Abstract
Soil invertebrates have limited defences against moisture stress but little is understood of how irrigation influences their community. This paper investigates the influence of five summer irrigation schedules on earthworms over a 15-month period in a long-term pasture irrigation study. The influence of the two extreme rates of irrigation on other soil invertebrates was also examined. The effects of frequent drought periods under dryland pasture favoured small and short-lived nematodes and oribatids. During wetter months, earthworm abundances were similar between dryland and irrigated treatments, while earthworms tended to migrate down the soil profile and aestivate in response to soil water deficits during summer. The higher abundance of earthworms in the summer months under irrigation may partly explain the lower soil carbon found under irrigation, due to enhanced rates of soil organic matter turnover, despite higher primary production and inputs of carbon. It is thus important to consider irrigation schedules not only to optimise plant growth, but also to optimise the invertebrate community and its activity.
Keywords:
Introduction
Water availability is a key determinant of plant growth (Snaydon Citation1987; Goh & Bruce Citation2005). By limiting primary production, drought conditions limit food resources in the soil food-web, influencing the soil fauna and the soil services they contribute to (Lee Citation1985; Edwards & Bohlen Citation1996; Coleman et al. Citation2004). Soil fauna are further influenced by moisture deficit as they are adapted to a high-humidity interstitial environment (Lee Citation1985); regular dry periods may influence life histories, with shorter-lived microarthropods, potentially opportunistic, r-strategists dominating drought-affected areas (Lindberg & Bengtsson Citation2005). Other soil fauna such as earthworms have adapted to surviving drought through their behaviour. Some species retreat deeper into the soil and become less active during dry periods (aestivate), others simply move to an area where there is more moisture present, while others die after producing cocoons that hatch when conditions become more favourable for activity (Edwards & Bohlen Citation1996).
Irrigation, used to overcome the soil water deficit in drought-prone areas to sustain pasture growth, moderates the abiotic environment of the soil fauna, reducing the risk of dehydration and anhydrobiosis. The increase in pasture growth through the removal of moisture limitations by irrigation and the associated higher returns of livestock excreta also provide additional organic matter inputs into the soil food-web. Irrigation of pasture enables earthworms to remain active in the topsoil for longer into the summer months where their activity might otherwise be limited (Barley & Kleinig Citation1964; Nordstöm & Rundgren Citation1974; Yeates Citation1976; Tisdall Citation1985; Blackwell & Blackwell Citation1989; Bezborodov & Khalbayeva Citation1990; Baker Citation1992; Baker et al. 1993b; Garnsey & Buckerfield Citation1993). However, it can also affect the composition of earthworm communities, for example decreasing Aporrectodea caliginosa and increasing Lumbricus rubellus abundance (Lobry de Bruyn & Kingston Citation1997). Nematodes, Acari and Collembola also respond positively to irrigation used to reduce the soil water deficit (Yeates Citation1978; Lindberg & Bengtsson Citation2005).
This study investigated the impact of alleviating the summer water deficit on the soil faunal community in a long-term irrigated sheep-grazed pasture study established in 1959 in the Canterbury region, New Zealand. It was hypothesised that dryland sheep-grazed pasture would have a higher proportion of earthworms aestivating, and there would be low mesofaunal and nematode abundances dominated by drought-tolerant, short-lived organisms. In contrast, irrigated sheep-grazed pastures would manage to maintain a lower soil water deficit and would have a greater abundance and diversity of earthworms and long-lived mesofauna and nematodes sustained throughout the year.
Materials and methods
Study sites
The study site is located at the AgResearch, Winchmore Irrigation Research Station (43°47′S 171°48′E), 150 m above sea level on the Canterbury plains, New Zealand. The soils are classified as a Cambisol (FAO) (NZSC=Brown, Lismore stony silt loam) (Hewitt Citation1992), with an average depth of topsoil approximately 25 cm, overlying stony gravels. The average annual air temperature is 11.1 °C and rainfall is 740 mm, with moisture deficits common from September through to March (Duncan et al. Citation1985). Pastures are dominated by Lolium perenne (ryegrass) but also contain Trifolium repens (white clover).
The long-term irrigation trial, established in 1959, receives irrigation from September to May when topsoil (0–100 mm) gravimetric soil moisture content falls below a pre-determined level. Irrigation is applied by the border-strip method, where bordered bays are periodically flooded (100 mm per irrigation event). The field experiment was set up in a randomised block design with four replicated plots of 0.09 ha each. The irrigation treatments were
| 1. | no irrigation (dryland) | ||||
| 2. | irrigated at 10% gravimetric soil moisture (10%M) | ||||
| 3. | irrigated at 15% gravimetric soil moisture (15%M) | ||||
| 4. | irrigated at 20% gravimetric soil moisture (20%M) | ||||
| 5. | irrigated at intervals of not less than 3 weeks during the dry season when evapotranspiration levels exceed rainfall (3W). | ||||
Treatments received 23 kg P ha−1 y−1 as single superphosphate (SSP). The plots were rotationally grazed by 6 (dryland) to 18 (20%M) stock units per hectare, where a ‘stock unit’ is defined as a ‘standard sheep’ consuming 550 kg dry matter (DM) per year. The plots were not grazed during winter. Further details of the study site are given by McDowell & Rowley (Citation2008).
