Abstract
The role of interspecific competition in regulating Cirsium arvense in newly sown pasture was investigated in two pot experiments in Canterbury, New Zealand, where it was grown in the presence and absence of Lolium perenne and Trifolium repens. In Experiment 1 (2006–07), where C. arvense was established as transplanted seedlings, it competitively excluded its neighbours when they were maintained between 20 and 60 mm in height. Coexistence occurred when the neighbour height was maintained between 100 and 150 mm, while competitive exclusion of C. arvense resulted when the neighbours were not trimmed. In Experiment 2 (2007–08), where C. arvense was established from small root cuttings, it was out-competed regardless of neighbour height. The results support the existence of a ‘zone threshold’ along a gradient of increasing pasture height (competitiveness) within which the weed and sown species can coexist, and below and above which the weed or the sown species exclude the other.
Introduction
Californian thistle (Cirsium arvense L. Scop.) occurs as an economically significant agricultural weed throughout much of the temperate world (Donald Citation1990; Skinner et al. Citation2000). Once established it is notoriously difficult to control, largely because of its extensive creeping root system (Leathwick & Bourdôt Citation2012). In New Zealand, a recent farmer survey indicates that in late summer when the weed’s ground cover peaks it excludes grazing on 6% of the country’s grazing land (Bourdôt et al. Citation2013). In these pastures, and depending on terrain limitations, control options include chemical herbicides (Hartley et al. Citation1984; Grekul & Bork Citation2007; Moyo Citation2008), mowing (Hurrell & Bourdôt Citation1996), grazing (Rolston et al. Citation1981; Hartley & Thomson Citation1982; Hartley et al. Citation1984; De Bruijn & Bork Citation2006) and biological control (Bourdôt et al. Citation1995; Drlik et al. Citation2000; Bourdôt et al. Citation2006; Cripps et al. Citation2011). All of these directly target C. arvense and reduce its ability to compete with its neighbouring pasture species, with varying degrees of success.
Enhancing the competitive ability of neighbouring plant species in a pasture through grazing management may be another option for controlling pasture populations of C. arvense. Although many studies have shown that competing crops can suppress the growth and survival of C. arvense (Hallgren Citation1976; Bacher & Schwab Citation2000; Edwards et al. Citation2000), fewer have focused on competition from sown pasture species (Bourdôt Citation1996; Edwards et al. Citation2000) and some of these indicate that C. arvense may be much less affected by its neighbours than are other thistle species (Bourdôt Citation1996). Nevertheless, several experiments that have manipulated the height of neighbouring pasture plant species show that shoot density and biomass in C. arvense decreases with increasing height of the simulated grazed pasture sward (Eerens et al. Citation2002; Cripps et al. Citation2010). A sensitivity of C. arvense to competition is also illustrated in a field experiment conducted in Alberta, Canada where the weed’s year-end biomass was lowest (4% of sward biomass) when neighbouring pasture species were not trimmed (a simulated deferred grazing regime) and highest (35% of sward biomass) when the neighbours were trimmed frequently to a low level during the growing season (a simulated set-stocking grazing regime) (De Bruijn et al. Citation2010).
Other evidence for the sensitivity of C. arvense to interspecific plant competition in a pasture comes from experiments with herbivorous insects. In a field experiment in Bern, Switzerland which manipulated a population of the beetle Cassida rubiginosa, at high beetle densities, 50% of C. arvense plants died in seeded herb plots and survivors were stunted. By contrast, there were no mortalities or stunting in non-seeded plots implying that competition from the seeded herbs predisposed C. arvense to the effects of herbivory from the beetle (Bacher & Schwab Citation2000). Other field and pot experiments with biological control agents also reveal that interspecific competition reduces the performance of C. arvense (Ang et al. Citation1994a,Citationb; CitationFerrero-Serrano et al. 2008; Knudson et al. Citation2012).
