Abstract
Maintaining and increasing legume abundance is a critical component of pastoral intensification, increasing nitrogen inputs to nitrogen deficient New Zealand high and hill country pastures and improving feed quality. Establishment and persistence of traditionally sown legume species white clover (Trifolium repens) and subterranean clover (T. subterraneum) is often limited in summer-dry high country. In contrast, naturalized, adventive annual pasture legume species such as cluster clover (T. glomeratum), haresfoot clover (T. arvense), striated clover (T. striatum) and suckling clover (T. dubium) persist on low fertility, summer-dry high and hill country slopes, although little is known about the edaphic requirements of these species. A glasshouse study was conducted to determine the response and efficiency of these four pasture legume species to increasing levels of available phosphorus (P) in a typical, low fertility, acidic New Zealand high country soil, comparing against white clover and subterranean clover as ‘reference’ species. Trifolium subterraneum was the most productive species (4.4 g dry matter [DM]/pot) and T. striatum yielded the least (0.8 g DM/pot). The order of greatest yield DM response was T. subterraneum > T. arvense > T. repens > T. dubium ≥ T. glomeratum > T. striatum, while the P application rates at which maximum yield occurred varied between species. Mean shoot P uptake was highest for T. subterraneum and lowest for T. striatum (24.1 and 3.8 mg P/pot, respectively). P-response efficiency by species was in the order of T. subterraneum > T. arvense > T. glomeratum > T. repens > T. dubium > T. striatum. Implications for low input, extensive grazing systems in high and hill country are discussed. Trifolium arvense and T. glomeratum show potential for further P response investigation under field conditions.
Introduction
Phosphorus (P) is second only to nitrogen (N) as the key macro nutrient driving the productivity of legume-based grazed pasture systems in New Zealand high and hill country (Haynes & Williams Citation1993). Often the sole N inputs to these systems are sourced from sward legumes (clovers) through biological N fixation. This N input, as the key driver of overall pasture yield, is strongly influenced by soil P fertility (Moir et al. Citation2000). In general, higher soil P fertility often allows for a larger proportion of pasture legume to be present and to persist in the sward, resulting in greater annual biological soil N inputs (Bowatte et al. Citation2006; Gillingham et al. Citation2008) and increased soil plant available N for sward growth (Moir et al. Citation1995, Citation1997). As such, highly productive legume species such as white clover, Trifolium repens L., (and pastures) are generally adapted to high fertility soil conditions and do not perform well in infertile and/or acid soil conditions (Haynes & Williams Citation1993). ‘Improved’ pastures therefore require annual maintenance fertilizer inputs, such as single superphosphate (SSP) to elevate soil P and sulphur (S) fertility and sustain high production. In contrast, annual fertilizer inputs to these farming systems are, in reality, low, driven by the economics of fertilizer inputs. As a result, many high and hill country soils in New Zealand have soil fertility levels far below optimum for many common pasture legume species, such as white clover. As such, the long-term sustainability of extensive agroecosystems may therefore depend on greater P efficiency, involving sustained production with reduced fertilizer P inputs (Gilbert Citation2009; Vaccari Citation2009).
An important component of pastoral intensification is to increase legume abundance, so as to provide increased feed and nitrogen inputs to N deficient, high country grassland. However, the establishment and persistence of sown legume species such as the perennial white clover (T. repens L.) and the annual subterranean clover (T. subterraneum L.) is often limited in summer-dry, low soil fertility areas (Knowles et al. Citation2003; Power et al. Citation2006). This is in contrast to the common presence of other naturalized, adventive and unsown annual legumes, such as cluster clover (T. glomeratum L.), haresfoot trefoil (T. arvense L.), striated clover (T. striatum L.) and suckling clover (T. dubium) (Boswell et al. Citation2003; Power et al. Citation2006) that may be more suited to the low soil fertility and microclimates that exist on these hill slopes (Boswell et al. Citation2003; Maxwell et al. Citation2010). Much research has been conducted investigating white clover (T. repens) dry matter (DM) and growth responses to added P in New Zealand (Caradus & Snaydon Citation1986; Caradus et al. Citation1995). Considerable research has also been conducted in Australia investigating subterranean clover (T. subterraneum) responses to added P (Paynter Citation1993; Bolland & Paynter Citation1994; Bolland Citation1995; Cayley & Hannah Citation1995; Cayley et al. Citation1998). The literature addressing P responses of T. subterraneum and naturalized adventive annual legumes under New Zealand conditions is limited (Beale et al. Citation1993; Dodd & Orr Citation1995; Maxwell et al. Citation2010, Citation2012). In a pot trial using two P rates, Caradus (Citation1980) reported a P response by striated, haresfoot and suckling clovers. Likewise, Hart & Jessop (Citation1984) reported that suckling clover responded to P, although the response was low.