Earthworm sampling April 1994 to June 1995
Earthworm abundance, biomass and species composition were assessed monthly for 15 months (April 1994 to June 1995). One field trial replicate was sampled each month, with four replicate soil samples (25×25×25 cm) extracted from each treatment. Sampling was rotated each month between a total of three of the field trial replicates. Between August 1994 and June 1995, the earthworm depth distribution was explored with each soil sample stratified into 0–5, 5–10 and 10–25 cm depths. Earthworms were hand-sorted (Gilyarov Citation1975), identified and weighed following gut voidance. Species were identified according to the key of Sims and Gerard (Citation1985) and classified as mature adults if they possessed a clitellum. Larger individuals without clitella were classified as large immatures and smaller individuals (<0.5 g) were classified as juveniles.
At each sampling date, four replicate soil samples (~10 g) were also taken from each of three depths (0–5, 5–10 and 10–25 cm) in each treatment for determination of gravimetric soil moisture content (soil dried overnight at 105 °C). Total soil nitrogen, total soil carbon (dry combustion using LECO TruSpec analyser, LECO Equipment Corp., St. Joseph, MI, USA) and pH (1:2 soil:water) were determined at each depth for the June 1995 sampling only. Daily rainfall was recorded at the trial site and soil temperature (at 10 cm depth) was measured periodically.
Invertebrate sampling October 2007
In a separate investigation at the same trial site, further samples were collected from the dryland and 20%M treatments in October 2007. From each plot, three earthworm cores (15.5 cm diameter, 15.5 cm depth), four mesofauna cores (5 cm diameter, 15 cm depth) and one composite sample for nematodes (five cores, each 2.5 cm diameter, 0–7.5 and 7.5–15 cm soil depths) were collected. Earthworm cores were hand-sorted and specimens were identified to species. Mesofauna were extracted using a modified Berlese–Tullgren funnel and identified to groups, except Oribatida, which were identified to species (for details see Schon et al. (Citation2008)). Nematodes were extracted using a modified tray method as described by Yeates (Citation1978) and identified to genus. The nematode channel ratio (NCR), maturity index (MI), plant parasitic index (PPI) and Σmaturity index (ΣMI) were calculated (Bongers Citation1990; Yeates Citation1994, Citation2003).
At the time of sampling, soil temperature (Checktemp, Hanna Instruments, UK) and moisture (TDR 300 Soil Moisture Probe, Spectrum Technologies Inc., USA) at 0–10 cm depth were recorded. After extracting the mesofauna, the soil cores were analysed for soil pH (1:2 soil:water), Olsen P (Olsen et al. Citation1954), total soil nitrogen and total soil carbon (dry combustion using LECO-2000, LECO Equipment Corp., St. Joseph, MI, USA). Bulk density was determined by collecting three intact soil cores (10 cm diameter, 7.5 cm depth) from each plot, drying (105 °C) and weighing.
Statistical analysis
April 1994 to June 1995 sampling
Due to the sampling regime for this experiment, there was one observation per treatment per month, taken from one of the three field trial replicates in a rotational fashion. Since the behaviour of the five treatments was likely to be similar in winter, the data were divided into two groups to represent ‘summer’ (October to March, the timeframe over which irrigation is commonly applied) and ‘winter’ (April–September) seasons. There was a total of six observations per treatment per season and it was assumed that the lag of 3 months between consecutive sampling on a single plot is sufficient to preclude influential correlation. By using consecutive month–month measurements as independent observations, we also assume that the main differentiating feature of interest within a ‘month’ is adequately explained by season. Each season is analysed separately, since season is not replicated (i.e. they were treated as two separate experiments).
Earthworm abundance data were analysed using analysis of variance (ANOVA) where residuals appeared homoscedastic. The variation represented shows variation between reps/months across the season. The importance of rainfall and mean temperature was also assessed for their effects on total earthworm abundance in summer. The effect of rainfall as a covariate was analysed using ANOVA. Since the effect of mean temperature was not independent of the treatments, it was assessed as a linear predictor in regression. For each analysis, estimated means are presented with a measure of the variation associated with them.
October 2007 sampling
The effects of irrigation as measured in the October 2007 sampling on soil and pasture properties, microbial respiration and the abundance of macrofauna, mesofauna and nematodes were analysed using PROC MIXED in SAS v.9.1 (SAS Institute Inc., USA). Two depths (not available for the macrofauna data) were considered separately. The data were log (x+1) transformed prior to analysis. The graphs and tables in this paper show untransformed arithmetic means. Error bars on figures show the standard errors of the means.
Results
Pasture and soil properties are listed in . Pasture production increased with irrigation. Dryland pastures had a lower proportion of grass than the irrigated pastures. Total soil nitrogen content did not vary significantly across treatments. Total carbon content and soil pH in the top 0–5 cm tended to decline with increasing levels of irrigation.
Table 1 Pasture and soil parameters of the sheep-grazed pastures under different irrigation treatments.
