Abstract
The nitrous oxide (N2O) molecule is both a greenhouse gas and a precursor to the formation of nitric oxide (NO) in the stratosphere, which is subsequently involved in catalytic destruction of ozone (O3). Tropospheric NO can form NO2 with both gases (NO
x
) able to react with volatile organic compounds to produce the detrimental O3 molecule. Information on how NO and N2O emissions respond to agricultural management is lacking. Limited data suggest N2O emissions following bovine urine deposition may vary with soil pH. This work hypothesised that soil liming would also reduce NO
x
emissions due to enhanced nitrification and reduced opportunity for HNO2 formation as soil pH increased. Bovine urine was applied (575 kg N/ha) to repacked soil cores (34% water-filled pore space) that ranged in pH from 4.4 to 7.6, and these were maintained at 21°C. With the exception of the pH 4.4 treatment, increases in NO–N fluxes occurred during the first 11 days, when nitrification was proceeding. Increasing the initial soil pH enhanced the net ammonium depletion rate. The loss of NO–N, expressed as a percentage of the net ammonium depletion rate, decreased with increasing initial pH during this first 11 days with NO–N fluxes reduced due to competition between abiotic and biological demands for . After 21 days, the optimal pH for the net NO–N flux was observed to be 5.7 to 6.0. However, cumulative NO–N fluxes after 35 days showed little variation due to initial soil pH and ranged from 0.02 to 0.05% of urine–N applied. The cumulative N2O–N flux ranged from 0.04 to 0.09% of urine–N applied with a linear increase from pH 5.7 to pH 7.6. Based on these results, where soils are relatively dry, liming acid soils from pH 5.5 to pH 6.5 will not affect cumulative NO fluxes from urine patches. However, cumulative N2O fluxes may be enhanced.
Introduction
Both nitric oxide (NO) and nitrous oxide (N2O) may be emitted following urine deposition onto grazed pasture soils (Galbally & Roy Citation1978; Maljanen et al. Citation2007) due to nitrification, nitrifier-denitrification, denitrification and abiotic mechanisms (Firestone & Davidson Citation1989; Russow et al. Citation2009). Abiotic production occurs due to the release of NO being a function of the presence of nitrous acid (HNO2), which increases as soil pH declines given sufficient nitrite (NO2 −) substrate (van Cleemput & Samater 1996; Venterea & Rolston Citation2000a). Nitrous oxide is a greenhouse gas that has a global warming potential of 298 over a 100-year time-frame (Forster et al. Citation2007). Stratospheric NO, of which terrestrial N2O emissions are the main source, is involved in the catalytic destruction of ozone (O3) (Crutzen Citation1970). Thus, the N2O molecule is currently the single most important O3-depleting substance (Ravishankara et al. Citation2009).
In the troposphere, NO is converted to NO2 via reactions with O3 and these NO x gases (NO+NO2) may also react, photochemically, with volatile organic compounds and enhance troposheric O3 concentrations (Crutzen Citation1979; Stohl et al. Citation1996). Ozone is detrimental to both plant and human health (Mauzerall et al. Citation2005; Cape Citation2008). Ultimately, tropospheric NO x predominately forms nitric acid, leading to deposition of N and changes in soil acidity downwind of the source (Crutzen Citation1981; Galbally & Roy Citation1983; Hargreaves et al. Citation1992). A review article by Davidson & Kingerlee (Citation1997) concluded that soil emissions could have a significant impact at the local level while at the regional or global level, the role of NO emissions from soils, compared with that of industrial processes, was uncertain. Matson (Citation1997) also noted that there were ‘missing pieces’ still required in order to address and understand the ecosystem–atmosphere interactions of NO x emissions. One of these pieces was the NO x response to agricultural management practices.
Information on the production of NO in intensively grazed ecosystems and the factors influencing this are scarce. A global inventory of soil NO emissions (Davidson & Kingerlee Citation1997) noted that, at first glance, temperate grasslands were well represented by 11 estimates. However, this dataset included six estimates from Colorado grasslands, which differ from intensively developed pastures, for which only one estimate was available. Relatively few studies have directly examined aspects of ruminant urine induced NO emissions (Colbourn et al. Citation1987; Watanabe et al. Citation1997; Williams et al. Citation1998; Bronson et al. Citation1999; Maljanen et al. Citation2007; Liua et al. 2009). The potential effect(s) of soil pH on NO x emissions from ruminant urine patches are unstudied and reports on the effect of soil pH on N2O emissions from urine patches are scarce.
In grazed pastures, soil pH change occurs due to lime application, ruminant urine deposition or fertiliser application (e.g. urea). Deposition of ruminant urine results in large and rapid shifts in soil pH, initially increasing as a result of hydrolysis reactions (Jarvis & Pain Citation1990) and then decreasing due to H+ production during the NH3 volatilisation and nitrification processes (Sherlock & Goh Citation1985). Venterea & Rolston (Citation2000b) clearly demonstrated that the soil pH decline during nitrification could result in a critical pH level being met, where nitrification was restricted and an increase in NO–N fluxes occurred.
Since N2O fluxes following urine deposition have been shown to be affected by initial soil pH (Clough et al. Citation2004) it was hypothesised that the initial soil pH, as affected by liming management of grazed pasture systems, would also affect the magnitude of NO x emissions from urine patches. This paper reports on a laboratory study where NO x and N2O fluxes were determined following urine application to soil columns of varying pH.