How these naturalized adventive legumes respond to increases in the plant-available soil P pool is therefore a crucial question. Further, establishing a ‘critical’ level of available soil P and plant P efficiency for these legume species is vital for extensive farming systems (Syers et al. Citation2008, Citation2010).
The objective of this study was to determine the influence of P supply on the DM yield and P response efficiency of six legume species grown in a typical low P fertility New Zealand high country soil under glasshouse conditions. This study is part of a larger suite of field and climate-controlled experiments examining the role of adventive annual clovers in extensively grazed high and hill country environments (Maxwell et al. Citation2010, Citation2012).
Materials and methods
Soil and growth conditions
The soil used was a ‘Cass Series’ High Country Southern Brown soil (New Zealand classification: Upland Allophanic Brown Soil—Hewitt [Citation1998]. USDA: Dystrochrept—Soil Survey Staff [Citation1998]). Soil (0–7.5 cm horizon) was collected from a high country site (43°19′02.88″S, 171°8′31.41″E) on Glenfalloch Station, a central Canterbury sheep and beef farm in the South Island, New Zealand in June 2008. The hill site (25° slope) has an altitude of 740 masl and a north-facing aspect. At most, a total of one or two fertilizer applications of SSP have been applied at this site since development (100 years; 30 kg P, 40 kg S ha−1). The field moist soil was prepared by passing through a 4 mm sieve, removing all plant material, then mixing thoroughly. Soil analyses were conducted before commencement of the experiment, with results confirming an initial low soil fertility status ().
Table 1 . Initial fertility status of a high country Allophanic Brown soil used in the glasshouse experiment. Topsoil sample (0–75 mm) was collected from a mid-altitude (740 m) central South Island, New Zealand high country site in June 2008.
The glasshouse experiment was conducted at the Lincoln University glasshouse facilities, Canterbury, New Zealand. Phosphorus, in the form of Ca(H2PO4).4H20, was applied at eight different rates; 0, 30, 60, 100, 250, 500, 1000 and 2500 mg P kg−1 soil. Treatments were replicated four times for each of the six pasture legume species. Basal nutrient addition prior to experiment commencement included sulphur (S) as calcium sulphate (CaSO4) to address the soil plant-available S deficiency, and potassium (K) as potassium chloride (KCl), applied at rates of 500 mg element kg−1 soil. All pots also received lime at a rate of 4 t ha−1 equivalent of laboratory grade CaCO3, which corrected soil pH to 6.1. The various P treatments and basal nutrients were added to 400 g of the air-dried soil and then mixed thoroughly. The soil was then lightly packed into 0.8 L (10 cm height×12 cm diameter) pots with saucers, at a bulk density (ρb) of 700 kg m−3 . Deionized (DI) water was slowly added to each pot to wet up the soil to a gravimetric water content of 40%. The pots were then moved to the glasshouse and arranged on tables in a randomized block design. The glasshouse was maintained within the range of 10–30 °C, with a mean temperature of 19.9 °C for the duration of the experiment.
Plant material and rhizobia inoculant
Volunteer seedlings were removed 10–14 days post wetting up of the soil. Seeds of the target pasture legume species were pre-treated by soaking and scarification and then sown directly, using a ‘grid’ sowing pattern using five sowing positions and two seeds at each position, on to the soil surface in the pots as monocultures, in late September (late spring). A small quantity (2 mm depth) of soil was sprinkled over the seed and gently packed. Soon after germination, all legume species were thinned to a final plant density of five plants per pot, to a plant density of 450 plants m−2, based on field observations (Maxwell et al. Citation2010). The pasture legume species evaluated were: white clover (T. repens cv. Nomad), subterranean clover (T. subterraneum cv. Mt Barker), striated clover (T. striatum), suckling clover (T. dubium), cluster clover (T. glomeratum) and haresfoot trefoil (T. arvense). Seeds of the traditionally sown pasture legume species investigated were sourced commercially. Seed of the naturalized legume species were collected from several hillslope farmland sites at Glenfalloch and Mt Grand Stations.