Seasonal response of earthworms to irrigation
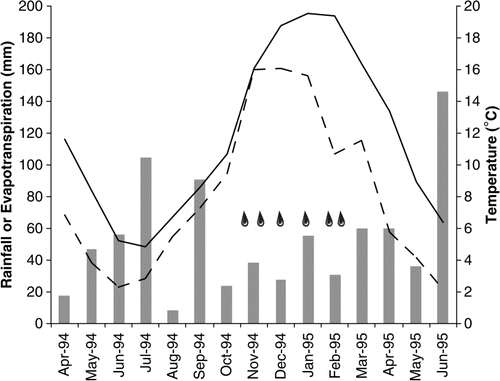
Between November 1994 and March 1995, the dryland plots received only natural rainfall of 173 mm, with a soil moisture deficit over the spring and summer period (). Over the 15-month duration of the trial, the soil moisture deficit equated to 395 mm (). During the study period, the 10%M treatment received two irrigations (100 mm per irrigation event), 15%M received four irrigations, 3W received six irrigations and the 20%M treatment received six irrigations over the spring and summer months. Soil temperature at 10 cm depth ranged from approximately 5 °C in mid-winter to around 19 °C in mid-summer. Soil temperature differed between treatments in summer only, with soil temperature in the 20%M treatment an average of 0.5 °C cooler than the dryland (data not shown).
Figure 1 Monthly rainfall (bars), Penman estimate of potential soil evapotranspiration (dashed line) and average monthly temperature (solid line, 0–10cm depth), as measured during the trial in 20%M treatment in 1994–1995. Timings of irrigation events are represented by raindrop symbols.
Five species of earthworms were found at the trial site ()—Aporrectodea caliginosa (Savigny), by far the dominant species, as well as Lumbricus rubellus (Hoffmeister), Aporrectodea trapezoides (Duges), Octolasion cyaneum (Savigny) and Aporrectodea rosea (Savigny).
Table 2 Mean proportions of individual earthworm species in sheep-grazed pastures under different irrigation treatments from April 1994 to June 1995.
Abundance and biomass of the earthworm populations showed marked seasonal effects that were strongly correlated with soil moisture, and less so with soil temperature (). The relationship with soil moisture was more apparent where either no or low levels of irrigation were applied (dryland, 10%M and 15%M). In the dryland pasture, higher earthworm abundance and biomass were observed in winter (600 m−2) than in the summer months (average 30 m−2) (). There was no evidence of a difference between irrigation treatments in the winter season (p=0.807), but there was some evidence of a treatment effect in summer (p=0.012; ). Over the ‘summer’ months (October to March), average earthworm populations ranged from around 130 m−2 in the dryland treatment and increased with increasing amounts of irrigation to an average of 530 m−2 in the 20%M treatment (). Irrespective of earthworm species (not shown here) or irrigation treatment, the majority of specimens present in summer tended to be smaller juvenile or immature earthworms (), especially in the most frequently irrigated plots (e.g. 20%M). The abundance and biomass (not shown) of earthworms remaining in the topsoil through the summer period increased with increasing levels of irrigation, with abundances above 300 m−2 and biomass above 60 g m−2 in the 20%M irrigation treatment (). This compares with zero in December in the dryland. Maximum biomass of earthworms during the late winter and early spring was approximately 140 g m−2 for both the dryland and 20%M irrigation treatments.
Figure 2 Seasonal changes in earthworm abundance (solid line) and fresh weight biomass (dashed line) in the dryland plots.
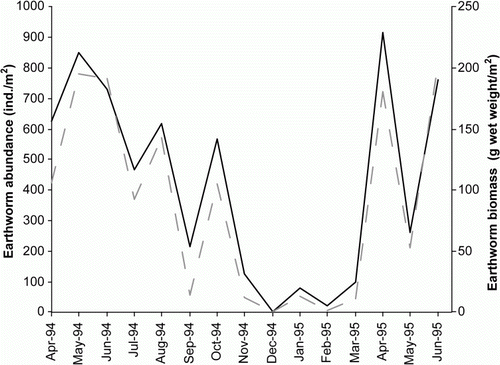
Figure 3 Effect of irrigation treatment on total earthworm abundance. Bars represent 5% LSD for winter (upper) and summer (lower) seasons. Summer is defined as the period from October to March (the time frame over which irrigation is commonly applied) and winter refers to the April–September period.
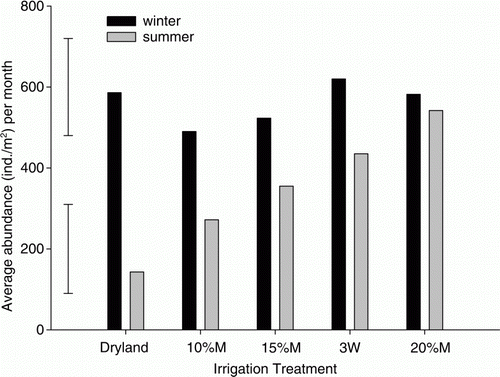
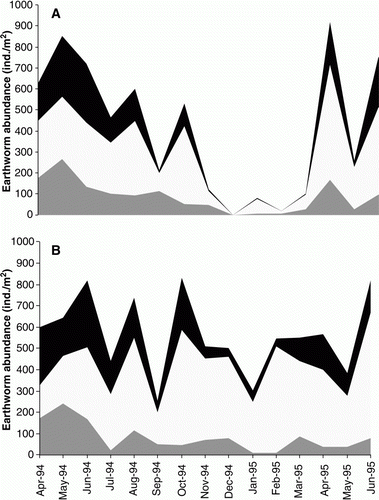
Figure 5 Changes in the summer earthworm population sizes (0–25cm depth) in response to five levels of irrigation.