Method
Treatments and experimental design
A Paparua Templeton silt loam soil (Pallic typic soil, New Zealand Soil Classification (Hewitt Citation1992)) was collected from 0–10 cm depth under a ryegrass (Lolium perenne L.) and white clover (Trifolium repens) pasture. The soil had a pH of 5.2 (10 g air-dried soil: 25 ml deionised water (Blakemore et al. Citation1987). Other physical and chemical soil characteristics are provided in . This soil was air-dried in a drying cabinet over 1 week at 15 °C, sieved (<2 mm) and then amended with quick-lime [Ca(OH)2], 2 weeks prior to the experiment, at rates equal to either 0, 0.63, 0.94, 1.88, 3.12 or 5.63 g/kg of soil. To provide a lower soil pH, a further aliquot of air-dried soil was treated with 20 ml of 0.05 M HCl/160 g of soil. After 2 weeks, at 20 °C, the soil pH values were 4.4, 5.2, 5.7, 6.0, 6.8, 6.9 and 7.6: a range that encompassed the desired pH range of intensively managed pasture systems (c. 6.0). These amended soils were then wetted up with deionised water and repacked into polyvinyl chloride (PVC) cores (5 cm internal diameter, 9 cm depth) to a depth of 7.5 cm. Re-wetting the soils did not change the initial soil pH values. The resulting water-filled pore space (WFPS) and bulk density (ρ b) were 34% and 1.0 g/cm respectively. A relatively low WFPS was selected in order to maximise the opportunity of seeing a treatment effect on NO emissions, since under wet soil conditions the likelihood of NO being consumed increases since it is an obligate intermediary in the denitrification pathway. Soil moisture was maintained by watering the soil cores, to a set mass every second day, using a hand-held sprayer. This may, of course, have led to slightly higher soil moisture contents at the soil surface.
Table 1 Soil chemical characteristics.
Cow urine was collected from Lincoln University dairy farm, during milking, from cows that had been grazing ryegrass/white clover pasture. This was immediately analysed for its total-N concentration (7.0 g N/l). The N concentration was then raised to 11.3 g N/l using reagent grade urea (AnalaR®), and the urine was pipetted onto those soil core treatments receiving urine (10 ml/core, 575 kg N/ha, 770 µg N/g soil); the exception was non-urine-treated cores at pH 5.2, which received deionised water. This initially raised the WFPS but it was subsequently maintained at 34%. Treatments, replicated three times, were destructively sampled on five occasions. There were thus 105 cores in total (seven initial soil pH treatments× three replicates×five sample times) maintained at a constant temperature of 21 °C. Ideally, it would also have been desirable to have as treatments all the limed soils, but without urine application. However, resources precluded the use of more than 200 soil cores.
Sampling and analytical procedures
On day 1, and at each destructive soil sampling time (7, 14, 21, 28 and 35 days after urine application, prior to extruding the soil and extracting it with 2 M KCl (1:10) for 1 h), the soil surface pH was measured using a flat surface Ag/AgCl/liquid-filled pH electrode (12 mm outer diameter, part no. C2404A-17, Broadley-James Corporation, Irvine, CA, USA). In order to do this, the soil surface was moistened with a drop of deionised water prior to placing the electrode on the soil surface, with the pH value taken after the reading stabilised. Measuring soil surface pH provided a better measure of soil pH dynamics resulting from urine application, since under the relatively dry soil conditions it was expected that most urine would remain near the soil surface. The soil –N and
–N concentrations in the extracts were determined using colorimetric flow injection methods (Keeney et al. Citation1982). Soil
–N concentrations were determined after extracting the soil for 1 min with 2 M KCl, buffered at pH 8.0 and using colorimetric analysis as described by (Stevens & Laughlin Citation1995). Theoretical concentrations of HNO2 were calculated using the measured concentrations of
–N, the surface soil pH and the acid dissociation constant (pKa=3.3) according to van Cleemput and Samater (1996).
Gas flux measurements were made prior to any water applications with NO x fluxes measured on days 0, 1, 2, 4, 7, 11, 14, 15, 16, 18, 21, 22, 23, 25, 28, 29, 30 and 32 and N2O fluxes measured on days 0, 4, 7, 11, 18, 21, 25, 28, 30 and 35. A chemiluminescence technique was used to determine NO x fluxes using a LMA-3D analyser (Unisearch Associates Inc., Ontario). Using an NO standard (1.02±2% µl NO/l in O2-free N2; Scott-Marrin Inc., CA, USA) a standard curve was constructed by sequentially diluting the NO standard with ambient air that was first passed through a NO x Purafil®scrubber (Purafil Inc.) to remove ambient NO x . The NO standard gas flow was varied from 2 to 30 ml/min using a mass flow controller so that the total gas flow was kept constant at 1 l/min, resulting in a maximum 500-fold dilution of the standard NO gas and giving a standard curve range of 2–3 1 nl/l. A similar method was used to calibrate for NO2 but using a NO2 standard (5.07±2% µl/l in O2-free N2; Scott-Marrin Inc., CA, USA). The NO x fluxes were then determined by placing the soil core, base-down so only the upper surface emitted gas, into a 0.59 l Mason jar sealed with a Perfit seal (Unilever, NZ), fitted with septa. The 0.46 l headspace was flushed with air for 1 min and then the headspace was connected to the LMA-3D analyser via the septa. The LMA-3D analyser circulated air in a closed circuit by drawing air out of the headspace, analysing it and then returning the air via a Drierite®and Purafil®scrubber to remove water and NO x respectively. Headspace NO concentrations were recorded every 5 s over a 120 s period. Calculation of the NO flux was performed as follows:
whereF NO is the NO flux (µg NO–N/m2 per hour), dC/dt is the slope of the linear regression of NO concentration versus time (µg NO–N/m3 per hour), V is the internal chamber volume (m3), A is the surface area of the soil core (m2), C A is the average NO concentration during the time interval of regression (µg NO–N/m) and Q is the recirculating air flow rate (m3/h). Fluxes of NO2 were determined in a similar manner.
The headspace was then flushed with air prior to subsequent N2O flux determinations. Headspace samples (10 ml) for N2O were taken manually using glass syringes equipped with three-way valves, at 0, 15 and 30 min, which were placed into 6 ml Exetainer®tubes, creating an overpressure, thus preventing back-diffusion of ambient air. These were re-equilibrated immediately prior to analysis on the gas chromatograph equipped with a 63Ni ECD detector as described by Sherlock et al. (Citation2002). The N2O flux was calculated according to Hutchinson & Mosier (Citation1981).
Statistical analyses were carried out using Minitab®(V15) with repeated measure analysis of variance to test for differences at the 0.05 level of significance. Pearson correlation and multiple linear regressions were performed, following tests for normality (Anderson–Darling) with data log-transformed [ln(flux+1)] as required. Nitric oxide fluxes were used as dependent variables while treatments and measured parameters (initial soil pH, –N,
–N,
–N, surface pH, HNO2, N2O flux and N2O–N:NO–N ratio) were used as independent variables to predict NO fluxes. Following the procedure used by Venterea & Rolston (Citation2000b), NO and N2O flux data were log transformed and are subsequently termed log10NO and log10N2O respectively.