Commercial rhizobia inoculant (Nodulaid, Group B and C, Becker Underwood), Group B for all white clover pots and Group C for all annual clover pots, was added 5 weeks after seed germination to ensure that an active soil rhizobia population was present. At 5 weeks post germination of seeds, a small quantity of N (30 kg N ha−1) nutrient solution, in the form of ammonium nitrate (NH4NO3), was applied to all pots in order to overcome any plant N deficiencies during the seedling establishment phase. Beyond this point, plants were dependent on N sourced from N fixation or soil N for growth (plants of all species were later confirmed to have nodulation on visual inspection). In addition, a nutrient solution (Booking Citation1976; Caradus & Snaydon Citation1986) containing trace elements only was applied on a regular basis (twice monthly over winter, then weekly thereafter during rapid growth in spring and early summer), to ensure adequate trace element nutrition. Total quantities of trace elements applied were 500, 200, 40 and 1000 g element ha−1 for boron (B), cobalt (Co), molybdenum (Mo) and zinc (Zn), respectively. Throughout the experiment, all pots were watered with DI water daily to maintain a gravimetric soil moisture content of 40%, and watered to weight twice weekly.
Yield and herbage analyses
Seven herbage harvests were conducted from late November 2008 to early May 2009, representing 9, 11, 13, 15, 20, 27 and 30 weeks post germination. The total duration of the experiment was 229 days. Clover plants were harvested by cutting 2 cm from the crown of each plant. Plant material from each pot was then dried at 70 °C for 48 h and weighed for DM yield. Samples were then ground, acid digested (Kjeldahl digest procedure; Blakemore et al. Citation1987) and analysed for total P concentration by molybdenum blue using an FIA (Flow Injection Analyser, Tecator Inc, Sweden). This information was then combined to give total P uptake for the duration of the experiment. Individual Olsen P tests were run on soil from all pots to determine the level of plant-available P at the conclusion of the experiment (). Further soil analyses were conducted on a bulked soil sample from all P treatment pots.
Table 2 . Values of total accumulated shoot yield, P concentration and P uptake by six pasture legume species, grown under glasshouse conditions in a New Zealand high country soil supplied with increasing rates of P (eight levels of P; increasing from 0 to 2500 mg P kg−1 soil) and Olsen P values of soil in which plants were grown.
Statistical analyses
The effects of applied P on DM yield, plant shoot P concentration, plant shoot P uptake and final soil Olsen P was analysed by an analysis of variance (ANOVA) of a randomized block design, using GenStat 12.2 (Lawes Agricultural Trust, Rothamsted, UK). The model included P rate, legume species and the P rate×species interaction as fixed effects. Highly significant (P <0.001) interaction effects were observed and therefore regression analysis and curve fitting was undertaken for all species individually in order to better interpret the response of species to P rate.
Results
Yield response
Plant growth response to applied soil P differed among pasture legume species, and between P application rates (, , P rate×Sp interaction, P<0.001). Averaged across P rates, mean total accumulated dry matter (TDM) yield ranged from 0.8 to 4.4 g DM/pot. Trifolium subterraneum was the most productive species (4.4 g DM/pot) and T. striatum yielded the least (0.8 g DM/pot) ().
Figure 1. Total accumulated shoot dry matter (DM) yield response of pasture legume species—A, Trifolium glomeratum; B, T. arvense; C, T. subterraneum; D, T. dubium; E, T. striatum; and F, T. repens—to increasing levels of soil phosphorus (eight levels of P; ranging from 0 to 2500 mg P kg−1 soil), grown in a New Zealand high country soil. Data are mean values ± SEM (n =4), with R 2 and P values for fitted curve showing data trend.
