Abstract
Nitrogen (N) fertilizer increases pasture production in New Zealand in a near linear manner and affects pasture composition, soil below-ground communities and N losses. We monitored N fertilized plots established in long-term low-fertility pasture over different time periods to compare changes in N availability on below-ground soil communities (particularly ammonia-oxidizing archaea [AOA] and ammonia-oxidizing bacteria [AOB] as they appear to be sensitive to change). Although the most significant effects were seen in the 30 gN (300 kg/ha) treatment, there were indications that even 5 gN had effects on archaea. AOA gene copies dominated in control and 5 gN treatments but decreased in the 10 gN treatment. The ratio of AOA to AOB in 10 gN was lower and more similar to 30 gN suggesting that the AOA/AOB ratio may be a sensitive indicator of change in N status. In the 30 gN treatment, both the soil C/N ratio and the fungal phospholipid fatty acids were reduced (consistent with changes in DNA profiles) and microbes were suppressed. The number of AOB gene copies significantly increased in this treatment and corresponded to a switch from ammonium-N to nitrate-N as the dominant inorganic form of N in the 56-day incubation. This was consistent with increased soil ammonium-N and nitrate-N concentrations, leading to increased nitrate-N leaching that occurred at a threshold of between 10 gN and 30 gN, and suggesting that, with 30 gN, nitrification and nitrate leaching are influenced more by AOB than archaea. Generally there was no significant change in mesofauna, microfauna or bacterial DNA profiles with the treatments. In a high-fertility pasture, DNA profiles for bacterial, fungal and archaeal groups clustered away from low-fertility pasture suggesting that changes in soil communities, with increased soil fertility, take more time to be fully expressed.
Introduction
Increased demand for animal protein and increased production from sheep and beef farms often involve greater use of fertilizers and agrichemicals on grazed pastures, which enables farmers to carry higher densities of livestock. Pasture production in New Zealand is generally limited by soil nitrogen (N) (Steele & Vallis Citation1988), therefore N fertilizer has been added in increasing amounts since 1990 to increase pasture production (MacLeod & Moller Citation2006). This has expanded the size of the labile N pool in soil, changed grass species, suppressed N fixation by white clover, and increased nitrate-N leaching (Ledgard et al. Citation1996, Citation2001; Parfitt et al. Citation2010).
Grazed pastures occurring on low-nutrient status soils have a diverse mixture of grasses, legumes and forbs that produce a wide range of substrates that lead to a high diversity of soil biota (Bardgett et al. Citation1999; Wardle Citation2006; Parfitt et al. Citation2010). Although pasture production and quality improve with N fertilizer, below-ground diversity may decrease as the quantity and quality of litter entering the soil food web changes (Donnison et al. Citation2000). In grassland soils, both the bacterial and fungal communities may experience a reduction in diversity in response to N fertilizer (Kennedy et al. Citation2004, Citation2005). Further, both soil microbial biomass and the soil fungal:bacterial ratio generally decrease with N fertilizer, (Bardgett et al. Citation1999, Citation2001; Grayston et al. Citation2001).
Within the N cycle, ammonia oxidizers are key organisms, converting ammonia to nitrite, that is the initial and rate-limiting step in nitrification and denitrification (Wang et al. Citation2009). These organisms include the important ammonia-oxidizing bacteria (AOB) as well as the ammonia-oxidizing archaea (AOA). Several studies have suggested the AOA play a significant role in ammonium oxidation in soil (Leininger et al. Citation2006; Francis et al. Citation2007; Prosser & Nichol Citation2008). For a N rich soil, however, AOA did not respond to application of ammonium-N, in contrast to the response of the AOB; therefore AOA may prefer low ammonium-N conditions (Di et al. Citation2009, Citation2010).
A permanent low-fertility pasture grazed continuously by sheep in hill country near Palmerston North has been managed with no fertilizer application since 1980. Previously we found that long-term increases in soil phosphate fertilizer gave increases in N fixation by clovers, and disadvantaged the fungal energy channel (Parfitt et al. Citation2005, Citation2010). When 30 gm−2 N (300 kg ha−1) was added each year, nitrate-N leaching increased within 3 years from 0.1 g m−2 y−1 to 1.4 g m−2 y−1 (1 to 14 N kg ha−1) (Parfitt et al. Citation2009). Extra N treatments have been established and this has allowed us to study the effect of different rates of N on soil N pools (e.g. ammonia-N, nitrate-N, net N mineralization), microbial community structure (e.g. soil fungal:bacterial ratio, and the DNA and RNA profiles of specific microbial groups) and larger soil invertebrates. We hypothesized that, because these pasture systems respond readily to N, changes in the below-ground system would also change in concert with increased soil N availability, particularly the abundances of AOB which appear to be sensitive to labile N. We test whether AOB and AOA respond differently to N additions in the field, as suggested in laboratory studies (Di et al. Citation2010). Implications for N leaching under grazed pastures are discussed.
Methods
Study site
The study was carried out on fields in farmlets that had been under continuous pasture for >50 years in hill country at AgResearch Hill Country Research Station, Ballantrae, New Zealand (40°18′S 175°50′E). The low-fertility farmlet (LF) had not received any fertilizer after 2.5 g m−2 y−1 phosphorus (P) and 3.0 g sulphur (S) (as superphosphate, SSP) were applied during 1973–75 and 1.2 g m−2y−1 P and 1.4 g S applied during 1977–80. An adjacent high-fertility farmlet (HF) had been under the same management as LF until 1975; 5–9 g m−2 y−1 P and S were applied during 1975–79; subsequently, 3.4 g P m−2 y−1 and 4.1 g S m−2 y−1 were applied from 1980 to 2009. Sheep stock densities were eight and 16 ewe equivalents per ha at LF and HF, respectively. Long-term mean annual precipitation at the sites is 1200 mm and mean air temperature is 16.6 °C in summer (November to May) and 7.9 °C in winter (June to October).
Plant species in the low-fertility pastures contained five principal grasses: Agrostis capillaris Sibith.>Anthoxanthum odoratum L.>Cynosaurus cristatus L.>Holcus lanatus L.>Lolium perenne L.; legumes were Trifolium repens L., Trifolium dubium Sibth., Trifolium micranthum A. Gray, and Lotus species; forbs included Hypochaeris radicata L. (Catsear) and Leontodon taraxacoides Vill. (Hawkbit). The high-fertility pastures included Lolium perenneL.>Agrostis capillaris Sibith.>Annual poa>Holcus lanatus L. and Trifolium repens L.
The soils in both fields are similar and are classified as Acidic Orthic Brown Soils (New Zealand soil classification); they are formed in silty mudstones and quartzo-feldspathic loess. The topsoils have a silt loam texture. The following properties have been previously described: nutrient transfer, S and N leaching (see Parfitt et al. Citation2010), carbon (C) inputs, soil organic matter (Lambert et al. Citation2000), microfauna (Parfitt et al. Citation2005) and earthworms and microarthropods (Schon et al. Citation2008). The pH values were in the range 5.3–5.6 at both sites, and the Olsen P values were 6–8 mg kg−1 at LF, and 48–53 mg kg−1 at HF (Parfitt et al. Citation2009).
