Abstract
A boroscope and mini-rhizotrons were used to explore root growth patterns in five different ryegrasses grown outdoors under simulated field edaphic conditions including drought. Few major differences in root counts were found among the ryegrasses. The seasonal patterns of root counts in well-watered treatments showed an autumn peak that was later (or even suppressed) as autumn soil temperatures increased in consecutive years. Seasonal patterns were not measurable at more than 7 cm depth down the profile. The summer drought resulted in an increase in root counts right down the profile, which started about a month after the drought began. This was followed by rapid death of roots in the top 15 cm of soil but lower death rates deeper in the soil. After rewetting of the soil, there was a delay of approximately 1 month before a rapid increase in root production occurred. This overcame the apparent high soil temperature inhibition of autumn root growth in the well-watered control treatment.
Keywords:
Introduction
Under New Zealand (NZ) conditions, the typical seasonal pattern of nodal root formation in perennial ryegrass (Lolium perenne L.) is for high rates of new root formation in the autumn, declining over winter to minimum rates in spring and summer (Jacques & Schwass Citation1956; Caradus & Evans Citation1977). Declining soil temperatures are associated with new root formation in ryegrass (Garwood Citation1968; Caradus & Evans Citation1977), and low rates of root formation over spring/summer are likely to be caused by diversion of photosynthate to reproductive tissues and soil moisture deficits that impede the establishment of new roots (Soper Citation1958). There is an apparent synchrony between leaf ageing and root initiation in ryegrass (Yang et al. Citation1998), and Hunt & Thomas (Citation1985) suggested that rates of ryegrass root appearance were determined by leaf appearance rates rather than rates of tillering. Growing conditions could therefore influence root formation patterns indirectly through effects on leaf development.
On deep, well-drained river silt at Palmerston North, Jacques (Citation1943) found ryegrass roots down to 1.8 m depth with 73% of root dry weight (DW) between 0 and 30 cm and only 5% of root DW below 90 cm. Under Canterbury conditions, ryegrass roots reached 80 cm depth, with approximately 60% of root DW in the top 20 cm (Gibbs Citation1986). Repeated samplings from mid-summer to late autumn of pastures growing on three different Waikato soils showed negligible effect of soil type on root distribution (Houlbrooke Citation1996). On average 75% of root DW was at 0–7 cm depth, 11% at 7–14 cm, 9% at 14–21 cm and 5% at 21–28 cm. Rooting depth of perennial ryegrass swards seems to be strongly influenced by subsoil physical conditions. On a Tokomaru silt loam soil at Palmerston North, ryegrass root production between 25 and 60 cm depth declined from 1.3 kg DW ha−1 day−1 (18% of total root production) in mid-January to 0.1 kg DW ha−1 day−1 (4% of total root production) in mid-May as the soil became wet and anoxic at depth (Matthew Citation1992).
The number of ryegrass phytomers bearing roots may be double the number bearing leaves, i.e. there is a slower turnover time for roots than for leaves (Yang et al. Citation1998). The slower turnover time for roots may be appropriate to a gradual seasonal change in root distribution—more surface-rooted in winter, deeper-rooted in summer (Matthew Citation1992). Death of ryegrass roots was apparent 144 days after sowing on a Wakanui silt loam near Lincoln (Gibbs & Reid Citation1992) with root longevities of 36–55 days, and the root decomposition rate in the top 10 cm of soil had a half-life of 254 days (Gibbs Citation1986). Estimates of ryegrass root production derived from instantaneous measurements can underestimate gross root production by 45% depending on sampling frequency because of death and decay of roots between samplings (Gibbs & Reid Citation1992).
