Abstract
Germination of ‘Cefalu’ arrowleaf, ‘Bolta’ balansa, ‘Prima’ gland and ‘Mihi’ Persian clovers was determined from constant temperature (5–40 °C) from incubator experiments. A ‘broken stick’ regression of germination rates against temperature was used to quantify thermal time (Tt) requirements and cardinal (base, optimum and maximum) temperatures. Tt requirements for 75% germination were higher for ‘Cefalu’ arrowleaf (34 °Cd) than ‘Bolta’ balansa (32 °Cd), ‘Prima’ gland (28 °Cd) and ‘Mihi’ Persian (25 °Cd) clovers. All species had a base temperature of 0 °C. ‘Mihi’ Persian clover had an optimum temperature (Topt) of 33 °C and a maximum temperature (Tmax) of 45 °C, while all the other species had Topt between 16–20 °C and Tmax of c. 34 °C. The maximum final germination percentage was above 60% for ‘Cefalu’ arrowleaf clover and 80% for the other three species.
Introduction
Annual clovers are used in dryland pastures (<800 mm annual rainfall) to provide sheep grazing during late winter and spring. They grow vigorously during spring, set seeds, then die in summer. These annuals then re-establish from seeds in the autumn. Thus, annual clovers avoid summer drought as seeds. Subterranean clover (Trifolium subterraneum L.) is the most widely used annual legume species in New Zealand. It is capable of producing large quantities of seed even under hard grazing (Smetham Citation2003). However, its seed burrs means specialized machinery is required for seed harvesting. This is not a problem for top flowering annual clovers. Arrowleaf clover (T. vesiculosum Savi) grows well during summer (Evans et al. Citation2003) and can be used to extend the grazing period for lamb finishing and improve soil fertility for future cropping (Evans & Mills Citation2008). Cultivar ‘Cefalu’, which flowers early, is suitable to fill in a feed gap during early spring in New Zealand. Balansa clover (T. michelianum Savi) produces high dry matter yield and has the capacity to generate a large seed bank that can be managed for regeneration in subsequent years (Monks et al. Citation2008). This research showed that when cultivar ‘Bolta’ was sown in a mixture with cocksfoot (Dactylis glomerata L.) in lowland Canterbury (<650 mm annual rainfall), it produced an annual dry matter yield of 3 t/ha or 30% of the total herbage. This indicates its ability to thrive in a dryland pasture. ‘Prima’, which is the only cultivar available of gland clover (T. glanduliferum Boiss), is highly resistant to pests such as red-legged earth mite (Halotydeus destructor), blue-green aphids (Acyrthosiphon kondoi) and cowpea aphids (Aphis craccivora) (Dear et al. Citation2001). It has a delayed pattern of hard-seed breakdown, which prevents the germinating seedlings from a ‘false break’ (Dear et al. Citation2002). Persian clover (T. resupinatum L.) tolerates water-logging and has high dry matter production during spring (Charlton & Stewart Citation2003). Cultivar ‘Mihi’, which belongs to subspecies majus, has an erect habit, large leaflets and is soft-seeded. This cultivar matures late and is used primarily as a specialist crop for fodder production. Presently, these top flowering annual clovers are not widely used in New Zealand and little is known about their potential performance in New Zealand pastures.
The persistence of annual pasture species depends on the population of seedlings that regenerate each autumn. Therefore, it is important to know the timing and rate of germination because these factors determine the number of seedlings that emerge from the ground. As long as moisture is adequate, seed germination is driven by the accumulation of thermal time (Tt) or growing degree-days (°Cd). Thermal time is defined as cumulative heat units above a base temperature on a daily basis. Quantification of thermal time for germination requires cardinal temperatures, namely base (Tb), optimum (Topt) and maximum (Tmax) to be calculated (Angus et al. Citation1981). The base temperature (Tb) is the temperature below which no germination occurs. The optimum temperature (Topt) is the temperature at which the germination rate occurs at its maximum capacity. The maximum temperature (Tmax) is the highest temperature at which seeds will germinate. Given that germination is a function of temperature, then the thermal time requirement for germination can be quantified (Arnold & Monteith Citation1974).
