Abstract
Grassland net primary productivity, carbon (C) allocation to roots, root production and subsequent turnover time in grazed pastures have significant implications for modelling landuse effects on global carbon dynamics. The objective of this work, using the decline of assimilated 13C, was to quantify C allocation to roots, root production and turnover time in response to long-term fertiliser and irrigation treatments. The treatments on the pastures on a long-term research site at Winchmore, Canterbury, New Zealand were 0 or 375 kg ha−1 y−1 superphosphate under irrigation and, on a nearby irrigation experiment, unirrigated or irrigated (with fertilisation of 250 kg ha−1 y−1 superphosphate) when the soil water content fell to 20% w/w (50% available moisture). The pasture treatments were pulse-labelled using 13CO2 within portable gas-tight enclosures. Separate micro-plots were 13CO2 pulse-labelled in late spring, summer and autumn. Below ground net primary production (206 g C m−2 y−1) was similar in unfertilised, unirrigated, fertilised and irrigated pastures, despite marked differences in above ground production. Unfertilised/irrigated and unirrigated/fertilised treatments had greater root biomass, root C allocation and longer root turnover time (1.9 y and 2.0 y, respectively) than fertilised and irrigated treatments (1.3 y). These root turnover times appeared to be consistent with the improved substrate quality attributed to the species found in the irrigated and fertilised treatments.
Introduction
Grazed pasture covers approximately 40% of the world's land surface (LeCain et al. Citation2002) and temperate grassland soils account for approximately 16% of all terrestrial soil carbon (C) (Houghton Citation1995). These large areas of grassland and amount of C in grassland soils suggests any changes in soil C dynamics from landuse changes need to be considered (Litton & Giardina Citation2008). In New Zealand, pastoral agriculture has been intensifying with increased livestock stocking rates and pasture utilisation from increased fertiliser use and irrigation of dryland areas. In intensively grazed pasture, a large proportion of the above ground production is consumed by livestock and, although approximately 30% of ingested C is excreted by livestock, below ground net primary production (BNPP—gross biomass root C production less autotrophic respiration) is a significant C input to soil organic matter (SOM) (Rasse et al. 2005). However, BNPP is poorly quantified.
Root production in grasslands may vary from 26 to 93% of NPP depending on management and environmental conditions (Williams Citation1977; Meurk Citation1978; Sims & Singh Citation1978; Crawford et al. Citation1997; Ni Citation2004; McCulley et al. Citation2005; Hui & Jackson Citation2006; Frank Citation2007; Wu et al. Citation2011). Furthermore, the large range in reported proportion of net primary production (NPP) allocated to BNPP is accompanied by the considerable uncertainty of any one calculation of BNPP (Lauenroth et al. Citation2006; Milchunas Citation2009). It is necessary to quantify the influence of pastoral system management on BNPP and the turnover of root C in order to accurately model these ecosystems and to determine their influence on C storage in relation to the global C cycle.
On low-altitude sites in mid-Canterbury New Zealand, estimates of 0–15 cm BNPP for unfertilised and fertilised pastures were 859 and 647 g DM m−2 y−1, respectively (Nguyen & Goh Citation1992). However these estimates of BNPP are not very precise as they were calculated from root mass using an assumed root turnover rate from the literature. Low- or high-fertility sites (Saggar et al. Citation1997), sowing improved species (Conant et al. Citation2001), topographical features (Saggar et al. Citation1999), grazing management (Svejcar & Christiansen Citation1987) and soil moisture differences (Davidson Citation1969; Frank Citation2007) have been shown to influence root C production. However, many studies have suffered from a variety of problems such as short-term measurements to estimate long-term production and methodological difficulties (Milchunas Citation2009).
Various methods have been used to measure BNPP in grasslands, including recording individual root appearance and disappearance, harvest maxima–minima, root ingrowth, nitrogen budget approaches, calculating from root mass, root length and C isotope dilution, decrease or turnover (Dahlman & Kucera Citation1965; Meurk Citation1978; Gibbs & Reid Citation1992; Milchunas & Lauenroth Citation2001; Gill et al. Citation2002a; Gill et al. Citation2002b; Ni Citation2004; Pucheta et al. Citation2004; McCulley et al. Citation2005; Frank Citation2007). Some methods require assumptions that may be difficult to quantify and may not be very accurate (Bliss & Mark Citation1974; Gibbs & Reid Citation1992; Milchunas & Lauenroth Citation1992; Lauenroth et al. Citation2006; Milchunas Citation2009). Milchunas & Lauenroth (Citation1992) compared harvest and C isotope dilution and turnover methods for measuring belowground NPP in a shortgrass steppe using 14C. They found C isotope turnover to be the most reliable of the three methods for measuring BNPP, providing a relatively unbiased integration of root turnover. Consequently, root production can be considered the inverse of the decrease in the isotope label. Root turnover can thus be estimated by regression to the time of zero remaining isotope (Milchunas & Lauenroth 2001). Although Milchunas (Citation2009) noted that the actual BNPP can never be known, the isotope turnover method is based on the least severe assumptions.