Trial layout and management
25 plots (4×4 m), consisting of five treatments in each of five blocks (replicates) contained within 0.5 ha at LF, were set out on a north-facing slope (1–8°). Three treatments were imposed in 2004: (a) control (LFC, unfertilized); (b) fertilizer applied to each plot as 5.0 g m−2 (50 kg ha−1) urea-N in each of six applications each year over 2004–09, which equates to 30 g N m−2 y−1—a total of 150 g N m−2 (30 N). Three additional plots were added to each block in November 2007 giving further treatments of 5 (5 N) and 10 g N m−2 y−1 (10 N); urea was added in split dressings in July and October 2008, and in July and September 2009. This gave a total of 10 g N m−2 and 20 g N m−2. The treatments were randomly assigned to each of the three plots within each block. At HF, control plots in each of five blocks (HFC, fertilized with P and S for HF as per section 2.1) were set out on a north-facing slope 500 m away from the LF plots. Plots were open to continuous grazing by sheep throughout the study.
Plant and soil chemical analyses
Herbage net accumulation was measured at different positions in each plot at different sampling times under a 0.5 m2 exclusion cage over 40–120 days depending on the season, after initial trimming, by cutting to 30 mm. Cages were moved to a new location after each cut. In 2008, there were six herbage cuts at the LF plots and five in 2009. Herbage N, P, K, Ca and Mg were analysed for each bulked set of summer and winter cuts as reported by Parfitt et al. (Citation2009).
We collected soils on 7 October 2009 from each plot at 0–100 mm depth by pooling 16 cores (25 mm in diameter) taken on a grid in the centre 2×2 m of the plot. The samples were sieved (<5.6 mm) and stored at moist 4 °C. For each sample, we measured soil water content and pH (in water). A subsample was dried and sieved (<2 mm) and Olsen P measured as described in Landcare Research (Citation2007). Total C and N were determined by combustion in a Leco FP-2000 CNS analyser (LECO Corp, St Joseph, MI, US). Three further soil samples (100 mm diameter, 100 mm depth) were taken for bulk density determination at each sampling occasion in each plot; bulk density was measured by dividing the oven-dry (105 °C) weight by the volume for each core.
Microbial biomass C and N, and composition
Microbial C and N were measured on the moist soils within 3 days, by using the fumigation-extraction procedure with soil adjusted to 60% water-holding capacity, according to Ross et al. (Citation1995, Citation2004). The k-factors used for converting extractable C and N flush to microbial C and N were, respectively, 0.41 (Sparling et al. Citation2000) and 0.45 (Jenkinson Citation1988).
The composition of the microbial community was assessed in moist samples (0–100 mm depth), by using phospholipid fatty acid (PLFA) analysis. PLFAs were estimated by the method of Bligh & Dyer (Citation1959), as modified by White et al. (Citation1979). Lipids were extracted from 1.5 g of fresh soil, fractionated and methylated, and the resulting fatty acid methyl esters analysed using gas-chromatography-mass-spectrometry (Agilent 7890A GC with Agilent 5975C VL MSD). Resulting peaks were identified using retention times relative to two added internal standards (C13 and C19) and a bacterial methyl ester standard mixture (Supeloc Bacterial Acid Methyl Esters CP Mix 47080-U). Peak size was quantified using the FAME 19:0 internal standard and the abundance of each of the individual fatty acids extracted expressed as relative ηmoles per g of dry soil using standard nomenclature. PLFAs used to calculate bacterial biomass were: i-15:0, a-15:0, 15:0, i-16.0, i-17:0, cy-17:0, 18:1ω7c and cy-19:0. For saprophytic fungal biomass (no ectomycorrhizal species were present), the 18:2ω6,9 marker was used (Parekh & Bardgett Citation2002). The biomarkers for actinomycetes (10Me16:0, 10Me17:0 and 10Me18:0) were also measured.
The ratio of fungal PLFAs to bacterial PLFAs was used as an estimate of the relative importance of the bacterial and fungal energy channels (Parekh & Bardgett Citation2002). All chemical and biochemical soil data are reported on an oven-dry (105 °C) basis.
Soil net N mineralization
We measured potential net N mineralization on the moist soils 2 days after their collection by incubating 5 g (equivalent oven-dry weight) of mineral soil at 60% of water-holding capacity in 125 ml polypropylene cups covered with polyethylene (30 µm) for 56 days at 25 °C (see Parfitt et al. Citation2005). Mineral-N (ammonium-N and nitrate-N) was determined at 0 and 56 days by extracting the soil with 50 mL of 2 M KCl, shaking for 1 h, and then filtering. Extractable ammonium-N and nitrate-N were measured colorimetrically with a Lachat Quickchem FIA 8000 analyser (Zellweger Analytics, Milwaukee, WI, US). Net N mineralization was calculated as the difference in ammonium-N and nitrate-N values between 0 and 56 days. The % nitrate-N after 56 days was calculated using these nitrate-N and ammonium-N values.
DNA and RNA
Subsamples of sieved moist soils were processed immediately; 0.3 g of each were weighed into DNA and RNA extraction tubes and stored at −80 °C. Tubes containing the soil subsamples were removed from storage and allowed to defrost on ice before processing. Bulk DNA was extracted using the Bio101 FastDNA Spin Kit for Soil (QBIOgene) as per the manufacturer's instructions. Total DNA was quantitated using the PicoGreen dsDNA Quantitation Kit (Molecular Probes Inc). Bulk RNA was extracted from 0.3 g soil using the Bio101 FastRNA Pro Soil-Direct Kit as per the manufacturer's instructions and quantitated using RiboGreen RNA Quantitation Kit (Molecular Probes Inc).
RNA was treated with 2 U of TURBO DNase according to the manufacturer's instructions (Ambion) to remove residual DNA and a PCR using 16S primers was performed to confirm the absence of DNA. RT-PCR was used to create cDNA from 0.5 µg RNA using Superscript III RT Kit (Invitrogen) according to the manufacturer's instructions. Two reactions were performed for each sample, using unlabelled primers specific to bacterial and fungal genes in one and primers specific to archaea and rhizobia in the other (). Resulting cDNA was then used for T-RFLP analysis.
T-RFLP
T-RFLP analysis was performed separately using the cDNA from the extracted RNA and from the extracted DNA. T-RFLP is commonly used to profile microbial communities. Because T-RFLP allows the examination of non-culturable organisms (Thies Citation2007), it enables investigation of the effects of environmental factors on the whole microbial community structure. Two multiplex PCR reactions were performed for each sample, each using two primer pairs (). The first amplified target DNA from the bacterial 16S gene and the fungal ITS region. The second amplified regions from archaea and rhizobia. Each reaction contained 25 µl 2× Multiplex PCR Mastermix (Qiagen); 200 nM each primer; 1 µl Q solution (Qiagen); 20 ng template DNA; and DNase-free H2O to a total volume of 50 µl.