Results
Soil inorganic-N, soil pH and theoretical HNO2 concentrations
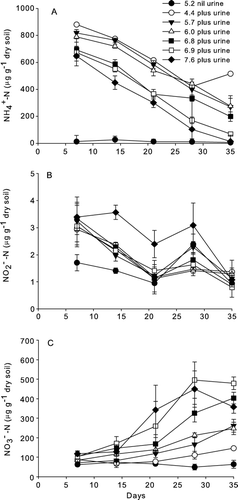
At pH 5.2, without urine, concentrations of –N were ≤27 µg/g soil for the duration of the experiment. However, they increased after urine application ranging from 880 to 647 µg/g soil by day 7 (A). At each destructive sampling time, the soil
–N concentrations where urine was applied were negatively correlated with initial soil pH (r=−0.57 to−0.88, P<0.01). Concentrations of
–N ranged from 0.9 to 2.4 µg/g soil without urine at pH 5.2 and 0.8 to 3.6 µg/g soil after urine application (B). The initial soil pH had no effect on
–N concentrations at day 7, but by days 14 and 21, with urine present, higher
–N concentrations occurred at initial pH 7.6. Soil
–N concentrations correlated with soil
–N concentrations (r=0.453, P<0.01) when data were pooled over all sampling times. Soil
–N concentrations were ≤74 µg/g soil without urine at pH 5.2. With urine, concentrations of
–N increased over time (C) and correlated positively with initial soil pH on all days except day 7 (r=0.58–0.81, P<0.01) and negatively with soil
–N concentrations when data were pooled over all sample dates (r=−0.75, P<0.01).
Figure 1 Mean soil inorganic-N concentrations over time for non-urine soil (pH 5.2) and urine-treated soils with varying initial soil pH values (n=3, error bars are ±SEM). A, Ammonium (–N) concentrations. B, Nitrite (
–N) concentrations. C, Nitrate (
–N) concentrations.
The net –N depletion rates () were calculated by regression of
–N concentrations versus time, as performed by Venterea & Rolston (Citation2000b), with a linear relationship occurring between net
–N depletion rate and initial soil pH (y=84x+296; r
2=0.59, P=0.06).
Table 2 Effect of initial pH on net ammonium ( –N) depletion rates.
–N) depletion rates.
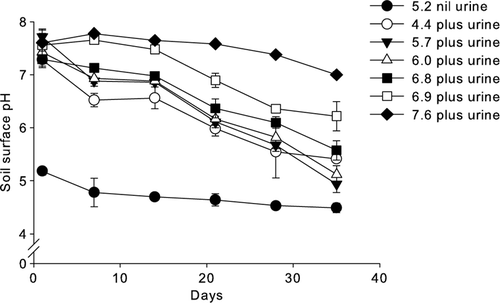
Twenty four hours after urine application, soil surface pH values had increased (mean of urine-treated soils at 24 h equalled 7.5±0.17 stdev) and they then decreased over time (). By day 21, the soil surface pH values had decreased most in the initial soil pH treatments ≤6.8. After 35 days, the highest initial soil pH treatment had a surface pH of 7.0, below its initial pH of 7.6, while in the lowest initial pH treatment where urine was applied the soil surface had still to reach its initial soil pH value of 4.4 and remained at 5.4 ().
Figure 2 Mean soil surface pH over time (n=3, error bars are ±SEM). Treatments were applied at time zero.
On day 7, the theoretical HNO2 concentrations, hereafter called HNO2 concentrations, did not differ due to initial soil pH and equalled 2 ng/g at initial soil pH 4.4 and ≤0.4 ng/g in all other urine-treated soils. By day 14, HNO2 concentrations had decreased to 1.5 ng/g in the initial soil pH 4.4 treatment and ≤0.7 ng/g in all other urine-treated soils. However, by day 21, HNO2 concentrations had begun to increase and peaked at initial soil pH 4.4 on day 28, and in the other initial soil pH treatments on day 35. However, there was no initial soil pH treatment effect although HNO2 concentrations were negatively correlated with initial soil pH on days 7, 14, 21 and 28 (r=0.66 to −0.41, P<0.05).
NOx and N2O fluxes
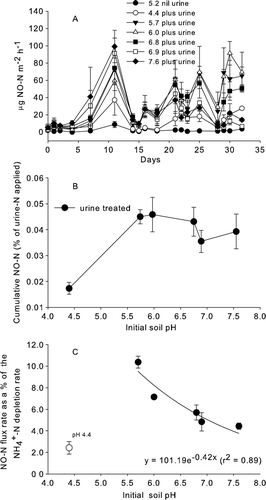
There was no NO2 detected during the flux measurement periods. Without urine (initial soil pH 5.2) the NO–N fluxes were lowest (0–14.3 µg NO–N/m per hour). With urine application, the NO–N fluxes increased (A), peaking on day 11 when the maximum NO–N flux was recorded at an initial soil pH of 7.6 (99.3 µg NO–N/m per hour). Generally, over the first 11 days, the NO–N fluxes correlated positively with initial soil pH in the urine-treated soils. After day 21, NO–N fluxes were greater in the interim soil pH treatments (5.7–6.0) with lower NO–N fluxes in the initial soil pH treatments at pH 4.4 and pH 6.8–7.6 (A).
Figure 3 NO–N dynamics over time. A, Mean NO–N flux over time for non-urine-treated soil (pH 5.2) and urine-treated soils with varying initial soil pH values. Mean (n=3, error bars are ±SEM) B, Cumulative NO–N flux (n=3, error bars are ±SEM) as a percentage of urine-N applied after 35 days versus the initial soil pH. Mean (n=3, error bars are ±SEM). C, NO–N flux rate (mean between days 7 and 11) as a percentage of the mean net –N depletion rate (days 7–14) versus initial soil pH treatments. The regression line excludes the initial soil pH 4.4 treatment. Mean (n=3, error bars are ±SEM).