An important component of NPP is the proportional allocation of C between roots and shoots. Poorter & Nagel (Citation2000) defined allocation as the amount of biomass that is present in various plant organs relative to total plant mass. However, Stewart & Metherell (Citation1999) defined allocation on the basis of C input not mass; they determined C allocation to roots by a similar labelling method to that used by Milchunas & Lauenroth (Citation1992), but with the stable C isotope 13C to label micro-plots on pasture ecosystems resulting from the long-term application of irrigation, superphosphate fertiliser and grazing management treatments. This study extends Stewart & Metherell's (Citation1999) work on short-term C allocation to roots, by giving estimates of root turnover and production from their study site that will provide additional information on NPP for the national C balance (Tate et al. Citation2000). There is good agreement between simulated and site-specific NPP amounts (Tate et al. Citation2000) although large uncertainties exist in the national NPP estimate due in part to limited information on below ground C allocation and therefore root C production.
It was hypothesised that fertiliser and irrigation would change root to shoot C allocation with consequent effects on root C production. The objective of this work was thus to quantify C allocation to roots and subsequent turnover and production in contrasting pastoral ecosystems that developed under irrigated, unirrigated, phosphate fertilised and unfertilised treatments.
Materials and methods
Site description
The treatments, pulse-labelling method, sampling procedure and initial calculations have been described in detail by Stewart & Metherell (Citation1999). However, relevant aspects to this study are now presented.
Winchmore Irrigation Research Station (latitude 43°47′S, longitude 171°48′E, altitude 160 m above sea level, 741 mm mean annual precipitation, mean winter temperature 5 °C, mean summer temperature 16 °C), is a flat, border-strip irrigated pasture site on a Lismore stony silt loam soil (Udic Ustochrept [USDA]; Pallic Firm Brown [New Zealand]) where fertiliser rate and irrigation frequency experiments have run since 1952.
The fertiliser experiment comprised fenced border strips (64 m×11 m) with five treatments in a randomised complete block design with four replicates. The 0 and 375 kg ha−1 y−1 single superphosphate (SSP) treatments were used in this study. All fertiliser treatments were irrigated when the gravimetric soil moisture content (w/w) in the top 100 mm was 15–20% (Smith et al. Citation2012). The SSP treatments will be referred to as SSP/irr.
The irrigation experiment, which was several hundred metres from the fertiliser experiment, received annual dressings of 250 kg SSP y−1. It comprised five irrigation treatments within separately fenced border strips with four replicates used in a completely randomised design (McBride Citation1994). The irrigation experiment treatments used in this study were (1) unirrigated and (2) irrigated when the soil water concentration in the surface 100 mm fell to 20% w/w. This irrigation treatment was based on irrigating at 50% available moisture, which was approximately equivalent to a 31 mm deficit in the top 300 mm of this stony soil. The irrigation treatments will be referred to as IRR/ssp. The sites were sown with perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.) in 1950, but the species composition at the time of this study (in 1995–1996) reflected botanical changes induced by the treatments. The nil SSP/irr pasture contained much lower proportions of ryegrass and white clover and more low-fertility grasses and weeds than the SSP/irr pasture (i.e. Agrostis capillaris, Anthoxanthum odoratum, Holcus lanatus and Cynosurus cristatus and weeds Hypochaeris radicata, Plantago lanceolata, Achillia millefolium and moss). Nil IRR/ssp pasture contained less white clover and more subterranean clover (Trifolium subterraneum L.) Agrostis capillaries and Erodium cicutarium than IRR/ssp pasture. Each treatment with four replicates (each 0.08 ha in size) was rotationally grazed by sheep during spring, summer and autumn. The 1996–1997 hogget stocking rates were 9, 27, 11 and 32 hoggets ha−1 (one 55 kg breeding ewe equivalent is one stock unit and a hogget is 0.7 of a stock unit), respectively, for nil SSP/irr, SSP/irr, nil IRR/ssp and Irr/ssp treatments.
Pulse-labelling method
Micro-plots were labelled with 13CO2 at Winchmore on 14–17 November 1995, 13–26 February 1996 and 13–23 May 1996 (i.e. ‘spring’, ‘summer’ and ‘autumn’ seasons, respectively). Repeating the labelling in separate micro-plots over three seasons gives a much better estimate of annual root production. One micro-plot was 13C labelled within each treatment replicate, when there was bright sunlight. Plots were labelled within cylindrical steel frames (1.95 m diameter) covered with ethyl-vinyl alcohol film (a gas-proof film) (EVAL, Europe) and sealed to the ground with bags of sand. The air within the canopies was circulated with an electric fan within a transparent cylinder. Containers holding aqueous solutions of either 2.5 or 4 g Na2 13CO3 (99 atom%) and 1.0 g of Na2 12CO3 were mounted below the cylinder. The total CO2 concentration within the canopy was continuously monitored during labelling with a ‘Binos’ infra-red gas analyser (Leybold-Heraeus, Germany). After the canopy was sealed and CO2 concentration decreased to equilibrium, a pulse of 13CO2 was released into the chamber by injecting 3 M H2SO4 into the Na2 13CO3 solution. After the CO2 concentration had again decreased to equilibrium a pulse of 12CO2 was released to increase label uptake by the pasture and the canopy was removed once the CO2 decreased to equilibrium (Stewart & Metherell Citation1999).
Measurements and sampling
The soil bulk density was measured by gamma ray attenuation (Strata Gauge, MC-S-36, CPN Corporation, California, USA) with allowance made for the content of stones greater than 2 mm in diameter.
