Abstract
Biofortification—with either the application of zinc and iron fertilizer or the application of amendments to increase their bioavailability in soil—is a possible strategy to tackle worldwide micronutrient malnutrition. We investigated the effect of hydrolysed wool on the uptake of zinc and iron by wheat (Triticum aestivum var. Greina). We performed pot experiments in which either hydrolysed wool or mineral fertilizer of the same elemental composition was incorporated into a loamy-sand collected from an agricultural field. Zinc grain concentrations were 37.7 mg kg−1 (control), 45.5 mg kg−1 (mineral fertilization) and 54.1 mg kg−1 (hydrolysed wool). In addition, hydrolysed wool application increased grain yield 2-fold and grain protein content 1.5-fold, compared with 1.4-fold and 1.3-fold, respectively, by the mineral fertilizer. We propose that hydrolysed wool could be used to supplement other fertilizers, enhancing the latter with an easily available N source as well as promoting zinc and iron uptake in plants.
Introduction
Zinc (Zn) and iron (Fe) deficiency are widespread micronutrient deficiencies in crop plants, animals and humans. FootnoteZn deficiency has adverse effects on human health, such as stunted growth, weakened immune system, increased morbidity and increased mortality (Hotz & Brown Citation2004; Black et al. Citation2008). Nearly half of the cereal-growing land in the world is affected by low soil Zn content and/or availability of soil Zn to plant roots, due to various adverse chemical and physical soil conditions, such as high pH, low organic matter turnover, water-logging or drought (Cakmak Citation2008). Nutritional iron deficiency is estimated to still affect 1.5–2 billion people worldwide (Lynch Citation2011). Iron deficiency impairs physical growth, mental development and learning capacity in children, reduces productivity in adults and represents the most common cause of anaemia (Kutman et al. Citation2011). Wheat grains are the most important food staple globally, but wheat is inherently too low in easily absorbable Zn and Fe to supply the recommended dietary intake of these micronutrients (Cakmak et al. Citation2010).
One strategy for combating micronutrient malnutrition is to ‘biofortify’ plant-based food through increased accumulation of critical elements in the edible parts of crop plants (Bouis Citation1996; Welch & Graham Citation1999; Frossard et al. Citation2000; Welch Citation2002). Generally, it is not the lack of those elements in soil that is the problem, but their insufficient availability for plant uptake (Cakmak Citation2008). Thus, one approach to ‘biofortify’ plant-based food with Zn and Fe is to enhance the bioavailability of the elements in soils e.g. by decreasing soil pH, addition of fertilizers or amendments that enhance their phytoavailability (Rengel et al. Citation1999; Frossard et al. Citation2000; Welch Citation2002). Zinc fertilization, e.g. applied as zinc sulphate (ZnSO4), is an effective method to increase Zn plant uptake, but its effect is often of short duration, especially on calcareous soils (Marschner Citation1993). Similarly, decreasing pH, e.g. by addition of elemental sulphur (S), has limited scope on well-buffered calcareous soils (Sims Citation1986; Kayser et al. Citation2000). In addition, acidification can reduce plant growth and the bioavailability of some plant nutrients. As an alternative, the application of organic Zn and Fe complexes or of solubilizing organic ligands may be considered (Nowack et al. Citation2008). The amendment of soils, in order to solubilize and desorb trace elements from the soil matrix, with synthetic chelating agents is very expensive (Cakmak Citation2008), and in case of low biodegradability, as for EDTA, it is also questionable from an environmental point of view (Evangelou et al. Citation2007). The application of biodegradable amendments could be more promising (Evangelou Citation2007; Evangelou et al. Citation2008). In particular, amino acids, peptides and proteins have the ability to complexate metals such as Zn and Fe. In fact, they play important roles in the uptake, transport and storage of these elements in plants (White & Broadley Citation2009). In addition, amino acids and peptides contain easily available nitrogen (N) that could positively affect grain yields as well as increasing protein content which would also positively affect baking quality. Rich sources of amino acids and peptides are manure or mammal hairs, such as sheep wool or even human hair, which has a protein content of more than 90% (Zahn et al. Citation2000). Manure has high contents of microbially-bound faecal proteins, but low contents of amino acids and oligo-peptides, which is considered the reason for the low transfer of N from manure to crops (Bosshard et al. Citation2011). From sheep wool, amino acids and oligo-peptides can be obtained rapidly by non-specific enzymatic hydrolysis (Evangelou et al. Citation2008). Sheep wool is also a source of Zn itself and may contain up to 195 mg of Zn kg−1 (Scott Citation1991). In New Zealand, concentrations from 80 to 300 mg kg−1 have been measured and may vary due to changes in Zn nutrition as well as the application of zinc oxide (ZnO) as an oral drench to prevent the occurrence of facial eczema (Grace & Lee Citation1992). Compared with other mammal hair, sheep wool has the advantage of being cheap and available in abundant quantities. In addition to biofortification and fertilization, the use of sheep wool as a soil amendment would also serve to recycle Zn and N from wool wastes and byproducts of wool production in a cost effective and environmentally beneficial way.