Table 1 Primers used for T-RFLP and qPCR analysis.
Reactions were heated to 95 °C for 15 min, followed by 30 cycles of 94 °C for 30 s, 56 °C for 45 s, 72 °C for 90 s and a final extension of 72 °C for 10 min using a Palmcycler (Corbett Life Science) thermocycler.
The products from the two multiplex PCR reactions were combined and purified using a QIAquick PCR Purification Kit (Qiagen). Restriction enzyme digestion was performed using MspI (Roche Molecular Biochemicals) as per the manufacturer's instructions with a 3 h incubation at 37 °C followed by a 20 min deactivation at 60 °C. The MinElute Enzymatic Reaction Cleanup Kit (Qiagen) was used as per the manufacturer's instructions to remove components of the enzymatic reaction before fragment analysis.
The resulting T-RFs were analysed by the Allan Wilson Centre for Biodiversity (Palmerston North) using an ABI3700 Genetic Analyser (Applied Biosystems). The raw T-RFLP profile data generated were collated and analysed using Genemapper v.4 software (Applied Biosystems). Only T-RFs that fell within the accurate sizing range (50–500 bp) were included in analysis. Any T-RFs with fluorescence units of less than 100 were also discarded to minimize the effect of artefacts, as were any T-RFs that contributed less than 1% of total fluorescence after exclusion of those under 100 fluorescence units. Mean fluorescence across all samples was calculated for each dye channel. Profiles that contained less than 20% of the mean fluorescence in any dye channel were sent for re-analysis. If the subsequent profile also fell below this threshold, that dye channel was excluded from further analysis.
qPCR of ammonia oxidizers
T-RFLP is a semi-quantitative method and gives relative, not absolute, quantification of community components. Therefore, quantitative polymerase chain reaction (qPCR) was also employed to determine whether the different treatments had an effect on AOB and AOA gene copy numbers.
Copy numbers of archaeal and bacterial amoA genes were determined by quantitative PCR using SYBR Green-based chemistry and a RotorGene 6000 instrument (Corbett Life Science). The bacterial primers used () were AmoA-1F* (Stephen et al. Citation1999) and AmoA-2R (Rotthauwe et al. Citation1997). The archaeal primers were Arch-amoAF and Arch-amoAR (Francis et al. Citation2005). Reactions were prepared as per the manufacturer's instructions with 10 U of SYBR GreenER (Invitrogen), 10 µM of each primer and 20 ng template DNA. BSA was added to the archaeal reaction. Cycling consisted of 95 °C for 5 min followed by 45 cycles of 95 °C for 10 s, 53 °C for 15 s and 72 °C for 15 s. Standard curves were generated using serial dilutions of linearized plasmids containing either a cloned archaeal or bacterial amoA gene.
A melting curve analysis was performed after each assay to ensure that only products of the desired melting temperature were generated.
Results of qPCR assays were analysed using the RotorGene software (Corbett Life Science), including defining the threshold value and baseline for analysis of the raw data. Standard curves of known copy numbers of each gene were generated by cloning into the pGEM-T cloning vector. Plasmid containing the correct insert was harvested from the recombinant E. coli host using a Midi-Plasmid prep kit (MoBio Inc) and quantified using PicoGreen. Serial dilutions of plasmid DNA were prepared to obtain a standard curve for each gene. Data were linear over at least eight orders of magnitude during qPCR. For each gene, amplification efficiencies were 92–96%, and no signal was observed in negative controls.
Microfauna and mesofauna
Five cores (25 mm in diameter, 0–100 mm depth) were collected from each plot in October 2009 for the extraction of microfauna. Microfauna were extracted using the Whitehead and Hemming tray method, counted and fixed, with an average of 130 nematodes per sample identified to nominal genus, and allocated to feeding groups, as described by Yeates et al. (Citation1993a, Citationb). Using the abundance data for all genera, a range of indices of richness (SR), evenness (J’), diversity (H’) and the nematode channel ratio (NCR) was calculated following Yeates (Citation1984, Citation2003). The NCR is the proportion of microbial-feeding nematodes that are bacterial (B) feeding rather than fungal (F) feeding (NCR = B/[B + F]), and values tending to 1 indicate an increasing proportion of microbial-mediated decomposition passing through the bacterial channel.
Two cores (50 mm in diameter, 0–100 mm depth) were collected from each plot in October 2009 for the extraction of mesofauna. These were extracted using a modified Berlese-Tullgren apparatus with a mesh size of ~2 mm for 7 days. The organisms were collected in 70% ethanol, counted and identified to major groups. Adult oribatid mites were cleared with lactic acid (70%), identified to species level and classified into trophic groups (fungivores, herbivores or herbofungivores), as described by Schon et al. (2008). Using the abundance data for all groups, a range of indices of richness (SR), evenness (J’) and diversity (H’) was calculated following Yeates (Citation1984).
Data analysis
All soil data were converted to an area basis using soil bulk density values for each plot. Response variables were analysed by ANOVA, testing for the effects of treatment. The five blocks within LF were the basis of replication for the analyses. These analyses were performed for LF through the program GenStat, using log-transformed data for fauna, and untransformed data for the other properties. LSD were obtained after ANOVA.
We recognize that field type was not replicated in our study. Although true replication is often not feasible for studies performed at large spatial scales such as this, it has been argued that statistical analysis among systems for which replication is not tractable may still be legitimate provided sufficient caution is applied (see Carpenter Citation1989; Oksanen Citation2001). As such, we maintain that major differences between LF and HF fields for our response variables should serve as an authentic measure of the effects of soil fertility long term, because climatic conditions, soil, slope and aspect are effectively the same at both fields. Further, at the time of commencement of the HF and LF treatments over 1972–76 (i.e. when the land was first subjected to different management), there were no distinguishable differences between areas in either annual herbage yields (Lambert et al. Citation1983) or soil chemical properties (Lambert et al. Citation2000). This strongly suggests that any differences currently present are a consequence of the different fertilizer treatments and sheep stock densities over the previous 30 years, and not of any major initial difference between the two farmlets. ANOVA were also performed for LFC and HFC alone through the program GenStat.
Multi-dimensional scaling (MDS) analysis was performed using PRIMER 6 (Plymouth Marine Laboratory) software (Clarke Citation1993). Data were square root transformed. A Bray-Curtis Similarity matrix was constructed for each dataset and the MDS plot generated from this, using 50 restarts to ensure the best solution, was presented. Statistical significance of groupings was performed using the ANOSIM function in PRIMER. MDS was used to examine the similarity between DNA profiles. This method allows profiles to be plotted spatially, depending on how similar they are to each other and is based on a similarity matrix of all the profiles.
Results
Above-ground pasture biomass and composition
Average total herbage accumulation increased significantly (P < 0.001) with N (from 2.2 up to 3.8 g m−2 day−1) (A). The response to N was linear with R2=1.00 for 2008 and R2=0.94 for 2009. The mean values of N, P, K, Ca and Mg for summer and winter are reported in , and the mean uptake of N for each treatment is shown in B.