When data were pooled over the course of the experiment, the NO–N fluxes correlated with –N concentrations (r=−0.29, P<0.01) and soil surface pH (r=−0.25, P<0.05) but not with
–N or
–N concentrations. On any individual soil sampling day, the NO–N fluxes correlated with
–N on day 7 (r=0.83, P<0.01);
–N (r=−0.49, P<0.05) and
–N (r=0.58, P<0.01) on day 14; and soil surface pH (r=−0.70, P<0.01), N2O flux (r=−0.55, P<0.05) and HNO2 concentrations (r=0.62, P<0.01) on day 35.
The cumulative NO–N fluxes ranged from 0.02 to 0.05% of the urine-N applied. The cumulative NO–N flux as a percentage of urine-N applied, after 35 days, was lower (P<0.01) in the most acidic soil pH treatment (4.4) compared with the other urine-treated initial soil pH treatments where no differences occurred (B).
As noted above, where urine was applied, the NO–N flux data up until day 11 correlated with initial soil pH, as did the net –N depletion rates over this time (days 7 to 14). When the NO–N flux rates between days 7 and 14 were expressed as a percentage of the
–N depletion rate and then plotted against the initial soil pH treatment, the percentage declined exponentially (r
2=0.89) with increasing initial soil pH, while excluding the initial pH 4.4 treatment (C).
In the absence of urine at pH 5.2, the N2O–N fluxes ranged from 1.5 to 32 µg N2O–N/m2 per hour (A). With urine application, the N2O fluxes increased (2.7 to 115.6 µg N2O–N/m2 per hour), particularly from day 11 onwards. The N2O fluxes had decreased by day 28, but remained elevated at pH ≥ 6.9 after this time (P<0.01; A). When the N2O–N flux data from all the sampling dates were pooled, N2O–N fluxes were significantly correlated with the initial soil pH, soil surface pH, –N and
–N concentrations (r=0.45, P<0.01; r=0.51, P<0.01; r=0.56, P<0.01; r=0.29, P<0.01 respectively). There was no significant correlation between the N2O–N and NO–N fluxes when all data were pooled over time but, on day 35, these gas fluxes were negatively correlated (r=−0.55, P<0.05).
Figure 4 Daily and cumulative N2O–N fluxes as a function of initial soil pH. A, Mean N2O–N flux over time for non-urine-treated soil (pH 5.2) and urine-treated soils with varying initial soil pH values (n=3, error bars are ±SEM). B, mean cumulative N2O–N flux as a percentage of urine-N applied after 35 days versus the initial soil pH (n=3, error bars are ±SEM).
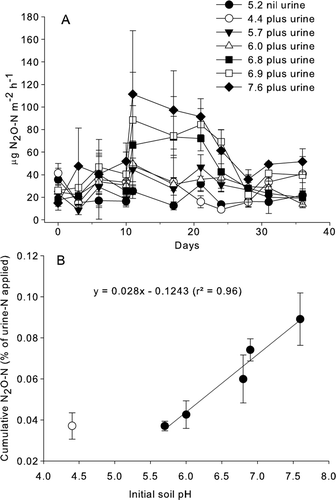
Between initial pH 5.7 and 7.6, the cumulative mean N2O–N fluxes after 35 days (0.04–0.09% of N applied) increased in a linear manner with increasing initial soil pH (B), with no difference between the cumulative N2O–N fluxes at initial soil pH 4.4 and 5.7 (B).
Regression analysis
On days 7, 14, 21, 28 and 35 the best predictors of log10NO were , (N2O+
+
), no variables, initial soil pH and (surface soil pH+
) respectively (). When these relationships were used to compare predicted NO fluxes with measured NO fluxes, the regression coefficients ranged from 0.26 to 0.61. Relationships between log10NO and the measured soil variables were then assessed for each initial soil pH treatment but, again, no variable consistently produced the ‘best-fit’ relationships with r
2 values of 0.50–0.68 at initial soil pH values ≤6.8 and no relationship established above this initial soil pH value (). Pooling data across all sample dates and initial soil pH treatments for the urine-treated soils showed that the multiple regression analysis of log10NO against surface soil pH, N2O flux,
,
HNO2 and initial soil pH only explained 26% of the variability in the data.
Table 3 Results of multiple regression analysis for log10NO–N (µg/m2 per hour) of urine-treated soils at individual sampling occasions across all initial soil pH treatments.
Table 4 Results of multiple regression analysis for log10NO–N (µg/m2 per hour) of urine-treated soils at different initial soil pH treatments over time.
Multiple regression analysis of log10N2O–N, with data pooled across initial pH treatments best explained the variability in the data on days 14 and 21 (r
2=0.34–0.80), with no significant relationships on days 28 and 35 (). When pooling data over time, variability in the log10N2O–N flux for the initial soil pH treatments was consistently a function of soil –N concentration in the best-fit multiple regressions at pH 5.7, 6.9 and 7.6 ().
Table 5 Results of multiple regression analysis for log10N2O–N (µg/m2 per hour) of urine-treated soils at individual sampling occasions across all initial soil pH treatments.
Table 6 Results of multiple regression analysis for log10N2O–N (µg/m2 per hour) of urine-treated soils at different initial soil pH treatments over time.
Discussion
Inorganic-N transformations, soil pH and HNO2 concentrations
Inorganic-N concentrations and transformations were typical of those previously reported for ruminant urine soil core studies (e.g. Clough et al. Citation2004). Ammonium depletion and –N accumulation occur via nitrification, with
–N an intermediate in the nitrification process (Wrage et al. Citation2001). Gleeson et al. (Citation2010) found that between 25 and 65% WFPS nitrification rates (both net and gross) in a sandy soil did not change as a function of WFPS. Thus, nitrification was unlikely to have been limited by the 35% WFPS used in the current study. Noteworthy were the relatively low
–N concentrations when compared with the rates of
–N depletion or
–N accumulation, indicating a high rate of
–N turnover (). The
–N concentrations at day 7 were higher than what was theoretically able to be supplied from the urine alone, and this may have been due to a urine-induced priming effect since the high soil pH resulting from urea hydrolysis results in solubilisation of soil organic matter (Clough et al. Citation2003); a fraction could possibly have come from N released as a consequence of soil liming.
