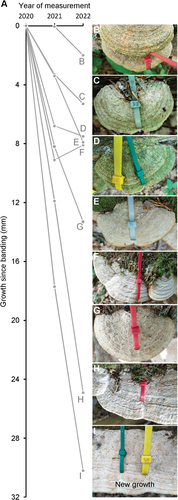
Of the 48 experimentally banded basidiocarps, after one year, 41 were still situated in the original position and had the bands attached (33 single band; 8 double band). One trunk with five banded basidiocarps had fallen over, and bands had been ripped off two basidiocarps; these amounted to the seven lost basidiocarps. Of the 41 remaining basidiocarps, 29 exhibited growth at the margin since banding () and 12 exhibited no growth. The growth of the 29 basidiocarps was on average 6.5 mm in the period July 2020 to July 2021 and with considerable variation between basidiocarps (range 0.3–17.7 mm, standard deviation 4.4 mm). The basidiocarp shown in grew 6.6 mm. In 19 of the 29 growing basidiocarps, the strips appeared to become partially covered by the hymenophore (). This shows geotrophic growth of the hymenophore and suggests that the hymenophore has a capacity to form new hymenophore at some distance from the pore field. The following year, by July 2022, four of the 29 basidiocarps had grown less than 0.5 mm and were consider to have not grown. The remaining 25 basidiocarps had on average grown 10.5 mm from July 2020 to July 2022 and with much variation between basidiocarps (range 1.0–30.2 mm, standard deviation 7.6 mm) (different growth patterns are illustrated in ). In the basidiocarp shown in , the parts that have grown since banding are similar to those of the basidiocarps that have grown around natural obstacles (). Also, that the perturbation to growth is affected by the size of the obstacle () was reproduced in the basidiocarps with bands of two different sizes (). Taken together, these data document that it can be expected that a large fraction of basidiocarps will not respond to perturbations and that there is much variation among the basidiocarps that do respond.
Figure 9. Observations on experimentally banded basidiocarps. A. Basidiocarp with attached 4.8-mm-wide plastic strip (July 2020). The insertion of the plastic strip resulted in a hole in the hymenophore. B. One year later (July 2021), the outward growth was 6.6 mm, such that the margin now embraced the plastic strip. Notice that new hymenophore has appeared in the insertion hole, whereas the surrounding hymenophore did not change configuration. C. After two years (July 2022), the outward growth was 11.6 mm but the merged margin was still perturbed. D. Basidiocarp banded with a standard-size strip (green, 4.8 mm) and a wide strip (yellow, 7.6 mm), 13 months after banding (July 2020 to August 2021). Outward growth (21.5 mm) was most perturbed distal to the wider strip. Lamellate tubes were found distal to the standard-size strip, showing that a poroid configuration is not the default configuration to perturbation. A zone of apposition was more evident distal to the wide strip, indicating wider obstacles imposes greater perturbations.

Figure 10. Variation in magnitude of growth. A. Examples of growth curves to illustrate the variation and magnitude of growth of the monitored basidiocarps. B exemplifies growth in the second year only. C and E exemplify relative slow growth, whereas D and F exemplify little or no growth in the second year. G and H exemplify very substantial growth of basidiocarps from three different trunks. I. The basidiocarp that grew the most of all monitored basidiocarps.
DISCUSSION
That basidiocarps with a fully resected pore field can grow new hymenophore, and pileus, is perhaps the strongest argument against the pore field being necessary for the formation of pores. In vitro experiments, however, document the tremendous capacity of any part of the basidiocarp to deliver totipotent hyphae (Butler Citation1992; Moore Citation2005) and the regeneration of the pore field may be the first response to its resection. It is therefore interesting that basidiocarps with resected sides did not show preferential growth from the intact pore field, but rather a similar growth response across the entire cut surface, irrespective of whether the cut surface is within the hymenophore or pore field. This indicates that the capacity to configure the hymenophore resides in the basidiocarp at large. In support hereof is that the new hymenophore of flipped basidiocarps was not predominantly close to the pore field, even though the pore field was intact, but rather it appeared in spots on the pileus and not infrequently close to the basidiocarp base. Also, appearance of a new hymenophore was often found in the insertion hole of the banded basidiocarps, far from the largely unperturbed pore field. Likely, this reflects the capacity of the basidiocarp to deliver totipotent hyphae. Additionally, the presented data do not even suggest a preferential role of the pore field to drive hymenophore configuration. Instead, the pore field may be a manifestation of growth rather than a driver of it.
It is interesting that the oak mazegill hymenophore configuration appears to be easy to perturb. The default configuration appears to be poroid, since the tubes closest to the margin often take the appearance of pores, but lamellate features are frequent (Ames Citation1913). In cultured Formitopsis cajanderi, the hymenophore configuration may transition from poroid to daedalean (Kennedy and Wong Citation1978). Given the phylogenetic position of oak mazegill within Polyporales (Nagy et al. Citation2016) and the presence of a mostly poroid hymenophore in other species of Daedalea (Han et al. Citation2015; Lindner et al. Citation2011), a poroid configuration is also the primitive condition. Additionally, the data presented here on the flipped basidiocarps show that the initial appearance of the second geotrophically grown hymenophore is invariably poroid. This poroid configuration, however, can be superseded by a more developed and “broken” configuration that is not normally found. The duration of light exposure can perturb the poroid configuration in in vitro experiments on Phellinus contiguus (Butler and Wood Citation1988), but given the in situ setting reported here, duration of light is not a likely explanation for the “broken” configuration. Also, the poroid configuration is not the inevitable response to perturbation, since the hymenophores that grew from cut surfaces tended to be lamellate. Likely, then, lamellate tubes form under the influence of an additional factor or factors, one of which may be the rate of outward or centrifugal growth. It is conceivable that the presence and length of lamellate tubes has a relation to growth rate and that a predominantly poroid hymenophore reflects a slowly grown basidiocarp. Gills compared with tubes have a greater hymenial surface (Clémençon Citation2012), and to the extent that lamellate tubes resemble gills, then the formation of lamellate tubes may be correlated to particular spore-releasing conditions. If the factors can be identified that lead to particular hymenophore configurations such as poroid, broken, and lamellate, the growth history of each basidiocarp is written in its hymenophore, which in turn likely relates to the surrounding environment.