Above ground pasture production was measured by harvesting herbage each 1–2 months from two 4 m2 cages (one of which had been cut close to ground level at the previous harvest) within each replicate (Nguyen et al. Citation1989).
Sampling, one to two monthly (7–10 sampling events) to perform 13C measurements, involved five subsamples per plot that were collected and bulked. Subsamples comprised 0.01 m2 of herbage material and 25 mm diameter by 200 mm deep soil cores containing both live and dead roots. The soil was washed from herbage and root samples. All samples were dried at 70 °C, the weights of herbage and root samples were determined and then ground (<0.25 mm). Total C and δ 13C were determined with a Europa Scientific Tracermass mass spectrometer. The soil C concentration was measured on composite (12–15 subsamples per sample), replicated samples taken in May/June 1997 with approximately 1 g of air-dried, sieved (2 mm) soil being analysed by combustion/infra-red detection of CO2 using a LECO CNS-2000 analyser (LECO, Michigan, USA). The relationship between titanium and C concentrations in herbage samples can be used to account for soil contamination (Cary Citation1992). The titanium concentration of selected herbage and root samples was measured following nitric/perchloric acid digestion with an induction coupled plasma technique (Stewart & Metherell Citation1999).
Calculations and statistical analysis
Consistent with the work of Stewart & Metherell (Citation1999), herbage and root masses were corrected for any remaining soil using the regression relationships between titanium and C concentrations for each type of sample (Cary Citation1992). Root δ 13C values were corrected for soil contamination using the measured root C concentration. The ratios and amounts of 13C in soil were calculated from root and below ground values and soil bulk density. The mass of 13C was determined from the fractional abundance (F) and the total C content using the absolute isotope ratio of the Pee Dee Belemnite (V-PDB) standard (R standard) sample (Boutton Citation1991).
First, root δ 13C was determined from the measured 13C and 12C isotope ratios of samples and the V-PDB standard
where δ 13C is the difference between sample and standard 13C content in parts per thousand, or per mil (%) and R (the absolute ratio) is the 13C/12C mass ratio of sample and standard.
To determine the fractional abundance of 13C in a sample, EquationEquation 1 can be transformed to calculate R sample
The fractional abundance of 13C is determined from
Calculations used the long-term treatment mean per plot values of root total carbon.
Background root δ 13C measurements were made on four occasions (13 November 1995, 14 February 1996, 9 March 1996 and 29 October 1996) in pasture adjacent to the labelled micro-plots and were used (along with interpolation for each plot between these data) with enriched root δ 13C measurements to determine the root enrichment (g 13C m−2). The root 13C enrichment data were used from their maximum enrichment (at 23–28 days after labelling) until the end of the experiment. The enriched root 13C values for each plot were linearly regressed against the time of peak enrichment (t=0) to the end of each sampling period for each season. The y intercept was used as the notional peak enriched 13C. The efficiency of 13C enrichment varied with the environmental conditions and the existing herbage mass at labelling. Thus absolute 13C enrichment data over time were normalised with respect to the notional peak enriched 13C. Consequently, each plot for all treatments and seasons had a common value of normalised peak 13C enrichment. This allowed an analysis of all seasons and treatments together. The x intercepts (time of root turnover) of the linear regressions of absolute 13C values for each plot were used to determine treatment differences. In the figures produced, means of data were presented with the regression lines for simplicity, however all regressions were calculated using per plot replicated data. Root production (g C m−2 y−1) was calculated by dividing mean root biomass (g C m−2) by root turnover time (years).
Analysis of variance was performed with Genstat 5 (VSN International Ltd, Oxford, UK) with P treatments in a randomised block design, the irrigated treatments in a completely randomised design and season of labelling within a sub-plot stratum. Long-term C allocation to roots was determined by the ratio of root C production to total plant C production (R/(R+S)) determined from annual DM and root production was calculated from 45.9% C in roots (R) and 40.0% C in shoots (S). To present a complete picture, some 21-day C allocation results from Stewart & Metherell (Citation1999) have been recalculated (due to a small arithmetic error associated with the soil contamination correction) and are presented in this paper.
Results
Root turnover time
Root turnover time (, ) was longer without irrigation and without fertilisation, although the difference was not quite significant (P=0.08) in the latter case. Over both experiments, there was no season of labelling effect on the estimate of turnover time, although there were some treatment interactions. Without fertilisation, root turnover time from spring labelling of 1.6 y, was less than 2.1 y with autumn labelling. It is important to note that the season of labelling refers to results from approximately 12 months of data from the time of labelling; consequently, they represent estimates of the same treatment merely with a different commencement time.
Figure 1 Relative 13C enrichment of roots with time following 13C pulse-labelling (normalised data means from peak enrichment) determined from the influence of long-term irrigation and superphosphate fertiliser. The fertiliser rate experiment was irrigated and the irrigation experiment was fertilised.
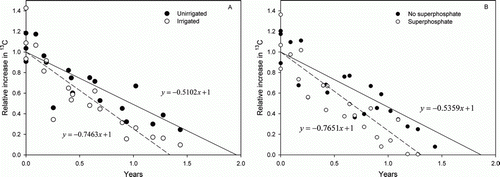
Table 1 Effect of irrigation, phosphate fertiliser and season of pulse-labelling on root turnover time (in years).
