Abstract
Cirsium arvense (Californian thistle) populations in pastures throughout New Zealand were surveyed in November–December 2005 (30 sites) and January–March 2006 (94 sites) to identify potential biological control agents for this weed. Fungi were isolated from healthy shoots and shoots showing leaf yellowing/browning, stunting or localized lesions. Verticillium dahliae was isolated most frequently, being detected at 30% of the sites in 2005 and at 51% in 2006. Other pathogenic/saprophytic fungi isolated included Sclerotinia sclerotiorum, Plectosphaerella cucumerina and species of Cylindrocarpon, Rhizoctonia and Phoma. Inoculating cut shoots of C. arvense with conidia of V. dahliae, or cutting shoots with a wetted blade previously used to cut infected shoots resulted in yellowing of leaves and shoot death. Spread of V. dahliae infection among plants facilitated by cutting when wet may explain the reported demise of C. arvense populations mown during rainfall and the fungus may have potential as a bioherbicide.
Introduction
Cirsium arvense (L.) Scop. (Californian thistle, Canada thistle, creeping thistle, perennial thistle) is a tenacious agricultural weed throughout much of the temperate world (Donald Citation1990; McClay et al. Citation2001; Skinner et al. Citation2009). In New Zealand, it is a widespread (Bourdôt et al. Citation2009) and persistent problem in pastures despite concerted attempts at cultural, chemical and biological control (Bourdôt et al. Citation1995; Bourdôt et al. Citation2006).
Biological control of C. arvense remains a favoured option, and despite recently expressed reservations (Müller & Nentwig Citation2011), the search continues for insects, fungi and bacteria that might have a substantial impact on populations of the weed. Several insects have been introduced into New Zealand for the classical biological control of C. arvense (Cripps et al. Citation2011) in which the agent is expected to establish self-sustaining populations and spread throughout areas of weed infestation. An alternative bioherbicide approach, whereby a pathogenic microbial biological control agent is applied repeatedly and inundatively to the target weed population, has also been widely investigated with candidate bacteria Pseudomonas (Bailey et al. Citation2000) and fungi from several genera including Alternaria (Gannibal & Berestetskiy Citation2008), Aschochyta (Gasich et al. Citation2007), Colletotrichum (Bailey et al. Citation2000), Fusarium (Ratajkiewicz et al. Citation2009), Phoma (Cimmino et al. Citation2008), Phomopsis (Leth et al. Citation2008), Phyllosticta (Evidente et al. Citation2008a), Puccinia(Voelker & Boyle Citation1994), Septoria (Hershenhorn et al. Citation1993), Sclerotinia (Bourdôt et al. Citation2006), Stagonospora (Evidente et al. Citation2008b) and Verticillium (Leth & Haas Citation1984).
The development of any one of these agents as a bioherbicide involves exhaustive evaluation and improvement to reach the levels of efficacy, safety and economy required for commercial release. This evaluation process has recently been described as the bioherbicide innovation chain (Bailey & Falk Citation2011). Unfortunately, no microbial biological control agent for C. arvense has progressed beyond the initial discovery or proof-of-concept stage to the technology development and later stages of this evaluation process. A key reason for this lack of progress has been that most candidates exhibited poor or variable efficacy against populations of C. arvense. Part of the explanation for this could be that the life history stage of the weed damaged by the potential microbial biocontrol agent has little influence on population growth. Agents that attack flowers, for instance, are almost certain to be ineffective in pastures because, here, seeds have little role in population growth of C. arvense (Bourdôt et al. Citation2006). Foliar pathogens, by contrast, could be effective, but only if their damage reduces the overwintering root biomass sufficiently to diminish shoot populations in the subsequent growing season (Bourdôt et al. Citation1998). Given the importance of overwintered root biomass for population growth in C. arvense (Bourdôt et al. Citation2006), a more effective microbial biological control agent might be one that either directly attacks these roots, or achieves systemic distribution within the plant that debilitates shoots sufficiently to interfere with the production and transfer of the photosynthate required to support root growth. To that end, we report here on: two disease surveys in New Zealand pastures designed to elucidate pathogenic fungi present in subterranean tissue of C. arvense; and related experiments with one promising fungus, Verticillium dahliae Kleb.
Materials and methods
Disease survey in spring 2005
A national survey of diseases occurring in pasture populations of C. arvense in New Zealand was conducted in spring 2005 using a stratified random sampling design. Areas of low- and high-producing grassland in each region were identified using The Land Cover Database of New Zealand (Terralink Citation2009), versions LCDB1 and LCDB2 and the corresponding land cover classes (MFE Citation2005). The two (grassland) land cover sub-classes of Class 10 (primarily pastoral) of LCDB1 used for this study were distinguished within LCDB2 as Class 40 (high-producing exotic grassland) and Class 41 (low-producing grassland). Topographical maps of the NZMS260 series (LINZ Citation2008) were produced with the areas of grassland (Class 40 + Class 41) overlaid by GIS analysis. These details were then plotted on to regional maps along with significant geographical features such as major roads, regional boundaries, rivers and mountain ranges. Random sampling coordinates falling within ‘grassland’ were generated for each of the 15 individual geo-political regions of New Zealand (Nelson and Marlborough combined). The number of sample sites per region was made proportional to the area of pasture and the expected frequency of C. arvense in each region. Samples were obtained from 30 sites during the spring 2005 survey ().
Figure 1 The geographical locations of the 30 and 94 sample sites of the spring (November–December 2005) (left) and summer/autumn (January–March 2006) (right) disease surveys in pasture populations of Cirsium arvense in New Zealand. The sample sites are labelled 1–30 and 31–124, respectively, as in Tables 1–3 and 4–6, respectively.
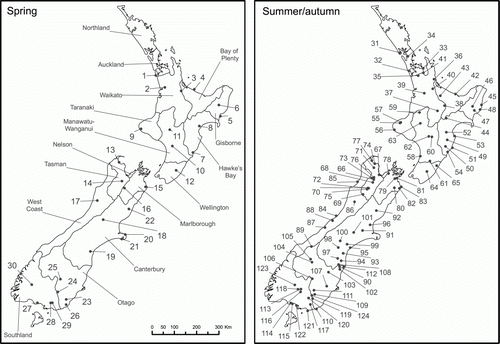
Each regional map shows the locations of the sampling sites, along with each point's sample-identifying number and grid coordinates (eastings/northings). Two ‘contingency’ sites per region were also plotted as replacements for inaccessible/unsuitable ‘primary’ sites. GPS units and local scale maps were used to assist in finding the random sampling sites. The sampling was conducted between 9 November and 23 December 2005.
On arrival at the location of the primary sampling site, the nearest pastoral farm was identified and the farmer contacted. The most appropriate area for sampling was determined on the basis of the farmer's recollection of the occurrence of diseased C. arvense, pasture age (older pastures selected over newer pastures) and density of the thistle stand (higher density selected over lower density). If there was no C. arvense on the property, or the farmer could not be found, the next nearest farm was chosen at random, or from a contingency site.
At each site, five shoots representative of C. arvense in a population identified by the farmer as being ‘unhealthy’ were dug up with a spade, along with their creeping roots. A further three ‘healthy’ shoots were removed for comparison. When the individual shoots at a particular site all appeared either healthy or unhealthy, eight shoots and their roots were collected at random from within the population. Collectors recorded the location of the farm, the GPS coordinates of the actual sampling site, details of the predominant growth stage of the C. arvense, and any symptoms of disease.