Consistent with earlier studies (e.g. Goodroad & Keeney Citation1983), liming increased the nitrification rate of –N as evidenced by the increased rate of net
–N depletion with increasing initial soil pH treatment. For
to be oxidised it must first be converted to NH3, and the equilibrium between these two compounds is pH dependent, favouring NH3 as pH increases (Sherlock & Goh Citation1984). However, the specific mechanism by which NH3 oxidisers respond to changes in soil pH remains an enigma (Kowalchuk & Stephen Citation2001). Interestingly, the net
–N depletion rates (225–800 ng N/g per hour) were of a similar magnitude to those measured by Venterea & Rolston (Citation2000b) in a NO–N related study following application of
fertiliser.
Increases in soil surface pH occurred in the urine-treated soils due to hydrolysis reactions (Jarvis & Pain Citation1990) while decreases in soil pH, over time, occurred due to H+ release during NH3 volatilisation (Sherlock & Goh Citation1984) and oxidation to
(Wrage et al. Citation2001). As initial soil pH increased, the soils had relatively lower active acidities and thus the rates of decline in soil pH, due to further production of H+ production, were reduced.
Despite the trend for the concentrations to decrease over time, HNO2 concentrations increased over time in all treatments, due to the declining surface soil pH. These theoretical HNO2 concentrations (≤0.03 µg/g dry soil) were low when compared with those reported in Venterea & Rolston's (2000b) NO–N study, where HNO2
−N concentrations ranged from 0.1 to 0.5 µg/g soil. However, in the study by Venterea & Rolston (Citation2000b), the periods of peak HNO2 concentrations coincided with higher
–N concentrations (0.4–10 µg/g soil) and soils of lower pH (pH 4.2–5.3 [0.01 M CaCl2]) than used here.
NO–N and N2O–N fluxes
It has been previously suggested that NO–N fluxes are a consequence of NO–N ‘leakage’ during nitrification (e.g. Firestone & Davidson Citation1989). The strong exponential relationship between NO–N flux rates as a percentage of –N depletion and the initial soil pH demonstrates for the first time that the relative magnitude of this NO–N ‘leakage’ from urine-treated soil can be pH dependent, due to the increasing soil acidity reducing nitrification rates and thus biological demand for
. In the current study, nitrification rates increased with increasing pH, thus the
pool was consumed faster, and subsequently the
pool turned over faster with little difference in
–N concentration as initial soil pH increased. It is the fate of this
pool that determines the NO flux. Underhill & Prosser (Citation1987) showed that Nitrosomonas, which oxidise NH3 to
, and Nitrobacter, which oxidise
to
, preferentially grew on cation and anion exchange regions respectively. Thus, as noted by Venterea & Rolston (Citation2000b), prior to the consumption of
by oxidising or reducing processes, any diffusion of nitrification derived
may provide an opportunity for abiotic HNO2 formation and subsequent NO production. Therefore, the relationship between ‘NO–N flux as a percentage of the
–N depletion rate’ versus initial soil pH shows higher percentage values at lower soil pH values because there is a lower nitrification rate and any biological formation of
is susceptible to abiotic transformation to NO. Conversely, at high soil pH values, the percentage is reduced because the nitrification rates are higher. While the biological formation of
occurs faster under such conditions, there is less abiotic transformation to NO because of both the strong biological competition for
and the reduced H+ concentrations.
Thus, increasing soil acidity enhances the opportunity for abiotic NO production while simultaneously slowing the –N oxidation rate, as seen in the current study, leading to greater abiotic competition for
–N and a higher proportion of
–N ‘leaking’ as NO–N. Thus, when the relatively constant
–N pools and varying H+ ion concentrations in the current study are considered, the data agree with the conclusions of Venterea & Rolston (Citation2000b) who found that gross rates of
oxidation did not have a major influence on NO production and, in addition, their data did not support models that calculated NO production rates as a fraction of gross or net nitrification rates. Nitric oxide–N production rates of 2.6–5.2 ng N/g soil per hour were reported by Venterea & Rolston (Citation2000b) during high-nitrification phases (0.3–2.3% of the gross
–N oxidation rate). In the current study, the NO–N fluxes were comparable (0.7–2.1 ng/g soil per hour on days 7–14), but they ranged from 4.4–10.0% of the
–N depletion rate. The differences in these results could be due to other biochemical or physical characteristics of the soils utilised in the separate studies. For example, Venterea & Rolston (Citation2000b) found a strong correlation between the rate coefficient, relating NO production to HNO2, and soil organic matter content.
The authors propose that the reason for the NO–N fluxes peaking on day 11 was due to the dominance of in the soil and the fact that
oxidisers had not become fully functional, thus there was less competition from biological sources for the
available. The study by Clough et al. (Citation2009) measured soil inorganic-N concentrations in situ, following urine deposition, and showed that the
oxidation rate was low in the first 5 days, and that
concentrations peaked on days 6–8. This peak was due to the
oxidation rate increasing and the resulting
pool building up, until the
oxidisers were functioning at a high enough rate to remove it through to
. It is proposed that a similar series of events occurred in the current study.
At pH 4.4, the soil –N and
–N concentrations were comparable to the other initial soil pH treatments, but less
–N accumulated and the NO–N flux rate was lower as a percentage of the
–N depletion rate. While the acidic soil preparation altered the rate of
–N depletion it did not halt the process (). The formation of HNO2 is favoured when the soil pH is <5. However, there was no enhanced decomposition of HNO2 to yield NO (Pauling Citation1970) and reactions of HNO2 with organic constituents, which would have yielded both NO and N2O, are also unlikely to have been significant (Chalk & Smith Citation1983) since this treatment produced relatively little N2O. Another possible mechanism for the removal of
from soil solution, and one that is enhanced with increasing soil acidity, is
fixation (Chalk & Smith Citation1983). Furthermore, a gas not measured in this study was N2, and reactions between soil amino groups and HNO2, known as the van Slyke reaction (van Slyke 1911), can potentially form N2 when the soil pH is <5.0. Thus, the lower than expected NO fluxes at pH 4.4 were possibly due to non-NO producing processes, competing for
and thus reducing the
pool available for HNO2 formation and its spontaneous decomposition to NO.