There has been little research on roots of NZ perennial ryegrasses, but interest in root systems is increasing because of their importance in drought tolerance (Kemp & Culvenor Citation1994), nitrate uptake (Nichols & Crush Citation2007) and the carbon balance of pastoral soils (Ghani et al. Citation2010). Houlbrooke et al. (Citation1997) compared the cultivar Yatsyn and two breeding lines in a glasshouse experiment and showed that root growth of Yatsyn ryegrass was less sensitive than two ryegrass breeding lines to increased soil bulk density. Matthew et al. (Citation1998) found evidence of differences in nodal frequency of root production between two ryegrass breeding populations grown in plots at Ruakura, and Crush et al. (Citation2009) reported differences in root/shoot DW ratios among ryegrass breeding lines in a glasshouse investigation. These results show that there are differences in root production and observed root mass among ryegrasses, and it seems likely that environmental factors have a major influence on this variation. This paper reports on root growth patterns in five different ryegrasses grown outdoors under simulated field edaphic conditions.
Materials and methods
The experiment was conducted in three bins 7.85×1.13×0.6 m depth. Details of the system, soils and planting are given in Wedderburn et al. (Citation1989a). Each bin was filled with 46 cm of an acid Kaawa yellow brown earth subsoil, and overlain with a 5 cm layer of the subsoil mixed with a Naike brown granular loam topsoil and a 7 cm layer of Naike topsoil. Soils were packed to field volume weights. The topsoil pH was 5.0 with 33 ppm exchangeable Al and 32 ppm exchangeable Al in the subsoil (Wedderburn et al. Citation1989a). The five ryegrass populations were:
-
hill ryegrasses grown from seed from a polycross of 1500 plants collected from North Island hill pastures (Wedderburn et al. Citation1989b)
-
a Whatawhata selection derived from crossing plants from the hill country population that had demonstrated tolerance to moisture stress (Wedderburn et al. Citation1990)
-
the cultivar Yatsyn
-
a ryegrass ecotype selected from dairy pastures at least 20 years old
-
a ryegrass selected for tolerance of aluminium in hydroponic culture (Edmeades et al. Citation1991) and showing superior performance under drought stress in root bins with a reconstructed acid profile (Wheeler et al. Citation1992).
The mini-rhizotrons (viewing tubes) were clear acrylic (25 mm external diameter, 19 mm internal diameter and 1000 mm length) with graduation lines scribed onto the outside wall with a lathe. Each tube had four equidistant longitudinal lines, and, commencing 250 mm from the top end, six circumferential lines at 25 mm spacing, three at 50 mm spacing and four at 100 mm spacing. The graduation lines were colour coded using Stabilo overhead transparency pens. The bottom of each tube was sealed with a rubber bung and co-polymer sealant and the 250 mm above-ground part of each tube was covered with black adhesive tape to exclude light. Between measurements, each tube was closed with a rubber bung. Two tubes were inserted into each replicate plot at an angle of 45° into a hole made with a 25 mm external diameter soil corer. The orientation of the colour-coded longitudinal lines was the same for all the tubes.
Roots were viewed through the tubes using an Olympus MKII borescope attached to an Olympus KMI KLS-301L cold light source. Each longitudinal line was followed and the number of roots intersecting the line was recorded for successive depth increments. Readings (which took 4 h for the 60 tubes) were taken fortnightly. Measurements commenced in November 1992 when the plants were 7 months old and continued until December 1995.
Sward height was maintained between 50 and 75 mm by regular clipping. Urea was applied monthly at 25 kgN ha−1 to all bins. Tiller counts were done on five ryegrass plants growing directly in line with each tube, on eight occasions between 19 March 1993 and 28 April 94. The tiller count data were analysed by analysis of variance (ANOVA) for individual sampling dates.
Soil moisture was kept at non-limiting levels at all times prior to the drought treatment. Rainout shelters (Wedderburn et al. Citation1989a) were installed over two bins in December 1994 until March 1995 while the third bin was kept well-watered.
The root count data were analysed using a Bayesian smoothing technique (flexi, Upsdell Citation1994). Graphs summarising the results were produced using Microsoft Excel, but significance of treatment differences is based on the flexi analysis.