The eight stem/root samples taken from each site for pathological investigation were freed from soil, placed separately in plastic bags and stored in a chilled bin until dispatched to AgResearch Grasslands, Palmerston North. Most samples (>70%) arrived within 3 days of collection and were stored at 4 °C for less than 1 week before examination and processing to isolate fungi. In the majority of samples, when plants were removed from the bags for examination, the symptoms noted in the field at the time of sampling were still evident.
The stems and root samples were assessed for disease symptoms. The above-ground portion of stems was examined for wilting or die-back, progressive rotting or brown necrotic lesions. Below-ground stem tissue was inspected for physical damage, symptoms of rotting or lesions. Creeping roots were inspected for rotting or discoloration and internal discoloration of the vascular cylinder (stele).
Pieces of tissue were cut from shoot samples showing symptoms of interest. Above- and below-ground stem material was washed with a jet of tap water, dried and then surface sterilized in 0.3% sodium hypochlorite for 2 min. The material was then rinsed in sterile water, dried on filter paper and the outer cortical tissues removed with a sterile scalpel. Vascular tissue was cut into 1–2 mm segments, five pieces of which were put on either of two plates of antibiotic water agar (AWA; 2% water agar containing 10 µg mL−1 streptomycin and chloramphenicol). Roots were washed in the same way as stems but the stele was removed before surface sterilization. Isolation plates were incubated at 20 °C until fungi could be identified or transferred to potato dextrose agar (PDA, Difco). Representative isolates were further subcultured on to PDA as single spores or hyphal tips (for non-sporulating fungi) and stored in sterile distilled water at 4 °C for subsequent pathogenicity studies.
Disease survey in summer/autumn 2006
This survey was conducted between 31 January and 23 March 2006 with samples obtained from 94 sites (). The sampling protocol was as used in the spring 2005 survey but included observations on the height of the sampled stems and the percentage of their length with leaves that were: dead or missing; showing chlorosis or necrosis; or green and healthy. The protocol for isolation of fungi was also modified so that all suitable plant samples, whether classified as healthy or unhealthy, were processed for isolation of fungi from the vascular tissue of the underground stem and creeping root. As previously, tissue showing discrete localized symptoms (e.g. stem lesions) was also processed.
Inoculation studies
Seed was collected from C. arvense from farms in Canterbury and dusted with 0.5 g of a 1:1 mixture of benomyl and captan fungicides g−1 seed prior to sowing in fine grade Vermiculite in perforated plastic propagating trays (370×230×55 mm). The trays were placed in plant growth cabinets (Conviron, Canada) at 20±3 °C (12 h photoperiod; light intensity approximately 105 µmol s−1 m−2). Seedlings emerged over a 3–4 week period and were transplanted, when their cotyledons were fully expanded, into a composted peat/bark/sand potting mix containing Osmocote slow release fertilizer and then transferred to an unheated glasshouse (8–28 °C). Some plants used for infection experiments were propagated from creeping root sections obtained from glasshouse-grown plants and grown in compost in black plastic planter bags (PB 1½).
Inoculation of roots with V. dahliae
Two forms of inoculum were used, conidia and microsclerotia. Conidial inoculum was prepared by flooding 2-week-old cultures of V. dahliae on PDA with sterile water, then scraping the surface with a spatula. The resulting suspension was filtered through two layers of nylon mesh and centrifuged at 600 relative centrifugal force for 15 min. The pellets were resuspended in sterile water and adjusted to a concentration of 106 conidia mL−1 with the aid of a haemocytometer. Microsclerotial inoculum was prepared by processing the fungal and agar substrate material from 10 plates of 1-month-old cultures of V. dahliae in 700 mL sterile water for 2 min in a blender (Moulinex, France). The resulting suspension was passed through a nest of sieves with apertures of 295, 180 and 38 µm. The microsclerotia retained on the 180 and 38 µm sieves were rinsed in running tap water to wash away any remaining conidia, resuspended in sterile water and adjusted to a concentration of 103 microsclerotia mL−1. Three isolates of V. dahliae (CT 322, 330 and 386) obtained from separate sites were used.
C. arvense plants were inoculated with V. dahliae when 12 weeks old. They were removed from their pots and their roots gently freed from potting mix in a bucket of water, then flushed with a stream of tap water. Sterile water (control) or suspensions of conidia or microsclerotia were poured into five beakers (replicates) and one individual test plant placed in each for 5 min during which the inoculum was stirred twice. Inoculated plants were repotted in fresh potting mix, transferred to a glasshouse compartment containing a mist generator and maintained at high humidity for 48 h before resuming normal glasshouse conditions. Plants were examined at intervals over several months for the appearance of symptoms and various measurements made to compare the growth/health of plants receiving different treatments. To confirm the presence of V. dahliae in symptomatic plants, pieces of vascular tissues from roots, above- and below-ground stems, and occasionally leaf midribs and blades were surface sterilized in 0.3% sodium hypochlorite, rinsed in sterile water, dried on filter paper, cut into 1–2 mm segments and plated on to AWA. These ‘isolation plates’ were incubated at 20 °C until V. dahliae could be recognized using a stereomicroscope (×10 to×40 mag.) by the presence of dark microsclerotia within the agar and of conidiophores with whorls of phialides.
Inoculation of shoots with V. dahliae
Two experiments were carried out with glasshouse-grown C. arvense plants in which stems and leaves were cut with scissors to simulate mowing. Inoculum of V. dahliae was applied either on the scissor blades that had been dipped in a suspension of conidia, or by spraying a suspension of conidia on to the cut surfaces. In both cases, conidial suspensions were made up with or without the addition of an organosilicone surfactant to promote uptake of the spore-containing liquid by the internal tissues of the cut stems.
Experiment 1
This investigated the effects of conidial concentration, addition of surfactant, and method of inoculum application on the development of wilt disease caused by V. dahliae. Six-week-old vegetatively propagated C. arvense plants in the rosette form were used. There were three levels of conidial concentration (0, 104 and 2×105 conidia mL−1) and two of Pulse (Nufarm; 0 and 0.05% v/v) surfactant. Two means of inoculum application were used: ‘Blade’, where leaf tissue 5 cm above soil level was removed by cutting in 3–5 passes with scissors that had been dipped in water or conidial suspension; and ‘Spray’, where plants were cut as above then sprayed with water or conidial suspension using a hand-held, trigger-operated atomizer (30 mL applied to the five replicate plants). After inoculation, plants of each of the five replicates were placed randomly in individual lidded translucent plastic storage bins (380×330×250 mm), sprayed lightly with a mist of water to maintain high humidity and incubated in a Conviron growth cabinet at 20 °C for 48 h (12 h dark/12 h light; intensity approximately 105 µmol s−1 m−2). The plants were then removed from the bins and randomly arranged in blocks on a glasshouse bench.
The plants were observed for the onset of symptoms. Shoot tissue above soil level was removed for determination of dry weight 8 weeks after inoculation. Herbage from the inoculated stem was collected and processed separately from that of new shoots that had emerged since inoculation. Since the plants were to be kept growing to allow further symptoms and growth suppression to become evident, care was taken to prevent cross contamination from infected to uninfected plants by cutting control plants first and sterilizing scissors in alcohol between plants. Plant samples were dried in an oven for 24 h at 80 °C. After removing their tops, the plants were returned to the glasshouse using a different randomization of treatments within blocks.