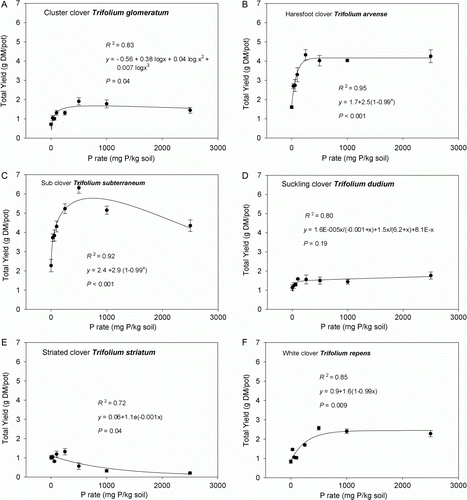
The soil P level at which maximum DM yield occurred varied among species (, ). Three species (T. subterraneum, T. repens and T. glomeratum) showed a clear rise to maximum DM yield at 500 mg P kg−1, followed by a decline (excluding T. repens) in yield at higher P rates (). Two species (T. arvense and T. dubium) showed a rapid rise in yield up to 250 mg P kg−1 soil, then plateaued, with no decline in yield. In the case of T. dubium, yield increased again slightly at the highest soil P level (). Trifolium striatum rose quickly to a maximum yield response at 250 mg P kg−1 soil, then showed a steady decline in yield as soil P level increased.
Table 3 . Rate of P application and shoot P concentration at which 95% maximum yield was observed for the pasture legume species.
Shoot P concentration and uptake
Mean shoot P concentration was highest for T. subterraneum and T. dubium, intermediate for T. striatum and lowest for T. glomeratum, T. arvense and T. repens (). All species, except T. striatum, showed the general pattern of increasing shoot P concentration as soil P availability increased (). Shoot P concentration increased at a similar rate from 0 to 2500 mg P kg−1 soil for T. arvense, T. subterraneum, T. dubium and T. repens. Shoot P concentration of T. glomeratum increased more rapidly between 0 and 250 mg P kg−1 soil before flattening off and steadily increasing in a similar pattern to the other species. Trifolium striatum shoot P concentration was high with no added P, but showed a general decline and then minimal change as soil P level increased. The latter trend was weakest of all the species (R 2=0.64; ).
Figure 2. Comparison of shoot P concentration of pasture legume species—A, T. glomeratum; B, T. arvense; C, T. subterraneum; D, T. dubium; E, T. striatum; and F, T. repens—grown in a New Zealand high country soil supplied with increasing levels of soil phosphorus (eight levels of P; ranging from 0 to 2500 mg P kg−1 soil). Data are mean values ± SEM (n =4), with R 2 and P values for fitted curve showing data trend.
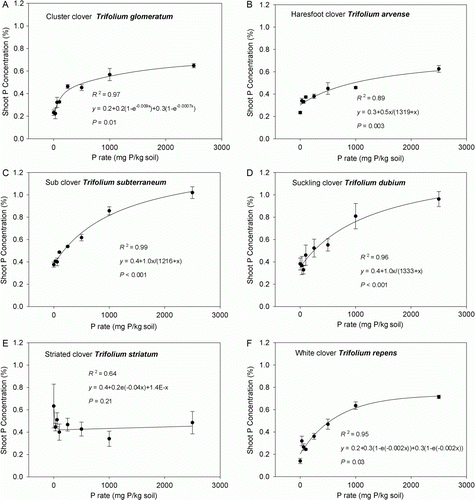
The relationship between shoot P concentration and 95% max yield varied among the species (). P concentration at which 95% maximum yield was achieved was highest for T. dubium (0.96%) and lowest for T. arvense (0.38%) (). P uptake also varied among the six pasture legume species (P rate×Sp interaction, P<0.001). The mean level of P uptake ranged from 3.8 mg P/pot in T. striatum to 24.0 mg P/pot in T. subterraneum ().