Given the evidence for a sensitivity in C. arvense to interference from neighbouring pasture plants it seems plausible that its neighbours, and how their competitive ability is modified under alternative grazing systems, may explain the variation in the ground cover of this weed between different pastoral farming systems in New Zealand (Bourdôt et al. Citation2013). By chance, C. arvense-free pastures may have been grazed in a manner that has promoted the growth of the sown species resulting in the competitive exclusion of C. arvense. On the other hand, pastures supporting monoculture populations of the weed, where the sown pasture species have not persisted within the areas now occupied by C. arvense clones, might be explained by high-intensity/high-frequency grazing management (e.g. heavy set stocking) that has inadvertently reduced the competitive ability of the sown species to an extent that has enabled C. arvense to exclude the sown species.
In the current two experiments we test the hypothesis that ‘interspecific competition from neighbouring Lolium perenne and Trifolium repens, species typically sown for pasture improvement, regulates population size and biomass in C. arvense’.
Methodology
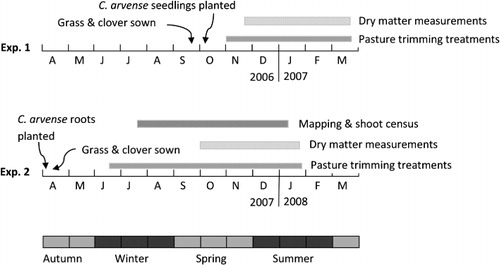
Two experiments, one in each of two consecutive years, were conducted. They simulated different levels of competition from a pasture towards C. arvense as might occur under different selective pasture grazing management regimes by manipulating the average height of sown neighbours during the growing season.
Experiment 1
Design
During the 2006–07 growing season, an experiment was conducted in plastic crates on an open area on the AgResearch campus at Lincoln, Canterbury, New Zealand (−43.6375°S, 172.4796°E) to determine the effect of interspecific competition on the growth of C. arvense and associated pasture species Lolium perenne (perennial ryegrass) and Trifolium repens (white clover). The experimental design was a split-plot with four pasture trimming heights, five times of harvest and six replicates (laid out in blocks) with the pasture trimming heights as the main-plots and the times of harvest as the sub-plots giving 120 sub-plots (crates) in total (4 trimming heights × 5 harvests × 6 replicates = 120 sub-plots). Each main-plot was a group of five adjacent crates. The pasture trimming height treatments were: ‘No pasture’ (C. arvense monoculture); ‘Short pasture’ (C. arvense + pasture maintained between 20 and 60 mm tall); ‘Long pasture’ (C. arvense + pasture maintained between 100 and 150 mm tall); and ‘Hay’ (C. arvense + pasture allowed to grow freely without trimming). The ‘Short pasture’ and ‘Long pasture’ treatments simulated sheep and dairy cattle grazing, respectively (Cosgrove & Edwards Citation2007), whereas the ‘Hay’ treatment simulated a pasture that is not grazed at all during the growing season.
Plant establishment
The seeds of C. arvense used to establish the plants for the experiment came from two sources: a farm at Templeton, in Canterbury (−43.5545°S, 172.4497°E) where the seeds were collected in 1999 (Line A) and in 2000 (Line B) and stored at 5 °C; and from a farm at Tai Tapu in Canterbury (−43.6549°S, 172.5648°E) (Line C) where seeds were collected in late summer 2006. On 25 August 2006, seeds from each of the three lines were dusted with a 50/50 w/w mix of benomyl (Benlate, 500 g ai/kg) and captan (Captan, 800 g ai/kg) fungicides and sown into plastic trays containing moistened vermiculite (two, four and two trays of seed lines A, B and C, respectively). The trays were placed in a Contherm incubator at 20 °C in the dark and on 18 September 2006 seedlings (in excess of the 600 [120 plots × 5 plants/crate] required for the experiment) were ‘pricked out’ as cotyledonary individuals into 25 mL Paperpot (Lannen Tehtaat Oy) root trainers and grown on until they had the first pair of true leaves.