The aims of the present study were to assess the suitability of hydrolysed wool to (i) act as a Zn and Fe fertilizer by applying these elements in a form that increases their bioavailable pool in the soil and (ii) to biofortify wheat plants by improving grain Zn and Fe concentrations. In addition, economic aspects on the potential applicability of hydrolysed wool in the field are discussed.
Materials and methods
Soil, plant and wool material
The experimental soil was a loamy sand, taken from the topsoil (0–20 cm) of an agricultural field near the town of Lalden, located in the upper Rhône valley in Switzerland (Canton Wallis). The soil was air dried at 40 °C, sieved to 2 mm and then thoroughly mixed. General soil properties are given in . Wheat seeds (Triticum aestivum var. Greina) were purchased from Delley Samen und Pflanzen AG (CH-Delley). The seeds were surface-sterilized in 10% H2O2 for 10 min, germinated for 3 d in the dark on water-presoaked filter papers and then exposed to ambient light for 1 d before planting. All plant experiments were carried out at 60% humidity in a climate chamber with a 16 h light period (light intensity of max. 20,000 lux) and a 22/15 °C light/dark temperature regime.
Table 1 Selected properties and trace element concentrations of the soil used in the experiments.
Wool hydrolysis
Wool hydrolysates (WH) were prepared from Merino sheep wool fibres obtained from Die Wollfabrik GmbH, Mönchengladbach (Germany). In a 1000 mL Schott-Bottle with a Teflon cap, 20 g of wool was incubated with 1000 mL of 1 M NaHSO3 for 30 min at 60 °C to reduce disulphide bonds to thiol groups (Nolte et al. Citation1996). After incubation, the NaHSO3 solution was discarded and the wool was washed for 5 min with 500 mL of a 0.1 M NaHCO3 solution, which was then replaced by 500 mL of 0.01 M NaHCO3. The final wool concentration was 40 g L−1. A commercially available thermo-stable and alkali-resistant protease from Bacillus sp. (Esperase 8.0, 8 Ug−1, Sigma-Aldrich, D-Steinheim) was used to hydrolyse the fibres due to its high proteolytic activity on wool. The reaction was started with the addition of 4 mL protease and carried out for 5 d at a temperature of 60 °C. At that point no further reaction was visible. As an indicator of an ongoing reaction, the decrease of wool remnants on the bottom of the bottle was chosen. The WH properties and trace element (TE) concentrations are shown in .
Table 2 Composition (in mg L−1 WH and mg kg−1 of wool) and pH of wool hydrolysate used in the experiments (n=3).
Experimental setup
We investigated the short-term Zn and Fe mobilization potential of WH for different application rates (0–300 mL WH kg−1 soil). To achieve these rates, 20 g aliquots of dry, sieved soil were weighed into 100 mL Erlenmeyer flasks and mixed with 8 mL (corresponding to maximum water holding capacity, ) of the following mixtures of WH and Millipore water: 0 +8 (corresponding to 0 mL WH kg−1 soil), 1+7 (50 mL WH kg−1 soil), 2+6 (100 mL WH kg−1 soil), 3+5 (150 mL WH kg−1 soil), 4+4 (200 mL WH kg−1 soil), 6+2 (300 mL WH kg−1 soil). All treatments were performed in triplicate. The mixtures were incubated for 2 h at room temperature. After drying at 40 °C, 10 g samples were suspended in 25 mL 0.1 M NaNO3 and shaken for 2 h (FAL et al. Citation1996). After filtration, TE concentrations were determined in the extracts by means of ICP-OES (Varian, Vista-MPX CCS simultaneous). For quality assurance, we analysed two WEPAL (Wageningen Evaluating Programmes for Analytical Laboratories) referenced soils (Wageningen, Netherlands, no. 989). Recoveries for Zn and Fe were >90%. The WH solution in our experiments contained approximately 2 mg Zn L−1 and 0.23 mg Fe L−1. To estimate the mobilization potential of the WH, we subtracted the amounts of TE that were added to the soil with the different WH:water mixtures, resulting in the TE concentration that would be expected due to TE-free WH addition. The slope of the resulting plot indicated the mobilization potential for the respective TE.
Finally, we investigated the effect of WH application on grain yield, Zn and Fe accumulation and protein content. Prior to this experiment, a preliminary pot experiment was conducted to assess possible phytotoxic effects of WH. Application rates up to 75 mL kg−1 of soil—which equals 3 g of hydrolysed wool per kg of dry soil—were tested. No toxic effects were observed.