Results
There were significant differences in tiller numbers among the ryegrass types at each sample date (). The Hill ryegrass ecotypes had more tillers than Yatsyn or the aluminium selection. Tiller numbers for the Whatawhata ryegrass selection and the dairy ecotype were intermediate between the Hill ecotype and Yatsyn. This pattern and the numbers of tillers were consistent over the measurement year.
Table 1 Tiller numbers of the five ryegrass types counted on eight occasions over the first year from establishment of a closed sward. Values are means for five plants/type.
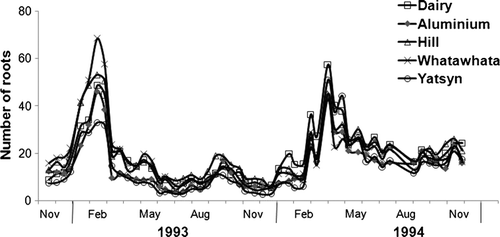
Over the first 2 years of the experiment, when there was no moisture stress, all the ryegrasses displayed very similar temporal patterns for total root counts (). The only statistically significant (P<0.05) difference in total root counts among the ryegrasses was recorded in November and December 1992, when the Whatawhata ryegrass had higher root counts than Yatsyn. Average monthly root counts at this time were 19±14 and 9±7 for the Whatawhata and Yatsyn ryegrasses, respectively. Root numbers peaked in early February in year 1, with minimum values in June–July (), while in year 2 maximum counts occurred in late March with minima in August.
Fig. 1 Average fortnightly total root counts between November 1992 and November 1994 for five ryegrasses grown under well-watered and nitrogen-fed conditions in experimental root bins.
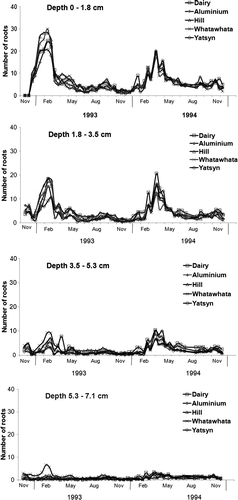
Total root count numbers and seasonal variation in counts declined rapidly with increasing depth () during the well-watered phase of the experiment. On average, only 21% of root counts were recorded below 7 cm depth. Between 7 and 42 cm depth, there was no measurable seasonal pattern in total root counts.
Fig. 2 Average fortnightly total root counts for 0–1.8, 1.8–3.5, 3.5–5.3 and 5.3–7.1 cm soil depths for five ryegrasses grown from November 1992 to November 1994 under well-watered and nitrogen-fed conditions in experimental root bins.
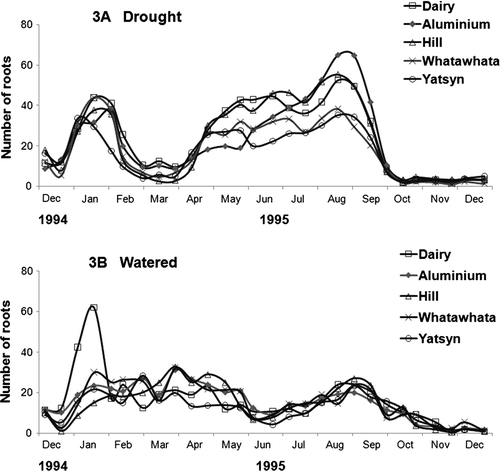
Imposition of moisture stress resulted in an increase in root counts in all five ryegrasses in the second month of the treatment (A), followed by a rapid decline in the number of roots counted. After rewetting, there was an initial delay in new root production in the surface soil followed by a rapid recovery of root numbers with counts peaking in August–September followed by a rapid decline. The well-watered ryegrass controls had lower root counts than the drought-stress plants, except in the immediate post-drought period, and did not display any strong autumnal seasonal pattern. Under moisture stress, the aluminium selection had significantly (P<0.05) higher root counts between June and October than both Yatsyn and the Whatawhata ryegrass. The dairy ecotype had higher (P<0.05) root counts in March–June than Yatsyn. In the well-watered controls, root counts did not vary much month to month, apart from decreases in June–July and from September to December (B). There were no significant differences in total root count among the ryegrass types in the well-watered treatment and the high values during January for the dairy ecotype arose from two of the six replicate tubes recording unusually high root numbers.