To determine whether the plants were infected with V. dahliae, 20 mm long pieces of stem were cut from below the soil surface of one plant from each replicate of the Blade control (0 conidia, 0 Pulse) and a corresponding inoculated treatment (104 conidia mL−1, 0 Pulse). The stem pieces were surface sterilized, plated on AWA and any colonies of V. dahliae present were identified as described previously.
Experiment 2
This investigated whether V. dahliae could be transmitted from infected to uninfected plants on a cutting blade, and the effect of wetness on this transmission. Ten, 6-week-old vegetatively propagated C. arvense plants at the rosette stage were used as test plants. Five test plants received a ‘dry’ treatment and five a ‘wet’ treatment. The plants providing the source of V. dahliae were those from Experiment 1 that had become infected after receiving the high level of conidial inoculum (2×105 conidia per mL, 0 Pulse). During the process of removing shoot tissue from an infected plant for the determination of shoot dry weight 8 weeks after inoculation in Experiment 1, sterile scissors were used to cut the shoots of each infected plant. For the dry treatment, scissors with dry blades were used to cut through stems of infected plants 3–5 times before being used to remove leaf tissue of a test plant 5 cm above the potting medium. For the wet treatment, the scissors were dipped in water before cutting the infected stems, and a mist of water was sprayed over the test plant after cutting. The plants were incubated at high humidity for 24 h before being transferred to the glasshouse bench and grown for a further 10 weeks.
Inoculation of shoots with other fungi
Selected isolates of other fungi collected in the 2005–06 disease surveys were tested for their ability to rot tissues of the stem and creeping root and to prevent the regrowth of new shoots following application to cut stems. Sclerotinia sclerotiorum Lib. (de Bary) (six isolates), Rhizoctonia solani J. G. Kühn (three isolates), Plectosphaerella cucumerina (Lindf.) W. Gams (one isolate), Cylindrocarpon spp. (three isolates) and Phoma sp. (two isolates) were cultured on plates of PDA. Plugs (4 mm2) from these cultures were placed on the wounded surface of glasshouse-grown C. arvense plants in which the main stem had been cut through with scissors. Plants were maintained at 20 °C in a growth cabinet (12 h photoperiod; under high humidity in a covered plastic bin for the first 48 h) for up to 13 days before assessing the progression of stem rotting.
Results
Disease survey in spring 2005
The occurrence of unhealthy stems in stands of C. arvense varied among and within sampling sites (). At 17 of the sites the collectors were unable to find individual stems that appeared less healthy than others in the stand, hence they submitted only healthy samples. Healthy stems did not always appear uniformly green and often their lower leaves were chlorotic, partially or completely necrotic, or were absent (A). These effects were considered to be a normal part of the process of ageing in a natural environment. At the remaining 13 sites, the collectors identified stems that showed similar, but more extensive and severe symptoms and sometimes distinct stunting or wilting; these were classified as unhealthy (B). Eight of these sites provided samples of relatively healthy stems for comparison with distinctly unhealthy stems. At the remaining five sites all the plants appeared unhealthy.
Figure 2 Appearance of Cirsium arvense plants in the two disease surveys. A, ‘Healthy’ stems that had just flowered showing dead (brown) leaves at their base. B, ‘Unhealthy’ stem from which Verticillium dahliae was isolated from the vascular tissue of a branch with yellow leaves and the underground stem and the stele of the creeping root. C, Blackening of stem as an extensive stem lesion. D, Rotting outer tissues at stem base exposing vascular strands.

Table 1 Spring survey (November–December 2005). Collectors' designation of Cirsium arvense stems as ‘healthy’ or ‘unhealthy’. The geographic locations of the sites are shown in .
Rust caused by Puccinia punctiformis (F. Strauss) Röhl., and aphids, were often seen on leaves. Areas of necrotic tissue likely to have been caused by fungal pathogens were seen on roots and stems (). Characteristics of these necrotic symptoms were:
| 1. | Sclerotinia stem rot—soft watery rot associated with an obvious white mycelium and dark sclerotia. | ||||
| 2. | Stem lesions (discrete dark brown patches)—often found on the lower portion of the stem where leaves had senesced and died, had been severely damaged by rust, or had been detached mechanically (C). It was observed that stem tissue rapidly underwent browning/blackening if leaves were torn from C. arvense plants in a way that pulled vascular strands from the stem out through the stem cortex and epidermis. | ||||
| 3. | Root rot—dieback at the end of an old root or near a dying stem. | ||||
| 4. | Stem base deterioration (browning and/or rotting of the region of stem between the creeping root and the zone of production of adventitious roots just below soil level)—often associated with desiccation or disappearance of the stem cortex which exposed vascular strands and gave a ‘skeletonized’ appearance to the stem base (D). | ||||
Table 2 Spring survey (November–December 2005). Disease symptoms and invertebrate infestations observed on the Cirsium arvense samples and fungi isolated from symptomatic tissue. The geographic locations of the sites are shown in .
Considering the isolations from the subterranean vascular tissues from the 25 sites where healthy stems were available, most pieces of root/stem vascular tissue failed to develop fungal colonies. Fungi were isolated from only 45% of stems/roots from the 17 sites where all stems appeared healthy, and from 17% of healthy stems/roots of the eight sites where both healthy and unhealthy stems were sampled. Where fungi did grow from vascular tissues of healthy stems/roots (), P. cucumerina was found at seven sites, whereas V. dahliae and several other fungi commonly found as parasites/saprophytes on plant tissues (e.g. R. solani and species of Cylindrocarpon, Fusarium and Phoma) were isolated less frequently.
Table 3 Spring survey (November–December 2005). Fungi isolated from the vascular tissue of the above- and below-ground stem sections and attached creeping roots of stems sampled from 30 sites. The geographic locations of the sites are shown in .
Fungi were isolated from 47% of unhealthy stems from the eight sites where healthy stems were available for comparison, and from 79% of unhealthy stems from the five sites at which all plants appeared unhealthy. V. dahliae was isolated from the most sites () with the range of other fungi present being similar to that from healthy stems.
We now consider the fungi isolated from rotting tissue or from discrete lesions. Rotting subterranean sections of the stems with white mycelium and dark sclerotia yielded colonies of S. sclerotiorum only (). Deteriorating subterranean sections of the stems yielded P. cucumerina and species of Fusarium, Ulocladium and sterile fungi. In many instances, no fungi were cultured from tissue showing stem lesions. When fungal isolates were obtained, they were a range of species similar to those isolated from the vascular tissues ().
Disease survey in summer/autumn 2006
As with the survey conducted in spring, the types of sample that could be collected from individual sites varied (). At 43 of the 94 sites only healthy stems were collected while, at 42 sites both healthy and unhealthy were collected. At nine sites only unhealthy stems were collected. Unhealthy stems were shorter (mean height 44 cm) compared with healthy stems (mean height 51 cm) and had a higher percentage of their leaves chlorotic, necrotic or missing (91%) compared with healthy stems (43%).
Table 4 Summer/autumn survey (January–March 2006). Collectors' designation of Cirsium arvense stems as ‘healthy’ or ‘unhealthy’. The geographic locations of the sites are shown in .