Once soil –N concentrations were significantly elevated, after day 21, the initial soil pH continued to play a significant role in defining the magnitude of the NO–N fluxes. The optimum pH (5.7–6.0) observed for the net NO–N flux after day 21 was due to low NO–N emissions at pH 4.4, for reasons noted above, and possibly due to enhanced consumption of NO–N at initial soil pH >6.0. A previous study by Murray & Knowles (Citation2001) found a strong linear relationship between pH and NO consumption (r
2=0.97) in anaerobic slurries of a sandy loam soil where first-order NO consumption rate constants at pH 8.0 were two-fold higher than at pH 6.0. In addition, at the initial pH values of 6.9–7.6,
–N depletion was almost complete by day 35; thus, the rate of NO–N production may have been lower at this time in addition to the enhanced denitrification consumption of NO–N in these treatments, as indicated by the observed negative correlation between NO–N and N2O–N fluxes on day 35.
The cumulative NO–N fluxes after 35 days were of a similar magnitude to those previously reported by Maljanen et al. (Citation2007) in a field experiment (WFPS 36–90%) where cumulative fluxes were 0.16% of urine-N applied (583 kg N/ha) to a soil at pH 6.0 after 110 days. The cumulative NO–N fluxes after this time were also consistent in magnitude with values reported by Colbourn et al. (Citation1987) of 0.03% of N applied. Despite the initial soil pH treatment producing varying intensities in the NO–N fluxes over periods of the experiment, there was no difference in cumulative fluxes, due to low- and high-flux periods compensating for each other over the course of the experiment. The presence of only one true control treatment theoretically places limitations on these results. However, at a soil pH of 5.2 without urine, NO fluxes were extremely low compared with those of the soil adjusted to pH 5.7 with urine applied (A). Therefore, any significant contribution of antecedent N to NO fluxes at higher soil pH values, in the presence of urine, is considered unlikely given cumulative NO fluxes in the presence of urine decreased as soil pH increased (C).
Two previous liming studies (pH range 4.4–7.7) utilised soil cores, silt loam soils and synthetic urine (500 kg N/ha) to examine the effect of soil liming on N2O emissions. In the first study, optimal cumulative N2O production (0.82% of N applied) after 60 days, at field capacity, occurred at pH 6.1 (Clough et al. Citation2003). In the second study, elevating the pH to ≥5.9 reduced N2O fluxes at field capacity (54% WFPS; 0.06–0.40% of N applied) while, under saturated (80% WFPS) soil conditions, cumulative N2O fluxes peaked at pH 6.6 (1.66% of N applied) after 85 days (Clough et al. Citation2004). The lack of any decrease in cumulative N2O emissions due to increasing soil pH in the current study is contrary to these earlier findings. This may be due to the lower soil moisture content (34% WFPS) invoking different N2O production mechanisms, reductions in production rates as a result of the lower WFPS or differences created by the use of real urine versus synthetic urine. However, Galbally et al. (Citation2010) also recorded no decrease in N2O emissions following the in situ application of sheep urine to an Australian pasture, after liming an acidic soil (pH 4.0) to pH 5.5.
In the current study, the maximum cumulative N2O–N flux (0.1% of N applied) was low when compared with earlier studies where optimal pH values were established for N2O fluxes (Clough et al. Citation2003,
Citation2004). Nitrification certainly occurred and, at 34% WFPS, it might be expected to be the dominant N2O production mechanism. This could explain the relatively low cumulative fluxes and why N2O production did not decrease with increasing pH. However, anaerobic microsites exist even in well-aerated soils and denitrification has been demonstrated under aerobic conditions (Müller et al. Citation2004). While denitrification cannot be discounted as an N2O source, it seems unlikely that anaerobic microsites with ≤5% O2, as required for denitrification (Venterea Citation2007; Russow et al. Citation2009), were prevalent. Given this and the negative correlation between NO–N and N2O–N fluxes on day 35, it is likely that nitrifier-denitrification was operating especially at the end of the experiment, since the NO molecule is converted to N2O in this process (Wrage et al. Citation2001; Kool et al.Citation2011). However, Wrage et al. (Citation2004) found no effect of soil pH (3.5–6.3) on nitrifier-denitrification when soil cores were extracted from fertilised field plots and incubated with inhibitors, but N rates were lower than the urine-N rate applied here. Abiotic production of N2O may also have occurred. Venterea (Citation2007), using soils collected from long-term tillage and woodland sites (pH 5.1–5.6 (1:1 M KCl)), found that the rate constants for abiotic production of N2O at concentrations below 60 µg N/g soil were negatively related to soil pH, with increased rates of abiotic N2O production occurring as soil pH decreased. In the current experiment, the cumulative fluxes of N2O increased with initial soil pH, indicating any abiotic N2O production mechanism(s) became less important as initial soil pH increased. As a percentage of urine-N applied, the cumulative N2O losses after 35 days were relatively low (0.04–0.09%), but similar to those reported in other studies after urine application to relatively dry soils (e.g. Van Groenigen et al. 2005; Galbally et al. Citation2010).
The prediction of the NO–N flux was most successful on day 7, using soil –N concentrations, when nitrification dominated and NO–N fluxes were increasing towards their peak on day 11. However, in general, the relatively poor predictions of net NO–N flux were confounded due to the simultaneous occurrence of production and consumption processes and mixing of the soil core prior to sub-sampling. Another confounding factor may have been adjustment of soil water contents. Although performed after gas analyses, this may have promoted NO consumption and possibly explains some of the variance in the NO–N flux between days 15 and 35.Venterea & Rolston (2000b) have produced one of the best datasets to date for examining the mechanism and kinetics of gross NO–N production in soils. They used (
)SO4 fertiliser as the N source. Thus, the confounding effects of urea hydrolysis and the large shifts in soil pH that occur as a result, along with subsequent NH3 production, did not occur as in the present study. These factors confound the ability to establish relationships between NO–N flux and associated soil variables. In the future, it would be useful to delineate the differences between net and gross NO
x
fluxes, following urine deposition, with an experimental system such as that described by Venterea & Rolston (Citation2000b).