Thermal time has been used to quantify germination of some annual clover species (Moot et al. Citation2000; Boswell et al. Citation2003; Lonati et al. Citation2009; Monks et al. Citation2009). In most cases, the thermal time was species dependent and unaffected by cultivar. However, the range of temperatures used was inadequate to accurately define all three cardinal temperatures and gland clover was not included in any of these studies. Thus, this study aims to define cardinal temperatures and quantify thermal time requirements for germination of ‘Cefalu’ arrowleaf, ‘Bolta’ balansa, ‘Prima’ gland and ‘Mihi’ Persian clovers through a series of incubator experiments.
Materials and methods
Three replicates of 50 scarified bare seeds of ‘Cefalu’ arrowleaf, ‘Bolta’ balansa, ‘Prima’ gland and ‘Mihi’ Persian clovers were placed on wetted blotting paper in sealed plastic containers and germinated in unlit incubators at constant temperatures of 5, 8, 10, 12, 15, 18, 20, 25 or 30 °C. Additional temperatures of 22.5, 35, 37.5 and 40 °C were added later, to increase the number of data points below and beyond the optimum temperature (Topt) and to assist determination of Tb and Tmax. Distilled water was added as required to ensure moisture was non-limiting for germination. Germinated seeds were counted and removed twice daily during periods of rapid germination and daily at other times until germination ceased (ISTA Citation2004). Seeds were considered germinated when the radical exceeded the small diameter of the seed. A Gompertz model was fitted to the cumulative percentage germination against days:
Data analysis
Data for each species were plotted as the reciprocal of the duration (in days) to 75% germination against the mean temperature (T). The inverse of duration (1/days) represents the germination rate. Least squares regression analysis was used for both the positive (sub-optimal) and the negative (supra-optimal) linear portions of the response whereby:
The standard error of Tb and Tt were calculated according to (Campbell et al. Citation1974) as:
Data were analysed using the statistical software Genstat 12.2. For each measured variable, maximum standard errors were reported. In most analyses, a single thermal time accumulation target is reported. This assumes the cardinal temperatures are representative of a normal distribution that is symmetrical and centred around Topt (Bonhomme Citation2000). However, where the data show a skewed or non-symmetrical relationship, a separate thermal time target may be required for sub- and supra-optimal temperatures.
Results
Final germination percentage
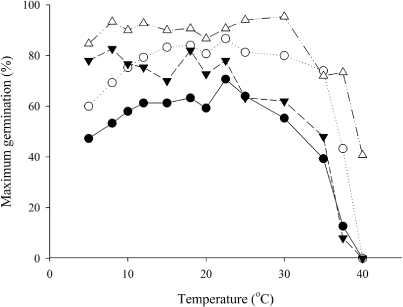
Gompertz functions described the cumulative germination over time for each species (). Specifically, for each species by temperature combination, there was a distinct linear phase before the final germination percentage was determined. The maximum final germination percentage was above 60% for arrowleaf clover and 80% for the other three species. However, at the higher and lower end of the temperature range, the final germination percentage was below these maximum values (). For example, ‘Cefalu’ arrowleaf had a final germination percentage of 58%–71% from 10–25 °C but this dropped to less than 50% at 5 °C and 35 °C with no germination at higher temperatures. ‘Mihi’ Persian had the highest final germination of 90%–95% at 30 °C but this decreased to 80% at 5 °C and 41% at 40 °C. Germination was not observed for ‘Cefalu’ arrowleaf, ‘Bolta’ balansa or ‘Prima’ gland clovers at 40 °C.