In the main experiment, a WH treatment with an according application rate was compared with a mineral fertilizer (MF) treatment in which we added a solution with the same elemental composition, but containing and
as the N source instead of amino acids and peptides. We filled 1000 g of dry and sieved soil into 1000 mL plastic pots. The area of each pot was 133 cm2. Four wheat seedlings were planted per pot. When the plants were 27 d old, one of the following solutions were applied at a rate of 75 mL once per week: deionized water as a control treatment (C), concentrated WH solution (WH treatment) and a mineral fertilizer (MF) treatment with a pH of 7.43 containing sodium (Na) (230 mg L−1), calcium (Ca) (17.5 mg L−1), potassium (K) (12 mg L−1), Fe (0.23 mg L−1), Zn (2 mg L−1) and N (2 g L−1) (N and cation concentrations as in concentrated WH, see ). Nitrogen was supplied as (NH4)2SO4, Ca as Ca(NO3)2, K as KNO3, Fe as FeSO4 and Zn as ZnSO4. Sulphur supply was covered by the salts applied for N, Fe and Zn. All treatments were applied in four replicates for duration of 4 weeks. Plants were harvested 63 d after the last application of the solutions (final age of the plants: 90 d) by cutting the shoots below 1 cm above ground. Grains were then separated from the shoot and both were rinsed with deionized water, dried between paper tissues, oven-dried at 60 °C until constant weight was achieved, and weighed. The dry material was ground using a ZM200 Titanium mill (Retsch GmbH, D-Haan) and a MM200 Mixer mill (Retsch GmbH, D-Haan). Then 0.2 g subsamples were taken and digested in 15 mL of HNO3 (65%) for 1 h at 120 oC. The extracts were diluted to 25 mL with H2O and analysed for TE by means of ICP-OES (Varian, Vista-MPX CCS simultaneous). For quality assurance, a certified reference plant sample of Virginia tobacco leaves (CTA-VTL-2) was analysed with each batch of extracts. We obtained recoveries of >90% for Zn and 80%–90% for Fe. The total nitrogen content of the grain samples was determined after dry combustion using a CNS-2000 Analyzer (Leco, US-Saint Joseph, Michigan). The grain protein contents were determined using the N:protein conversion factors given by Mosse (Citation1990).
Statistical analysis
Treatment effects were analysed using one-way analysis of variance (ANOVA). Differences at the P<0.05 level were considered statistically significant.
Results
Soil metal mobilization by addition of WH
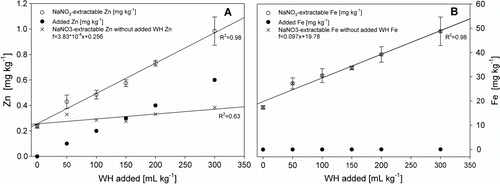
There were linear relationships between WH dosage and NaNO3-extractable soil Zn and Fe concentrations, as shown in Figs. 1A and B, respectively. Assuming that the entire TE input due to WH addition remained soluble, then the difference between the determined NaNO3 extractable TE concentration (, ○) and the calculated TE increase due to WH input (, •) gives the TE concentration that would be expected to have resulted from TE-free WH addition (, ×). Thus, the slope of the latter indicates the mobilization potential for the respective TE. For Fe, the determined concentration and the calculated concentration resulting from addition of Fe free WH is identical, indicating that the increase of soluble Fe is attributable nearly solely to the mobilization potential of the WH. For Zn, the mobilization potential of the WH contributed only slightly to the increase up to an addition of 150 mL kg−1. With higher additions, the Zn added with the WH played the major part. After addition of 50 mL kg−1 WH, the NaNO3 extractable Zn concentration was almost doubled from 0.24 to 0.43 mg kg−1, whereas the NaNO3 extractable Fe concentration increased only 1.5 fold.
Figure 1 Relationships between added amount of WH per kg soil and NaNO3 extractable soil Zn (A, ○) and Fe (B, ○). • corresponds to the amount of TE added to the soil with the WH solution per kg of soil.×represents the TE concentration that would be expected to result from TE-free WH addition. Error bars represent±standard deviation of triplicates (n=3).
Pot experiment
Shoot and grain yields
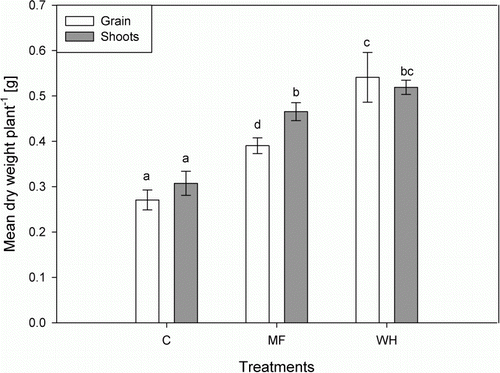
Grain yields doubled in the WH treatment (0.54±0.05 g plant−1) compared with the controls (0.27±0.02 g plant−1). Compared with the MF treatment (0.39±0.02 g plant−1) the yield in the WH treatment was also significantly (P<0.05) higher and increased approximately 1.4 fold (). Similar effects were observed in the shoots: the largest biomass was produced by plants grown in the WH treatment (0.51±0.02 g plant−1), being significantly higher (P<0.05) than the MF treatments (0.47±0.02 g plant−1). The controls reached 0.31±0.03 g plant−1. The harvest index (grain:grain+shoot ratio) of the WH treated plants was higher (0.51) compared with that of the MF treated plants (0.45). Furthermore, it was observed that the plants treated with MF or WH solution formed grains later than the control plants.