Fig. 3 Average fortnightly total root counts between December 1994 and December 1995 for five ryegrasses with no water applied for the first three months (A) and well-watered (B).
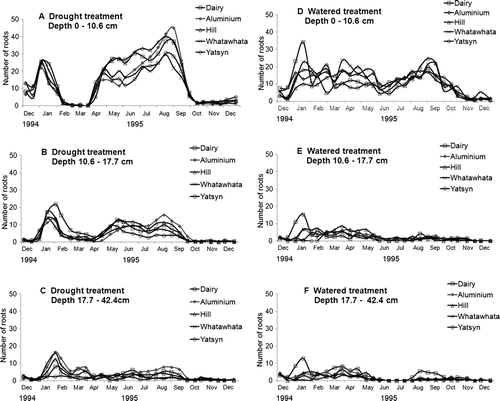
The increase in root counts observed in the second month after imposition of the drought treatment was most pronounced in the 0–15 cm soil depth, but was measurable at all depths down the profile. Peak root count numbers during this phase of the drought treatment occurred later at successive measurement depths (). Root counts decreased to lower levels in the 0–15 cm zone in February and March than in the 15–25 and 25–60 cm layers. In the drought recovery phase, root counts increased at all depths in the drought treatment, with higher counts at all depths than in the non-drought controls ().
Fig. 4 Total root counts between December 1994 and December 1995 at 0–15, 15–25 and 25–60 cm depth for five ryegrasses with an initial 3 month drought treatment (A, B and C) or well-watered (D, E and F).
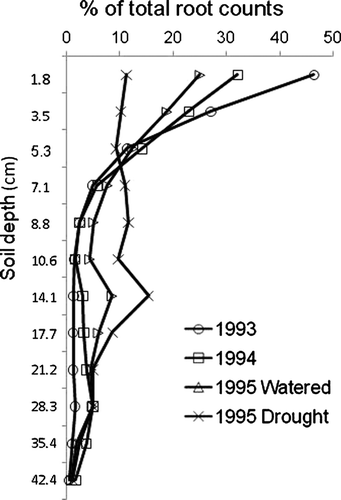
Average annual root system shape, expressed as percentage of root counts at each sample depth, changed over time in the well-watered bins (). As the swards aged, there was a change in the proportion of root numbers found down the soil profile over successive years with less counted in the shallow layers and more at depth. Annual average root system shape in the drought treatment was characterised by a low percentage of roots in the top 4 cm of soil and a greater percentage of roots at 5–20 cm than in the watered treatments ().
Discussion
The frequent defoliation that maintained sward height between 50 and 75 mm effectively simulated a continuous grazing regime. This would have advanced the Hill and Whatawhata ryegrass ecotypes over a more erect, low tiller density cultivar like Yatsyn (Bluett et al. Citation1999). The present study was not designed to examine whether root growth patterns were affected by possible ryegrass type×defoliation frequency interactions, but other research has shown that root mass and new root production are relatively insensitive to grazing management (Matthew et al. Citation1991). Tiller numbers per plant did vary among the ryegrass populations, and confirmed that the unselected hill country ryegrass has more tillers per plant than Yatsyn (Wedderburn et al. Citation1989b). At each of the eight measurements, the unselected dairy ecotype had higher tiller numbers than Yatsyn or the aluminium selection ryegrass, and in most instances this was statistically significant (P<0.05). For well-watered bins, the only difference in total root counts among the ryegrasses occurred in the first 2 months of the experiment. At this time, the Whatawhata selection, which was intermediate for tillers per plant, had higher total root counts than Yatsyn, but the highest tiller-number ryegrasses (hill and dairy types) did not differ from Yatsyn. This suggests that tiller size/density compensation processes (Matthew et al. Citation1996) were being expressed and, as reported by Matthew et al. (Citation1991), the results for root counts were not confounded by plant type×defoliation effects. Other work (Crush et al. Citation2004) suggests that any differences in endophyte strains among the ryegrasses would have only very minor effects on root distribution. The two-weekly root counts would have been sufficiently frequent to minimise errors associated with root death and decay between successive samplings (Gibbs & Reid Citation1992). This makes us confident that the temporal root count patterns were not influenced by the methodology. The rapid increases in root counts in the first two autumns and during drought recovery, and rapid decreases in September following summer drought, demonstrate how dynamic the root population of ryegrass is under a very frequent and regular defoliation regime.