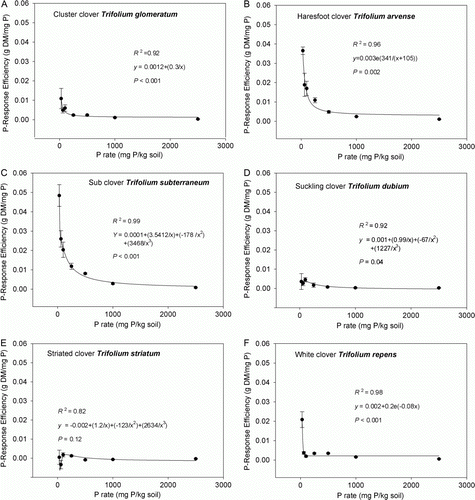
P-response efficiency
The P rate at which the highest P-response efficiency (defined as P applied g−1 DM grown; Syers et al. Citation2008) was observed varied among species (P rate×Sp interaction, P<0.001). Trifolium glomeratum, T. arvense, T. subterraneum and T. repens all showed the highest P-response efficiency at 30 mg P kg−1 soil (). In contrast, the highest P-response efficiency for T. dubium and T. striatum occurred at 100 mg P kg−1 soil. In general, the order of P-response efficiency by species was as follows: T. subterraneum>T. arvense>T. repens>T. glomeratum>T. dubium >T. striatum ().
Figure 3. Comparison of P-response efficiency of pasture legume species—A, T. glomeratum; B, T. arvense; C, T. subterraneum; D, T. dubium; E, T. striatum; and F, T. repens—grown in a New Zealand high country soil supplied with increasing levels of soil phosphorus (eight levels of P; ranging from 0 to 2500 mg P kg−1 soil). Data are mean values ± SEM (n =4), with R 2 and P values for fitted curve showing data trend.
Discussion
P yield response
Total DM yields differed between species, in the order of T. subterraneum>T. arvense>T. repens>T. dubium≥T. glomeratum>T. striatum. Also, the annual T. arvense out-yielded the perennial T. repens cv. Nomad at every soil P level. This suggests that T. arvense may have a stronger agronomic potential than T. repens cv. Nomad, a cultivar selected for increased persistence in dry environments prone to low summer moisture levels (Agricom Citation2010). Trifolium glomeratum and T. dubium yields did not differ significantly from each other, although both out-yielded T. striatum as soil P levels increased.
The superior yield performance of T. subterraneum cv. Mt Barker can be explained by its large seed size, with greater endosperm reserves relative to all the other species. This enabled rapid early seedling development, and subsequent exhibition of its good agronomic potential, under the optimum soil and climatic glasshouse conditions. Caradus (Citation1980) reported on the responsiveness of T. repens cv. Huia, T. subterraneum cv. Woodenellup, T. striatum, T. arvense and T. dubium to added P, in terms of shoot dry weight and total shoot P content, grown at relatively high P rates of 300 and 2000 mg P kg−1, in a P-deficient soil (volcanic Stratford coarse sandy loam) over 24 weeks. Similar to our study, T. striatum was the lowest yielding least P responsive annual legume species. The yield results for T. arvense and T. dubium, however, contrast our results, as T. arvense yielded less and T. dubium yielded more in that study. This was possibly due to ecotypic variation in germplasm used between the two experiments. Trifolium subterraneum was the most productive species across all P rates. Differences in cultivar of T. subterraneum and T. repens grown by Caradus (Citation1980) to those in our study may explain the contrasting trends in yield responses between the two comparable P rates; both species had greater yields at 2000 mg P kg−1 soil than at 300 mg P kg−1 soil which contrasts the yield response trends for these species in our study. However, the two relatively high P rates used by Caradus (Citation1980) do not allow for the examination of P response of these species at low soil P fertility, which is a critical focus of our experiment for low-P high country environments. Additionally, the volcanic soil examined by Caradus (Citation1980) contrasts with the sedimentary high country Allophanic Brown soil of our study, in terms of soil parent material (high allophane content and very high P retention capacity) and environmental soil-forming conditions.