Figure 1 A, Mean total herbage accumulation (g dry matter m−2 y−1). B, Mean uptake of N (gN m−2 y−1) by herbage at LF in the control (C) and urea-amended (5 N, 10 N, 30 N) plots in 2008 and 2009. Error bars show LSD of treatments at 5% level. Multiply by 10 to get kg ha−1.
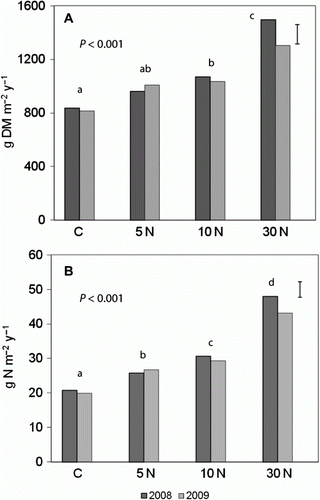
Table 2 Mean concentration of N, P, K, Ca and Mg in herbage for each treatment in summer (October to March) and winter (April to September).
Soil properties, microbial biomass and net N mineralization for 2009
Soil carbon at LFC ranged from 4.7 at 10 N to 5.1 kg m−2 at LFC and the values were not significantly different; at HFC carbon was 5.3 kg m−2 (). Average soil C/N ratios were significantly lower (P < 0.001) in the 30 N (11.7) than in the LFC plots (12.6). Average soil pH values were higher (P < 0.001) with 30 N urea treatment and at HFC (both values were 5.3) compared with 5.2 in the other plots.
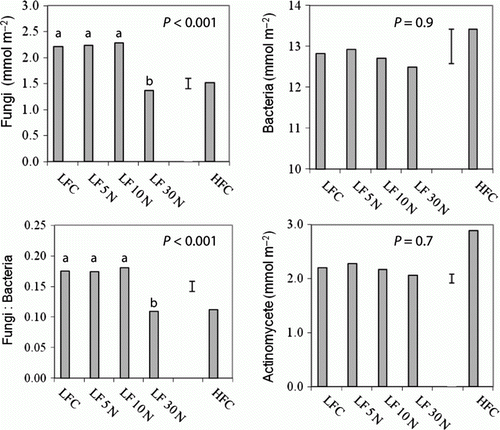
Figure 2 Selected soil properties in the control (LFC) and urea-amended (5 N, 10 N, 30 N) plots at LF and HFC. Error bars show LSD of treatments. Net N min = Net N mineralization (aerobic) for 56 days;% nitrate-N after 56 days’ incubation.
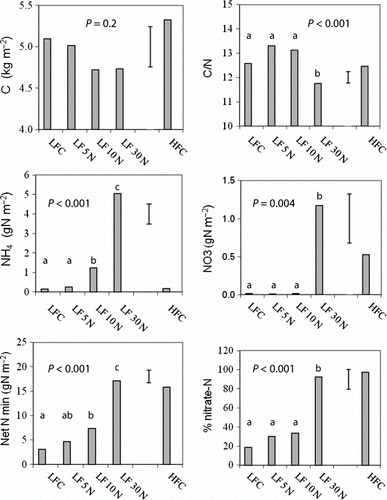
Soil ammonium-N in the fresh soil at 30 N was 5.0 g m−2 and nitrate-N was 1.2 g m−2; both were significantly higher than for the other treatments (). Soil net N mineralization (after 56 days) increased (P < 0.001) with application of N, and was considerably higher at 30 N and HFC than at the other plots. Nitrate-N (%) produced during the incubation, increased with N from 20% in the LFC plots to more than 90% with 30 N.
Average total herbage accumulation was linearly related to soil net N mineralization in both 2008 (R2=0.98) and 2009 (R2=0.91).
Soil microbial C and N measured by fumigation did not differ with treatment but the ratio of microbial N/total N (P = 0.02) was significantly lower at 30 N (and HFC) than at the other plots (data not shown). When microbial C at LFC and 30 N were compared directly there was a weak (P = 0.1) decrease at 30 N.
Soil microbial biomass measured by PLFA was lowest in the 30 N plots (data not shown). The fungal biomass (as indicated by 18:2ω6,9 marker) was significantly lower at 30 N and HFC (P < 0.001), and the ratio of fungi to bacteria was consequently lower (P<0.001) than at the other plots; the actinomycetes (P=0.001) were greater at HFC than at LFC ().
DNA and RNA
T-RFLP
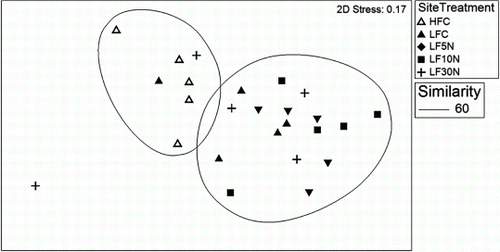
T-RFLP DNA data for all microbe groups combined (bacteria, fungi, archaea and rhizobia) showed no significant differences between treatments at LF, but there was a clear difference between HFC and the other plots (P < 0.05) (A). Three profiles were excluded from some subsequent analyses due to low fluorescence in the bacterial dye channel.
Figure 4 DNA profiles of: A, all four microbe groups; B, fungi; C, archaea plotted using MDS. Treatments are control (C) and urea-amended (5 N, 10 N, 30 N) plots at LF and HFC. Sample data were standardized by total fluorescence and square root transformed prior to similarity matrix construction using the Bray-Curtis Similarity Index. 70% similarity is shown by large ‘circles’ enclosing data points. The 2D stress value for this ordination is 0.15 which means that the data are reasonably well represented in two dimensions.
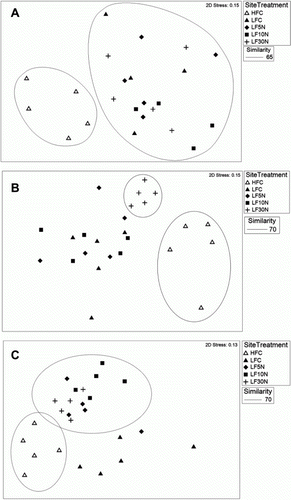
When individual microbe groups were examined, greater differences became apparent. The fungal profiles (B) showed clustering of the 30 N plots away from the other plots, and there was also a clear separation of the HFC samples. The archaeal profiles (C) showed a separation of HFC plots from the LF plots treated with N. There was some variability within the LFC plots and although they do not cluster significantly as a group, they (along with a single 5 N plot) were clearly outside the clustering of the LF-N treated plots.
No significant differences were seen between treatments in the MDS plots of the bacterial or rhizobial DNA profiles (data not shown). The HFC samples did group together, more so than any treatment group from the LF site, but were not significantly more similar to each other than to the other samples.
The RNA T-RFLP profiles did not show any significant clustering for any of the treatments within any of the microbial groups. In the overall profiles that included all four microbial groups (), the HFC samples did group away from the majority of the LF samples, but not significantly.