In the absence of moisture stress, ryegrass showed a pronounced peak in total root counts in February 1993 and April 1994. This conforms to the generally accepted pattern of high autumn rates of new root formation in ryegrass (Jacques & Schwass Citation1956; Caradus & Evans Citation1977) or, more precisely, a strongly positive balance for root development/senescence processes. January and February soil temperatures were 2°C higher in 1994 than in 1993 () and these elevated temperatures probably explain the delay in peak root counts in 1994 because declining soil temperatures are associated with higher rates of new root formation in ryegrass (Garwood Citation1968; Caradus & Evans Citation1977). In 1995, ryegrasses in the well-watered treatment showed no sharp autumn peak in total root counts. Instead, root counts remained relatively constant from January to May at about one-third of the number counted in the autumn peaks of the previous two years. Soil temperatures during March and April 1995 were 4–5°C higher than in the first two years; this probably explains the absence of an autumn peak in root counts in 1995, despite adequate soil moisture.
Table 2 Average monthly screen air and 10 cm soil temperatures recorded at the Whatawhata meteorological station during the experiment.
Total root counts in the well-watered bins in November and December seemed to be closely related to the previous autumn root count patterns. This suggests that, depending on the previous autumn, ryegrass root systems could be in variable condition to cope with the stresses of flowering and/or an early dry spell. For example, average November/December total counts varied from 7.0 in 1993 to 15.3 in 1994 and 2.9 in 1995 (LSD0.05 between years = 1.5). The highest November/December root counts followed a later autumn peak root count and persistence of roots through winter and into spring. The lowest November/December counts were in 1995 when no autumn peak in root counts occurred. Year to year variation in ryegrass yields and persistence will be influenced by these changes in root system development.
The apparent effect of autumn soil temperatures on the balance of root development/senescence in these well-watered soils suggests that seasonal patterns of root frequencies are going to vary with slope, aspect and region. As a result, root development patterns based on one year of NZ data (Jacques & Schwass Citation1956) or northern hemisphere (lower temperature) sites should not be extrapolated generally throughout NZ. The same caveat applies to the results of short-term studies of carbon partitioning and translocation to roots in pastures (e.g. Saggar et al. Citation1997), which will be very time bound and site specific.