Root C production
There were no consistent seasonal trends in root biomass so long-term mean biomass was used. shows the influence of irrigation and superphosphate fertiliser on the average above ground herbage C production, root C production and root mass. Above ground C production increased considerably with IRR/ssp and SSP/irr. In contrast, the overall mass of root C declined with IRR/ssp and SSP/irr while annual root C production was unaffected by the treatments.
Table 2 Effect of long-term irrigation or superphosphate fertiliser application on above ground herbage C production, root C production and root C mass.
Root allocation
shows the proportion of 13C allocated to roots after 21 days, determined from long-term biomass samples and root turnover time. The data originally presented by Stewart & Metherell (Citation1999) were reanalysed, but trends in the results are the same. Root allocation after 21 days declined with the addition of superphosphate and was also lower in summer compared with the spring and autumn labelling. In contrast, under the IRR/ssp experiment, there was no main irrigation effect while both spring and summer main effects were less than the autumn labelling. However, the allocation of C to roots determined from long-term biomass samples and root turnover time indicated a decline with both added irrigation water and superphosphate.
Table 3 The effect of long-term irrigation, superphosphate fertiliser and season of pulse-labelling on the proportion of plant 13C recovered in roots 21 days after labelling and determined from annual shoot and root production.
Discussion
Root turnover time
By determining root turnover, it is possible to more accurately calculate BNPP from root biomass measurements (Milchunas Citation2009). In the current study, root turnover time was determined by 13C decrease. However, other workers have determined root turnover from various below ground biomass and below ground biomass production measures (Dahlman & Kucera Citation1965; Sims & Singh Citation1978; Pucheta et al. Citation2004; Wu et al. Citation2011) and from minirhizotron tube observations (Kätterer & Andrén Citation1999; Frank Citation2007). Milchunas (Citation2009) showed that root production based on sequential biomass measurements generates erroneous results. For example, decreases in biomass between sampling dates are ignored. Consequently, using such a method to determine root turnover time would be expected to also produce erroneous estimates of root turnover. The evaluation of methods to estimate root production conducted by Milchunas (Citation2009) concluded that minirhizotron and isotope decrease methods are the most suitable, albeit the isotope method tends to overestimate root turnover times (and therefore underestimate BNPP) whereas minirhizotron methods underestimate root turnover times. Differences between the minirhizotron and isotope decrease may be attributed, for example, to incomplete soil isotope removal or extra root growth over the curved tube surface and tube bending or movement.
With the proviso then that our study possibly overestimated root turnover time (Milchunas Citation2009), we determined that root turnover time with SSP/irr was 1.3 y but not quite statistically significantly different (P=0.08) from nil SSP/irr at 1.9 y, (, ). However, when autumn labelling season is considered, root turnover with fertiliser (both IRR/ssp and SSP/irr) at 1.1 y was significantly different than nil SSP/irr at 2.1 y. All treatments in the SSP/irr experiment were irrigated, indicating plant moisture stress was not a factor to consider. Furthermore, residual herbage after each grazing was similar across treatments, ensuring differences in stocking rate between treatments would also not be a factor influencing root turnover time or BNPP or C allocation from above ground to below ground. However, the SSP/irr treatment compared with the nil SSP/irr contained a greater proportion of ryegrass and clovers whereas the nil SSP/irr treatment contained greater proportions of low-fertility grasses and weeds (i.e. Agrostis capillaris, Anthoxanthum odoratum, Holcus lanatus and Cynosurus cristatus and weeds Hypochaeris radicata, Plantago lanceolata, Achillia millefolium and moss). Furthermore, there were more upright pasture species with fibrous roots in the fertilised treatment and many flat weeds with tap roots in the unfertilised treatment. Plant material in the unfertilised treatment had lower N concentration than the fertilised treatment and there was more lignin in unirrigated and unfertilised treatments (Metherell Citation2003). This lower quality plant material will decompose more slowly (Parton et al. Citation1987). Root turnover times of individual species were not studied, but it is likely that both species differences and plant nutritional effects would contribute to the observed results.
The finding in this work, that root turnover time under low fertility was longer than under high-fertility conditions, is consistent with the findings of other workers (Aerts et al. Citation1992; Ryser Citation1996; Gill et al. Citation2002a; Van der Krift & Berendse Citation2002). Van der Krift and Berendse (2002) found root life spans of grasses adapted to high-fertility sites were less than grass species from low-fertility sites. Although they did not measure root mass, they concluded that grass species from fertile habitats lose more biomass and nutrients by root turnover, and input more C and nutrients into the soil system than species that inhabit less fertile soils. Furthermore, they and Gill et al. (2002a) found root diameter was positively correlated to root life span. In addition, root diameter declined with root age and the decline rate was lower in plants from lower fertility habitats. Ryser (Citation1996) studied fast- and slow-growing grass species in low soil nutrient conditions. Species characteristic of nutrient-rich sites had low leaf and root tissue density compared with the higher tissue density of the slow-growing species characteristic of low-nutrient sites. After two growing seasons, the species from nutrient-poor sites were larger. The life span of both leaves and roots was correlated with tissue density. Species with low tissue density had a faster turnover of leaves and roots. Thus, the physiological attributes of plants growing in high- and low-fertility sites observed by Gill et al. (Citation2002a) and Ryser (Citation1996) indicate that one would expect root turnover under the nil SSP/irr treatment to be longer than under the SSP/irr treatment. On dry heath land, Aerts et al. (Citation1992) found turnover times decreased from 1.3 y for low-fertility species to 0.7 y for high-production species preferring high-fertility sites; this decreased to 0.4 y on a wet site (Aerts et al. Citation1989).