Rotting and discrete lesions were often found on the lower regions of stems (). Rust and aphids were again commonly encountered on leaves. Galls of root knot nematode (Meloidogyne sp.) were seen on adventitious roots.
Table 5 Summer/autumn survey (January–March 2006): Disease symptoms and invertebrate infestations observed on Cirsium arvense samples and fungi isolated from symptomatic tissue. The geographic locations of the sites are shown in .
Of the 85 sites where healthy stems were available for examination, only 18% of the 450 subterranean sections of the stems processed yielded fungal colonies on isolation plates. V. dahliae and P. cucumerina were the most frequently isolated () along with a wide range of parasitic/saprophytic fungi as had been found in the spring survey. Fungi were isolated from 59% of the 243 stems/roots processed from the 51 sites where unhealthy stems were identified. As with the spring survey and healthy samples of this survey, V. dahliae was isolated from most sites (36; ), with P. cucumerina and a range of other species also present.
Table 6 Summer/autumn survey (January–March 2006). Fungi isolated from the vascular tissue of the above- and below-ground stem sections and attached creeping roots of stems sampled from 94 sites. The geographic locations of the sites are shown in .
The fungus S. sclerotiorum was consistently isolated from rotting subterranean sections of the stems (). The range of fungi isolated from deteriorating subterranean sections of the stems was similar to that isolated from discrete lesions and included many of the fungal saprophytes or facultative parasites found in tissues showing these symptoms in the spring 2005 survey.
Inoculation studies
Inoculation of roots with V. dahliae
After 9 weeks, significant reductions in the height of plants and increases in the percentage of stems and leaves that were dead were apparent in plants inoculated with either the conidia or microsclerotia of V. dahliae (). The impact of the conidial inoculation treatment on these same parameters was significantly greater than that of microsclerotial treatment. There was no indication that any one isolate of V. dahliae was more damaging than any other. At 19 weeks, the majority of the inoculated plants were dead while uninoculated plants all remained alive and healthy.
Table 7 Extent of growth and death of shoot tissue 9 and 19 weeks after the roots of 12 week-old glasshouse-grown Cirsium arvense plants had been inoculated with suspensions of conidia (106 per mL) or microsclerotia (103 per mL) from three isolates of Verticillium dahliae. Contrasts included in the analyses of variance are (1) control versus treatments 1–6, and (2), treatments 1–3 versus treatments 4–6. For the last two columns, these contrasts were tested using chi-squared tests.
Inoculation of shoots with V. dahliae
In experiment 1, leaves began to show characteristic symptoms of infection by V. dahliae about 1 month after inoculation, with most inoculated plants showing symptoms by 6 weeks (12 of 20 treated with 104 conidia mL−1 and 19 of 20 receiving 2×105 conidia mL−1). Symptoms, which usually appeared first on those leaves that had been cut during the inoculation, started as yellowing (chlorosis) of part of one or more leaves followed by browning (necrosis) within chlorotic areas which spread until entire leaves were killed. Shoots often appeared stunted and eventually died. Later, the emergence of new shoots, which were below soil level at the time of inoculation, reduced the apparent severity of the disease on plants until they themselves developed symptoms. All uninoculated plants remained green and healthy except for a little chlorosis in one or two of the oldest leaves that were beginning to senesce.
Significant effects of inoculation treatments on the dry weight of shoots were evident after 8 weeks (). Inoculated shoots had produced significantly less dry weight than uninoculated ones; those receiving 2×105 conidia mL−1 (53% reduction) produced less than shoots receiving 104 conidia mL−1 (20% reduction). As well as inoculated shoots, new shoots that had emerged from beneath the soil were also present in pots, as noted above. The dry weights of these new shoots were measured separately and found to be significantly higher in plants inoculated with 2×105 conidia mL−1 than the uninoculated ones, although not significantly so in the comparison of the two rates of inoculum. The effect of this apparent compensatory growth of new shoots was to reduce the overall impact of inoculation on total shoot dry matter production to 4.4% for the low conidial concentration and 17% for the high concentration. There was no significant effect on shoot dry weight of adding the surfactant Pulse. The dipped blade method of inoculation did give a significantly greater reduction in dry weight than the spray method, but only for the inoculated shoots.
Table 8 Main effects of treatments on the dry weight of Cirsium arvense shoots, 8 weeks after inoculation with Verticillium dahliae, and on disease score, 23 weeks after inoculation (15 weeks after shoots were removed for dry weight determination) in Experiment 1.
The additional new shoots that emerged from inoculated plants following the removal of foliage for dry weight determination progressively developed chlorotic and necrotic symptoms. As plants grew older and nutrients in the potting compost became exhausted, signs of senescence became more prominent in shoots of both uninoculated and inoculated plants. Older leaves became yellow, died and fell off and some shoots died. These effects were more severe in inoculated plants, particularly those receiving the higher rate of conidial inoculum. By 23 weeks after inoculation (15 weeks after foliage removal), the effects of treatments were clearly reflected in the disease scores (). Disease scores were significantly higher on inoculated than uninoculated plants () but there were no significant differences between inoculum rates. There was also no significant effect on disease score of surfactant or inoculation method used. V. dahliae was cultured from each of the five plants that had been inoculated with the fungus by the blade method but not from any control plant.
In experiment 2, all test plants that had been cut with scissors that had previously been used to cut plants infected with V. dahliae developed symptoms of verticillium wilt. The symptoms were more severe when scissors were wetted prior to cutting the infected plant than when dry scissors were used ().
Inoculation of shoots with other fungi
Only S. sclerotiorum and the two Phoma isolates caused a progressive stem rot. The most aggressive was S. sclerotiorum which had rotted 10 to 20 mm of the stem after 3 days and had reached leaves and underground tissue by 9 days. This not only killed the plants but also prevented regrowth of shoots from root and stem buds. The fungus was reisolated from rotting roots and subterranean sections of the stems. The two Phoma isolates caused a dark stem rot which had progressed 12 mm after 13 days. Although rotting spread to some petiole bases, the fungus failed to kill leaves or progress into the roots. Browning was observed at the cut ends of stems inoculated with isolates of P. cucumerina, R. solani and Cylindrocarpon spp. but this did not extend more than 1 mm down the stem.
Discussion
Selecting microbial agents for the biological control of C. arvense has usually focused on organisms responsible for disease symptoms on aerial parts of the plant (Popay & Cheah Citation1990; Berestetskiy Citation1997; Bailey et al. Citation2000; Bithell & Stewart Citation2001; Gronwald et al. Citation2002; Waipara Citation2003; Zhang et al. Citation2004; Kluth et al. Citation2005; Gasich & Berestetskiy Citation2006; Leth et al. Citation2008). In an attempt to target the most significant stages of the plant's lifecycle, our study has concentrated instead on pathogenic fungi that might affect the regenerative capacity of the weed's invasive creeping root system. This contribution by subterranean tissues to the development of the often dense populations of new shoots in the spring/summer growing season is a key factor in the weed's ability to compete effectively with desirable pasture and crop species. To infect these subterranean tissues, it would be preferable to use a pathogen that can invade them following application to aerial shoots rather than to the soil.