Figure 5 RNA profiles of all four microbe groups plotted using MDS. Treatments are control (C) herbicide-amended (H) and urea-amended (5 N, 10 N, 30 N) plots at LF and HFC. Sample data were standardized by total fluorescence and square root transformed prior to similarity matrix construction using the Bray-Curtis Similarity Index; similarity is shown by large ‘circles’ enclosing data points.
qPCR of ammonia oxidizers
The number of AOB gene copies increased significantly (P < 0.001) (A) with application of 30 N, and were also 24 times higher at HFC than at LFC (P < 0.001). AOA gene copies were lower in 5 N and 10 N than LFC (30.9) but only significantly so for the 10 N treatment (7.6) (P = 0.02). Additionally AOA gene copies were five times higher (P < 0.001) higher at HFC than LFC.
Figure 6 A, Gene copies of amoA from ammonia oxidizing bacteria (AOB) and from ammonia oxidizing archaea (AOA) per ng of extracted DNA in the control (C) and urea-amended (5 N, 10 N, 30 N) plots at LF and HFC. Error bars (15) show LSD for AOA. LSD for AOB is 14. B, Ratio AOA/AOB calculated from 6A.
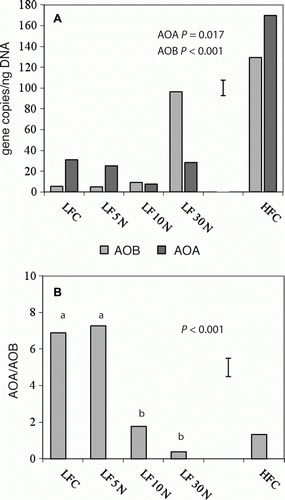
The ratio of AOA/AOB copy numbers at the 5 N plots was similar to those at LFC plots (B). The plots treated with 10 N showed significantly reduced ratios (P < 0.001), with lower AOA levels. At 30 N, the AOA/AOB ratio was smaller again, but with significantly more AOB gene copies than AOA copies. At HFC, although AOA gene copies were greater than AOB gene copies, the ratio was only slightly greater than 1 and more similar to LF 10 N and 30 N than LFC.
Microfauna and mesofauna
A total of 38 nematode taxa were found in the study. Total nematodes varied between 0.4 million and 1.2 million m−2 (data not shown). Generally the numbers were variable and few significant differences were found between the treatments. For the fungal feeders, the LFC plots appeared to be anomalously low, and if they were excluded, the fungal feeders appeared to decrease with N, but the numbers were not statistically different. The NCR appeared to show higher values (P = 0.06) at HFC (0.96) than at LFC (0.92), and thus showed a greater relative contribution of the bacterial-based energy channel relative to the fungal-based channel.
The numbers of juveniles of the root-inhabiting Heterodera, an obligate parasite of white clover (Trifolium repens) were 12-fold higher at HFC than at LF (P = 0.01), and the nematode plant parasitic index (PPI) was lower (P = 0.04) at HFC (0.89) than at the other plots (mean = 1.2).
Enchytraeid numbers did not differ among treatments but tardigrade numbers appeared to be lower at HFC (600) than at the other plots (mean = 18,000).
The average total numbers of mesofauna were about 100,000 m−2 but numbers of mesofauna were highly variable and there were no significant differences. Shannon-Wiener diversity (H’) of all mesofauna appeared to be higher at HFC but this was not statistically different from H’ at the other plots.
Discussion
Nitrogen treatment effects at LF
Nitrogen fertilizer increased pasture production at LF in a near linear manner, and the average total herbage accumulation also was linearly related to soil net N mineralization in both 2008 and 2009. This linear response to N fertilization was observed despite differences in duration of N treatments but is consistent with previous work on N for pasture soils (Parfitt et al. Citation2005). All levels of N addition increased soil labile N and nitrification as measured by aerobic incubation. The soil ammonium-N measured also generally increased with N fertilizer (). While the effects on below-ground invertebrate communities were much less evident in the 5 N and 10 N treatments than in the 30 N treatment, and may take longer than 2 years to respond to low levels of N addition, the soil microbiology was more sensitive to N fertilizer.
The archaea DNA profiles changed with N application suggesting that the overall composition of the community was affected by N addition although we did not see large changes in AOA gene copies. In contrast, while the bacterial DNA profiles suggested no change in overall community composition, as hypothesized the abundance of AOB gene copies responded positively to N application and the increasing amounts of ammonium-N (, A). This is in keeping with other studies that found that AOB was stimulated by N inputs when ammonium-N is available under conditions where AOA may not be as responsive (He et al. Citation2007; Di et al. Citation2009; Schauss et al. Citation2009; Jia & Conrad Citation2010). Thus, the ratio of AOA to AOB gene copies appeared to be a sensitive indicator of increase in N status for low-fertility soils, with no change in AOA/AOB ratio observed in the 5 N treatment, but being reduced with 10 N and 30 N (B).
The 30 N treatment had received 150 g N m−2 in total over 5 years. Since the plots were continuously grazed by sheep, however, more than 70% of this N was removed from the plots in the grazed herbage (Parfitt et al. Citation2009). Within the 30 N treatments, the fungal DNA profiles were significantly different from other LF treatments () and the fungi, as measured by PLFA, were suppressed (). Previously, we showed that fungal feeding nematodes decreased in the 30 N plots in 2006 after 30 g N m−2 y−1 had been added to the soil over 2 years (Parfitt et al. Citation2010). In 2009, the numbers of fungal-feeding nematodes were lower and more variable than in 2006 and 2007, but there was some indication that the numbers were decreasing with N. These results suggest that, in our soil, the fungal population is not suppressed until after higher rates than 10 g N m−2 have been applied. This is higher than with other studies showing that fungi and fungal-feeding organisms are often reduced when soil N fertility and recycling increases (Coleman et al. Citation1983; Bardgett et al. Citation1999; De Vries et al. Citation2006, Citation2007; Lauber et al. Citation2008; Krumins et al. Citation2009). The mechanism is unknown but both are direct effects of fertilizer itself, and changes in the plant community and litter associated with fertilizers could be involved (Donnison et al. Citation2000; Manning et al. Citation2006). Fungal DNA profiles can also undergo a reduction in diversity in response to fertilizer (Allison et al. Citation2007), but others found that the bacterial communities (but not fungi) can be altered by high rates of fertilizer (Gray et al. Citation2003; Kennedy et al. Citation2004, Citation2005).
The ratios of soil microbial N/total N, and soil C/N decreased with the 30 N treatment, and soil microbial C appeared to be lower with 30 N. DNA profiles suggested the bacterial communities were not affected by the treatments; the archaeal communities, however, were affected by N. T-RFLP is primarily a ‘fingerprinting’ technique and is subject to the same inherent biases as all PCR-based community analysis methods. Species that contribute less than 1% of PCR product in a mixture are not always reliably detected, thus, it is possible that heterogeneities within the soil environment affected the discriminating power of the method (and more extraction replicates could decrease the variability). Given that gene copy numbers vary between species, the technique is only semi-quantitative. While T-RFLP has been shown to accurately detect abundance differences in simulated microbial communities (Hartmann & Widmer Citation2008), the technique is most discriminatory when comparisons are made between samples with distinct communities. The lack of difference in the T-RFLP data would suggest changes to the bacterial community due to fertilization were more the result of an abundance of organisms present rather than significant changes in composition of the bacterial community.