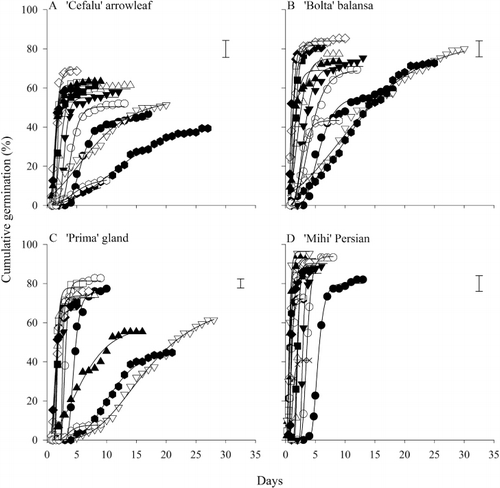
Time to 75% germination and germination rate
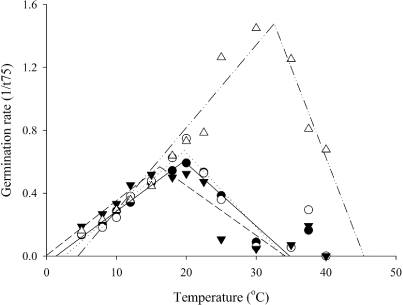
The number of days to reach 75% of final germination decreased as temperature increased up to 20 °C for both ‘Cefalu’ arrowleaf and ‘Bolta’ balansa, 15 °C for ‘Prima’ gland and 30 °C for ‘Mihi’ Persian clovers (). There was then an increase in the duration until the maximum effective temperature was reached: 35 °C for ‘Cefalu’ arrowleaf and ‘Bolta’ balansa, and 40 °C for ‘Mihi’ Persian clover. For ‘Prima’ gland clover, the time to germination remained the same from 15–20 °C before it increased up to the maximum effective temperature of 30 °C. The reciprocals of these data were used to define the cardinal temperatures for each species.
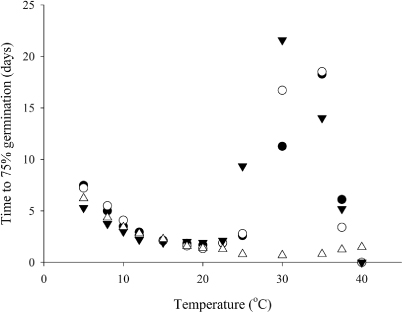
In all species, the germination rate showed a positive linear trend from the minimum temperature (Tb) up to the optimum temperature (Topt), and then a negative linear decline until no germination occurred at the maximum temperature (Tmax) (). Split-line regression analysis estimated a Tb of 1.4 °C for ‘Cefalu’ arrowleaf, 2.6 °C for ‘Bolta’ balansa, −0.1 °C for ‘Prima’ gland and 4.5 °C for ‘Mihi’ Persian clover. For all species, the 95% confidence interval showed Tb estimates for germination included 0 °C. ‘Cefalu’ arrowleaf, ‘Bolta’ balansa and ‘Prima’ gland clovers had a maximum germination rate of between 0.6–0.7 per day across a Topt range of 16.2–19.7 °C and Tmax of c. 34 °C. ‘Mihi’ Persian clover had the highest Topt of 32.6 °C (1.5 per day) and a Tmax estimate of 45.4 °C.
The Tt requirement for 75% of final germination for temperatures in the sub-optimal range was 25( ±1.0) °Cd for ‘Mihi’ Persian and 34 (±0.9) °Cd for ‘Cefalu’ arrowleaf above a Tb of 0 °C (). In the supra-optimal range, Tt requirement (from Topt to Tmax) was similar to the sub-optimal range except for ‘Mihi’ Persian clover (9 [±1.7] °Cd).
Table 1 Base (Tb), optimum (Topt) and maximum (Tmax) temperatures and thermal time (Tt) requirements for 75% germination of ‘Cefalu’ arrowleaf, ‘Bolta’ balansa, ‘Prima’ gland and ‘Mihi’ Persian clovers.