The key steps in this experiment are illustrated in . On 22 September 2006, seeds of Certified Basic Nui perennial ryegrass (L. perenne) and Certified Basic Huia white clover (T. repens) were mixed at a ratio of 0.87/0.13 w/w and broadcast at 0.5 g/crate (equivalent to 20 kg/ha and 3 kg/ha for the L. perenne and T. repens, respectively, typical sowing rates for these species) on to the surface of 90 crates filled with a bark-based potting mix containing Osmocote 6-month slow-release fertiliser. After sowing, a thin layer of the potting mix was sprinkled to cover the seeds, bird scarers were placed in the vicinity and an overhead irrigation system prevented any moisture deficit in the pasture swards. The crates measured 335 (length) × 335 (width) × 280 (height) mm (31.4 L volume and 0.112 m2 surface area) and contained nine bottom drain holes. Each was filled to the top with a bark-based potting mix containing Osmocote® 6-month slow release fertiliser.
On 6 October 2006, five C. arvense seedlings were planted into each of the 120 crates. The seedlings were at the full first-true-leaf stage and L. perenne and T. repens seedlings were beginning to emerge. The crates of replicate blocks 1, 3 and 5 received seedlings from Line C seeds and those of replicate blocks 2, 4 and 6 received seedlings from Line A and B seeds. A plywood planting guide was used to position the five seedlings in a cross pattern with the four outer ones in the four corners of the crate, 70 mm from both edges of the crate, with the fifth seedling in the centre of the crate. Each seedling was therefore 138 mm from its nearest neighbour.
Trimming
The two treatments in which the height of the L. perenne/T. repens component of mixed-species swards was manipulated (‘Short pasture’ and ‘Long pasture’) were achieved by regular trimming using scissors and a cutting height guide from November 2006 until late March 2007 (). The L. perenne/T. repens in the ‘Short pasture’ treatment was trimmed to a height of 20 mm when the average height of the tillers reached 60 mm, while for the ‘Long pasture’ treatment, the trimming was less frequent and maintained the L. perenne/T. repens component between 100 and 150 mm in height. The trimmings were discarded. C. arvense was not trimmed in any of the treatments in order to simulate completely selective grazing (De Bruijn et al. Citation2010) and neither component was trimmed in the ‘Hay’ treatment in order to simulate a system in which grazing does not occur.
Data collection
Five destructive harvests were made at 22–25 day intervals from late spring until early autumn (on 23 November 2006, 12 December 2006, 4 January 2007, 29 January 2007 and 22 March 2007). At each harvest, the aerial shoots of C. arvense were counted and, along with the aerial shoots of L. perenne and T. repens (combined), were cut off at the point of emergence from the potting mix, dried at 60 °C for 48 h and weighed. The creeping roots and subterranean shoots of C. arvense were separated manually from the potting mix, washed with water to remove any potting mix debris, and then dried at 60 °C for 48 h prior to weighing. The roots of L. perenne and T. repens could not be retrieved due to fragmentation and losses during processing and were discarded. From the third harvest on, replicates 1 and 6 were removed from the experiment to keep within resource limits but the 40 crates were grown on to provide roots for establishing Experiment 2.
Data analysis
The dry mass data for the creeping roots, subterranean shoots and aerial shoots of C. arvense, the combined L. perenne/T. repens component, and the aerial shoot mass of C. arvense, expressed as a percentage of the total sward aerial dry mass, were analysed using an analysis of variance for a split-plot design. Data from only replicate blocks 2, 3, 4 and 5 were used in the analysis since replicates 1 and 6 were discontinued as explained in the previous paragraph. Any difference between the two seed lines was removed as part of the block term in the analysis of variance. Log10 transformation was necessary for all data sets prior to analysis to ensure that the homogeneity of variance assumption of the analysis of variance was satisfied. Mean separation was carried out using the unrestricted least significant difference (LSD) procedure (Saville Citation1990).