The fungi we investigated were cultured mainly from the vascular tissue of creeping roots and below-ground sections of the stems ( and ), although leaves and aerial portions of those stems showing specific symptoms were also investigated ( and ). It was our expectation that pathogens affecting subterranean tissues would impair the plant's absorption/conducting system and thus the symptoms displayed by unhealthy shoots might be less distinctive than those produced by foliar/stem pathogens. Furthermore, their causal agents might not be present in shoot tissues showing symptoms. The fungi isolated in culture were most likely to have entered the subterranean tissue by systemic colonization of vessels (e.g. vascular wilt fungi), by progressive invasion from aerial stems that had become rotten and/or damaged (e.g. by mowing), or from lesions in the adjacent root/stem cortex.
The symptoms observed by farmers and collectors in plants they regarded as unhealthy included: rotting, wilting, die-back or stunting of stems; yellowing (chlorosis), browning and withering (necrosis) of leaf tissue; or loss of leaves progressively from the stem base. Some signs of deterioration of foliage were seen on the majority of shoots collected, particularly as they became older and began to senesce. Thus a subjective judgment had to be made while sampling about which were healthy and which were unhealthy. Where possible, both classes of shoot were sampled and examined. By sampling sites on 124 farms throughout New Zealand over all seasons of growth activity in the thistle (spring, summer and autumn), our survey maximized the range of C. arvense-occupied environments sampled and hence also the probability of finding fungi of interest.
Many of the fungal cultures obtained from the vascular tissue of the roots and stems of both healthy and unhealthy samples (Tables 1, 3, 4 and 6) were of genera rarely known to contain plant pathogenic species e.g. Acremonium, Aureobasidium, Chaetomium, Cladosporium, Geotrichum, Mucor, Paecilomyces, Penicillium, Trichoderma and Ulocladium. They may have been present as saprophytes in the dead tissue that was often found in these underground organs or as endophytes within living plant tissue (Schulz et al. Citation1998; Gange et al. Citation2007; Dodd et al. Citation2010; Eschen et al. Citation2010). The entomopathogens Metarhizium anisopliae and Beauveria brongniartii obtained () may also have been endophytes or were derived from fragments of infected insects transferred to the isolation plates. Having discounted these genera, several other fungi remained of interest as pathogens of C. arvense that might have the capacity to debilitate the creeping root system. The most interesting of these (and those chosen for further investigation) were V. dahliae, P. cucumerina, R. solani, Cylindrocarpon spp. and pycnidial species (e.g. Phoma spp.). These pathogens were often found in subterranean sections of the stems that were deteriorating or had discrete lesions ( and ), while S. sclerotiorum was present where rotting subterranean sections of the stems were found covered with white mycelium. Although species of Fusarium were often present, F. oxysporum and several other species appear to be common indiscriminate invaders of subterranean tissue of plants in pasture (Skipp & Watson Citation1996).
When tested for their ability to rot stems and roots progressively, S. sclerotiorum was by far the most aggressive pathogen. This fungus has been extensively evaluated as a potential bioherbicide (Hurrell et al. Citation2001; Bourdôt et al. Citation2006) but as yet has not been deployed in farming practice. The Phoma species also caused a rot that progressed some way down the inoculated stem, but the isolates of the Cylindrocarpon spp., P. cucumerina and R. solani were restricted to the site of inoculation of cut stems.
V. dahliae has attributes that make it of particular interest with regard to its potential to colonize, and hence incapacitate, the creeping root system of C. arvense and the new shoots generated each year. It produces conidia within vessels of infected plants that enable it to develop systemically within below-ground and above-ground organs (Wright Citation1968; Alexander & Hall Citation1974). The fungus was extremely common in our survey, being found in C. arvense throughout New Zealand and throughout the growing season; 30% and 51% of sites sampled in spring and summer/autumn, respectively ( and ). It is also of interest that during the 2006 survey this fungus was found in the thistle at 27 of the 35 North Island sites (77%) and at only 21 of the 59 South Island sites (36%) (, ). This suggests that environmental conditions may be more conducive in the warmer North Island; V. dahliae is commonly regarded as a warm temperature pathogen (Smith Citation1965).
The widespread occurrence of V. dahliae in C. arvense throughout New Zealand pastures revealed by these surveys is particularly notable because C. arvense is not listed as a host of V. dahliae in major international collections and databases (Landcare Citation2008; Farr et al. Citation2011). However, the morphological characteristics (e.g. absence of yellow pigmentation, microsclerotia as resting structures and conidial length generally <6 µm) of the fungus found in our surveys correspond to those of published descriptions of V. dahliae (Smith Citation1965; Inderbitzin et al. Citation2011). Furthermore, there are other published reports of the isolation of V. dahliae from C. arvense in Europe (Leth & Haas Citation1984; Henriksson Citation1995; Demirci & Genc Citation2009) and New Zealand (Waipara et al. Citation1991; Dodd et al. Citation2010). The fungus has also been detected in C. arvense in New Zealand by PCR methods (Bourdôt et al. Citation2011) using primers specifically targeting the V. dahliae β tubulin gene (Atallah et al. Citation2007), or by closest sequence match with V. dahliae following a GenBank BLASTn search (Dodd et al. Citation2010). Recent research has indicted that, while plant pathogenic species of Verticillium can be distinguished by their morphological characters in culture (Goud et al. Citation2003), the phylogenetic relationship among isolates is complicated (Qin et al. Citation2006; Inderbitzin et al. Citation2011). Future studies on Verticillium spp. from plants, including C. arvense, will need to accommodate new understandings of the identification and taxonomy of Verticillium spp.
Our glasshouse experiments confirmed the pathogenicity of V. dahliae to C. arvense. Inoculated plants developed symptoms of a vascular wilt disease, including chlorosis, necrosis and death of stems ( and ) when inoculum was applied as conidia or microsclerotia to roots or as conidia to cut stems and leaves of propagated plants. These symptoms were similar to those that had been recognized by farmers and collectors as signs of unhealthy plants in pastures during the two surveys. Browning was detected in the stele of the roots of experimental plants showing these symptoms and V. dahliae was consistently reisolated from the roots, stems and leaves of symptomatic plants.
The vascular wilt disease of C. arvense caused by V. dahliae develops relatively slowly. Symptoms first became apparent about one month after inoculation of experimental plants, with significant effects on shoot height, death of stems and leaves () and dry matter production of infected shoots () being detected after about 2 months. An increasing proportion of inoculated plants died over the following 3–4 months. By this time, while uninoculated plants also showed senescence-related yellowing, withering and abscission of leaves, inoculated plants had sustained far more damage. The surveys demonstrated that shoots of C. arvense in many pastures throughout New Zealand carry infections of V. dahliae in the spring, thus the progressive development of wilt disease during summer and autumn is likely to have a real impact on the vigour and invasiveness of populations of this weed.