Figure 2 Mean grain versus shoot dry weight per plant of the controls (C), a treatment with the same elemental composition (MF) as in the WH and the WH treatment. Different letters indicate statistically significant difference (P<0.05). Error bars represent±standard deviation of four replicates (n=4).
Zn and Fe concentrations in grains and shoots
Grain Zn concentrations increased approximately 1.4 fold, after WH treatment (54.1±3.3 mg kg−1), compared with the controls (37.7±3.6 mg kg−1) (A). Furthermore, Zn grain concentrations in the WH treatment were significantly higher (P<0.05) than in the MF treatment (45.5±1.7 mg kg−1). Iron grain concentrations increased approximately 1.6 fold, after WH treatment (36.6±2.5 mg kg−1), compared with the controls (22.7±2.2 mg kg−1). Concerning Fe, WH and MF treatments were not significantly different.
Figure 3 Mean Zn and Fe concentrations in grains (A) and shoots (B) of the controls (C), a treatment with the same elemental composition (MF) as in the WH and the WH treatment. Different letters indicate statistically significant difference (P<0.05). Error bars represent±standard deviation of four replicates (n=4).
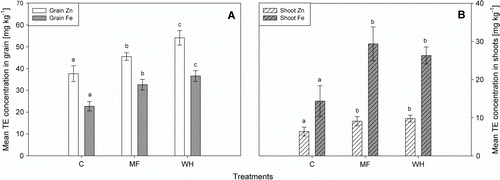
Zinc shoot concentrations were highest after application of concentrated WH (9.8±0.9 mg kg−1), followed by the MF treatment (9.1±1.2 mg kg−1). The two treatments were not significantly different from each other (P>0.05). The shoots of the controls reached a Zn concentration of 6.4±1.2 mg kg−1 Zn. Zinc harvest index (grain Zn uptake:biomass Zn uptake) was highest in the WH treatment (0.43), followed by the control (0.4) and the MF treatment (0.38). The Fe harvest index followed a similar pattern: WH (0.3)>controls (0.29)>MF (0.24). Calcium, K, magnesium (Mg), manganese (Mn) and S shoot and grain concentrations did not show any significant differences between the two treatments MF and WH (S). To exclude a pH dependent effect, the pH value (0.01 M CaCl2) of the respective soils was determined after the experiment, showing no significant difference between the treatments and the control. The pH value increased negligibly from 7.14 (C) to 7.19 (WH). The average pH of the MF treatments was 7.13.
Grain protein contents
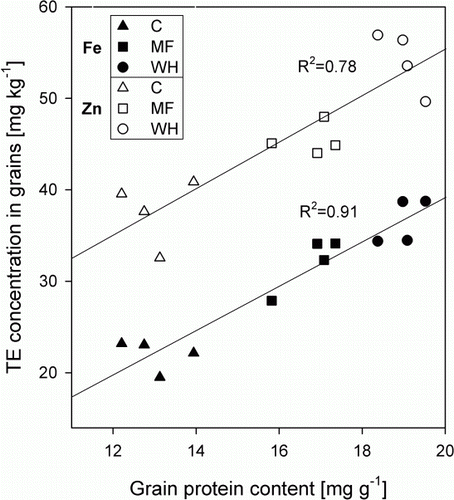
The N-concentration increased from 2.4% in the controls to 3.1% in the MF treatments and 3.5% in the WH treatments. The calculated grain protein contents of the WH treated plants were significantly higher than in the MF plants and the controls (P<0.05), reaching an average of 19%. This is approximately a 1.5-fold increase in protein content relative to the controls. Also, the grain protein content in the WH treatments surpassed the treatment containing inorganic nitrogen (MF) significantly (P<0.05, ). Grain Zn and Fe concentrations and grain protein content of the grains were positively correlated (R2=0.78 and 0.91, respectively ).
Figure 4 Relationship between Zn (○) and Fe (•) concentrations and protein content in the grains, determined using a N:protein conversion factor (Mosse Citation1990).