The growth rate was found to be on average some 5 mm, in the period July 2020 to July 2022. This suggests that large basidiocarps from the same localities, some of which clearly exceeds a base-to-margin distance of 5 cm, may be more than a decade old, and an age of two decades seems plausible for some basidiocarps. The growth rate varied more than an order of magnitude, between 0.5 and 15 mm per year, and if slowing-growing basidiocarps can reach large sizes, such basidiocarps can be several decades old. One caveat to the accuracy of the measured growth rates is that they were measured on basidiocarps that had been damaged by insertion of one or two plastic strips. Although it was not obvious that banded basidiocarps grew slower than the neighboring and intact basidiocarps, it was not ruled out that their growth was affected by the banding. It is more likely, perhaps, that the marked variation in growth rate among basidiocarps reflects the availability of nutrients, which itself may reflect the volume of available substrate and the number of basidiocarps growing from it. Analysis of the flipped basidiocarps showed that a new pileus appears faster than a new hymenophore. This fits the notion that in trimitic basidiocarps of polypores, such as the oak mazegill (Lindner et al. Citation2011), the pileus differentiates earlier than the hymenophore (Butler Citation1992).
CONCLUSION
Basidiocarps of the oak mazegill do not grow fast, but they can attain a very substantial size over time. Consequently, they can reach a considerable age. Despite their growth rate, they can exhibit a pronounced response to reorientation and resection within weeks. This response includes the formation of a new hymenophore, which seems to grow from the basidiocarp at large. Therefore, it is suggested that the pore field does not drive hymenophore configuration. Perhaps the pore field is a manifestation of growth rather than a driver of it.
Supplemental Material
Download Zip (5.3 MB)ACKNOWLEDGMENTS
The author expresses gratitude to the inhabitants of Nonbo hede 6 and to Irina Sergeeva for technical support and critical reading of a draft version of the manuscript.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00275514.2023.2227553
LITERATURE CITED
- Ames A. 1913. A consideration of structure in relation to genera of the Polyporaceae. Ann Mycol. 11:211–253.
- Butler GM. 1988. Pattern of pore morphogenesis in the resupinate basidiome of Phellinus contiguus. Trans Br Mycol Soc. 91:677–686. doi:10.1016/S0007-1536(88)80044-5.
- Butler GM. 1992. Location of hyphal differentiation in the agar pore field of the basidiome of Phellinus contiguus. Mycol Res. 96:313–317. doi:10.1016/S0953-7562(09)80944-1.
- Butler GM, Wood AE. 1988. Effects of environmental factors on basidiome development in the resupinate polypore Phellinus contiguus. Trans Bri Mycol Soc. 90(1):75–83. doi:10.1016/S0007-1536(88)80182-7.
- Clémençon H. 2012. Cytology and plectology of the Hymenomycetes. 2nd rev. ed. Stuttgart (Germany): Gebrüder Borntraeger Verlagsbuchhandlung. p. 520.
- Corner EHJ. 1932. The fruit-body of Polystictus xanthopus Fr. Ann Bot. 46:71–111. doi:10.1093/oxfordjournals.aob.a090319.
- Han ML, Vlasak J, Cui B-K. 2015. Daedalea americana sp. nov.(Polyporales, Basidiomycota) evidenced by morphological characters and phylogenetic analysis. Phytotaxa. 204(4):277–286. doi:10.11646/phytotaxa.204.4.4.
- Jensen B, Coolen BF, Smit TH. 2020. Hymenophore configuration of the oak mazegill (Daedalea quercina). Mycologia. 112:895–907. doi:10.1080/00275514.2020.1785197.
- Kennedy LL, Wong WM. 1978. Fomitopsis cajanderi: development in nature and in culture. Canad J Bot. 56:2319–2327. doi:10.1139/b78-280.
- Lindner DL, Ryvarden L, Baroni TJ. 2011. A new species of Daedalea (Basidiomycota) and a synopsis of core species in Daedalea sensu stricto. N Am Fungi. 6:1–12. doi:10.2509/naf2011.006.004.
- Mo W, Jensen A, Liu PL. 2013. Plunging solitary wave and its interaction with a slender cylinder on a sloping beach. Ocean Eng. 74:48–60. doi:10.1016/j.oceaneng.2013.09.011.
- Money NP. 2002. Mushroom stem cells. Bioessays. 24:949–952. doi:10.1002/bies.10160.
- Moore D. 2005. Principles of mushroom developmental biology. Int J Med Mushrooms. 7:79–101. doi:10.1615/IntJMedMushr.v7.i12.90.
- Nagy LG, Riley R, Tritt A, Adam C, Daum C, Floudas D, Sun H, Yadav JS, Pangilinan J, Larsson K-H, et al. 2016. Comparative genomics of early-diverging mushroom-forming fungi provides insights into the origins of lignocellulose decay capabilities. Mol Biol Evol. 33:959–970. doi:10.1093/molbev/msv337.
- Wang Z, Oron E, Nelson B, Razis S, Ivanova N. 2012. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 10:440–454. doi:10.1016/j.stem.2012.02.016.