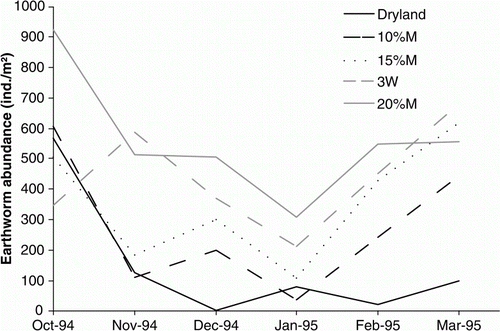
Table 3 Correlation coefficients between earthworm populations (abundance and biomass per m2) and soil moisture or temperature in sheep-grazed pastures under different irrigation treatments (n=15).
During the winter period, the majority of the earthworms were present in the upper 5 cm of soil, while during the summer months the majority of earthworms were present at >10 cm soil depth (). During the summer, treatment differences in earthworms were observed, with no earthworms present in the top 0–10 cm in the dryland pasture, but found in all three depths in the 20%M irrigation treatment (). Earthworm species differences in depth distribution were also observed. Throughout the year, A. caliginosa dominated the earthworm population, with the majority found at 0–10 cm depths during winter and 10–25 cm depths in the summer, regardless of irrigation treatment (). Lumbricus rubellus remained close to the soil surface (0–10 cm) throughout the year, with the exception of the dryland treatment during summer where they were detected deeper in the soil profile (10–25 cm). A. rosea declined during the summer in the two replicates where it was found, but the proportion of A. rosea close to the soil surface (0–5 cm) increased with irrigation. Octolasion cyaneum was in low numbers in all treatments. Its presence tended to increase with increasing levels of irrigation, with specimens recovered in summer or early autumn mostly located at 10–25 cm soil depth. In contrast to other species, in general, A. trapezoides responded negatively to irrigation, only being present in the 0% dryland, 10%M and 15%M irrigation treatments. During the summer period, A. trapezoides individuals were sometimes found at 10–25 cm depths or not found at all (December to February). Mature and immature specimens of A. trapezoides were again found in the subsequent autumn and winter (data not shown), which suggests that A. trapezoides was able to retreat to a depth greater than 25 cm during the dry, hot summer months.
Figure 6 The distribution of the earthworm abundance (average total for all species) within the soil profile between August 1994 and June 1995 in A, the dryland treatment and B, the 20%M treatment, where ▪= 0–5 cm; □=5–10 cm; and ![]()
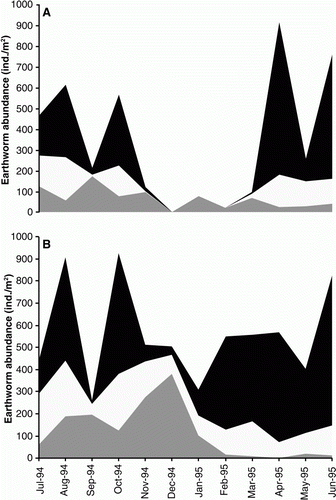
Table 4 Distribution down the soil profile of each earthworm species (% of population by number) averaged for those found present in the summer (average for Dec–Feb) and winter months (average for Jun–Aug) in sheep-grazed pastures under different irrigation treatments (dominant results are in bold).
Response of soil invertebrates to irrigation
The abundance and biomass of earthworms were not significantly different under dryland and irrigated pastures in mid-spring (October 2007; data not shown). Mesofauna were more abundant in irrigated than in dryland pastures (0–7.5 cm; , ), with Thysanoptera, Mesostigmata, Collembola and Protura particularly high in abundance. Astigmata and Oribatida were more abundant in dryland pastures, although the abundance of Oribatida was low (<1000 m−2). Deeper in the soil (7.5–15 cm; ), the mesofauna showed similar trends to the 0–7.5 cm depth, with higher abundances of Mesostigmata and Collembola under irrigation than dryland.
Figure 7 Invertebrates in sheep-grazed pastures under different irrigation treatments at 0–7.5 cm soil depths. A, abundance of mesofauna trophic groups and B, abundance of nematode trophic groups.
*predacious and omnivorous nematodes, **plant-feeding and plant-associated nematodes.
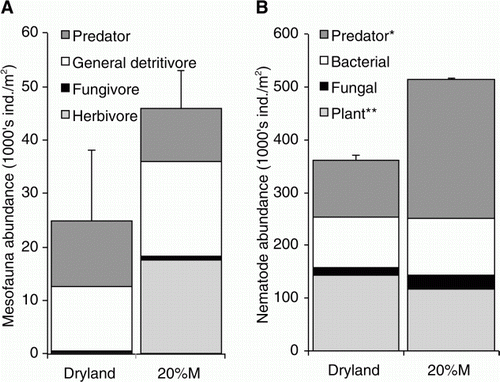
Table 5 Mesofauna abundance (1000 m−2), including Oribatida species, at 0–7.5 cm soil depth in sheep-grazed pastures under dryland and 20%M irrigation treatments in 2007. Values shown in bold: P value significant at α=0.05 between treatments. Neelipleona, Coleoptera, Hymenoptera, Psocoptera, Chilopoda, Pauropoda and Symphyla were found at abundances of less than 200 individuals m−2 in both treatments and are not reported in the table.