Long-term effects of superphosphate fertilizer and sheep stock density
The HFC plots in an adjacent field to the LF plots had a higher level of soil fertility, greater sheep stock density and greater herbage accumulation than at LFC (Parfitt et al. Citation2009). The data from HFC are included to compare the effects of increases in soil fertility, long term, on the soil communities. Generally, there were more pasture species at LFC (11.9 species), with more low-fertility grasses and higher-than-average lignin and fibre concentrations, than at HFC (9.0 species) (Parfitt et al. Citation2010).
Fungi were lower in the HFC and LF 30 N plots than at the LFC plots (P = 0.05), which is consistent with the higher N status. The DNA profiles of the HFC samples clustered away from other treatment samples more consistently than any other group, showing that the microbial communities differed from those at LF. Both the LF 30 N and HFC fungal T-RFLP plots clustered away from the other LF treatments. This is in agreement with Suzuki et al. (Citation2009), who found that fungi responded more strongly to a fertilizer regime than bacteria.
The AOB gene copies were 24 times higher at HFC than at LFC, and they were probably responding to the higher N status at HFC in the same way that the AOB responded to 30 N at LF. The bacterial community structure (and nitrifier activity) can respond rapidly to changes in management, such as increased grazing and additions of animal urine (Le Roux et al. Citation2008; Rooney & Clipson Citation2008; Singh et al. Citation2009; Di et al. Citation2010). The actinomycetes (a group of gram+ bacteria) measured by PLFA were higher at HFC (P = 0.01) and this is generally consistent with other studies showing that gram+ bacteria often increase with increased intensity of grazing (Bardgett et al. Citation1999; Klumpp et al. Citation2009).
Di et al. (Citation2009), however, suggested that AOA do not increase with N, but in our study there were significantly higher numbers of both AOA and AOB at HFC compared with LFC. In contrast, Schauss et al. (Citation2009) observed that both AOA and AOB are involved in ammonia-oxidization in soils, and that the contribution of AOA and AOB is dependent on abundance and per-cell oxidation rates. Their study showed growth of AOA in an organic stimulated soil and suggested a ‘substantial proportion’ of ammonia-oxidization was carried out by AOA. Schauss et al. (Citation2009) concluded that AOA are able to compensate for their low per-cell oxidation rates by their higher abundance, indicating a possible key role for the overall ecosystem function when the growth conditions of AOB are disturbed. They may provide a ‘back-up function’ to enable the soil to continue ammonia oxidation under variable conditions. The higher numbers of AOA and AOB at HFC suggests that either specific land use practice, pasture species composition or continued addition of fertilizer may, in the long term, increase the quantities of both groups of organisms within the soil. Despite this, the response in our study in the LF 30 N plots would tend to agree with Di et al. (Citation2009, Citation2010), that AOB are driving ammonia oxidation rates in high-fertility systems because AOB abundance increased at the same time as the dominant form of mineralized N shifted from ammonium to nitrate.
These data have implications for N cycling for hill country under grazed pastures. Our data show that AOB are generally increasing, resulting in the oxidation of more plant available N. At the same site Parfitt et al. (Citation2009) observed an increase in plant available N. This was reflected in the uptake of N in the pasture from LFC to LF 30 N, and the leaching of N (0.1 to 1.4 g nitrate-N m−2 y−1 within 3 years of applying 30 N). At HFC the uptake of N was 70 g m−2 y−1 and the leaching loss was 1.8 g m−2 y−1. Using data from this study we speculate that when labile N in the soil reaches a threshold (in this case between 10 N and 30 N), soil ammonium increases, AOB multiply, conversion of ammonium to nitrate increases, and the microbial biomass and pasture are no longer able to retain an increasing proportion of labile N, and more nitrate-N is lost by leaching. Since AOA do not increase (relative to the control) with 30 N, it suggests nitrification and nitrate leaching are influenced more by AOB than by archaea.
Conclusions
Experimental treatments using different rates of N fertilizer, which increased primary production, were used to examine their influence on soil communities under sheep-grazed pastures. The low-fertility pasture, with low net primary production, had a high plant diversity that favoured a relatively high soil microbial biomass and the fungal-based energy channel. Application of urea increased the net primary production, and led to a change in the DNA profiles, with a lesser role of the fungal-based channel within the soil. We found that AOB (but not AOA) responded positively to N fertilizer and changes in AOB abundance corresponded to a shift from ammonium towards nitrate as the dominant form of mineralized N. The 10 gN m−2 y−1 treatment significantly reduced the ratio AOA to AOB gene copies, but did not differ in other measures of microbial community structure, suggesting the AOA/AOB ratio may be a sensitive indicator of increase in N status for low-fertility soils.
Our results show that increases in pasture production, the soil labile N pool and microbial community can occur with fertilizer at rates of up to 30 gN m−2 y−1, but large changes in below-ground invertebrate communities do not occur in our soil with lower rates of N, at least in the initial 2-year period, and it would be useful to continue to monitor these plots.
Acknowledgements
We are grateful to Phil Budding and Alec Mackay of AgResearch for pasture production data, and the late Des Ross for generous assistance with sample preparation and soil microbial biomass measurements. David DF Hunter performed PLFA analyses.
References
- Allison , SD , Hanson , CA and Treseder , KK . 2007 . Nitrogen fertilization reduces diversity and alters community structure of active fungi in boreal ecosystems . Soil Biology and Biochemistry , 39 : 1878 – 1887 .
- Bardgett , RD , Jones , AC , Jones , DL , Kemmitt , SJ , Cook , R and Hobbs , PJ . 2001 . Soil microbial community patterns related to the history and intensity of grazing in sub-montane ecosystems . Soil Biology and Biochemistry , 33 : 1653 – 1664 .
- Bardgett , RD , Mawdsley , JL , Edwards , S , Hobbs , PJ , Rodwell , JS and Davies , WJ . 1999 . Plant species and nitrogen effects on soil biological properties of temperate upland grasslands . Functional Ecology , 13 : 650 – 660 .
- Bligh , EG and Dyer , WJ . 1959 . A rapid method of total lipid extraction and purification . Canadian Journal of Biochemistry and Physiology , 37 : 911 – 917 .
- Carpenter , SR . 1989 . Replication and treatment strength in whole lake experiments . Ecology , 79 : 453 – 463 .
- Clarke , KR . 1993 . Non-parametric multivariate analyses of changes in community structure . Australian Journal of Ecology , 18 : 117 – 143 .
- Coleman , DC , Reid , CPP and Cole , C . 1983 . Biological strategies of nutrient cycling in soil systems . Advances in Ecological Research , 13 : 1 – 51 .
- De Vries , FT , Bloem , J , van Eekeren , N , Brussaard , L and Hoffland , E . 2007 . Fungal biomass in pastures increases with age and reduced N input . Soil Biology and Biochemistry , 39 : 1620 – 1630 .