Similar to the unfertilised treatment, the unirrigated treatment contained a greater proportion of low-fertility species. These low-fertility species would be expected to have contributed to a longer root turnover time under the drier unirrigated conditions. Without irrigation, turnover time was 2.0 y compared with irrigation at 1.3 y. The treatment effects on root turnover in our study (, ) are consistent with the findings of Hayes & Seastedt (Citation1987) who used root windows and cores in tall grass (C4) prairie in Kansas in wet and dry years. They found root disappearance at 0–40 cm depth declined in the dry period of a dry year. Root disappearance increased in the wetter season the following year. Frank (Citation2007), under high-elevation grazed grassland (1900 m above sea level), also found that BNPP declined in a dry year, as did McCulley et al. (Citation2005) who found BNPP increased during a wet year.
The root turnover times in the current study (1.1–2.2 y) appear to be equal to or greater than other reported root turnover times in New Zealand. Gibbs & Reid (Citation1992) determined a mean perennial ryegrass root life of 46 days at Lincoln, New Zealand, which was similar to that reported by Garwood (Citation1967) in the United Kingdom. However, as roots aged, Gibbs & Reid (Citation1992) had difficulty distinguishing whether roots were still alive. Furthermore, they did not monitor roots between 0 and 5 cm depth. In contrast, Dahlman & Kucera (Citation1965) from soil cores taken to a depth of 86 cm from undisturbed Missouri prairie, estimated turnover time to be 4 y and Pucheta et al. (2004), using the same method as Dahlman & Kucera (Citation1965), found grazed central Argentinean grassland root turnover to be 0.95 y. Using 14C pulse-labelling, Milchunas & Lauenroth (Citation1992) estimated, to 20 cm depth, root turnover of Colorado range land to be 5 y. However, a follow-up study (Milchunas & Lauenroth Citation2001) indicated a second slower phase of decay in isotope concentration 5–10 y post-labelling. Under a high-fertility New Zealand dairy farm, Saggar & Hedley (Citation2001) obtained root turnover periods of approximately 400 days. Gill & Jackson (Citation2000) noted that the variability in root turnover time estimates by various workers may relate to the variety of methods used, especially when turnover is greater than 1 y. The 13C pulse-labelling approach used in this study did not suffer from the constraints and problems illustrated by Gill & Jackson (Citation2000) and has been found to be more reliable than traditional coring methods (Milchunas & Lauenroth Citation2001; Milchunas Citation2009).
There was no general difference in root turnover with time of labelling (). Gibbs & Reid (Citation1992), however, observed the shortest root longevity in ryegrass roots in spring and summer, and greatest longevity in the autumn and winter, suggesting that optimum soil moisture and temperature conditions for root decomposition occurred at this time. Saggar & Hedley (Citation2001) and Eissenstat & Yanai (Citation1997) also found root turnover time was lower in spring than autumn and winter.
Root production
Fertiliser and irrigation greatly increased herbage production but root C production was not significantly affected by the addition of irrigation or superphosphate fertiliser (). Furthermore, the overall mass of root C declined with the addition of irrigation and fertilisation. This is consistent with the findings of others (Davidson Citation1969, Tate et al. Citation1991, Crawford et al. Citation1997, Kätterer & Andrén Citation1999). Moreover, these results are consistent with the surface (0–10 cm) total soil C concentrations of 4.20% nil IRR/ssp, 3.59% under IRR/ssp (LSD0.05 0.19) and 3.66% nil SSP/irr and 3.64% with SSP/irr (LSD0.05 0.23) (Metherell Citation2003; Condron et al. 2006). The higher % C without irrigation is attributed to lower mineralisation rates under dryer conditions and the higher lignin content in dryland litter. The similar root C inputs with and without fertilisation, lower quality litter C without fertiliser and similar environmental conditions are reflected in the similar soil C levels in the fertiliser trial (Metherell Citation2003).
Saggar et al. (Citation1997) found root C production in hill pasture in the North Island 35 days after 14C pulse-labelling was 245, 384 and 443 g C m−2 y−1 for low-, medium- and high-fertility sites, respectively. They estimated annual C flux from annual shoot growth, % C content and % of net assimilated 14C from spring pulse-labelling. This assumes that C concentration and allocation between plant and soil components and respiration were the same throughout the year, which may not have been the case. In addition, root production has been found to be greater in the spring (Saggar & Hedley Citation2001). Consequently, it is likely their values are overestimates. Thus, the BNPP values of 191 and 225 g C m−2 y−1 (not statistically significant at P<0.05) under unfertilised and fertilised conditions, respectively, in the current study appear to be credible estimates. It is noted that estimates of root production at Winchmore in 1985–1986 by Nguyen & Goh (Citation1992) were limited by the use of mean annual root biomass from soil coring and a rough estimate from the literature of an annual root turnover rate.