Discussion
Wool hydrolysate as a fertilizer
The WH had a strong fertilizer effect. Although the macro- and micronutrient amounts applied were approximately the same in WH and MF, the grain and shoot biomass as well as the N concentration of the grains was significantly higher in the WH treatments. A possible cause for this effect could lie in the form in which N is applied as a fertilizer and its subsequent phytoavailability. Bosshard et al. (Citation2011) showed the importance of the form of N for the uptake by plants, when applied as a fertilizer. The authors conducted an incubation experiment to assess N release from faeces and its subsequent uptake by plants. They concluded that a low content in and amino acids, a low rate of N release from faeces during incubation and a relatively high faecal protein content, particularly the hard to mineralize undigested and microbially bound forms, were responsible for the low transfer of N from faeces to crops. In our experiment, N was applied as WH (amino acids and peptides) and as MF (
and
). At the time of incorporation into the soil, the major part of the WH-N was in form of amino acids as well as di- and tripeptides (determined via Electrospray Ionization-Quadrupole Time-of-Flight Mass Spectrometry (ESI-QTOF-MS), S). In soils, amino acids are known to be transformed into
and
(Jan et al. Citation2009). While directly applied inorganic N can be lost due to nitrification (leaching) or denitrification, WH could act as a slow-release fertilizer for N, defined by its mineralization kinetics. Besides, different plants within a community may have different preferences for organic and inorganic N forms. Nashölm et al. (Citation2001) showed that at least 91%, 64% and 42% of N applied as glycine was also taken up as glycine by the dwarf shrub Vaccinium myrtillus, the grass Deschampsia flexuosa and the trees Pinus sylvestris and Picea abies, respectively. Some plants can even take up peptides as the sole N source (Schmidt et al. Citation2003). Concerning these options, the reason for the higher uptake of N by plants treated with WH could be that either amino acids are directly taken up by wheat, that slower release of N through WH-mineralization sustains availability of N in comparison to inorganic N, or a combination of both. Clarification of these mechanisms should be the objective of future work and could be achieved e.g. through measuring potassium chloride-extractable
and
in the system over time.
Zinc and Fe biofortification of wheat
Applying micronutrients directly by fertilization and increasing their phytoavailability in soil by mobilizing amendments are two common strategies of biofortification. Application of WH combines both. Zinc was applied as a fertilizer together with the WH; in addition the WH mobilized native soil Zn to a lesser and Fe to a greater extent. Sheep wool is a source of Zn and Fe, which are complexed in the various protein chains of the wool surface and also enclosed in the fibres (Grace & Lee Citation1992). Some amino acids such as histidine play an important role in the uptake of Zn and Fe by plants. In addition, amino acids and peptides can mobilize TE in soil (Evangelou et al. Citation2008). Therefore, through the application of hydrolysed wool, it is likely that Zn and Fe were applied to the soil in a complexed and phytoavailable form. These Zn- and Fe-amino acid complexes may enter the root and be taken up by the plant, just as in the case of Zn-phytosiderophore complexes which have been shown to be partly taken up as entire complexes (vonWiren et al. Citation1996; White & Broadley Citation2009). In our experiment, WH-induced mobilization was inferred in the case of Fe, whereas the effect was small for Zn. In contrast to Fe, the supply of Zn with the WH played a much greater role than the mobilization of native soil Zn for the enrichment of the wheat plants in our study with Zn.
Grain nitrogen status and Zn and Fe biofortification
The N status of plants is a critical factor in agronomic biofortification of wheat with Zn and Fe (Cakmak et al. Citation2010; Kutman et al. Citation2010; Shi et al. Citation2010). The combined application of N and Zn fertilizer has a synergistic effect on grain Zn concentration (Kutman et al. Citation2011). Nitrogen application and N availability also positively affects wheat grain protein concentration (Dupont et al. Citation2006). Cakmak et al. (Citation2010) found that grain Zn and Fe concentrations were strongly correlated with grain protein. Very low levels of grain protein have been suggested as a major reason for low concentrations of Zn and Fe in grains. Uauy et al. (Citation2006) hypothesized that the genes affecting the accumulations of Zn, Fe and protein in grains are closely linked and that an improved N nutritional status of wheat may enhance the abundance of transporter proteins and nitrogenous chelators involved in the uptake, translocation, remobilization and grain allocation of Zn and Fe. In our study, WH application resulted in a higher grain protein concentration, which correlated positively with Zn and Fe grain concentration. As in the study of Cakmak et al. (Citation2010), grain proteins could represent a sink for Zn and Fe. A further benefit of the high grain protein concentrations is that it could contribute to higher bioavailability of micronutrients in the diet, as shown by House et al. (Citation1996) and Lönnerdal (Citation2000).