Table 6 Mesofauna and nematode abundance (1000 m−2) in sheep-grazed pasture under the dryland and 20%M irrigation treatments at 7.5–15 cm soil depth in 2007. Values shown in bold: P value significant at α=0.05 between treatments
At 0–7.5 cm depth, nematode abundance was similar under dryland and irrigated pastures (; ). The proportion of short-lived (CP2) nematodes was higher under dryland, resulting in a lower maturity indices (MI and ΣMI), but higher PPI. The NCR was similar between dryland and irrigated pastures (0.87 vs. 0.81), suggesting no difference in the dominance of bacterial-feeding or fungal-feeding nematodes. Nematode assemblages were different in the two treatments, with short-lived CP2 Cephalenchus more abundant under dryland pastures, and longer-lived CP4 Dorylaimus and Pungentus more abundant under irrigated pastures. At the 7.5–15 cm depth, nematode abundance tended to be higher under irrigation than under dryland pasture (). Similar to 0–7.5 cm depth, CP2 nematodes were more abundant under dryland than under irrigation (data not shown).
Table 7 Nematode abundance (1000 m−2) in sheep-grazed pastures (0–7.5 cm soil depth) under dryland and 20%M irrigation treatments in 2007. Values shown in bold: P value significant at α=0.05 between treatments. Ditylenchus, Pratylenchus, Seinura, Acrobeles, Monhystera, Chromadoridae and Doryllium were found at <2000 individuals m−2 in both treatments and are not included in the table.
Discussion
Earthworms
Earthworm abundance and biomass declined (to very low numbers) during the summer months, due to the combined effect of a soil water deficit (Duncan et al. Citation1985) and elevated soil temperatures that approached 20 °C. Just as Baker (Citation1998) observed the importance of rainfall for earthworm populations in South Australia, we observed the influence of a reduction in soil water deficit had on the diversity and abundance of the earthworm community. Apart from A. trapezoides, the other four earthworm species recorded at this site responded positively to irrigation, most likely through a combination of improved soil moisture, temperature and increased food resources in the form of plant-derived soil organic matter. Even at the highest level of irrigation, earthworm abundance still declined from winter to summer. That earthworms were inhibited even under the highest level of irrigation is not surprising if you consider the size of the water deficit and hence moisture stress the earthworms would still have been exposed to in the 20%M irrigation treatment. At this threshold of irrigation (20% w/w), pores larger than 3 µm in diameter are drained of water between irrigation events. The average soil moisture 33% (R. Moss, pers. comm.) of the 20%M irrigation treatment is less than field capacity (38%) (McDowell & Rowley Citation2008). Previous studies (Watt & Burgham Citation1992; Baker et al. Citation1993) have shown that if pores>3 µm diameter are drained for extended periods, earthworm activity ceases.
Earthworms have various mechanisms to survive moisture stress (Edwards & Bohlen Citation1996), all of which were observed in the present study. As well as aestivating during summer, earthworms moved deeper in the soil profile (10–25 cm) than in winter (0–5 cm). This trend was particularly apparent in the dryland soil, with no earthworms detected in the upper 10 cm of soil as soil moisture plummeted to 6%. The depth distribution varied between earthworm species, reflecting their temperature preferences with endogeic A. caliginosa preferring temperatures of 10–15 °C (Daughberger Citation1988) and moving deeper in the soil profile than the epigeic L. rubellus, which prefers temperatures closer to 25 °C (Edwards Citation1988). Lumbricus rubellus are reported to survive moisture stress by laying cocoons that hatch when conditions become more favourable (Parmelee & Crossley Citation1988; Edwards et al. Citation1995; Fraser & Piercy Citation1998). Indeed, there was a flush of juvenile L. rubellus in autumn. However, there were also a number of mature L. rubellus that reappeared in the soil profile in autumn, particularly in the wetter treatments, suggesting that aestivation also occurs in this species, especially with the mature component of the population. Few mature earthworms of any species were detected in the summer, with none detected in the top 25 cm of soil.
While overall earthworm abundance tended to respond favourably to irrigation (and the consequent increases in stocking rates) in the current sheep study, it is interesting to note that A. trapezoides was not found in the two irrigation treatments that received 600 mm of water (3W and 20%M). With this observation also made by Baker (Citation1998), it would suggest that A. trapezoides appears to be adapted to lower soil moistures. These results contrast with those of other authors (Barley & Kleinig Citation1964; Reineke & Visser Citation1980) who reported an increase in earthworm diversity with irrigation. It is likely, however, that the base climate under which irrigation is applied will have an over-riding influence on the biological effects observed.