Conclusion
Initial soil pH can influence daily fluxes of NO–N and N2O–N from urine-affected soil when the WFPS is relatively low (34%). Elevating soil pH enhances nitrification, thus increasing nitrifier competition for –N and reducing the opportunity for abiotic NO production. A negative exponential relationship was observed between the NO–N flux (expressed as a percentage of the
–N depletion rate) and the initial soil pH during a period dominated by nitrification activity. However, at the end of the 35-day experiment, the NO–N fluxes were correlated with N2O fluxes and an optimum pH of 5.7–6.1 was observed for NO–N production, indicating that either nitrifier-denitrification or denitrification were influencing NO–N fluxes. Cumulative NO–N fluxes over 35 days were not affected by initial soil pH at pH>4.4. Under the soil moisture conditions utilised in this study, the soil pH aimed for in grazed temperate pastures (pH 5.5–6.5) will not affect cumulative NO fluxes but may enhance cumulative N2O emissions.
References
- Blakemore LC, Searle PL, Daly BK 1987 . Methods for chemical analysis of soils , vol. 80 . Lincoln, , New Zealand , Manaaki-Whenua Press .
- Bronson , KF , Sparling , GP and Fillery , IRP . 1999 . Short term N dynamics following application of N15-labelled urine to a sandy soil in summer . Soil Biology & Biochemistry , 31 : 1049 – 1057 .
- Cape , JN . 2008 . Surface ozone concentrations and ecosystem health: Past trends and a guide to future projections . Science of the Total Environment , 400 : 257 – 269 .
- Chalk PM, Smith CJ 1983 . Chemodenitrification . In : Freney JR, Simpson JR Gaseous loss of nitrogen from plant–soil systems. Developments in Plant and Soils Sciences , vol. 9 . Martinus Nijhoff, Boston . Pp. 65 – 89 .
- Clough , TJ , Sherlock , RR and Kelliher , FM . 2003 . Can liming mitigate N2O fluxes from a urine-amended soil? . Australian Journal of Soil Research , 41 : 439 – 457 .
- Clough , TJ , Kelliher , FM , Sherlock , RR and Ford , CD . 2004 . Lime and soil moisture effects on nitrous oxide and dinitrogen emissions from a pasture soil . Soil Science Society of America Journal , 68 : 1600 – 1609 .
- Clough , TJ , Ray , JL , Buckthought , LE , Calder , J , Baird , D , O'Callaghan , M , Sherlock , RR and Condron , LM . 2009 . The mitigation potential of hippuric acid on N2O emissions fromurine patches: An in situ determination of its effect . Soil Biology and Biochemistry , 414 : 2222 – 2229 .
- Colbourn , P , Ryden , JC and Dollard , GP . 1987 . Emissions of NO x from urine treated pasture . Environmental Pollution , 46 : 253 – 261 .
- Crutzen , PJ . 1970 . The influence of nitrogen oxides on the atmospheric ozone content . Quarterly Journal of the Royal Meteorological Society , 96 : 320 – 325 .
- Crutzen , PJ . 1979 . The role of NO and NO2 in the chemistry of the troposphere and stratosphere . Annual Review of Earth and Planetary Sciences , 7 : 443 – 472 .
- Crutzen , PJ . 1981 . “ Atmospheric chemical processes of the oxides of nitrogen, including nitrous oxide ” . In Denitrification, nitrification and atmospheric nitrous oxide , Edited by: Delwiche , CC . 17 – 44 . New York : Wiley .
- Davidson , EA and Kingerlee , W . 1997 . A global inventory of nitric oxide emissions from soils . Nutrient Cycling in Agroecosystems , 48 : 37 – 50 .
- Firestone , MK and Davidson , EA . 1989 . “ Microbiological basis of NO and N2O production and consumption in soil ” . In Exchange of trace gases between terrestrial ecosystems and the atmosphere , Edited by: Andreae , MO and Schimel , DS . 7 – 21 . New York : Wiley .
- Forster , P , Ramaswamy , V , Artaxo , P , Berntsen , T , Betts , R , Fahey , DW , Haywood , J , Lean , J , Lowe , DC , Myhre , G , Nganga , J , Prinn , RG , Raga , G , Schulz , M and Van Dorland , R . 2007 . “ Changes in atmospheric constituents and in radiative forcing ” . In Climate change 2007: The physical basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Solomon , S, . 129 – 234 . Cambridge, , UK : Cambridge University Press .
- Galbally , IE and Roy , CR . 1978 . Loss of fixed nitrogen from soils by nitric oxide exhalation . Nature , 275 : 734 – 735 .
- Galbally IE, Roy CR 1983 . The fate of nitrogen compounds in the atmosphere . In : Freney JR, Simpson JR Gaseous loss of nitrogen from plant soil systems. The Hague, Martinus Nijhoff . Pp. 265 – 284 .
- Galbally , IE , Meyer , MCP , Wang , YP , Smith , CJ and Weeks , IA . 2010 . Nitrous oxide emissions from a legume pasture and the influence of liming and urine addition . Agriculture Ecosystems and Environment , 136 : 262 – 272 .
- Gleeson , DB , Müller , C , Banerjee , S , Ma , W , Siciliano , SD and Murphy , DV . 2010 . Response of ammonia oxidizing archea and bacteria to changing water filled pore space . Soil Biology & Biochemistry , 42 : 1888 – 1891 .
- Goodroad , LL and Keeney , DR . 1983 . Nitrous oxide production in aerobic soils under varying pH, temperature and water content . Soil Biology & Biochemistry , 16 : 39 – 43 .
- Hargreaves , KJ , Fowler , D , Storeton-West , RL and Duyzer , JH . 1992 . The exchange of nitric oxide, nitrogen dioxide and ozone between pasture and the atmosphere . Environmental Pollution , 75 : 53 – 59 .
- Hewitt AE 1992 . New Zealand Soil Classification . Maanaki Whenua Press , New Zealand
- Hutchinson , GL and Mosier , AR . 1981 . Improved soil cover method for field measurement of nitrous oxide fluxes . Soil Science Society of America Journal , 45 : 311 – 316 .
- Jarvis , SC and Pain , BF . 1990 . Ammonia volatilization from agricultural land . Proceedings of the Fertilizer Society , 298 : 1 – 35 .
- Keeney , DR , Nelson , DW and Ladd , JN . 1982 . “ Nitrogen-inorganic forms ” . In Methods of soil analysis, part 2.Chemical and microbiological properties , Edited by: Page , AL , Miller , RH and Keeney , DR . 699 – 709 . Madison, WI : American Society of Agronomy .