Feasibility analysis
For using hydrolysed wool as a fertilizer, costs will be a critical factor. The rate of 3 g of wool per kg of soil applied in this study is equivalent to a rate of more than 2 t ha−1. Worldwide, the price of coarse wool has almost tripled since 2008, rising from US$0.43 kg−1 to US$1.207 kg−1. This trend has two reasons: the first reason being the falling supply due to i) very low prices for wool over the decades that have made wool production unprofitable and b) the impact that the 2011 floods had on wool production in the world's largest wool-producing nation (Australia). The second reason is the rising demand for expensive wool suits in emerging markets such as China and India. However, it is important to note that with regard to the use of hydrolysed wool as a soil amendment, wool quality does not play an important role. On the market there are other wool wastes that can be utilized. In Switzerland, for example, more than 400 tons of wool are burned as waste every year (Meschenmoser Citation2001). Some wastes could be purchased for approximately US$0.4 kg−1. The prices of these wool wastes are rising, however, as they can be used in the insulation of homes, packaging materials, etc. Nevertheless, although the prices are high at the moment, it is very likely that wool production will follow the phenomenon of the pork cycle/cattle cycle, thus falling again in the near future. In addition, human hair could be used as a supplement or alternative to wool. It is comparable in its biochemical properties to sheep wool (Zahn Citation1989).
The protease enzyme used to hydrolyse the wool is quite inexpensive. The cost is approximately US$2.2 kg−1 for protease with an activity of 50,000 U g−1 (Zhao Dong Sun Shine Enzyme Co, China, via email). At a WH application rate of 2 t ha−1 protease costs would be approximately US$157 ha−1 and for the wool itself US$800 ha−1, when calculated with the rate of US$0.4 kg−1. When expressed as costs per kg of N, at the moment WH is 14 times more expensive than urea. The cost of urea dropped from US$1.7 per kg N in 2008 to US$0.8 per kg N in 2012 (Barrientos & Soria Citation2013). Preparing hydrolysed wool with a nitrogen content of about 4% () would result in costs of US$10.8 per kg−1 N.
In spite of these currently high costs, one has to assess the life cycle of a product in order to consider its environmental impact. The production of urea in the Middle East consumes 23.93 MJ kg−1 and in China 33.08 MJ kg−1 (Ledgard et al. Citation2011). Energy consumption for wool production ranges between 8 MJ kg−1 for greasy wool and 38 MJ kg−1 for fine wool, which includes scouring, drying and combing (Barber & Pellow Citation2006). For the production of WH, the use of greasy wool or even wool wasted would be sufficient, thus making the use of wool as a fertilizer more environment friendly than urea. Furthermore, WH supplies additional nutrients as well as N, and it could be produced and used locally where it is needed due to its easy production process. In addition, the distance from the place of production to the place of use would then be small, saving additional transport and packaging costs.
Conclusions
With hydrolysed wool we present an alternative amendment for biofortification of Zn and Fe in wheat that combines the aspects of a slow release N fertilizer as well as a micronutrient fertilizer. This study has shown that WH is capable of increasing grain yield, grain protein content and grain Zn and Fe concentrations in wheat. We propose that hydrolysed wool could be used as an additive to common fertilizer products, enhancing the latter with an easily available N source and promoting Zn and Fe plant uptake. The N accumulation mechanism of WH compared with MF is not clear, therefore future work would be worthwhile to focus on the availability and the persistence of N in the system.
Supplementary data
Supplementary file 1: Table S1. ESI-QTOF mass spectrum of a WH solution employed in the experiments. The crossed out signals correspond to internal standards.
Supplementary file 2: Table S2. Metal concentrations (mean values±standard deviation) in grains and shoots (n=4). Different letters indicate statistically significant difference (P<0.05).
Table S1. ESI-QTOF mass spectrum of a WH solution employed in the experiments. The crossed out signals correspond to internal standards.
Download JPEG Image (2 MB)Table S2. Metal concentrations (mean values ± standard deviation) in grains and shoots (n=4). Different letters indicate statistically significant difference (P < 0.05).
Download MS Word (35.5 KB)Acknowledgements
We would like to acknowledge Dr Andrea Körner, Björn Studer and Fabio Ugolini for their help during the experiments and the MS-Service at the Laboratory of Organic Chemistry at ETH Zurich for the ESI-QTOF-MS measurements. Furthermore, we would like to thank the IDEA League for supporting this study financially.
Notes
Supplementary data available online at www.tandfonline.com/10.1080/00288233.2013.775165
Supplementary file 1: Table S1. ESI-QTOF mass spectrum of a WH solution employed in the experiments. The crossed out signals correspond to internal standards; Supplementary file 2: Table S2. Metal concentrations (mean values±standard deviation) in grains and shoots (n=4). Different letters indicate statistically significant difference (P<0.05).
References
- Barber , A and Pellow , G . 2006 . Life cycle assessment: New Zealand merino industry—Merino wool total energy use and carbon dioxide emissions , 19 – 20 . Pukekohe , Auckland : The AgriBusiness Group .
- Barrientos M , Soria C 2013 . Indexmundi—urea monthly price—US dollars per metric ton . http://www.indexmundi.com/commodities/?commodity=urea&months=120 (accessed 24 January 2013) .