Root allocation
Although there are differences in absolute values of 13C allocation to roots between Stewart & Metherell (Citation1999) and the reanalysed values presented here (), the relative treatment effects did not generally change. However, the proportion of 13C allocated to roots after 21 days showed little or no treatment effects () but allocation to roots was lower than previously presented by Stewart & Metherell (Citation1999). In contrast, C allocation determined from long-term production data () showed a number of clearly significant treatment effects. The difference in the C allocation generated by the two methods may reflect different conditions on the day of labelling (Stewart & Metherell Citation1999), whereas the biomass harvesting of the latter method reflects influences over the whole growing season. Niklaus et al. (Citation2001) showed that estimates of C allocation from 13C labelling were dependent on season and interval between application and recovery of the label. Swinnen et al. (Citation1994) also reported that allocation can be affected by temperature, light intensity, soil moisture, soil texture, nutrient status, stage of plant development and O2 tension. Consequently, in this work, the labelling was carried out over three seasons in order to improve estimates of root turnover time and annual root production. Complete allocation has been observed by day 19 after 14C labelling (Swinnen et al. Citation1994), while other workers observed similar or shorter time periods (Gordon et al. Citation1977; Warembourg et al. Citation1982; Keith et al. Citation1986; Meharg & Killham Citation1990).
Although above ground herbage C production increased with the addition of fertiliser, both root C mass and root allocation (determined 21 days after labelling) declined, while root C production was unaffected (see and ). With the application of irrigation, above ground herbage C production increased, while root C mass declined, but both root C production and root allocation were unaffected ( and ). However, C allocation to roots determined from long-term production DM harvests, which did not suffer from the short-term environmental effects on the day of labelling, showed allocation was greater without irrigation or fertiliser (). This is consistent with the findings of Saggar et al. (Citation1997) who found progressively greater allocation to roots with decreasing fertility under North Island hill pastures. Poorter & Nagel (Citation2000) analysed data from the literature to characterise biomass allocation in response to resource limitation and found increased allocation to roots when water or nutrients were limiting. These findings are consistent with the longer root turnover times determined in the current study for unirrigated and unfertilised pastures.
Conclusions
The decline of pulse-labelled 13C enabled BNPP, root C allocation and root turnover time to be determined under different pastoral ecosystems resulting from long-term fertiliser and irrigation treatments. Although unfertilised (but with irrigation) and unirrigated (but fertilised) treatments had greater root biomass and root C allocation, the BNPP of 206 g C m−2 y−1 was similar in unfertilised, unirrigated, fertilised and irrigated pastures, despite marked differences in above ground production. Landuse intensification with added irrigation and superphosphate fertiliser produced substantial increases in above ground herbage C production but no significant difference in BNPP. The longer root turnover times of 1.9 y and 2.0 y under unfertilised (but irrigated) and unirrigated (but fertilised) pasture, respectively, than 1.3 y under fertilised and irrigated treatments was a contributing factor. These root turnover times appeared to be consistent with the attributes of the species found in the distinct plant communities that developed in response to the imposed treatments.
Acknowledgements
Experimental work and data analysis were conducted while the authors were employed by AgResearch. We thank Peter Carey and Yin Chow Woo for helping with field work and sample preparation, Anjana Rajendram of the University of Waikato Stable Isotope Unit for carbon analyses, Roger Cresswell for soil C analysis, David Whitehead of Landcare Research for the loan of a gas analyser, the staff of Winchmore, David Baird for statistical advice, the New Zealand Foundation for Research Science and Technology for funding this research. Finally, thanks go to both the Agricultural and Marketing Research and Development Trust and the New Zealand Fertiliser Manufacturer's Research Association for their support, which enabled this work to be completed after the sad and untimely death of Dr Dean Stewart.
References
- Aerts , R , Berendse , F , Klerk , NM and Bakker , C . 1989 . Root production and root turnover in two dominant species of wet heathlands . Oecologia , 81 : 374 – 378 .
- Aerts , R , Bakker , C and De Caluwe , H . 1992 . Root turnover as a determinant of the cycling of C, N, and P in a dry heathland ecosystem . Biogeochemistry , 15 : 175 – 190 .
- Bliss , LC and Mark , AF . 1974 . High-alpine environments and primary production on the rock and pillar range, Central Otago, New Zealand . New Zealand Journal of Botany , 12 : 445 – 483 .
- Boutton TW 1991 . Stable carbon isotope ratios of natural materials: 1. Sample preparation and mass spectrometric analysis . In : Coleman DC , Fry B . Carbon isotope techniques San Diego , Ca , Academic Press . 155 – 171 .
- Cary , EE . 1992 . Correction of elemental analysis of soil-grown wheat roots contaminated with soil . Journal of Plant Nutrition , 15 : 857 – 869 .
- Conant , RT , Paustian , K and Elliott , ET . 2001 . Grassland management and conversion into grassland: effects on soil carbon . Ecological Applications , 11 : 343 – 355 .
- Condron LM Sinaj S McDowell RW Dudler-Guela J Scott JT Metherell AK 2006 Influence of long term irrigation on the distribution and availability of soil phosphorus under permanent pasture Australian Journal of Soil Research 44 127 133
- Crawford , MC , Grace , PR , Bellotti , WD and Oades , JM . 1997 . Root production of barrel medic (Medicago truncatula) pasture, a barley grass (Hordeum leporinum) pasture, and a faba bean (Vicia faba) crop in southern Australia . Australian Journal of Agricultural Research , 48 : 1139 – 1150 .