The glasshouse experiments also demonstrated that V. dahliae is readily transmitted between cut shoots, particularly when moisture is applied during cutting (). Such transmission probably occurs by transfer of conidia present within the xylem vessels of infected shoots on to the surfaces of vascular elements of previously uninfected shoots in droplets of moisture on the cutting blades. Mowing has long been used to manage infestations of C. arvense in pastures in New Zealand (Mitchell & Abernethy Citation1995; Hurrell & Bourdôt Citation1996; Mitchell & Davis Citation1996) and it is likely that this has also become a means by which V. dahliae has been disseminated within stands. The apparent increase in efficacy of infection using moistened blades found in the present study () may suggest a possible explanation for the anecdotes of some New Zealand farmers that the spring regrowth of C. arvense stands is far less vigorous when mowing the previous year had been done during rainfall rather than in dry conditions. There is now direct support for this proposition from field experiments carried out on farms in the North and South Islands where the effects of different mowing regimes (‘not mown’, ‘dry’ and ‘during rainfall’) were evident in the percentage ground cover of the thistle in the following spring. Mowing in the rain achieved a 21%–32% greater reduction compared with mowing during dry weather (Bourdôt et al. Citation2011). It was not established in these experiments whether V. dahliae and/or other pathogenic microbes contributed to this effect.
Wilt disease caused by V. dahliae is of major economic importance for a diverse range of crops such as brassicas, cotton, pepper, potato, strawberry, tomato, watermelon, a number of fruit, nut and forest trees, and woody or herbaceous ornamentals (Bhat & Subbarao Citation1999). V. dahliae has been recorded on these and many other plant species in New Zealand (Landcare Citation2008) and on more than 700 plant species worldwide (Farr et al. Citation2011), some of which could become significant new hosts in New Zealand if the fungus were to spread to them.
The ability of V. dahliae to cause substantial losses to so many plant species is partly attributable to its ability to produce resistant ‘microsclerotia’ within infected tissue as plants senesce and die (Goud & Termorshuizen Citation2003). Microsclerotia eventually become incorporated into the soil and are the major form of inoculum infecting subsequent crops via their roots (Evans & Gleeson Citation1973; Olsson & Nordbring-Hertz Citation1985). Microsclerotia may live for up to 13 years when kept dry in the laboratory (Wilhelm Citation1955) but they are likely to die much sooner in field soil (Hawke & Lazarovits Citation1994) especially if a suitable host is absent. V. dahliae appears to maintain reservoirs of microsclerotial inoculum in rotations of non-susceptible species by associating with roots of ‘symptomless carriers’ such as grass species and dicotyledonous weeds (Thanassoulopoulos et al. Citation1981; Krikun & Bernier Citation1990). In New Zealand pastures, maintenance of a base level of microsclerotia in the soil may also enable infection and reinfection of C. arvense.
Verticillium wilt has never been considered to be a problem that threatens the productivity of desirable species in pastures. Grasses do not develop symptoms of verticillium wilt although their roots may be infected by the fungus (Thanassoulopoulos et al. Citation1981; Krikun & Bernier Citation1990). White clover (Trifolium repens) is not susceptible to verticillium wilt but red clover (Trifolium pratense) is (Milton & Isaac Citation1976; Skipp et al. Citation1986). The fungus has been found in the roots of red clover in New Zealand (Skipp & Christensen Citation1990) but only at sites where red clover had been grown as a monoculture for several years or where it had been grown in rotation with potato, a crop known to be very susceptible to V. dahliae (Viljanen-Rollinson & Anderson Citation2010). Forage brassicas, chicory and lucerne are hosts for V. dahliae and while wilt disease can be severe on brassicas and chicory (Ciccarese et al. Citation1987), it is the related species V. albo-atrum that is responsible for the important verticillium wilt disease of lucerne.
In the absence of a more efficient mode of transmission, it is likely that the reservoirs of inoculum, and the incidence of infection of C. arvense shoots by V. dahliae, remain reasonably constant between years. If V. dahliae were to be utilized for biological control (indirectly via mowing practices, or directly through inundative application as a bioherbicide), over time this could lead to an increase in levels of this highly plurivorous plant pathogenic fungus in the pasture environment. This may bring some added risk of adverse consequences. These might include effects on: 1) pasture production, persistence or botanical composition; 2) an adjacent crop of a susceptible species; or 3) susceptible species in a subsequent crop in a rotation involving a pasture in which the fungus has been used to control C. arvense. With respect to 1), adverse effects would be minimized by the natural resilience of pasture achieved by compensatory growth of competing species. Furthermore, pasture productivity would be expected to increase if the growth of C. arvense were curbed by vascular wilt. With respect to 2), risks to an adjacent crop susceptible to V. dahliae would be minimal because the fungus does not produce a profusion of conidia that are disseminated by wind or rain-splash. Conidia of Verticillium spp. are short-lived and do not readily infect uninjured plant shoot tissues (Jiménez Díaz & Millar Citation1986). With respect to 3), there may be a risk of greater disease levels in susceptible crop species associated with any increase in microsclerotial inoculum in the soil. This risk would need to be assessed as part of any programme evaluating the potential of V. dahliae as a bioherbicide for controlling C. arvense in pasture.
Conclusion
In this paper we have shown that pasture populations of C. arvense in New Zealand host a great variety of fungi in their subterranean root and stem tissues. Among these, V. dahliae may have the most potential for biological control of this troublesome weed. The capacity of V. dahliae for biological control may inadvertently be realized already as a contributing factor in the enhanced demise of C. arvense populations following mowing during rainfall. The wide geographic distribution of this pathogen within C. arvense populations and the ease with which infection can be spread among shoots by cutting with wet blades supports this possibility. With suitable safeguards for susceptible species in the vicinity, it may also be feasible to achieve more effective control by applying formulated preparations of conidia of the fungus during the wet mowing process. We conclude that the significance of V. dahliae as a pathogen of C. arvense has been underestimated and further research is likely to be rewarding.
Acknowledgements
We thank Meat & Wool New Zealand (now Beef + Lamb NZ) for funding this project under a pastoral industry levy scheme, the New Zealand Foundation for Research, Science and Technology for funding the underpinning population dynamics studies on Cirsium arvense (C10X0318 and C10X0811) and the farmers who helped us carry out the surveys.
References
- Alexander , SJ and Hall , R . 1974 . Verticillium wilt of chrysanthemum: anatomical observations on colonization of roots, stem, and leaves . Canadian Journal of Botany , 52 : 783 – 789 .
- Atallah , ZK , Bae , J , Jansky , SH , Rouse , DI and Stevenson , WR . 2007 . Multiplex real-time quantitative PCR to detect and quantify Verticillium dahliae colonization in potato lines that differ in response to verticillium wilt . Phytopathology , 97 : 865 – 872 .
- Bailey KL , Boyetchko SM , Derby J , Hall W , Sawchyn K , Nelson T , Johnson DR 2000 . Evaluation of fungal and bacterial agents for biological control of Canada thistle . In : Spencer NR Proceedings of the X International Symposium on Biological Control of Weeds , Bozeman , Montana , US , 4–14 July 1999 . Bozeman, Montana State University . Pp. 203 – 208 .
- Bailey , KL and Falk , S . 2011 . Turning research on microbial bioherbicides into commercial products—A Phoma story . Pest Technology , 5 ( Special Issue 1 ) : 73 – 79 .
- Berestetskiy , AO . 1997 . Mycobiota of creeping thistle and related species in the territory of the European part of Russia . Mikologiya i Fitopatologiya , 31 : 39 – 45 .
- Bhat , RG and Subbarao , KV . 1999 . Host range specificity in Verticillium dahliae . Phytopathology , 89 : 1218 – 1225 .
- Bithell , SL and Stewart , A . 2001 . Evaluation of the pathogenicity of Phoma exigua var. exigua on Californian thistle . New Zealand Plant Protection , 54 : 179 – 183 .