Discussion
In all species, the germination rate increased linearly with increased temperatures up to an optimum. Further increases in temperature then decreased the germination rate until there was no further germination (). The relationship between germination rate and temperature enabled the cardinal temperatures (base, optimum and maximum) to be defined. A two-piece ‘broken stick’ regression model was appropriate for describing the germination response of these annual clovers at the sub-optimal and supra-optimal range of temperatures. In most cases, a single thermal time target of a phenophase is appropriate, based on the assumption that the slopes of the two regression lines are symmetrical. In this case, a single thermal time target can be used to quantify germination of ‘Cefalu’ arrowleaf, ‘Bolta’ balansa and ‘Prima’ gland clovers. However, for ‘Mihi’ Persian clover, the asymmetric relationship meant thermal time requirements differed at sub-optimal and supra-optimal temperatures. Also, in the supra-optimal range, germination rate at 37.5 and 40 °C for ‘Cefalu’ arrowleaf, ‘Bolta’ balansa and ‘Prima’ gland clovers deviated from the negative linear model () and were excluded from analysis. These were outside the species' optimal thermal range (Angus et al. Citation1981). The germination rate deviated because at these high temperatures, seeds either germinated rapidly or died and the germination rate was overestimated, based on the 75% germination duration of the surviving seeds. At 37.5 °C, the seeds of ‘Cefalu’ arrowleaf, ‘Bolta’ balansa and ‘Prima’ gland clovers took six, three and five days, respectively, to germinate. However, the seed population that survived at 37.5 °C was <20% for both ‘Cefalu’ arrowleaf and ‘Prima’ gland clovers and <45% for ‘Bolta’ balansa clover (). Except for ‘Mihi’ Persian clover, no germination occurred at 40 °C. ‘Mihi’ Persian clover had greater tolerance to high temperatures with its fastest germination rate at 33 °C and 40% of its seed population germinated at 40 °C. The ability of ‘Mihi’ Persian clover to germinate at high temperature is unknown. ‘Mihi’, a soft seeded cultivar that belongs to a subspecies majus, is developed for use in irrigated farm for forage production. Cultivars from subspecies majus may have been selected for early germination under warm conditions, as they are often sown under irrigation in late summer (February), when temperatures can still be high (Anonymous Citation2003).
In the moist temperate climate of New Zealand, the soil temperatures that seeds would experience were within the sub-optimal range. However, during dry summer, near soil surface temperature has been reported to exceed 30 °C in the Bay of Plenty (Watson et al. Citation1996) and >40 °C at the ‘Ashley Dene’ dryland farm, Canterbury (R. Sim, unpubl. data). The ability of ‘Mihi’ Persian clover to germinate at 40 °C suggests that in any given population, there is a small percentage of seeds that can germinate outside the optimum temperature range. From an ecological perspective, this may be an adaptive strategy for the survival of an annual species under extreme (>37.5 °C) soil temperature conditions.
Cardinal temperatures for germination of ‘Cefalu’ arrowleaf, ‘Bolta’ balansa, ‘Prima’ gland and ‘Mihi’ Persian clovers showed that these species are adapted to a broader range of temperatures compared with subterranean clover and the adventive annual clovers namely, haresfoot (Trifolium arvense), suckling (T. dubium), cluster (T. glomeratum) and striated (T. striatum) clovers (Boswell et al. Citation2003; Lonati et al. Citation2009). This means that ‘Cefalu’ arrowleaf, ‘Bolta’ balansa, ‘Prima’ gland and ‘Mihi’ Persian clovers could inhabit the same ecological niche as the subterranean clover and adventive clovers, and also germinate in warmer areas where some other species cannot. In addition, ‘Cefalu’ arrowleaf, ‘Bolta’ balansa, ‘Prima’ gland and ‘Mihi’ Persian clovers germinate quicker than subterranean and white clovers (Moot et al. Citation2000). The thermal time requirements for germination of ‘Cefalu’ arrowleaf, ‘Bolta’ balansa, ‘Prima’ gland and ‘Mihi’ Persian clovers were similar () to the adventive clovers (Boswell et al. Citation2003; Lonati et al. Citation2009). Their similarity in temperature response and germination rate suggests that ‘Cefalu’ arrowleaf, ‘Bolta’ balansa, ‘Prima’ gland and ‘Mihi’ Persian clovers may be suitable for over-sowing in hill and high country where the adventive clovers are abundant (Maxwell et al. Citation2010).
Within the range of sub-optimal temperatures, the final germination of each species was above 50%. The concept of thermal time summarized the range of individual temperature responses () into a single coefficient that can be used for all temperatures in the sub-optimal range. For example, thermal time calculated for germination of ‘Cefalu’ arrowleaf clover was 30 °Cd, hence at 5 °C ‘Cefalu’ arrowleaf could be expected to germinate in six days compared with two days at 15 °C. In all species, the Tbs calculated were ≤4.5 °C and were not different from 0 °C (). These values were consistent with those reported by Monks (Citation2009) and Lonati (Citation2009) and therefore suggest that future work in annual clover species could assume a Tb of 0 °C for germination.