- Dahlman , RC and Kucera , CL . 1965 . Root productivity and turnover in native prairie . Ecology , 46 : 84 – 89 .
- Davidson , RL . 1969 . Effects of soil nutrients and moisture on root/shoot ratios in Lolium perenne L. and Trifolium repens L . Annals of Botany , 33 : 571 – 577 .
- Eissenstat , DM and Yanai , RD . 1997 . The ecology of root life span . Advances in Ecological Research , 27 : 1 – 62 .
- Frank , DA . 2007 . Drought effects on above- and belowground production of a grazed temperate grassland ecosystem . Oecologia , 152 : 131 – 139 .
- Garwood , EA . 1967 . Seasonal variation in appearance and growth of grass roots . Journal of the British Grassland Society , 22 : 121 – 130 .
- Gibbs , RJ and Reid , JB . 1992 . Comparison between net and gross root production by winter wheat and by perennial ryegrass . New Zealand Journal of Crop and Horticultural Science , 20 : 483 – 487 .
- Gill , RA and Jackson , RB . 2000 . Global patterns of root turnover for terrestrial ecosystems . New Phytologist , 147 : 13 – 31 .
- Gill , RA , Burke , IC , Lauenroth , WK and Milchunas , DG . 2002a . Longevity and turnover of roots in the shortgrass steppe: influence of diameter and depth . Plant Ecology , 159 : 241 – 251 .
- Gill , RA , Kelly , RH , Parton , WJ , Day , KA , Jackson , RB , Morgan , JA , Scurlock , JMO , Tieszen , LL , Castle , JV , Ojima , DS and Zhang , XS . 2002b . Using simple environmental variables to estimate below-ground productivity in grasslands . Global Ecology and Biogeography , 11 : 79 – 86 .
- Gordon , AJ , Ryle , GJA and Powell , CE . 1977 . The strategy of carbon utilization in uniculm barley I. The chemical fate of photosynthetically assimilated 14C . Journal of Experimental Botany , 28 : 1258 – 1269 .
- Hayes , DC and Seastedt , TR . 1987 . Root dynamics of tallgrass prairie in wet and dry years . Canadian Journal of Botany , 65 : 787 – 791 .
- Houghton , RA . 1995 . “ Changes in the storage of terrestrial carbon since 1850 ” . In Soils and global change , Edited by: Lal , R , Kimble , J , Levine , E and Stewart , BA . 45 – 65 . Boca Raton , FL : CRC Press .
- Hui , DF and Jackson , RB . 2006 . Geographical and interannual variability in biomass partitioning in grassland ecosystems: a synthesis of field data . New Phytologist , 169 : 85 – 93 .
- Kätterer , T and Andrén , O . 1999 . Growth dynamics of reed canarygrass (Phalaris arundinacea L.) and its allocation of biomass and nitrogen below ground in a field receiving daily irrigation and fertilisation . Nutrient Cycling in Agroecosystems , 54 : 21 – 29 .
- Keith , H , Oades , JM and Martin , JK . 1986 . Input of carbon to soil from wheat plants . Soil Biology and Biochemistry , 18 : 445 – 449 .
- Lauenroth , WK , Wade , AA , Williamson , MA , Ross , BE , Kumar , S and Cariveau , DP . 2006 . Uncertainty in calculations of net primary production for grasslands . Ecosystems , 9 : 843 – 851 .
- LeCain , DR , Morgan , JA , Schuman , GE , Reeder , JD and Hart , RH . 2002 . Carbon exchange and species composition of grazed pastures and exclosures in the shortgrass steppe of Colorado . Agriculture Ecosystems and Environment , 93 : 421 – 435 .
- Litton , CM and Giardina , CP . 2008 . Below-ground carbon flux and partitioning: global patterns and response to temperature . Functional Ecology , 22 : 941 – 954 .
- McBride , SD . 1994 . Pasture yield response to irrigation in Canterbury . Proceedings of the New Zealand Grassland Association. , 56 : 165 – 168 .
- McCulley , RL , Burke , IC , Nelson , JA , Lauenroth , WK , Knapp , AK and Kelly , EF . 2005 . Regional patterns in carbon cycling across the Great Plains of North America . Ecosystems , 8 : 106 – 121 .
- Meharg , AA and Killham , K . 1990 . Carbon distribution within the plant and rhizosphere for Lolium perenne subjected to anaerobic soil conditions . Soil Biology and Biochemistry , 22 : 643 – 647 .
- Metherell , AK . 2003 . Management effects on soil carbon storage in New Zealand pastures . Proceedings of the New Zealand Grassland Association , 65 : 259 – 264 .
- Meurk , CD . 1978 . Alpine phytomass and primary productivity in Central Otago, New Zealand . New Zealand Journal of Ecology , 1 : 27 – 50 .
- Milchunas , DG . 2009 . Estimating root production: comparison of 11 methods in shortgrass steppe and review of biases . Ecosystems , 12 : 1381 – 1402 .
- Milchunas , DG and Lauenroth , WK . 1992 . Carbon dynamics and estimates of primary production by harvest, 14C dilution, and 14C turnover . Ecology , 73 : 593 – 607 .
- Milchunas , DG and Lauenroth , WK . 2001 . Below ground primary production by carbon isotope decay and long-term root biomass dynamics . Ecosystems , 4 : 139 – 150 .