Experiment 2
This experiment, conducted during the 2007–08 growing season immediately following Experiment 1, differed in that the C. arvense plants were established using root fragments and the dynamics of the C. arvense shoot population was measured through regular mapping and census. The key steps are illustrated in .
Design
The experimental design was a split-plot with the same four treatments used in Experiment 1, five times of harvest and four replicates (laid out in blocks), with the treatments as the main-plots and the times of harvest as the sub-plots, giving 80 sub-plots (crates) in total (4 treatments × 5 harvests × 4 replicates).
Plant establishment
Root fragments of C. arvense containing root buds, taken from plants growing in the spare replicate 1 and 6 crates from Experiment 1, were planted on 3 April 2007 () by placing two 1.25 g fragments per crate, separated by 50–100 mm and covered by potting mix, in a 75 mm deep trench in the central third of each of the 80 crates. It was estimated by counting the buds on several root fragments that 2.5 g of fresh root would yield on average about five initial adventitious shoots, the same initial shoot density as in Experiment 1. The planted root fragments varied in length from 50–150 mm and in diameter from 1.5–5 mm. The roots used in replicate blocks 1 and 3 were from Tai Tapu plants (Line C) and those in replicate blocks 2 and 4 were from Templeton plants (Lines A and B). Eight days later, seeds from the L. perenne/T. repens seed mixture used in Experiment 1 were broadcast on to the surface of the potting mix in 60 of the crates and lightly raked in (the remaining 20 becoming the C. arvense monoculture treatment).
Trimming
Trimming of the L.perenne/T. repens components of the swards began on 18 June 2007 using the same procedure as in Experiment 1. The interval between cutting was 2–2.5 weeks from early August until the end of October and then approximately weekly until the end of the experiment in January 2008 ().
Data collection
Two sets of measurements were made. The first was a series of destructive harvests in which the dry mass of the four variables measured in Experiment 1 and the percentage of the sward aerial biomass comprising C. arvense were obtained using the same methods as in Experiment 1. The harvests were made at 22–25 day intervals initially (on 1 and 23 October 2007, 15 November 2007, 10 December 2007); the last harvest was made on 15 January 2008, following a 36 day interval. The second set of measurements comprised a census of all C. arvense shoots on each of the 16 (4 treatments × 4 replicates) plots (crates) destined for destructive harvest on the final occasion. This census was done on each of 11 occasions (13 July 2007; 6 and 19 September; 4, 16 and 30 October; 15 and 29 November; 11 and 27 December 2007; 15 January 2008). At each census, the shoots from previous censuses were classified as alive or dead and any new shoots were tagged with a twisted coloured wire marker and recorded.
Data analysis
The dry mass data for each of the measured variables was analysed in the same way as for Experiment 1. For the additional shoot ‘census’ data collected, the final cumulative total births, deaths and net population size were square-root transformed and analysed using an analysis of variance for a randomised complete block design.
Results
Dry matter growth
In Experiment 1, growth of the roots and subterranean shoots of C. arvense decreased the further the trimming height of the neighbouring pasture (L. perenne/T. repens) was increased above the ground (–). Although growth in both components continued throughout the experimental period under all treatments, when the sward was never cut (‘Hay’) the subterranean shoots were reduced proportionately more than was the root mass for the first four harvests ( cf. 2A). In Experiment 2, effects of the neighbouring species were also evident and, proportionately, considerably greater than in Experiment 1 ( cf. 2A and 2D cf. 2B). The effects of the neighbouring species became evident earlier than in Experiment 1 and while the roots and subterranean shoots continued to grow throughout the experimental period in both the ‘Short pasture’ and ‘Long pasture’ treatments, the relative rates of growth were apparently lower under these treatments compared with the C. arvense monoculture (‘No pasture’) and were negative under the ‘Hay’ treatment (–).