All the ryegrasses responded to the onset of a summer drought treatment with an initial increase in root counts right down the profile. Prospecting for moisture is a short-term drought-avoidance strategy that has been observed in some genotypes of a perennial ryegrass mapping population (Crush et al. Citation2007), in a Mediterranean tall fescue cultivar (Assuero et al. Citation2002) and other grasses (DaCosta & Huang Citation2006; Degu et al. Citation2008). As the drought continued, root counts decreased rapidly to close to zero in the uppermost root zones, indicating high root death rates. Root death rates decreased down the profile where, presumably, there was still some moisture. The aluminium selection ryegrass was notable for having significantly higher root counts during the post-drought winter than Yatsyn, and this was particularly noticeable deeper in the soil (). Wheeler et al. (Citation1992) reported superior post-drought growth of the same selection, in the same acid soil, and speculated that the ability to explore a larger soil volume and moisture pool may have been responsible. The results from the present experiment support this proposition. After rewetting of the soil there was a lag of about 1 month before a rapid increase in root counts in the topsoil was observed. This delay may be a mechanism to prevent energy loss to the plant caused by premature growth of new roots into a possibly ephemeral soil moisture supply if the rewetting is not sustained. There are very few reports in the literature on early post-drought growth of ryegrass roots. Our results suggest that the delay in phosphorus uptake of 2–3 weeks after rewetting of droughted ryegrass reported by Jupp & Newman (Citation1987) may have been caused by a temporary inhibition of new root growth. The effect of drought on autumn root growth in rewetted soils completely overrode the apparent temperature controls on root initiation seen in the well-watered treatments. Root counts following drought were relatively high throughout winter, with a broad peak in September. This post-drought effect provides a further complication for any attempt to describe a typical seasonal pattern of ryegrass root growth in environments with variable summer soil moisture supply. The pattern of progressively less surface rooting and more roots at depth in successive years () was unexpected, and contradicts some older Finnish and Dutch results for unidentified swards reported in Troughton (Citation1957). When measurements began, the swards were 7 months old. In agricultural terms, these were initially young swards but were well past the seedling stage. This suggests that the changing annual pattern was not associated with the transition from seminal to nodal rooting.
The maximum observation depth in the experiment was 42.5 cm. This is much less than the maximum root depth observed for ryegrass recorded in Canterbury and the Manawatu (Jacques Citation1943; Gibbs Citation1986), but in those studies 60–70% of root DW was in the top 20–30 cm of soil. The proportion of root counts in the top 7 cm in this experiment agreed well with root DW distribution under Waikato pastures (Houlbrooke et al. 1996). Data from the Netherlands reviewed by Troughton (Citation1957) suggest that root counts may not be a good indicator of root mass in the topsoil. A high proportion of thicker roots close to the surface could lead to underestimation of root DW derived from root count data. Seasonal fluctuations in root counts in the well-watered treatments were much more pronounced in the surface soil than at depth, but the response to the onset of drought and recovery from drought were observable deeper in the profile where non-droughted root systems were relatively inert. These results emphasise the powerful effect of drought on ryegrass root systems, both during the drought and in drought recovery. Projections of the future NZ climate indicate an increased risk of drought and drought severity in the drier eastern and northern regions (Tait et al. Citation2008). The consequent damage to ryegrass root systems could mean that perennial ryegrass cultivars have to be replaced by other grasses.
Results for the first two years in the well-watered treatments agreed with the accepted pattern for ryegrass root development under NZ conditions (Jacques & Schwass Citation1956; Caradus & Evans Citation1977) of an autumn peak and early summer minimum rates. The autumn peak was displaced later in the second year, and largely suppressed in the third year, probably due to soil temperatures being higher later into the autumn. A severe summer drought treatment completely overrode the apparent high soil temperature inhibition of autumn root growth and this control mechanism would facilitate the recovery of ryegrass plants after a summer drought. The year-to-year variation and effect of drought on root counts, coupled with the extremely rapid decreases in root counts in spring, demonstrate that ryegrass root systems are dynamic. Soil moistures and temperatures are likely to vary with slope, aspect and locality, as well as over time, so extrapolation of root mass or function (e.g. carbon deposition) from short-term studies to annual or regional budgets could be fraught with errors.
Acknowledgements
The authors thank Dr Martin Upsdell for flexi analysis of the root count data, Lily Ouyang for preparation of the summary graphs and MA Tucker for technical assistance with the root bins and data collection.
References
- Assuero , SG , Matthew , C , Kemp , P , Barker , DJ and Mazzanti , A . 2002 . Effects of water deficit on Mediterranean and temperate cultivars of tall fescue . Australian Journal of Agricultural Research , 53 : 29 – 40 .
- Bluett , SJ , Hodgson , J , Kemp , PD and Barry , TN . 1999 . Survival, reproductive development and density of tillers in pure swards of Aries HD and Yatsyn 1 perennial ryegrass (Lolium perenne) . Proceedings of the New Zealand Grassland Association , 61 : 197 – 201 .