The use of 95% confidence interval to determine whether Tb was different from 0 °C () highlights the need to understand bases and limits of using linear relationships to describe development rate with temperature. This is because the linearity between development rate and temperature is only limited for a short range of temperatures. This relationship is practically exponential for temperatures approaching the Tb (Angus et al. Citation1981; Bonhomme Citation2000). In this study, the lowest temperature used for germination tests was 5 °C. At too low a temperature (<5 °C), the enzyme is insufficiently flexible to carry the reaction; hence seeds either took too long to germinate or eventually rotted and died (Toole et al. Citation1956). Given that the Tb is calculated from an extrapolation of the linear relationship between development rate and temperature, this method excludes the exponential relationship near the physiological Tb. Therefore, calculated Tb from extrapolation is often higher than the actual (physiological) Tb for which development is zero. For example, the Tb extrapolated for ‘Mihi’ Persian clover based on the linear relationship was 4.5 °C. In reality, there is likely to be some germination occurring below the calculated Tb because germination was still above 80% at 5 °C ().
Non-linear models such as Lactin (Lactin et al. Citation1995), Beta (Yan & Hunt Citation1999), line plus exponential and critical exponential (standard curves from Genstat) were also tested to define cardinal temperatures for germination in this study but none of these models gave a better fit to the data points (based on the R2 value) compared with the ‘broken stick’ linear model. Therefore, the use of the linear model was considered appropriate to describe development rate with temperature. It emphasizes the idea that Tb is only an approximation of the actual temperature threshold (Angus et al. Citation1981; Bonhomme Citation2000). Determining Tb is crucial to calculate thermal time accumulation between phenophases and when 95% confidence interval for Tb includes 0 °C, it is possible to re-analyse thermal time using a Tb of 0 °C to allow comparisons among species (Moot et al. Citation2000).
Conclusions
The use of a linear model was appropriate to define the cardinal temperatures and thermal time requirements for germination of annual clovers. The thermal time requirements for germination were highest for ‘Cefalu’ arrowleaf (34 °Cd) and lowest for ‘Mihi’ Persian (25 °Cd) clovers. ‘Mihi’ Persian clover had an optimum temperature (Topt) of 32.6 °C while all the other species had a Topt between 16–20 °C. The effect of temperature on germination showed that ‘Mihi’ Persian clover seeds can survive under extreme temperature beyond 35 °C.
Acknowledgements
H. Nori acknowledges the Ministry of Higher Education Malaysia for a scholarship. Part of this work was funded through the ‘Pastoral 21’ programme, supported by Beef + Lamb New Zealand.
References
- Angus JF, Cunningham RB, Moncur MW, Mackenzie DH 1981. Phasic development in field crops. I. Thermal response in the seedling phase. Field Crops Research 3: 365–378. 10.1016/0378-4290(80)90042-8
- Anon. 2003. Persian clover. Agfact P2.5.26. 4th Edition, Department of Primary Industry, Fisheries and Mines. Northern Territory Government. http://www.dpi.nsw.gov.au/agriculture/field/pastures-and-rangelands/species-varieties/factsheets/persian-clover (accessed xx xxxx xxxx).
- Arnold SM, Monteith JL 1974. Plant development and mean temperature in a teesdale habitat. Journal of Ecology 62: 711–720. 10.2307/2258951
- Bonhomme R 2000. Bases and limits to using ‘degree.day’ units. European Journal of Agronomy 13: 1–10. 10.1016/S1161-0301(00)00058-7
- Boswell CC, Lucas RJ, Lonati M, Fletcher A, Moot DJ 2003. The ecology of four annual clovers adventive in New Zealand grasslands. In: Moot DJ ed. Legumes for dryland pastures, Proceedings of a New Zealand Grassland Association (Inc.) Symposium, 18–19 November, Canterbury, New Zealand, Lincoln University. Pp. 175–184.