- Black , RE , Allen , LH , Bhutta , ZA , Caulfield , LE , de Onis , M , Ezzati , M , Mathers , C and Rivera , J . 2008 . Maternal and child undernutrition 1—Maternal and child undernutrition: Global and regional exposures and health consequences . Lancet , 371 : 243 – 260 . doi: 10.1016/S0140-6736(07)61690-0
- Bosshard , C , Oberson , A , Leinweber , P , Jandl , G , Knicker , H , Wettstein , HR , Kreuzer , M and Frossard , E . 2011 . Characterization of fecal nitrogen forms produced by a sheep fed with (15)N labeled ryegrass . Nutrient Cycling in Agroecosystems , 90 : 355 – 368 . doi: 10.1007/s10705-011-9437-1
- Bouis , H . 1996 . Enrichment of food staples through plant breeding: A new strategy for fighting micronutrient malnutrition . Nutrition Reviews , 54 : 131 – 137 . doi: 10.1111/j.1753-4887.1996.tb03915.x
- Cakmak , I . 2008 . Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? . Plant and Soil , 302 : 1 – 17 . doi: 10.1007/s11104-007-9466-3
- Cakmak , I , Pfeiffer , WH and McClafferty , B . 2010 . Biofortification of durum wheat with zinc and iron . Cereal Chemistry , 87 : 10 – 20 . doi: 10.1094/CCHEM-87-1-0010
- Dupont , FM , Hurkman , WJ , Vensel , WH , Tanaka , C , Kothari , KM , Chung , OK and Altenbach , SB . 2006 . Protein accumulation and composition in wheat grains: Effects of mineral nutrients and high temperature . European Journal of Agronomy , 25 : 96 – 107 . doi: 10.1016/j.eja.2006.04.003
- Evangelou , MWH . 2007 . Biochelators as an alternative to EDTA and other synthetic chelators for the phytoextraction of heavy metals (Cu, Cd, Pb) from soil. Fakultät für Mathematik, Informatik und Naturwissenschaften , Aachen : RWTH Aachen University .
- Evangelou , MWH , Ebel , M , Koerner , A and Schaeffer , A . 2008 . Hydrolysed wool: A novel chelating agent for metal chelant-assisted phytoextraction from soil . Chemosphere , 72 : 525 – 531 . doi: 10.1016/j.chemosphere.2008.03.063
- Evangelou , MWH , Ebel , M and Schaeffer , A . 2007 . Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents . Chemosphere , 68 : 989 – 1003 . doi: 10.1016/j.chemosphere.2007.01.062
- FAL, RAC, FAW 1996 . Extraktion von Schwermetallen mit Natriumnitrat (1:2.5) . Schweizerische Referenzmethoden der Eidgenössischen landwirtschaftlichen Forschungsanstalten. Eidgenössische Forschungsanstalt FAL, RAC, FAW .
- Frossard , E , Bucher , M , Machler , F , Mozafar , A and Hurrell , R . 2000 . Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition . Journal of the Science of Food and Agriculture , 80 : 861 – 879 . doi: 10.1002/(SICI)1097-0010(20000515)80:7%3C861::AID-JSFA601%3E3.0.CO;2-P
- Grace , ND and Lee , J . 1992 . Influence of high zinc intakes, season and staple site on the elemental composition of wool and fleece quality in grazing sheep . New Zealand Journal of Agricultural Research , 35 : 367 – 377 . doi: 10.1080/00288233.1992.10421345
- Hotz , C and Brown , KH . 2004 . International Zinc Nutrition Consultative Group (IZiNCG) technical document 1. Assessment of the risk of zinc deficiency in populations and options for its control . Food and Nutrition Bulletin , 25 : S94 – S203 .
- House , WA , VanCampen , DR and Welch , RM . 1996 . Influence of dietary sulfur-containing amino acids on the bioavailability to rats of zinc in corn kernels . Nutrition Research , 16 : 225 – 235 . doi: 10.1016/0271-5317(96)00007-3
- Jan , MT , Roberts , P , Tonheim , SK and Jones , DL . 2009 . Protein breakdown represents a major bottleneck in nitrogen cycling in grassland soils . Soil Biology and Biochemistry , 41 : 2272 – 2282 . doi: 10.1016/j.soilbio.2009.08.013
- Kayser , A , Wenger , K , Keller , A , Attinger , W , Felix , HR , Gupta , SK and Schulin , R . 2000 . Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: The use of NTA and sulfur amendments . Environmental Science and Technology , 34 : 1778 – 1783 . doi: 10.1021/es990697s
- Kutman , UB , Yildiz , B and Cakmak , I . 2011 . Effect of nitrogen on uptake, remobilization and partitioning of zinc and iron throughout the development of durum wheat . Plant and Soil , 342 : 149 – 164 . doi: 10.1007/s11104-010-0679-5
- Kutman , UB , Yildiz , B , Ozturk , L and Cakmak , I . 2010 . Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen . Cereal Chemistry , 87 : 1 – 9 . doi: 10.1094/CCHEM-87-1-0001
- Ledgard SF , Boyea M , Brentrup F 2011 . Life cycle assessment of local and imported fertilisers used on New Zealand farms . In : Currie LD , Christensen CL Adding to the knowledge base for the nutrient manager . http://flrc.massey.ac.nz/publications.html. Occasional Report No. 24 . Palmerston North , , New Zealand , Fertilizer and Lime Research Centre, Massey University .