- Bourdôt , GW , Harvey , IC , Hurrell , GA and Saville , DJ . 1995 . Demographic and biomass production consequences of inundative treatment of Cirsium arvense with Sclerotinia sclerotiorum . Biocontrol Science and Technology , 5 : 11 – 25 .
- Bourdôt , GW , Hurrell , GA , Saville , DJ and Leathwick , DM . 2006 . Impacts of applied Sclerotinia sclerotiorum on the dynamics of a Cirsium arvense population . Weed Research , 46 : 61 – 72 .
- Bourdôt , GW , Hurrell , GA , Skipp , RA , Monk , J and Saville , D . 2011 . Mowing during rainfall enhances the control of Cirsium arvense . Biocontrol Science and Technology , 21 : 1213 – 1223 .
- Bourdôt GW , Leathwick DM , Hurrell GA , Saville DJ 1998 . Relationship between aerial shoot and root biomass in Californian thistle . In : O'Callaghan M Proceedings of the 51st New Zealand Plant Protection Conference . Pp. 28 – 32 .
- Bourdôt GW , Skipp RA , Hurrell GA , Saville DJ 2009 . A search for a root-pathogen of Cirsium arvense in New Zealand . In : Morales-Payan JP IXth International Bioherbicide Group Workshop . Pp. 13 – 15 .
- Ciccarese , F , Frisullo , S and Cirulli , M . 1987 . Severe outbreaks of Verticillium wilt on Cichorium intybus and Brassica rapa and pathogenic variations among isolates of Verticillium dahliae . Plant Disease , 71 : 1144 – 1145 .
- Cimmino , A , Andolfi , A , Berestetskiy , A and Evidente , A . 2008 . Production of phytotoxins by Phoma exigua var. exigua, a potential mycoherbicide against perennial thistles . Journal of Agricultural and Food Chemistry , 56 : 6304 – 6309 .
- Cripps , M , Gassmann , A , Fowler , SV , Bourdôt , GW , McClay , AS and Edwards , G . 2011 . Classical biological control of Cirsium arvense: lessons from the past . Biological Control , 57 : 165 – 174 .
- Demirci , E and Genc , T . 2009 . Vegetative compatibility groups of Verticillium dahliae isolates from weeds in potato fields . Journal of Plant Pathology , 91 : 671 – 676 .
- Dodd S , Ganley R , Bellgard S , Than D 2010 . Endophytes associated with Cirsium arvense and their influence on its biological control—POSTER ABSTRACT [D.01] . Proceedings of the International Mycological Congress 9—The Biology of Fungi .
- Donald , WW . 1990 . Management and control of Canada thistle (Cirsium arvense) . Review of Weed Science , 5 : 193 – 250 .
- Eschen , R , Hunt , S , Mykura , C , Gange , AC and Sutton , BC . 2010 . The foliar endophytic fungal community composition in Cirsium arvense is affected by mycorrhizal colonization and soil nutrient content . Fungal Biology , 114 : 991 – 998 .
- Evans , G and Gleeson , AC . 1973 . Observations on the origin and nature of Verticillium dahliae colonizing plant roots . Australian Journal of Biological Science , 26 : 151 – 161 .
- Evidente , A , Cimmino , A , Andolfi , A , Vurro , M , Zonno , MC and Mottao , A . 2008b . Phyllostoxin and phyllostin, bioactive metabolites produced by Phyllosticta cirsii, a potential mycoherbicide for Cirsium arvense biocontrol . Journal of Agricultural and Food Chemistry , 56 : 884 – 888 .
- Evidente , A , Cimmino , A , Berestetskiy , A , Andolfi , A and Motta , A . 2008a . Stagonolides G-I and modiolide A, nonenolides produced by Stagonospora cirsii, a potential mycoherbicide for Cirsium arvense . Journal of Natural Products , 71 : 1897 – 1901 .
- Farr DF , Rossman AY , Palm ME , McCray EB 2011 . Fungal databases . Systematic Mycology and Microbiology Laboratory, ARS, USDA. http://nt.ars-grin.gov/fungaldatabases/ (accessed 1 July 2011) .
- Gange , AC , Dey , S , Currie , AF and Sutton , BC . 2007 . Site- and species-specific differences in endophyte occurrence in two herbaceous plants . Journal of Ecology (Oxford) , 95 : 614 – 622 .
- Gannibal , PB and Berestetskiy , AO . 2008 . Species of the genus Alternaria in the mycobiota of Canada thistle (Cirsium arvense), their toxigenicity and pathogenicity . Mikologiya i Fitopatologiya , 42 : 110 – 118 .
- Gasich , EL and Berestetskiy , AO . 2006 . Studies on soil application of Stagonospora cirsii, a candidate for biological control of Cirsium arvense . Agronomy Research , 4 ( Special Issue ) : 171 – 175 .
- Gasich EL , Khlopunova LB , Levitin MM 2007 . Evaluation of the fungus Ascochyta sonchi for biological control of Cirsium arvense and Sonchus arvensis . 14th EWRS Symposium . Pp. 29 .
- Goud , JC and Termorshuizen , AJ . 2003 . Quality of methods to quantify microsclerotia of Verticillium dahliae in soil . European Journal of Plant Pathology , 109 : 523 – 534 .
- Goud , JKC , Termorshuizen , AJ and Gams , W . 2003 . Morphology of Verticillium dahliae and V. tricorpus on semi-selective media used for the detection of V. dahliae in soil . Mycological Research , 107 : 822 – 830 .
- Gronwald , JW , Plaisance , KL , Ide , DA and Wyse , DL . 2002 . Assessment of Pseudomonas syringae pv. tagetis as a biocontrol agent for Canada thistle . Weed Science , 50 : 397 – 404 .
- Hawke , MA and Lazarovits , G . 1994 . Production and manipulation of individual microsclerotia of Verticillium dahliae for use in studies of survival . Phytopathology , 84 : 883 – 890 .
- Henriksson , M . 1995 . Host plant range of Swedish isolates of Verticillium dahliae . Vaxtskyddsnotiser , 59 : 92 – 96 .
- Hershenhorn , J , Vurro , M , Zonno , MC , Stierle , A and Strobel , G . 1993 . Septoria cirsii, a potential biocontrol agent of Canada thistle and its phytotoxin-beta nitropropionic acid . Plant Science , 94 : 227 – 234 .
- Hurrell GA , Bourdôt GW 1996 . Sclerotinia sclerotiorum and mowing independently reduce Californian thistle in a sheep pasture . In : O'Callaghan M Proceedings of the 49th New Zealand Plant Protection Conference . Pp. 225 – 228 .
- Hurrell , GA , Bourdôt , GW and Saville , DJ . 2001 . Effect of application time on the efficacy of Sclerotinia sclerotiorum as a mycoherbicide for Cirsium arvense control in pasture . Biocontrol Science and Technology , 11 : 317 – 330 .
- Inderbitzin P , Bostock RM , Davis RM , T Ui , Platt HW , Subbarao KV 2011 . Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species . PLoS ONE 6 : e28341 . doi: 10.1371/journal.pone.0028341
- Jiménez Díaz , RM and Millar , RL . 1986 . Lack of systemic colonization of alfalfa plants after inoculation of uninjured leaves with conidia of Verticillium albo-atrum . Plant Disease , 70 : 509 – 515 .