- Campbell A, Frazer BD, Gilbert N, Gutierrez AP, Mackauer M 1974. Temperature requirements of some aphids and their parasites. Journal of Applied Ecology 11: 431–438. 10.2307/2402197
- Charlton D, Stewart A 2003. Pasture and forage plants for New Zealand. Grassland Research and Practice Series 8. Dunedin, New Zealand, New Zealand Grassland Association and New Zealand Grassland Trust.
- Dear BS, Sandral GA, Nutt BJ, Wilson B, Rodham AC, Taylor J 2001. Gland clover – a new insect resistant legume. Proceedings of 16th Annual Conference Grassland Society of New South Wales. Wagga Wagga, New South Wales, Grassland Society of New South Wales. Pp. 63–64.
- Dear BS, Sandral GA, Wilson BCD, Rodham CA, McCaskie P 2002. Productivity and persistence of Trifolium hirtum, T. michelianum, T. glanduliferum and Ornithopus sativus sown as monocultures or in mixtures with T. subterraneum in the south-eastern Australian wheat belt. Australian Journal of Experimental Agriculture 42: 549–556. 10.1071/EA01138
- Evans PM, Mills A 2008. Arrowleaf clover: potential for dryland farming systems in New Zealand. Proceedings of the New Zealand Grassland Association 70: 239–243.
- Evans PM, Zhang XZ, Riffkin PA 2003. Annual pasture legumes for farming systems in cool-temperate areas with summer soil moisture deficits. In: Moot DJ ed. Legumes for dryland pastures, Proceedings of a New Zealand Grassland Association (Inc.) Symposium, 18–19 November, Canterbury, New Zealand, Lincoln University. Pp. 149–154.
- ISTA 2004. International rules for seed testing. Zurich, International Seed Testing Association.
- Lactin DJ, Holliday NJ, Johnson DL, Craigen R 1995. Improved rate model of temperature-dependent development by arthropods. Environmental Entomology 24: 68–75.
- Lonati M, Moot DJ, Aceto P, Cavallero A, Lucas RJ 2009. Thermal time requirements for germination, emergence and seedling development of adventives legume and grass species. New Zealand Journal of Agricultural Research 52: 17–29. 10.1080/00288230909510485
- Maxwell TMR, Moir JL, Edwards GR 2010. Influence of environmental factors on the abundance of naturalised annual clovers in the South Island hill and high country. Proceedings of the New Zealand Grassland Association 72: 165–169.
- Monks DP 2009. The vegetative and reproductive development of balansa clover. PhD thesis. Canterbury, Lincoln University.
- Monks DP, Moot DJ, Smith MC, Lucas RJ 2008. Grazing management for regeneration of balansa clover in a cocksfoot pasture. Proceedings of the New Zealand Grassland Association 70: 233–238.
- Monks DP, SadatAsilan K, Moot DJ 2009. Cardinal temperatures and thermal time requirements for germination of annual and perennial temperate pasture species. Agronomy New Zealand 39: 95–110.
- Moot DJ, Scott WR, Roy AM, Nicholls AC 2000. Base temperature and thermal time requirements for germination and emergence of temperate pasture species. New Zealand Journal of Agricultural Research 43: 15–25. 10.1080/00288233.2000.9513404
- Smetham ML 2003. A review of subterranean clover (Trifolium subterraneum L.): its ecology, and use as a pasture legume in Australasia. Advances in Agronomy. Florida, Academic Press. Pp. 303–350.
- Toole EH, Hendricks SB, Borthwick HA, Toole VK 1956. Physiology of seed germination. Annual Review of Plant Physiology 7: 299–324 10.1146/annurev.pp.07.060156.001503
- Watson RN, Harris SL, Bell NL, Neville FJ 1996. Deferred grazing to enhance white clover content in pastures. Agronomy Society of New Zealand Special Publication No. 11. Grassland Research and Practice Series No. 6. Dunedin, New Zealand, New Zealand Grassland Association and New Zealand Grassland Trust.
- Yan W, Hunt LA 1999. An equation for modelling the temperature response of plants using only the cardinal temperatures. Annals of Botany 84: 607–614. 10.1006/anbo.1999.0955