- Lönnerdal , B . 2000 . Dietary factors influencing zinc absorption . Journal of Nutrition , 130 : 1378S – 1383S .
- Lynch , SR . 2011 . Why nutritional iron deficiency persists as a worldwide problem . Journal of Nutrition , 141 : 763S – 768S . doi: 10.3945/jn.110.130609
- Marschner , H . 1993 . “ Zinc uptake from soils ” . In Zinc in soils and plants , Edited by: Robson , AD . 59 – 77 . Dordrecht , , The Netherlands : Kluwer Academic Publishers .
- Meschenmoser M 2001 . Schafwolle: Ein ökologischer Blödsinn . Saldo 20 .
- Mosse , J . 1990 . Nitrogen to protein conversion factor for 10 cereals and 6 legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and seed protein content . Journal of Agriculture and Food Chemistry , 38 : 18 – 24 . doi: 10.1021/jf00091a004
- Nashölm , T , Huss-Danell , K and Hogberg , P . 2001 . Uptake of glycine by field grown wheat . New Phytologist , 150 : 59 – 63 . doi: 10.1046/j.1469-8137.2001.00072.x
- Nolte , H , Bishop , DP and Hocker , H . 1996 . Effects of proteolytic and lipolytic enzymes on untreated and shrink-resist-treated wool . Journal of the Textile Institute , 87 : 212 – 226 . doi: 10.1080/00405009608659069
- Nowack , B , Schwyzer , I and Schulin , R . 2008 . Uptake of Zn and Fe by wheat (Triticum aestivum var. Greina) and transfer to the grains in the presence of chelating agents (ethylenediaminedisuccinic acid and ethylenediaminetetraacetic acid) . Journal of Agriculture and Food Chemistry , 56 : 4643 – 4649 . doi: 10.1021/jf800041b
- Rengel , Z , Batten , GD and Crowley , DE . 1999 . Agronomic approaches for improving the micronutrient density in edible portions of field crops . Field Crops Research , 60 : 27 – 40 . doi: 10.1016/S0378-4290(98)00131-2
- Schmidt , S , Mason , M , Sangtiean , T and Stewart , GR . 2003 . Do cluster roots of Hakea actities (Proteaceae) acquire complex organic nitrogen? . Plant and Soil , 248 : 157 – 165 . doi: 10.1023/A:1022352415728
- Scott , GE . 1991 . The sheepman's production handbook , Denver , Colorado : Abegg Printing .
- Shi , R , Zhang , Y , Chen , X , Sun , Q , Zhang , F , Roemheld , V and Zou , C . 2010 . Influence of long-term nitrogen fertilization on micronutrient density in grain of winter wheat (Triticum aestivum L.) . Journal of Cereal Science , 51 : 165 – 170 . doi: 10.1016/j.jcs.2009.11.008
- Sims , JT . 1986 . Soil-pH effects on the distribution and plant availability of manganese, copper and zinc . Soil Science Society of America Journal , 50 : 367 – 373 . doi: 10.2136/sssaj1986.03615995005000020023x
- Uauy , C , Distelfeld , A , Fahima , T , Blechl , A and Dubcovsky , J . 2006 . A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat . Science , 314 : 1298 – 1301 . doi: 10.1126/science.1133649
- vonWiren , N , Marschner , H and Romheld , V . 1996 . Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc . Plant Physiology , 111 : 1119 – 1125 .
- Welch , RM . 2002 . The impact of mineral nutrients in food crops on global human health . Plant and Soil , 247 : 83 – 90 . doi: 10.1023/A:1021140122921
- Welch , RM and Graham , RD . 1999 . A new paradigm for world agriculture: meeting human needs—Productive, sustainable, nutritious . Field Crops Research , 60 : 1 – 10 . doi: 10.1016/S0378-4290(98)00129-4
- White , PJ and Broadley , MR . 2009 . Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine . New Phytologist , 182 : 49 – 84 . doi: 10.1111/j.1469-8137.2008.02738.x
- Zahn , H . 1989 . Das Haar aus der Sicht des Chemikers . Chemie in unserer Zeit , 23 : 141 – 150 . doi: 10.1002/ciuz.19890230502
- Zahn H , Wortmann F-J , Wortmann G , Schäfer K , Hoffmann R , Finch R 2000 . Wool . In : Ullmann's encyclopedia of industrial chemistry . Wiley-VCH Verlag , Germany .