Blair & Cordero (Citation1978) also found that T. subterraneum out-yielded T. glomeratum in a 10-week pot trial. Caradus et al. (Citation1995) reported only minor yield responses of T. repens beyond 400 mg P kg−1, which is comparable to our study. Hart & Jessop (Citation1984) examined the growth responses to P of T. repens growing in an Egmont sandy loam, a very N deficient soil of high phosphate fixing capacity. Phosphorus was added to the soil at levels equivalent to those used in our study. In accord with our results, they observed T. repens responding more strongly to added P than T. dubium, with the latter having lower shoot dry weights. Steepest increases in shoot dry weight of both species occurred at lower P rates (0–500 mg P kg−1 soil). They concluded T. dubium to be a species that has a relatively small response to improvements in P availability, which is in agreement with the results of this experiment.
Maximum yield
The soil P level at which maximum yield was observed varied between species suggesting that the optimum P requirement of these species is different. Maximum yield response for T. subterraneum, T. glomeratum and T. repens occurred at an equivalent rate of 221 kg P ha−1. Beyond this point however, these three species were unable to utilize further increases in available P for shoot growth, suggesting that factors other than plant-available soil P were limiting yield. For T. dubium, maximum yield was observed at the highest soil P level treatment suggesting this species has the ability to take up P available in the soil at very high levels. This species appears to be able to continue to take up P over a wide range of availability, although remaining very unresponsive to P in terms of DM yield, suggesting perhaps a very low P requirement to reach its growth potential.
Shoot P concentration and uptake
In general, all pasture legume species, with the exception of T. striatum, showed steadily rising shoot P concentrations with increased availability of soil P. In agreement with this, Hart & Jessop (Citation1984) reported leaf P concentration of T. dubium and T. repens cv. Huia rose with increasing P supply. Blair & Cordero (1978) found that the shoot P concentration of T. subterraneum and T. glomeratum increased with increasing rates of applied P. However, shoot P concentration continued to increase beyond the point of maximum yield response for T. glomeratum, T. arvense, T. subterraneum and T. repens (), suggesting ‘luxury’ P uptake by these species. There are no reports suggesting P toxicity in pasture legumes grown under glasshouse conditions, which would cause a reduction in P yield response.
The shoot P concentration at which maximum yield was observed varied among the pasture legume species. Interestingly, T. arvense had a much lower shoot P concentration at maximum yield than T. subterraneum. The magnitude of P uptake varied between the clover species, with T. subterraneum showing the greatest mean level of P uptake from the soil across all P rates. As expected, P uptake reflected the DM yield of the clovers.
P efficiency
The ability to acquire P from the soil and use it efficiently for biomass production is an important characteristic for adaptation to soils low in available P (Pang et al. Citation2010). In general, at low-medium P levels, T. subterraneum was the most efficient species in utilizing applied P for biomass production (P required g−1 DM grown), followed by T. arvense and T. glomeratum. Beyond very low soil P level, T. repens became the least efficient out of the top three higher-yielding pasture legume species. These results clearly indicate the inefficiency of T. repens to utilize P at very low soil P levels, in contrast to T. subterraneum, T. arvense and T. glomeratum.
Practical implications and conclusions
Gaining an understanding of P requirements of plant species is important for the purposes of introduction, selection and breeding. Trifolium arvense, due to high yield and high P efficiency, showed the most promise as a species to be utilized, and its spread and abundance through grazing management encouraged in extensive high and hill country ecosystems. The critical P requirement for maximum yield for T. arvense was lower than T. repens cv. Nomad and T. subterraneum cv. Mt Barker. Trifolium arvense had almost twice the DM yield and greater P response efficiency at lower soil P levels on this soil than T. repens. In addition, being an annual pasture species, T. arvense is able to complete its life cycle before soil moisture deficits occur in late spring-early summer in summer-dry hill country. Although being less productive than T. subterraneum in this study, the herbage DM production of T. arvense could be improved by breeding and selection of better performing cultivars, and searching to find ecotypic variation within the large areas of New Zealand high and hill country where T. arvense is found to survive and persist (Maxwell et al. Citation2010). Trifolium glomeratum also shows promise with a comparable yield P response trend and greater P response efficiency than T. repens at very low soil P levels. The results of this 20-week glasshouse study need to be examined further in long-term field studies. Such studies, conducted under field climatic and environmental conditions, should identify how the more P responsive naturalized species such as T. arvense and T. glomeratum, when grown as monocultures or mixtures, perform when subjected to realistic P fertilizer rates in the field.