In this study, the differences in earthworm abundance and diversity between irrigation treatments observed during the dry summer months were reduced during the winter months, highlighting the importance of understanding seasonal fluctuations. Peak earthworm abundances are observed during the cooler and wetter months (Martin Citation1978; Fraser & Piercy Citation1996). Earthworms are often more abundant under more productive pastures with greater organic matter inputs (Curry et al. Citation2008) and numerous studies have shown that earthworms increase with application of fertiliser, following the associated increases in pasture productivity and organic matter inputs (Curry et al. 2008; Edwards & Lofty 1982; Fraser et al. 1994; Schon et al. 2008). For example, in another long-term, sheep-grazed pasture trial site (located adjacent to the current irrigation trial) where, at the time of sampling, rates of 0, 188 and 366 kg of superphosphate ha−1 had been applied annually for 37 years, earthworm populations in spring were 452, 715 and 820 m−2, respectively (Fraser et al. 1994). Interestingly, Stewart & Metherell (Citation1999) showed that despite the increase in herbage production under the 20%M irrigation treatment, this and the dryland pasture had similar root biomass, although root turnover was postulated to be slower in the dryland pasture. Despite the higher pasture production in the irrigated treatments, soil carbon declined. The decline in soil C may be partially attributed to the energy demands of the larger and more active earthworm community in the irrigated pasture soil. Since earthworms are primary decomposers, where earthworm populations increase, the rates of organic matter decomposition and soil and microbial respiration may also increase. With an average of 200 more earthworms per square metre under 20%M than dryland annually, and assuming 20 g of dung is consumed annually per earthworm (Edwards & Bohlen Citation1996), this could equate to the consumption of up to 1.4 kg more carbon annually per square metre by earthworms under this irrigation regime.
Mesofauna and Nematoda
Mesofauna and Nematode abundances in both the dryland and irrigated pastures when sampled in the early spring in this study were low for typical New Zealand grazed pastures (McMillan Citation1969; Adams Citation1971; Yeates Citation1984; Springett Citation1992; Schon et al. Citation2008), with a predominance of smaller, shorter-lived organisms. This finding contrasts with the findings for earthworms, where little difference was found in abundance between the dryland and 20%M treatments in spring.
Nematodes live in the water films of soils but only a few nematodes, such as Paratylenchus, have specific drought-resistant stages (Yeates Citation1978). As could be expected, plant-feeding CP2 Paratylenchus was most abundant under dryland in this study, despite limited pasture growth. With nematodes having few adaptations to drought, short-lived species were found to be better adapted to drought disturbances, with a higher proportion of the short-lived CP2 nematodes. Short-lived nematodes are also smaller (e.g. CP2 Tylenchus and Cephalenchus have adult body widths of only 25 and 22 µm respectively). Their lesser size allows for continued activity during periods of low soil moisture, as they can access smaller pores where water is held. Some bacterial-feeding species are able to reproduce when pores as small as 1 µm diameter contain water (Yeates Citation1987; Yeates et al. Citation2002). Treatment differences could not be observed at depth, although Verschoor (Citation2002) suggested that the effects of soil moisture deficit would decrease with depth.
Mesofauna reside in the air-filled pore spaces but are still influenced by moisture limitations. Thysanoptera, Mesostigmata, Collembola and Protura were more abundant in irrigated pastures than in dryland. In contrast, Astigmata and Oribatida were more abundant in dryland pastures, although the abundance of Oribatida was low. Not surprisingly, Acari and Collembola are likely to be particularly affected by moisture stress, with their lifespan, dispersal and reproductive abilities providing limited defence against periodic drying (Lindberg & Bengtsson Citation2005). Longer-lived Acari, such as Oribatida, may be especially vulnerable to drought (Lindberg & Bengtsson Citation2005). Of the oribatid species observed in this study, most were small (body widths<350 µm) and short-lived (<100 days) (Grishina Citation1991). The exception was large Platynothrus peltifer (lifecycle of 130–332 days), which was not found under the dryland pasture. Lindberg & Bengtsson (Citation2005) found Oppiella nova to be one of the first Oribatida to recover after drought in a boreal spruce stand. In the current study, Oppiella nova was found in higher abundance in dryland than the irrigated treatments. According to Lavelle and Spain (Citation2001), Collembola are physiologically less drought resistant than Oribatida. This was also apparent in the current study, with lower abundance under dryland than under irrigated pastures. The effects of drought were also evident at depth (7.5–15 cm), with the proportion of Acari and Collembola lower under dryland (6%) than irrigated pastures (27%).
Conclusions
Earthworm abundance and biomass fluctuated according to season with a typical peak in both abundance and biomass in winter and the early spring months and a decline during the summer months with the onset of the soil water deficit. Even in the 20%M irrigation treatment where the soil moisture deficit was relatively low, earthworm abundance and biomass declined in the summer months. Yet this decline was only a fraction of the decline found in the dryland pasture that relied solely on incident rainfall. High soil temperature was a driver of the lower earthworm activity observed in summer and all earthworm species migrated to depth and many aestivated as a mechanism to avoid soil moisture stress and increased temperatures. This was not an option for other invertebrates, with the smaller and shorter lived organisms among the Acari and Nematoda more abundant in the dryland pastures in spring.
To an extent, the soil invertebrate community under irrigation may be more characteristic of a dryland soil due to moisture stress between each irrigation event. The irrigation scheduling used in this study appeared to limit the abundance and activity of invertebrates by failing to sustain soil water contents for optimum faunal activity in the summer months, With nearly one million hectares of New Zealand under irrigation in 2006 (Aqualink Research Ltd Citation2006) and irrigated land area potentially increasing, it would be interesting to consider the benefits of an irrigation regime that would attain soil moisture in a range that supported invertebrate abundance and activity, rather than just optimising plant growth.
Acknowledgements
We are sincerely grateful to all past and present staff of Winchmore Research Station for initiating and maintaining the long-term irrigation trial at Winchmore and for allowing us access to the site. We are also very grateful to GW Yeates, MJ Hedley, R Gray, P Budding and S Lambie for their assistance. The projects were funded by the FRST contracts CO2XO405 and C02X0812 and an AGMARDT Doctoral Scholarship.