![Figure 2 Growth responses (log10[g dry mass/m2] averaged over four replicates) of the creeping roots (A, C) and subterranean shoots (B, D) of Cirsium arvense to the presence of neighbouring pasture species Lolium perenne and Trifolium repens maintained at different heights in Experiments 1 and 2: No pasture (Display full size); Short pasture (Display full size); Long pasture (Display full size); Hay (Display full size). The two vertical bars are the two LSD (5%) values for the trimming treatment by harvest time interaction table as output by an analysis of variance for a split-plot design. The right-hand bar is for any comparison between times of harvest means for a particular trimming treatment. The left-hand bar is for any other comparison of means, including the comparison of trimming treatment means for a particular time of harvest. The x-axis labels align with the first day of the month.](/cms/asset/30508cf8-4f32-47e8-84ab-5eb0e2a28ea8/tnza_a_941507_f0002_b.jpg)
The responses of the growth in dry matter of the aerial shoots of C. arvense to the neighbouring pasture trimming height treatments in Experiment 1 reveal a sharp dichotomy between the ‘Short pasture’ and ‘Long pasture’ treatments (). The C. arvense aerial shoot populations declined in dry mass from December onwards under both the ‘Long pasture’ and ‘Hay’ treatment. By contrast, the aerial shoot dry mass under the ‘Short pasture’ treatment continued to increase, by late March equalling that of the C. arvense monoculture (‘No pasture’) treatment (). The opposite effects were evident in the growth of the aerial parts of the neighbouring pasture plants (). The net effect of this competition was that under the ‘Short pasture’ trimming treatment, C. arvense rapidly dominated so that its aerial shoot population contributed 73% of the sward dry mass by mid-November and 99% by late January (). Under the ‘Long pasture’ and ‘Hay’ treatments, the opposite was true with the C. arvense contribution to the sward declining from 31% in mid-November to 16% in late March with the ‘Long pasture’, and from 13% to 1% under the ‘Hay’ treatment (). In Experiment 2, there was no evidence of the dichotomy in the response of C. arvense to the neighbouring pasture trimming height treatments. Here the dry mass of the C. arvense aerial shoot population was reduced under all three trimming regimes () and the converse applied to the growth of the aerial parts of the neighbouring pasture plants (). The net effect of this competition was that C. arvense remained a minor component of the sward under all three neighbouring pasture trimming treatments, although increasing during the October to mid-January period from 0.61% to 14% and from 0.02% to 1.8% of the sward under the ‘Short pasture’ and ‘Long pasture’ treatments, respectively (). Under the ‘Hay’ treatment, the C. arvense contribution was less than 0.05% throughout the experiment although this estimate involved many zero values in the underlying aerial shoot mass data and the ‘Hay’ treatment was therefore excluded both from the analysis and from .
![Figure 3 Growth responses (log10[g dry mass/m2] averaged over four replicates) of the aerial shoots of Cirsium arvense (A, D) and the neighbouring pasture species Lolium perenne and Trifolium repens (B, E) where the latter were maintained at different heights, and the percentage contribution that C. arvense made to the total sward (C, F) in Experiments 1 and 2: No pasture (Display full size); Short pasture (Display full size); Long pasture (Display full size); Hay (Display full size). In D and F, values for the ‘Hay’ treatment were mostly zeroes and are excluded from the analyses. The two vertical bars are the two LSD (5%) values for the trimming treatment by harvest time interaction table as output by an analysis of variance for a split-plot design. The right-hand bar is for any comparison between times of harvest means for a particular trimming treatment. The left-hand bar is for any other comparison of means, including the comparison of trimming treatment means for a particular time of harvest. In C and F, the error bars exclude the ‘No pasture’ treatment where the sward was always 100% C. arvense. The x-axis labels align with the first day of the month.](/cms/asset/31f356ed-fade-4955-8d1d-682b58997645/tnza_a_941507_f0003_b.jpg)
The effects of the treatments in Experiments 1 and 2 are illustrated in the photographs in .