- De Vries , FT , Hoffland , E , van Eekeren , N , Brussaard , L and Bloem , J . 2006 . Fungal/bacterial ratios in grasslands with contrasting nitrogen management . Soil Biology and Biochemistry , 38 : 2092 – 2103 .
- Di , HJ , Cameron , KC , Shen , JP , Winefield , CS , O'Callaghan , M , Bowatte , S and He , JZ . 2009 . Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils . Nature Geoscience , 2 : 621 – 624 .
- Di , HJ , Cameron , KC , Shen , JP , Winefield , CS , O'Callaghan , M , Bowatte , S and He , JZ . 2010 . Ammonia-oxidising bacteria and archaea grow under contrasting soil nitrogen conditions . FEMS Microbiology Ecology , 72 : 386 – 394 .
- Donnison , LM , Griffith , GS , Hedger , J , Hobbs , PJ and Bardgett , RD . 2000 . Management influences on soil microbial communities and their function in botanically diverse haymeadows of northern England and Wales . Soil Biology and Biochemistry , 32 : 253 – 263 .
- Francis , CA , Beman , JM and Kuypers , MM . 2007 . New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation . ISME Journal , 1 : 19 – 27 .
- Francis , CA , Roberts , KI , Beman , JM , Santoro , AE and Oakley , BB . 2005 . Ubiquity and diversity of ammonia-oxidising archaea in water columns and sediments of the ocean . Proceedings of the National Academy of Sciences , 102 : 14,683 – 14,688 .
- Gardes , M and Bruns , TD . 1993 . ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhiza and rusts . Molecular Ecology , 2 : 113 – 118 .
- Giovannoni , SJ , Delong , EF , Olsen , GJ and Pace , NR . 1988 . Phylogenetic group-specific oligonucleotide probes for identification of single microbial cells . Journal of Bacteriology , 170 : 720 – 726 .
- Gray , ND , Hastings , RC , Sheppard , SK , Loughnane , P , Lloyd , D , McCarthy , AJ and Head , IM . 2003 . Effects of soil improvement treatments on bacterial community structure and soil processes in an upland grassland soil . FEMS Microbiology Ecology , 46 : 11 – 22 .
- Grayston , SJ , Griffith , GS , Mawdsley , JL , Campbell , CD and Bardgett , RD . 2001 . Accounting for variability in soil microbial communities of temperate upland grassland ecosystems . Soil Biology and Biochemistry , 33 : 533 – 551 .
- Hartmann , M and Widmer , F . 2008 . Reliability for detecting composition and changes of microbial communities by T-RFLP genetic profiling . FEMS Microbiology Ecology , 63 : 249 – 260 .
- Hauben , L , Vauterin , L , Swings , J and Moore , ERB . 1997 . Comparison of 16S ribosomal DNA sequence of all Xanthomonas species . International Journal of Systematic Bacteriology , 47 : 328 – 335 .
- He , J , Shen , J , Zhang , L , Zhu , Y , Zheng , Y , Xu , M and Di , H . 2007 . Quantitative analysis of the abundance and composition of ammonia-oxidising bacteria and ammonia-oxidising archaea of a Chinese upland red soil under long-term fertilization practices . Environmental Microbiology , 9 : 2364 – 2374 .
- Jenkinson , DS . 1988 . “ Determination of microbial biomass carbon and nitrogen in soils ” . In Advances in nitrogen cycling in agricultural ecosystems , Edited by: Wilson , JR . 368 – 386 . Wallingford , , UK : CAB International .
- Jia , Z and Conrad , R . 2010 . Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil . Environmental Microbiology , 11 : 1658 – 1671 .
- Jurgens , G , Lindstrom , K and Saano , A . 1997 . Novel group within the kingdom Crenarchaeota from boreal forest soil . Applied Environmental Microbiology , 63 : 803 – 805 .
- Kennedy , N , Brodie , E , Connolly , J and Clipson , N . 2004 . Impact of lime, nitrogen and plant species on bacterial community structure in grassland microcosms . Environmental Microbiology , 6 : 1070 – 1080 .
- Kennedy , N , Connolly , J and Clipson , N . 2005 . Impact of lime, nitrogen and plant species on fungal community structure in grassland microcosms . Environmental Microbiology , 7 : 780 – 788 .
- Klumpp , K , Fontaine , S , Attard , E , Le Roux , X , Gleixner , G and Soussana , J-F . 2009 . Grazing triggers soil carbon loss by altering plant roots and their control on soil microbial community . Journal of Ecology , 97 : 876 – 885 .
- Krumins , JA , Dighton , J , Gray , D , Franklin , RB , Morin , PA and Roberts , MS . 2009 . Soil microbial community response to nitrogen enrichment in two scrub oak forests . Forest Ecology and Management , 258 : 1383 – 1390 .
- Lambert , MG , Clark , DA , Grant , DA , Costall , DA and Fletcher , RH . 1983 . Influence of fertiliser and grazing management on North Island moist hill country: I. Herbage accumulation . New Zealand Journal of Agricultural Research , 26 : 95 – 108 .
- Lambert , MG , Clark , DA , Mackay , AD and Costall , DA . 2000 . Effects of fertiliser application on nutrient status and organic matter content of hill soils . New Zealand Journal of Agricultural Research , 43 : 127 – 138 .
- Landcare Research 2007 . www.landcareresearch.co.nz/services/laboratories/eclab (accessed October 2007) .
- Lauber , CL , Strickland , MS , Bradford , MA and Fierer , N . 2008 . The influence of soil properties on the structure of bacterial and fungal communities across land-use types . Soil Biology and Biochemistry , 40 : 2407 – 2415 .
- Le Roux , X , Poly , F , Currey , P , Commeaux , C , Hai , B , Nicol , GW , Prosser , JI , Schloter , M , Attard , E and Klumpp , K . 2008 . Effects of aboveground grazing on coupling among nitrifier activity, abundance and community structure . ISME Journal , 2 : 221 – 232 .
- Ledgard , SF , Sprosen , MS , Penno , JW and Rajendram , GS . 2001 . Nitrogen fixation by white clover in pastures grazed by dairy cows: Temporal variation and effects of nitrogen fertilization . Plant and Soil , 229 : 177 – 187 .
- Ledgard , SF , Sprosen , MS and Steele , KW . 1996 . Nitrogen fixation by nine white clover cultivars in grazed pasture, as affected by nitrogen fertilization . Plant and Soil , 179 : 193 – 203 .
- Leininger , S , Urich , T , Schloter , M , Schwark , L , Qi , J , Nicol , GW , Prosser , JI , Schuster , SC and Schleper , C . 2006 . Archaea predominate among ammonia-oxidising prokaryotes in soil . Nature , 442 : 806 – 809 .
- MacLeod , CJ and Moller , H . 2006 . Intensification and diversification of New Zealand agriculture since 1960. An evaluation of current indicators of land use change . Agriculture, Ecosystems and Environment , 115 : 201 – 218 .