- Kluth , S , Kruess , A and Tscharntke , T . 2005 . Effects of two pathogens on the performance of Cirsium arvense in a successional fallow . Weed Research , 45 : 261 – 269 .
- Krikun , J and Bernier , CC . 1990 . Morphology of microsclerotia of Verticillium dahliae in roots of gramineous plants . Canadian Journal of Plant Pathology , 12 : 439 – 441 .
- Landcare 2008 . Landcare research database of fungi and microorganisms . http://www.landcareresearch.co.nz/databases/ (accessed 1 July 2011) .
- Leth V , Haas H 1984 . The potential for biological weed control in Denmark . First Danish Conference on Plant Protection: Weed, Statens Planteavlsforsoeg . Plantevaernscentret , Lyngby , , Denmark , Institut for Ukrudtsbekaempelse , Slagelse, Denmark . Pp. 221 – 244 .
- Leth , V , Netland , J and Andreasen , C . 2008 . Phomopsis cirsii: a potential biocontrol agent of Cirsium arvense . Weed Research , 48 : 1 – 9 .
- LINZ 2008 . Topographic maps . http://www.linz.govt.nz/core/topography/topographicmaps/index.html (accessed 1 July 2011) .
- McClay , AS , Bourchier , RS , Butts , RA and Peschken , DP . 2001 . “ Cirsium arvense (L.) Scopoli, Canada thistle (Asteraceae) ” . In Biological control programmes in Canada, 1981–2000 , Edited by: Mason , PG and Huber , JT . 318 – 330 . Wallingford , , UK : CABI Publishing .
- MFE 2005 . The land cover classes . http://www.mfe.govt.nz/issues/land/land-cover-dbase/classes.html (accessed 1 July 2011) .
- Milton , JM and Isaac , I . 1976 . Verticillium wilt of clover . Plant Pathology , 25 : 119 – 121 .
- Mitchell RB , Abernethy RJ 1995 . The effect of topping and repeat grazings on Californian thistle and pasture production . In : Popay AJ Proceedings of the 48th New Zealand Plant Protection Conference Pp. 189 – 193 .
- Mitchell RB , Davis LT 1996 . Effect of control treatments on the root reserves of Californian thistle (Cirsium arvense) . Proceedings of the 49th New Zealand Plant Protection Conference . Pp. 229 – 233 .
- Müller , E and Nentwig , W . 2011 . Plant pathogens as biocontrol agents of Cirsium arvense—an overestimated approach? . NeoBiota , 11 : 1 – 24 .
- Olsson , S and Nordbring-Hertz , B . 1985 . Microsclerotial germination of Verticillium dahliae as affected by rape rhizosphere . FEMS Microbiology Ecology , 31 : 293 – 299 .
- Popay , AI and Cheah , LH . 1990 . “ Californian thistle (Cirsium arvense)—a suitable case for mycoherbicides ” . In FRI Bulletin 155 , Edited by: Bassett , C , Whitehouse , LJ and Zabkiewicz , JA . 93 – 95 . Rotorua , , New Zealand : Ministry of Forestry .
- Qin , QM , Vallad , GE , Wu , BM and Subbarao , KV . 2006 . Phylogenetic analyses of phytopathogenic isolates of Verticillium spp . Phytopathology , 96 : 582 – 592 .
- Ratajkiewicz , H , Karolewski , Z and Werner , M . 2009 . Assessment of potential application of Canada thistle (Cirsium arvense [L.] Scop.) pathogens in biological control of this weed . Progress in Plant Protection , 49 : 878 – 882 .
- Schulz , B , Guske , S , Dammann , U and Boyle , C . 1998 . Endophyte-host interactions. II. Defining symbiosis of the endophyte-host interaction . Symbiosis (Rehovot) , 25 : 213 – 227 .
- Skinner , K , Smith , L and Rice , P . 2009 . Using noxious weed lists to prioritize targets for developing weed management strategies . Weed Science , 48 : 640 – 644 .
- Skipp , RA and Christensen , MJ . 1990 . Selection for persistence in red clover: Influence of root disease and stem nematode . New Zealand Journal of Agricultural Research , 33 : 319 – 333 .
- Skipp , RA , Christensen , MJ and Nan , ZB . 1986 . Invasion of red clover (Trifolium pratense) roots by soilborne fungi . New Zealand Journal of Agricultural Research , 29 : 305 – 313 .
- Skipp , R and Watson , R . 1996 . “ Disease complexes in New Zealand pastures ” . In Pasture and forage crop pathology , Edited by: Chakraborty , S , Leath , K , Skipp , R , Pederson , G , Bray , R , Latch , G and Nutter , F . 429 – 451 . Madison , Wisconsin : American Society of Agronomy, Crop Science Society of America, Soil Science Society of America .
- Smith , HC . 1965 . The morphology of Verticillium albo-atrum, V. dahliae, and V. tricorpus . New Zealand Journal of Agricultural Research , 8 : 450 – 478 .
- Terralink 2009 . Land cover database of New Zealand . http://www.terralink.co.nz/products_services/satellite/_cover_database_of_new_zealand/index.htm (accessed 15 February 2012) .
- Thanassoulopoulos , CC , Biris , DA and Tjamos , EC . 1981 . Weed hosts as inoculum source of Verticillium in olive orchards . Phytopathologia Mediterranea , 20 : 164 – 168 .
- Viljanen-Rollinson S , Anderson J 2010 . Potato early dying (Verticillium wilt)—Crop and Food Research Broadsheet 126 .
- Voelker , K and Boyle , C . 1994 . Bean rust as a model system to evaluate efficiency of teliospore induction, especially in the potential mycoherbicide Puccinia punctiformis . Weed Research , 34 : 275 – 281 .
- Waipara N , Bourdôt G , Harvey I 1991 . Microbial control of Cirsium arvense (L.) Scop . (Californian thistle)—Occurrence and pathogenicity of some disease-causing organisms in New Zealand . MAF Technology Report , April 1991 . 40 p.
- Waipara , NW . 2003 . “ Evaluation of Phoma exigua var. exigua as a biocontrol against Californian thistle (Cirsium arvense) ” . In Workshop on biocontrol of weeds with pathogens , Edited by: Bourdôt , G and Lamoureaux , S . 31 – 32 . Lincoln , , New Zealand : Canterbury Agriculture and Science Centre .
- Wilhelm , S . 1955 . Longevity of the Verticillium wilt fungus in the laboratory and in the field . Phytopathology , 45 : 180 – 181 .
- Wright , DSC . 1968 . Verticillium wilt of tobacco VIII. Movement of conidia of Verticillium dahliae Kleb. in tobacco plants . New Zealand Journal of Agricultural Research , 11 : 803 – 811 .
- Zhang , W , Sulz , M , Mykitiek , T , Li , X , Yanke , LJ , Kong , HS , Buyer , JS and Lydon , J . 2004 . “ A Canadian strain of Pseudomonas syringae causes white-colour disease of Cirsium arvense (Canada thistle) ” . In Proceedings of the XI International Symposium on Biological Control of Weeds , Edited by: Cullen , JM , Briese , DT , Kriticos , DJ , Lonsdale , WM , Morin , L and Scott , JK . 215 – 220 . Canberra , , Australia : CSIRO Entomology .