Shoot population growth
The analysis of the aerial shoot population data collected by the sequential censuses during Experiment 2 reveals that the birth of C. arvense shoots was reduced as the average height of the neighbouring pasture increased (; ). By contrast, there was no evidence that the death of these shoots was affected (; ). The reduction in shoot births became increasingly more pronounced under the ‘Long pasture’ and ‘Hay’ treatments (–). When neighbouring pasture was present, the shoot deaths began to occur early in the experiment (–), whereas the few deaths in the C. arvense monoculture occurred only late in the experiment (). Overall, based on the back-transformed square-root means, the death rates of the shoots arising during the experiment were 72%, 18%, 17% and 1% in the ‘Hay’, ‘Long’, ‘Short’ and C. arvense monoculture treatments, respectively ().
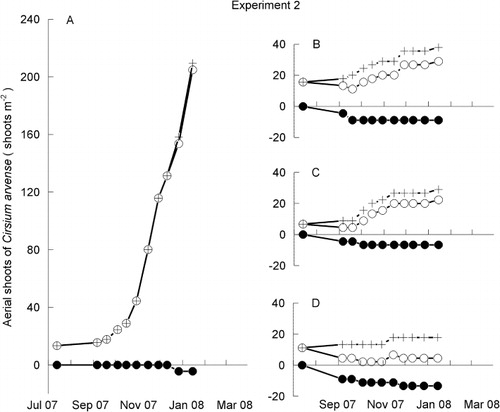
Table 1 Cumulative total numbers of aerial shoot births and deaths and net population size (shoots/m−2) at the end of Experiment 2 as illustrated in . For calculating the linear contrast, average pasture heights were taken to be 0, 40, 125 and 335 mm for the four treatments, respectively (in the order shown in the table).
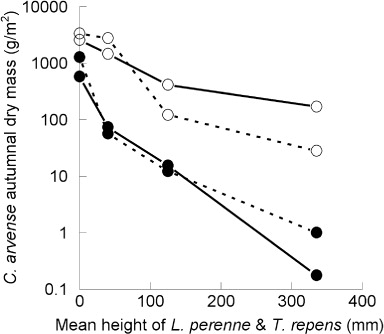
Relationship between C. arvense autumnal dry mass and pasture height
The effect of increasing mean neighbour height and end-of-experiment accumulated C. arvense shoot and root dry mass in both experiments is illustrated in . Evidently there was an exponential decline in both shoot and root mass in both experiments with increasing height of the neighbouring L. perenne and Trifolium repens.
Discussion
The results presented here indicate the existence of a ‘zone-type threshold’ along a gradient of increasing pasture height (competitiveness) within which C. arvense and sown pasture plant species can coexist, and below and above which the weed or the sown species will exclude the other, and provide support for the hypothesis that ‘interspecific competition from neighbouring L. perenne and T. repens, species typically sown for pasture improvement, plays a role in regulating population size and biomass in C. arvense’.
In Experiment 1, where the seedlings of C. arvense were planted in spring, 14 days after the sowing of neighbouring pasture seeds at a density equivalent to the seed sowing rate for pasture establishment in New Zealand, the growth in dry matter of all subterranean and aerial components of C. arvense declined with increasing height of the neighbouring pasture component of the sward (– and ), while the growth of the neighbouring pasture increased (). This result implies that these neighbouring pasture species competed more intensely with C. arvense the greater the height they were trimmed. This effect on the growth of C. arvense (–) is illustrated by the steep declines in end-of-experiment accumulated dry mass of the aerial shoots and roots in response to increasing mean height of the pasture species (). These results support the idea that deferred grazing or high-intensity/low-frequency rotational grazing systems where the grazed neighbours of the weed have time to recover and compete, can reduce the presence of C. arvense in a pasture as was demonstrated in natural field populations of the weed in Alberta, Canada (De Bruijn et al. Citation2010). The results also suggest that high-intensity/high-frequency grazing systems such as set stocking (continuous grazing) are likely to promote the weed through reducing the ability of the grazed neighbours to compete, as was also found in the Alberta experiment.