- Kool , DM , Dolfing , J , Wrage , N and Van Groenigen , JW . 2011 . Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil . Soil Biology and Biochemistry , 43 : 171 – 178 .
- Kowalchuk , GA and Stephen , JR . 2001 . Ammonia-oxidizing bacteria: a model for molecular microbial ecology . Annual Review of Microbiology , 55 : 485 – 529 .
- Liua , C , Holstb , J , Yaoa , Z , Brüggemannb , N , Butterbach-Bahlb , K , Hana , S , Hand , X and Zhenga , X . 1990 . Sheepfolds as ‘hotspots’ of nitric oxide (NO) emission in an Inner Mongolian steppe Agriculture . Ecosystems & Environment , 134 : 136 – 142 .
- Maljanen , M , Martikkala , M , Kopnen , HT , Virkajarvi , P and Martikainen , P . 2007 . Fluxes of nitrous oxide and nitric oxide from experimental excreta patches in boreal agricultural soil . Soil Biology & Biochemistry , 39 : 914 – 920 .
- Matson , P . 1997 . NO x emission from soils and its consequences for the atmosphere and biosphere: critical gaps and research directions for the future . Nutrient Cycling in Agroecosystems , 48 : 1 – 6 .
- Mauzerall , DL , Sultan , B , Kim , N and Bradford , DF . 2005 . NOx emissions from large point sources: variability in ozone production resulting health damages and economic costs . Atmospheric Environment , 39 : 2851 – 2866 .
- Müller , C , Stevens , RJ , Laughlin , RJ and Jäger , HJ . 2004 . Microbial processes and the site of N2O production in a temperate grassland soil . Soil Biology & Biochemistry , 36 : 453 – 461 .
- Murray , RE and Knowles , R . 2001 . Influence of pH on production and consumption of NO by slurries of an agricultural soil under denitrifying conditions . Biology and Fertility of Soils , 34 : 357 – 362 .
- Pauling L 1970 . General Chemistry . Dover , Mineola , NY .
- Ravishankara , AR , Daniel , JS and Portmann , RW . 2009 . Nitrous oxide (N2O): The dominant ozone-depeleting substance emitted in the 21st century . Science , 326 : 123 – 125 .
- Russow , R , Stange , CF and Neue , HU . 2009 . Role of nitrite and nitric oxide in the processes of nitrification and denitrification in soil: Results from 15N tracer experiments . Soil Biology & Biochemistry , 41 : 785 – 795 .
- Sherlock , RR and Goh , KM . 1984 . Dynamics of ammonia volatilization from simulated urine patches and aqueous urea applied to pasture . Fertilizer Research , 5 : 181 – 195 .
- Sherlock , RR and Goh , KM . 1985 . Dynamics of ammonia volatilisation from simulated urine patches and aqueous urea applied to pasture. II. Theoretical derivation of a simplified model . Fertilizer Research , 6 : 3 – 22 .
- Sherlock , RR , Sommer , SG , Khan , RZ , Wood , CW , Guertal , EA , Freney , JR , Dawson , CO , Cameron , KC and Sven , G . 2002 . Ammonia, methane, and nitrous oxide emission from pig slurry applied to a pasture in New Zealand . Journal of Environmental Quality , 31 : 1491 – 1501 .
- Stevens , RJ and Laughlin , RJ . 1995 . Nitrite transformations during soil extraction with potassium chloride . Soil Science Society of America Journal , 59 : 933 – 938 .
- Stohl , A , Williams , EGW and Kromp-Kolb , H . 1996 . A European inventory of soil nitric oxide emissions and the effect of these emissions on the photochemical formation of ozone . Atmospheric Environment , 30 : 3741 – 3755 .
- Underhill , SE and Prosser , JI . 1987 . Surface attachment of nitrifying bacteria and their inhibition by potassium ethyl xanthate . Microbial Ecology , 14 : 129 – 139 .
- van Cleemput , O and Samater , AH . 1996 . Nitrite in soils: Accumulation and role in the formation of gaseous N compounds . Fertilizer Research , 45 : 81 – 89 .
- Van Groenigen , JW , Kuikman , PJ , de Groot , WJM and Velthof , GL . 2005 . Nitrous oxide emission from urine-treated soil as influenced by urine composition and soil physical conditions . Soil Biology & Biochemistry , 37 : 463 – 473 .
- van Slyke , DD . 1911 . A method for quantitative determination of aliphatic amino groups. Application to the study of proteolysis and proteolytic products . Journal of Biological Chemistry , 9 : 185 – 205 .
- Venterea , RT . 2007 . Nitrite driven nitrous oxide production under aerobic soil conditions: kinetics and biochemical controls . Global Change Biology , 13 : 1798 – 1809 .
- Venterea , R and Rolston , D . 2000a . Mechanisms and kinetics of nitric and nitrous oxide production during nitrification in agricultural soil . Global Change Biology , 6 : 303 – 316 .
- Venterea , RT and Rolston , DE . 2000b . Nitric and nitrous oxide emissions following fertilizer application to agricultural soil: Biotic and abiotic mechanisms and kinetics . Journal of Geophysical Research , 105 : 15117 – 15129 .
- Watanabe , T , Osada , T , Yoh , M and Tsuruta , H . 1997 . N2O and NO emissions from grassland soils after the application of cattle and swine excreta . Nutrient Cycling in Agroecosystems , 49 : 35 – 39 .
- Williams , PH , Jarvis , SC and Dixon , E . 1998 . Emission of nitric oxide and nitrous oxide from soil under field and laboratory conditions Soil Biology & Biochemistry , 30 : 1885 – 1893 .
- Wrage , N , Velthof , GL , van Beusichem , ML and Oenema , O . 2001 . Role of nitrifier denitrification in the production of nitrous oxide . Soil Biology & Biochemistry , 33 : 1723 – 1732 .
- Wrage , N , Velthof , GL , Laanbroek , HJ and Oenema , O . 2004 . Nitrous oxide production in grassland soils: assessing the contribution of nitrifier denitrification . Soil Biology & Biochemistry , 36 : 229 – 236 .