Acknowledgements
This research was funded by the Miss EL Hellaby Indigenous Grasslands Research Trust. We thank Chas Todhunter of Glenfalloch Station and Brendan Malcolm, Carole Barlow, Qian Liang, Sho Kasuya and Fiona McConville of Lincoln University for technical assistance.
References
- Agricom 2010 . Agrinote: Grasslands Nomad white clover , Agricom; Pastures for Profit. http://www.agricom.co.nz/userfiles/files/Nomad/pdf (accessed 19 May 2011) .
- Beale PE , Bounejmate M , Lahlou A , Marx DB , Christiansen S 1993 . Distribution of annual Trifolium species in Morocco . Australian Journal of Agricultural Research 44 : 1303 – 1310 . doi: 10.1071/AR9931303
- Blair GJ , Cordero S 1978 . The phosphorus efficiency of three annual legumes . Plant and Soil 50 : 387 – 398 . doi: 10.1007/BF02107187
- Blakemore LL , Searle PL , Daly BK 1987 . Methods of chemical analysis of soils . New Zealand Bureau Scientific Report 80 . Pp. 21 – 29 .
- Bolland MDA 1995 . Yield response of subterranean clover to applications of superphosphate and rock phosphate measured in the year of application at the same site in different years . Journal of Plant Nutrition 18 : 47 – 63 . doi: 10.1080/01904169509364884
- Bolland MDA , Paynter BH 1994 . Critical phosphorus concentrations for burr medic, yellow serradella, subterranean clover and wheat . Communications in Soil Science and Plant Analysis 25 : 385 – 394 . doi: 10.1080/00103629409369045
- Booking IR 1976 . Soilless potting media for controlled-environment facilities . New Zealand Journal of Experimental Agriculture 4 : 203 – 208 . doi: 10.1080/03015521.1976.10425870
- Boswell CC , Lucas RJ , Lonati M , Fletcher A , Moot DJ 2003 . The ecology of four annual clovers adventive in New Zealand grasslands . In: Moot DJ Legumes for dryland pastures . Proceedings of the New Zealand Grassland Association, Grassland Research and Practice Series, No 11 . Pp. 175 – 188 .
- Bowatte S , Russell T , Carran A , Gillingham AG 2006 . Can phosphorus fertilisers alone increase levels of soil nitrogen in New Zealand hill country pastures? Nutrient Cycling in Agroecosystems 75 : 57 – 66 . doi: 10.1007/s10705-006-9011-4
- Caradus JR 1980 . Distinguishing between grass and legume species for efficiency of phosphorus use . New Zealand Journal of Agricultural Research 23 : 75 – 81 . doi: 10.1080/00288233.1980.10417847
- Caradus JR , Mackay AD , Dunlop J , Van Den Bosch J 1995 . Relationships between shoot and root characteristics of white clover cultivars differing in response to phosphorus . Journal of Plant Nutrition 18 : 2707 – 2722 . doi: 10.1080/01904169509365095
- Caradus JR , Snaydon RW 1986 . Response to phosphorus of populations of white clover, 2. Glasshouse and growth cabinet studies . New Zealand Journal of Agricultural Research 29 : 163 – 168 . doi: 10.1080/00288233.1986.10426969
- Cayley JWD , Hannah MC 1995 . Response to phosphorus fertilizer compared under grazing and mowing . Australian Journal of Agricultural Research 46 : 1601 – 1619 . doi: 10.1071/AR9951601
- Cayley JWD , Hannah MC , Kearney GA , Clark SG 1998 . Effects of phosphorus fertiliser and rate of stocking on the seasonal pasture production of perennial ryegrass-subterranean clover pasture . Australian Journal of Agricultural Research 49 : 233 – 248 . doi: 10.1071/A97113
- Dodd MB , Orr SJ 1995 . Seasonal growth, flowering patterns, and phosphate response of 18 annual legumes grown in a hill country soil . New Zealand Journal of Agricultural Research 38 : 21 – 32 . doi: 10.1080/00288233.1995.9513100
- Gilbert N 2009 . The disappearing nutrient . Nature 461 : 716 – 718 . doi: 10.1038/461716a
- Gillingham AG , Morton JD , Gray MH 2008 . Pasture responses to phosphorus and nitrogen fertilisers on east coast hill country: 2. Clover and grass production from easy slopes . New Zealand Journal of Agricultural Research 51 : 85 – 97 . doi: 10.1080/00288230809510438
- Hart AL , Jessop D 1984 . Leaf phosphorus fractionation and growth responses to phosphorus of the forage legumes Trifolium repens, T. dubium and Lotus pedunculatus . Physiologia Plantarum 61 : 435 – 440 . doi: 10.1111/j.1399-3054.1984.tb06352.x
- Haynes RJ , Williams PH 1993 . Nutrient cycling and soil fertility in the grazed pasture ecosystem . Advances in Agronomy 49 : 119 – 199 .