- Nguyen , L and Goh , KM . 1992 . Nutrient cycling and losses based on a mass-balance model in grazed pastures receiving long-term superphosphate applications in New Zealand. 1. Phosphorus . Journal Agricultural Science , 119 : 89 – 106 .
- Nguyen , ML , Rickard , DS and McBride , SD . 1989 . Pasture production and changes in phosphorus and sulphur status in irrigated pastures receiving long-term applications of superphosphate fertiliser . New Zealand Journal of Agricultural Research , 32 : 245 – 262 .
- Ni , J . 2004 . Estimating net primary productivity of grasslands from field biomass measurements in temperate northern China . Plant Ecology , 174 : 217 – 234 .
- Niklaus , PA , Glockler , E , Siegwolf , R and Korner , C . 2001 . Carbon allocation in calcareous grassland under elevated CO2: a combined 13C pulse-labelling/soil physical fractionation study . Functional Ecology , 15 : 43 – 50 .
- Parton , WJ , Schimel , DS , Cole , CV and Ojima , DS . 1987 . Analysis of factors controlling soil organic matter levels in Great Plains grasslands . Journal of the Soil Science Society of America , 51 : 1173 – 1179 .
- Poorter , H and Nagel , O . 2000 . The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review . Australian Journal of Plant Physiology , 27 : 595 – 607 .
- Pucheta , E , Bonamici , I , Cabido , M and Diaz , S . 2004 . Below-ground biomass and productivity of a grazed site and a neighbouring ungrazed exclosure in a grassland in central Argentina . Austral Ecology , 29 : 201 – 208 .
- Rasse DP Rumpel C Dignac MF 2005 Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation Plant and Soil 269 341 356
- Ryser , P . 1996 . The importance of tissue density for growth and life span of leaves and roots: a comparison of five ecologically contrasting grasses . Functional Ecology , 10 : 717 – 723 .
- Saggar , S and Hedley , CB . 2001 . Estimating seasonal and annual carbon inputs, and root decomposition rates in a temperate pasture following field 14C pulse-labelling . Plant and Soil , 236 : 91 – 103 .
- Saggar , S , Hedley , C and Mackay , AD . 1997 . Partitioning and translocation of photosynthetically fixed 14C in grazed hill pastures . Biology and Fertility of Soils , 25 : 152 – 158 .
- Saggar , S , Mackay , AD and Hedley , CB . 1999 . Hill slope effects on the vertical fluxes of photosynthetically fixed 14C in a grazed pasture . Australian Journal of Soil Research , 37 : 655 – 666 .
- Sims , PL and Singh , JS . 1978 . The structure and function of ten western North American grasslands III. Net primary production, turnover and efficiencies of energy capture and water use . Journal of Ecology , 66 : 573 – 597 .
- Smith , LC , Moss , RA , Morton , JD , Metherell , AK and Fraser , TJ . 2012 . Pasture production from a long-term fertiliser trial under irrigation . New Zealand Journal of Agricultural Research , 55 : 105 – 117 .
- Stewart , DPC and Metherell , AK . 1999 . Carbon (13C) uptake and allocation in pasture plants following field pulse-labelling . Plant and Soil , 210 : 61 – 73 .
- Svejcar , T and Christiansen , S . 1987 . The influence of grazing pressure on rooting dynamics of Caucasian Bluestem . Journal of Range Management , 40 : 224 – 227 .
- Swinnen , J , Van Veen , JA and Merckx , R . 1994 . 14C pulse-labelling of field-grown spring wheat: an evaluation of its use in rhizosphere carbon budget estimations . Soil Biology and Biochemitry , 26 : 161 – 170 .
- Tate , KR , Speir , TW , Ross , DJ , Parfitt , RL , Whale , KN and Cowling , JC . 1991 . Temporal variations in some plant and soil P pools in two pasture soils of widely different P fertility status . Plant and Soil , 132 : 219 – 232 .
- Tate , KR , Scott , NA , Parshotam , A , Brown , L , Wilde , RH , Giltrap , DJ , Trustrum , NA , Gomez , B and Ross , DJ . 2000 . A multi-scale analysis of a terrestrial carbon budget Is New Zealand a source or sink of carbon? . Agriculture Ecosystems and Environment , 82 : 229 – 246 .
- Van Der Krift , TAJ and Berendse , F . 2002 . Root life spans of four grass species from habitats differing in nutrient availability . Functional Ecology , 16 : 198 – 203 .
- Warembourg , FR , Montagne , D and Bardine , R . 1982 . The simultaneous use of 14CO2 and 15N2 labelling techniques to study the carbon and nitrogen economy of legumes grown under natural conditions . Physiologia Plantarum , 56 : 46 – 55 .
- Williams , PA . 1977 . Growth, biomass, and net productivity of tall-tussock (Chionochloa) grasslands, Canterbury, New Zealand . New Zealand Journal of Botany , 15 : 399 – 442 .
- Wu , Y , Wu , J , Deng , Y , Tan , H , Du , Y , Gu , S , Tang , Y and Cui , X . 2011 . Comprehensive assessments of root biomass and production in a Kobresia humilis meadow on the Qinghai-Tibetan Plateau . Plant and Soil , 338 : 497 – 510 .