References
- Adams , ECG . 1971 . Ecological studies of microarthropods in a New Zealand pasture soil with special reference to Collembola. 1 . Pedobiologia , 11 : 321 – 337 .
- Aqualink Research Ltd 2006 . Snapshot of water allocation in New Zealand . Christchurch, New Zealand, Aqualink Research Ltd, ME 782 . p. 64 .
- Baker GH 1992 . Optimising earthworm activity in soils . Proceedings of the XXXII Annual Conference of the Grassland Society of Victoria . Pp. 59 – 66 .
- Baker GH 1998 . Ecology, management and benefits of earthworms in agricultural soils . In: Edwards CA Earthworm ecology. Boca Raton . CRC Press LLC , FL , , USA . 229 – 257 .
- Baker , GH , Barrett , VJ , Carter , PJ , Williams , PML and Buckerfield , JC . 1993 . Seasonal changes in the abundance of earthworms (Annelida: Lumbricidae and Acanthodrilidae) in soils used for cereal and lucerne production in South Australia . Australian Journal of Agricultural Research , 44 : 1291 – 1301 .
- Barley , KP and Kleinig , CR . 1964 . The occupation of newly irrigated lands by earthworms . Australian Journal of Science , 26 : 290 – 291 .
- Bezborodov , Khalbayeva . 1990 . Effects of earthworms on the agrochemical and hydrophysical properties of irrigated sierozems . Soviet Soil Science , 22 : 30 – 35 .
- Blackwell , PS and Blackwell , J . 1989 . The introduction of earthworms to an ameliorated, irrigated duplex soil in South-eastern Australia and the influence on macropores . Australian Journal of Soil Research , 27 : 807 – 814 .
- Bongers , T . 1990 . The maturity index – an ecological measure of environmental disturbance based on nematode species composition . Oecologia , 83 : 14 – 19 .
- Coleman DC , Crossley DA , Hendrix PF 2004 . Fundamentals of soil ecology , Second edn Elsevier Academic Press , Los Angeles, CA , , USA . p. 205 .
- Curry , JP , Doherty , P , Purvis , G and Schmidt , O . 2008 . Relationships between earthworm populations and management intensity in cattle-grazed pastures in Ireland . Applied Soil Ecology , 39 : 58 – 64 .
- Daughberger , P . 1988 . Temperature and moisture preference of three earthworm species (Oligochaeta: Lumbricidae) . Pedobiologia , 32 : 57 – 64 .
- Duncan , MJ , Maidment , DR and Campbell , FO . 1985 . Water use in the Valetta Irrigation Scheme, Canterbury, New Zealand . New Zealand Journal of Experimental Agriculture , 13 : 395 – 402 .
- Edwards , CA . 1988 . Breakdown of animal, vegetable and industrial organic wastes by earthworms . Agricultural Ecosystems and Environment , 24 : 21 – 31 .
- Edwards CA , Bohlen PJ 1996 . Biology and ecology of earthworms . Chapman and Hall , London , , UK . 426 .
- Edwards , CA , Bohlen , PJ , Linden , DR and Subler , S . 1995 . “ Earthworms in agroecosystems ” . In Earthworm ecology and biogeography in North America , Edited by: Hendrix , PF . 185 – 213 . Boca Raton, FL , , USA : Lewis Publishers .
- Edwards , CA and Lofty , JR . 1982 . Nitrogenous fertilizers and earthworm populations in agricultural soils . Soil Biology Biochemistry , 14 : 515 – 521 .
- Fraser , PM , Haynes , RJ and Williams , PH . 1994 . Effects of pasture improvement and intensive cultivation on microbial biomass, enzyme activities, and composition and size of earthworm populations . Biology and Fertility of Soils , 17 : 185 – 190 .
- Fraser PM , Piercy JE 1996 . Effects of summer irrigation on the seasonal activity, population size, composition and biomass of lumbricid earthworms in a long-term irrigation trial at Winchmore, New Zealand. Proceedings of ASSSI and NZSSS Conference . Pp. 89 – 90 .
- Fraser , PM and Piercy , JE . 1998 . The influence of different straw management practices on lumbricid earthworm populations . Applied Soil Ecology , 9 : 369 – 373 .
- Garnsey R , Buckerfield JC 1993 . Management of earthworms in agriculture . In : Temple-Smith MG , Pinkard T The role of earthworms in agriculture and land management. Report of National Workshop , June 1993 Tasmania , Australia , Department of Primary Industry and Fisheries .
- Gilyarov MS 1975 . The evaluation of large soil invertebrates (mesofauna) . In: Gilyarov MS Methods of study in soil zoology . Nauka , Moscow , , Russia . 12 – 29 .
- Goh , KM and Bruce , GE . 2005 . Comparison of biomass production and biological nitrogen fixation of multi-species pastures (mixed herb leys) with perennial ryegrass-white clover pasture with and without irrigation in Canterbury, New Zealand . Agriculture Ecosystems & Environment , 110 : 230 – 240 .
- Grishina LG 1991 . Duration of the life cycle of oribatid mites (Sarcoptiformes, Oribatei) . Sibirskii Biologicheskii Zhurnal (Siberian Biological Journal) 3 : 38 – 47 (in Russian with English abstract) .