- Hewitt AE 1998 . New Zealand soil classification , 2nd edition. . Lincoln , , New Zealand , Manaaki Whenua Press . 133 p.
- Knowles IM , Fraser TJ , Daly MJ 2003 . White clover: loss in drought and subsequent recovery . In: Moot DJ ed. Legumes for dryland pastures. Proceedings of New Zealand Grassland Association symposium , Lincoln University 18–19 November , 2003. Grassland Research and Practice Series 11 : 37 – 42 .
- Maxwell TMR , Moir JL , Edwards GR 2010 . Influence of environmental factors on the abundance of naturalised annual clovers in the South Island hill and high country . Proceedings of the New Zealand Grassland Association 72 : 165 – 170 .
- Maxwell TMR , Moir JL , Edwards GR 2012 . Sulphur and lime response of four adventive annual clovers grown in a New Zealand high country soil under glasshouse conditions . New Zealand Journal of Agricultural Research 55 : 47 – 62 . doi: 10.1080/00288233.2011.643904
- Moir JL , Hedley MJ , Mackay AD , Garland CB 1995 . Climate, soil fertility and pasture production in Wairarapa hill country . In: Currie LD , Loganathan P Fertiliser requirements of grazed pasture and field crops: macro and micro nutrients . Occasional Report No 8 . Fertiliser and Lime Research Centre, Massey University , Palmerston North , , New Zealand . Pp. 254 – 260 .
- Moir JL , Hedley MJ , Mackay AD , Tillman RW 1997 . The effect of fertiliser history on nutrient accumulation and plant-available nutrient supply in legume-based pasture soils . In: Proceedings of XVIII International Grassland Congress, Winnipeg, Canada . Pp. 68 – 69 . http://www.internationalgrasslands.org/publications/browse-97/ (accessed 20 January 2013).
- Moir JL , Scotter DR , Hedley MJ , Mackay AD 2000 . A climate-driven, soil fertility dependent, pasture production model . New Zealand Journal of Agricultural Research 43 : 491 – 500 . doi: 10.1080/00288233.2000.9513445
- Pang J , Tibbett M , Denton MD , Lambers H , Siddique KHM , Bolland MDA et al. 2010 . Variation in seedling growth of 11 perennial legumes in response to phosphorus supply . Plant and Soil 328 : 133 – 143 . doi: 10.1007/s11104-009-0088-9
- Paynter BH 1993 . Effect of external phosphorus and seed phosphorus supply on the shoot and root growth of yellow serradella, burr medic, and subterranean clover . Journal of Plant Nutrition 16 : 2313 – 2331 .
- Power DR , Pollock KM , Lucas RJ , Moot DJ 2006 . Clover species cover on summer dry hill country in Central Otago . Proceedings of the New Zealand Grassland Association 68 : 343 – 347 .
- Soil Survey Staff 1998 . Keys to soil taxonomy , 8th edition. . Washington , DC , United States Department of Agriculture .
- Syers JK , Johnston AE , Curtin D 2008 . Efficiency of soil and fertilizer phosphorus use . Rome , FAO . 109 p.
- Syers JK , Johnston AE , Curtin D 2010 . A new perspective on the efficiency of phosphorus fertilizer use . Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World . Pp. 172 – 175 .
- Vaccari DA 2009 . Phosphorus: A looming crisis . Scientific American , June : 42 – 47 .