- Caradus , JR and Evans , PS . 1977 . Seasonal root formation of white clover, ryegrass, and cocksfoot in New Zealand . New Zealand Journal of Agricultural Research , 20 : 337 – 342 .
- Crush , JR , Popay , AJ and Waller , J . 2004 . Effect of different Neotyphodium endophytes on root distribution of a perennial ryegrass (Lolium perenne L.) cultivar . New Zealand Journal of Agricultural Research , 47 : 345 – 349 .
- Crush , JR , Easton , HS , Waller , JE , Hume , DE and Faville , MJ . 2007 . Genotypic variation in patters of root distribution, nitrate interception, and moisture stress response of a perennial ryegrass (Lolium perenne L.) mapping population . Grass and Forage Science , 62 : 265 – 273 .
- Crush , JR , Nichols , SN , Easton , HS , Ouyang , L and Hume , DE . 2009 . Comparisons between wild populations and bred perennial ryegrasses for root growth and root/shoot partitioning . New Zealand Journal of Agricultural Research , 52 : 161 – 169 .
- DaCosta , M and Huang , B . 2006 . Changes in carbon partitioning and accumulation patterns during drought and recovery for colonial bentgrass, creeping bentgrass, and velvet bentgrass . Journal of the American Society for Horticultural Science , 131 : 484 – 490 .
- Degu , HD , Ohta , M and Fujimura , T . 2008 . Drought tolerance of Eragrostis tef and development of roots . International Journal of Plant Science , 169 : 768 – 775 .
- Edmeades DC , Wheeler DM , Christie RA 1991 . The effects of aluminium and pH on the growth of a range of temperate grass species and cultivars . In: Wright RJ , Baligar VC , Murrmann RP Plant–soil interactions at low pH . Dordrecht , Kluwer . 913 924 .
- Garwood , EA . 1968 . Some effects of soil-water conditions and soil temperature on the roots of grasses and clover. 2. Effects of variation in soil-water content and temperature on root growth . Journal of the British Grassland Society , 23 : 117 – 128 .
- Ghani , A , Müller , K , Dodd , M and Mackay , A . 2010 . Soil carbon and nitrogen: dissolved organic matter movement in some contrasting New Zealand pasture soils . European Journal of Soil Science , 62 : 525 – 538 .
- Gibbs RJ 1986 . Changes in soil structure under different cropping systems . Unpublished PhD thesis , Lincoln College, University of Canterbury . 131 132 .
- Gibbs , RJ and Reid , JB . 1992 . Comparison between net and gross root production by winter wheat and by perennial ryegrass . New Zealand Journal of Crop and Horticultural Science , 20 : 483 – 487 .
- Houlbrooke DJ 1996 . Subsoiling and soil compaction effects on soil physical properties and pasture response . Unpublished MSc thesis , University of Waikato . 147 149 .
- Houlbrooke , DJ , Thom , ER , Chapman , R and McLay , CDA . 1997 . A study of the effects of soil bulk density on root and shoot growth of different ryegrass lines . New Zealand Journal of Agricultural Research , 40 : 429 – 435 .
- Hunt , WF and Thomas , VJ . 1985 . Growth and developmental responses of perennial ryegrass grown at constant temperature II. Influence of light and temperature on leaf, tiller and root appearance . Australian Journal of Plant Physiology , 12 : 69 – 76 .
- Jacques , WA . 1943 . Root-development in some common New Zealand pasture plants II. Perennial ryegrass (Lolium perenne) cocksfoot (Dactylis glomerata) and white clover (Trifolium repens) . New Zealand Journal of Science and Technology , A25 : 91 – 117 .
- Jacques , WA and Schwass , RH . 1956 . Root development in some common New Zealand pasture plants VII. Seasonal root replacement in perennial ryegrass (Lolium perenne) Italian ryegrass (L. multiflorum) and tall fescue (Festuca arundinacea) . New Zealand Journal of Science and Technology , 37A : 569 – 583 .