- Hewitt AE 1992 . New Zealand soil classification . Lower Hutt, DSIR Land Resources Scientific Report . p. 133 .
- Lavelle P , Spain AV 2001 . Soil ecology . Dordrecht , Kluwer , , Netherlands 654
- Lee K 1985 . Earthworms: their ecology and relationships with soils and land use . Academic Press , Sydney , , Australia . 411 .
- Lindberg , N and Bengtsson , J . 2005 . Population responses of oribatid mites and collembolans after drought . Applied Soil Ecology , 28 : 163 – 174 .
- Lobry de Bruyn , LA and Kingston , TJ . 1997 . Effects of summer irrigation and trampling in dairy pastures on soil physical properties and earthworm number and species composition . Australian Journal of Agricultural Research , 48 : 1059 – 1079 .
- Martin NA 1978 . Earthworms in New Zealand agriculture . Proceedings of the 31st New Zealand Weed and Pest Control Conference: 176–180 .
- McBride , SD . 1994 . Pasture yield responses to irrigation in Canterbury . Proceedings of the New Zealand Grasslands Association , 56 : 165 – 168 .
- McDowell , RW and Rowley , D . 2008 . The fate of phosphorus under contrasting border-check irrigation regimes . Australian Journal of Soil Research , 46 : 309 – 314 .
- McMillan , JH . 1969 . The ecology of the acarine and collembolan fauna of two New Zealand pastures . Pedobiologia , 9 : 372 – 404 .
- Nordstöm , Rundgren . 1974 . Environmental factors and lumbricid associations in Southern Sweden . Pedobiologia , 14 : 1 – 27 .
- Olsen SR , Cole CV , Wanatabe FS , Dean LA 1954 . Estimation of available phosphorus in soils by extraction with sodium hydrogen carbonate . Washington, DC, USA, US Department of Agriculture. Circular 939 .
- Parmelee , RW and Crossley , DAJ . 1988 . Earthworm production and role in the nitrogen cycle of a no-tillage agroecosystem on the Georgia Piedmont . Pedobiologia , 32 : 351 – 361 .
- Reineke AJ , Visser FA 1980 . The influence of agricultural land practices on the population densities of Allolobophora trapezoides and Eisenia rosea (Oligochaeta) in Southern Africa . In : LD Dindal Soil biology as related to land use practices . Environmental Protection Agency , Washington, DC , , USA . 310 – 324 .
- Rickard , DS , McBride , SD and Fitzgerald , PD . 1986 . The effect of soil moisture deficits on pasture yield . New Zealand Agricultural Science , 20 : 7 – 12 .
- Schon , NL , Mackay , AD , Minor , MA , Yeates , GW and Hedley , MJ . 2008 . Soil fauna in grazed New Zealand hill country pastures at two management intensities . Applied Soil Ecology , 40 : 218 – 228 .
- Sims RW , Gerard BM 1985 . Earthworms . Linnaean Society , London , , UK 171 .
- Snaydon RW 1987 . Managed grasslands: analytical studies. Ecosystems of the world . 17 . Elsevier , Amsterdam , , Netherlands 285 .
- Springett , JA . 1992 . Distribution of lumbricid earthworms in New Zealand . Soil Biology and Biochemistry , 24 : 1377 – 1381 .
- Stewart DPC , Metherell AK 1999 . Long-term influence of management practices on soil carbon of pastoral ecosystems . Proceedings of the Fertiliser and Lime Research Centre Conference 12 : 317 – 326 .
- Tisdall , JM . 1985 . Earthworms activity in irrigated red-brown earths used for annual crops in Victoria . Australian Journal of Soil Research , 23 : 291 – 299 .
- Verschoor , BC . 2002 . Carbon and nitrogen budgets of plant-feeding nematodes in grasslands of different productivity . Applied Soil Ecology , 20 : 15 – 25 .
- Watt JPC , Burgham SJ 1992 . Physical properties of eight soils of the Lincoln area, Canterbury . DSIR Land Resource Technical Record 103 .
- Yeates , GW . 1976 . Earthworm population of a pasture spray-irrigated with dairy shed effluent . New Zealand Journal of Agricultural Research , 19 : 387 – 389 .
- Yeates , GW . 1978 . Populations of nematode genera in soils under pasture. I. Seasonal dynamics in dryland and irrigated pastures on a southern yellow-grey earth . New Zealand Journal of Agricultural Research , 21 : 321 – 330 .
- Yeates , GW . 1984 . Variation in soil nematode diversity under pasture with soil and year . Soil Biology & Biochemistry , 16 : 95 – 102 .
- Yeates , GW . 1987 . Nematode feeding and activity – the importance of development stages . Biology and Fertility of Soils , 3 : 143 – 146 .
- Yeates , GW . 1994 . Modification and qualification of the nematode maturity index . Pedobiologia , 38 : 97 – 101 .
- Yeates , GW . 2003 . Nematodes as soil indicators: functional and biodiversity aspects . Biology and Fertility of Soils , 37 : 199 – 210 .
- Yeates , GW , Dando , JL and Shepherd , TG . 2002 . Pressure plate studies to determine how moisture affects access of bacterial-feeding nematodes to food in soil . European Journal of Soil Science , 53 : 355 – 365 .