- Manning , P , Newington , JE , Robson , HR , Saunders , M , Bradford , MA , Eggers , T , Ellis , RJ , Gange , AC , Marhan , S , Kandeler , E , Tscherko , D , Reid , E , Grayston , SJ , Bonkowski , M , Bardgett , RD , Godfray , HCJ and Rees , M . 2006 . Decoupling the direct and indirect effects of nitrogen deposition on ecosystem function . Ecological Letters , 9 : 1015 – 1024 .
- Marchesi , JR , Sato , T , Weightman , AJ , Martin , TA , Fry , JC , Hiom , SJ and Wade , WG . 1998 . Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA . Environmental Microbiology , 64 : 795 – 799 .
- Oksanen , L . 2001 . Logic of experiments in ecology: is pseudoreplication a pseudoissue? . Oikos , 94 : 27 – 38 .
- Parfitt , RL , Mackay , AD , Ross , DJ and Budding , PJ . 2009 . Effects of soil fertility on leaching losses of N, P and C in hill country . New Zealand Journal of Agricultural Research , 52 : 69 – 80 .
- Parfitt , RL , Yeates , GW , Ross , DJ , Mackay , AD and Budding , PJ . 2005 . Relationships between soil biota, nitrogen and phosphorus availability, and pasture growth under organic and conventional management . Applied Soil Ecology , 28 : 1 – 13 .
- Parfitt , RL , Yeates , GW , Ross , DJ , Schon , NL , Mackay , AD and Wardle , DA . 2010 . Effect of fertilizer, herbicide and grazing management of pastures on plant and soil communities . Applied Soil Ecology , 45 : 175 – 186 .
- Parekh , NR and Bardgett , RD . 2002 . “ The characterisation of microbial communities in environmental samples ” . In Interactions of microorganisms with radionucleotides , Edited by: Keith-Roach , MJ and Livens , FR . 37 – 60 . Amsterdam : Elsevier .
- Prosser , JI and Nicol , GW . 2008 . Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment . Environmental Microbiology , 10 : 2931 – 2941 .
- Rooney , DC and Clipson , NJW . 2008 . Impact of sheep urine deposition and plant species on ammonia-oxidizing bacteria in upland grassland soil . Canadian Journal of Microbiology , 54 : 791 – 796 .
- Ross , DJ , Newton , PCD and Tate , KR . 2004 . Elevated (CO2) effects on herbage production and soil C and N pools and mineralization in a species-rich grazed pasture on a seasonally dry sand . Plant and Soil , 260 : 183 – 196 .
- Ross , DJ , Speir , TW , Kettles , HA and Mackay , AD . 1995 . Soil microbial biomass, C and N mineralization and enzyme activities in a hill country pasture; influence of season and slow release P and S fertilizer . Soil Biology and Biochemistry , 27 : 1431 – 1443 .
- Rotthauwe , JH , Witzel , KP and Liesack , W . 1997 . The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations . Applied Environmental Microbiology , 63 : 4704 – 4712 .
- Schauss , K , Focks , A , Leininger , S , Kotzerke , A , Heuer , H , Thiele-Bruhn , S , Sharma , S , Wilke , BM , Matthies , M , Smalla , K , Munch , JC , Amelung , W , Kaupenjohann , M , Schloter , M and Schleper , C . 2009 . Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils . Environmental Microbiology , 11 : 446 – 456 .
- Schon , NL , Mackay , AD , Minor , MA , Yeates , GW and Hedley , MJ . 2008 . Soil fauna in grazed New Zealand hill country pastures at two management intensities . Applied Soil Ecology , 40 : 218 – 228 .
- Singh , BK , Nunan , N and Millard , PAF . 2009 . Response of fungal, bacterial and ureolytic communities to synthetic sheep urine deposition in a grassland soil . FEMS Microbiology Ecology , 70 : 109 – 117 .
- Sparling , GP , Shepherd , TG and Schipper , LA . 2000 . Topsoil characteristics of three contrasting New Zealand soils under four long-term land uses . New Zealand Journal of Agricultural Research , 43 : 569 – 583 .
- Steele , KW and Vallis , I . 1988 . “ The nitrogen cycle in pastures ” . In Advances in nitrogen cycling in agricultural ecosytems , Edited by: Wilson , JR . 274 – 291 . Wallingford , , UK : CAB International .
- Stephen , JR , Chang , YJ , Macnaughton , SJ , Kowalchuk , GA , Leung , KT , Flemming , CA and Whire , SC . 1999 . Effect of toxic metals on indigenous soil beta-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria . Applied Environmental Microbiology , 65 : 95 – 101 .
- Suzuki , C , Nagaoka , K , Shimada , A and Takenaka , M . 2009 . Bacterial communities are more dependent on soil type than fertilizer type, but the reverse is true for fungal communities . Soil Science and Plant Nutrition , 55 : 80 – 90 .
- Thies , J . 2007 . Soil microbial community analysis using terminal restriction fragment length polymorphisms . Soil Science Society of America Journal , 71 : 579 – 591 .
- Tom-Petersen , A , Leser , TD , Marsh , TL and Nybroe , O . 2003 . Effect of copper amendment on the bacterial community in agricultural soil analysed by T-RFLP . FEMS Microbiology Ecology , 46 : 53 – 62 .
- Wang , YA , Ke , XB , Wu , LQ and Lu , YH . 2009 . Community composition of ammonia-oxidizing bacteria and archaea in rice field soil as affected by nitrogen fertilization . Systematic and Applied Microbiology , 32 : 27 – 36 .
- Wardle , DA . 2006 . The influence of biotic interactions on soil biodiversity . Ecology Letters , 9 : 870 – 886 .
- White , DC , Davis , WM , Nickels , JS , King , JD and Bobbie , RJ . 1979 . Determination of the sedimentary microbial biomass by extractible lipid phosphate . Oecologia , 40 : 51 – 62 .
- White , TJ , Bruns , T , Lee , S and Taylor , J . 1990 . “ Analysis of phylogenetic relationship by amplification and direct sequencing of ribosomal RNA genes ” . In PCR protocol: A guide to method and applications , Edited by: Innis , MA , Gelfond , DH , Sainsky , JJ and White , TJ . 315 – 322 . New York : Academic Press .
- Yeates , GW . 1984 . Variation in soil nematode diversity under pasture with soil and year . Soil Biology and Biochemistry , 16 : 95 – 102 .
- Yeates , GW . 2003 . Nematodes as soil indicators: functional and biodiversity aspects . Biology and Fertility of Soils , 37 : 199 – 210 .
- Yeates , GW , Bongers , T , de Goede , RGM , Freckman , DW and Georgieva , SS . 1993a . Feeding habits in soil nematode families and genera—an outline for soil ecologists . Journal of Nematology , 25 : 315 – 331 .
- Yeates , GW , Wardle , DA and Watson , RN . 1993b . Relationships between nematodes, soil microbial biomass and weed management strategies in maize and asparagus cropping systems . Soil Biology and Biochemistry , 25 : 869 – 876 .