- Jupp , AP and Newman , EI . 1987 . Phosphorus uptake from soil by Lolium perenne during and after severe drought . Journal of Applied Ecology , 24 : 979 – 990 .
- Kemp , DR and Culvenor , RA . 1994 . Improving the grazing and drought tolerance of temperate perennial pastures . New Zealand Journal of Agricultural Research , 37 : 365 – 378 .
- Matthew C 1992 . A study of seasonal root and tiller dynamics in swards of perennial ryegrass . Unpublished PhD thesis , Massey University .
- Matthew C , Xia JX , Chu ACP , Mackay AD , Hodgson J 1991 . Relationship between root production and tiller appearance rates in perennial ryegrass (Lolium perenne L.) . In: Atkinson D Plant root growth . Oxford , Blackwell Scientific, British Ecological Society Special Publication No. 10 . 281 290 .
- Matthew , C , Hernandez-Garay , A and Hodgson , J . 1996 . Making sense of the link between tiller density and pasture production . Proceedings of the New Zealand Grassland Association , 57 : 83 – 87 .
- Matthew , C , Yang , JZ and Potter , JF . 1998 . Determination of tiller and root appearance in perennial ryegrass (Lolium perenne) swards by observation of the tiller axis, and potential application in mechanistic modelling . New Zealand Journal of Agricultural Research , 41 : 1 – 10 .
- Nichols , SN and Crush , JR . 2007 . Selecting forage grasses for improved nitrate retention – a progress report . Proceedings of the New Zealand Grassland Association , 69 : 207 – 211 .
- Saggar , S , Hedley , C and Mackay , AD . 1997 . Partitioning and translocation of photosynthetically fixed 14C in grazed hill pastures . Biology and Fertility of Soils , 25 : 152 – 158 .
- Soper , K . 1958 . Effects of flowering on the root system and summer survival of ryegrass . New Zealand Journal of Agricultural Research , 1 : 329 – 340 .
- Tait , A , Baisden , T , Wratt , D , Mullan , B and Stroombergen , A . 2008 . An initial assessment of the potential effects of climate change on New Zealand agriculture . New Zealand Science Review , 65 : 50 – 56 .
- Troughton A 1957 . The underground organs of herbage grasses . Commonwealth Bureau of Pastures and Field Crops , Bulletin 44 .
- Upsdell , MP . 1994 . Bayesian smoothers as an extension on non-linear regression . The New Zealand Statistician , 29 : 66 – 81 .
- Wedderburn , ME , Pengelly , WJ and Tucker , MA . 1989a . Simulated soil profiles as a plant screening technique . Proceedings of the New Zealand Grassland Association , 50 : 249 – 252 .
- Wedderburn , ME , Pengelly , WJ , Tucker , MA and di Menna , ME . 1989b . Description of ryegrass removed from North Island hill country . New Zealand Journal of Agricultural Research , 32 : 521 – 529 .
- Wedderburn , ME , Tucker , MA , Pengelly , WJ and Ledgard , SF . 1990 . Responses of a New Zealand North Island hill perennial ryegrass collection to nitrogen, moisture stress, and grass grub (Costelytra zealandica) infestation . New Zealand Journal of Agricultural Research , 33 : 405 – 411 .
- Wheeler , DM , Edmeades , DC , Smith , DR and Wedderburn , ME . 1992 . Screening perennial; rye-grass from New Zealand for aluminium tolerance . Plant and Soil , 146 : 9 – 19 .
- Yang , JZ , Matthew , C and Rowland , RE . 1998 . Tiller axis observations for perennial ryegrass (Lolium perenne) and tall fescue (Festuca arundinacea): number of active phytomers, probability of tiller appearance, and frequency of root appearance per phytomer for three cutting heights . New Zealand Journal of Agricultural Research , 41 : 11 – 17 .