The trajectories over time of the contribution that C. arvense made to the total sward aerial dry mass under the different trimming height treatments provide an unexpected insight into the interaction between C. arvense and its neighbours (). When the neighbour height was maintained between 20 and 60 mm (‘Short pasture’), C. arvense rapidly dominated the sward so that L. perenne and T. repens were almost excluded by the end of the growth season (). The latter had all but disappeared by the end of the experiment leaving a virtual monoculture of C. arvense with a root system, and thus a potential population growth rate (Leathwick & Bourdôt Citation2012) equal to that of the C. arvense monoculture (‘No pasture’) treatment. It therefore seems probable that competitive exclusion of the neighbouring sown pasture species would result within one or a few generations under the ‘Short pasture’ treatment. By contrast, under the ‘Hay’ treatment, where the neighbouring species were never trimmed, the downward trajectory of the C. arvense contribution to the sward () implies that competitive exclusion of C. arvense would result within one or a few generations. Between these two extreme outcomes—C. arvense ‘winning’ under the ‘Short pasture’ and ‘losing’ under the ‘Hay’ treatment—coexistence is implied under the ‘Long pasture’ treatment. Here the neighbouring pasture was maintained at 100–150 mm in height resulting in a relatively small decline in the C. arvense contribution to the total sward aerial dry mass (from 101.365 [23%] to 101.117 [13%]) during the course of the experiment ().
The results of Experiment 1 suggest that C. arvense may coexist in an unstable equilibrium in a pasture sward where there is a ‘zone-type threshold’ along a gradient of increasing mean pasture height within which the coexistence can occur. Above the threshold C. arvense is excluded and below the threshold the pasture species are excluded. In Experiment 2, C. arvense was a poor competitor regardless of the neighbour trimming height (–). Here it would seem that the pasture height below which C. arvense would exclude the neighbouring pasture plants is much lower than in Experiment 1 ().
Unfortunately, the experimental design was not able to explain the poorer competitive ability of C. arvense in Experiment 2. Although root cuttings of C. arvense have been observed to often produce rather weak shoots compared with those from seedlings (personal observation), in Experiment 2 the L. perenne and T. repens established root systems relatively earlier than the emerging adventitious shoots of C. arvense. It seems reasonable that this may have put C. arvense at a disadvantage compared with Experiment 1 where C. arvense was transplanted as rooted seedlings. The relatively deep planting (75 mm) of the root cuttings used to establish C. arvense plants in Experiment 2 may, through delaying shoot emergence, also have contributed towards the lower competitive ability of C. arvense in this experiment.
In a real pasture, the neighbour height threshold above which C. arvense is competitively excluded from the sward can be expected to vary. Based on the differing outcomes of Experiments 1 and 2, the relative times of emergence of the competing species may be important. On some farms it may be possible to manipulate this factor in favour of the sown pasture plant species using agronomic practices such as a ‘stale seedbed’ especially where new pastures are sown regularly. However, on many New Zealand farms this may be impossible. For example, many hill country properties have little or no opportunity for cultivation and re-grassing. Also, the emergence of C. arvense shoots in the spring coincides with peak demands for grazing over the period when stock are lactating so that lax or deferred grazing may not always be possible during this period. Emergence also coincides with peak pasture production, which normally outstrips the demands of the lactating animals, resulting in paddocks being closed for hay at this time. Selecting paddocks infested with C. arvense for hay may be a useful approach, as long as grazing is stopped early in the growing season to maximise the competitiveness of the sward against the thistle.
In summary, the results of these two experiments align with those of other simulated grazing experiments (Eerens et al. Citation2002; Cripps et al. Citation2010; De Bruijn et al. Citation2010) that indicate that competition from sown pasture species can regulate population growth in C. arvense. They lead to the inference that, under grazing systems where C. arvense itself is avoided (e.g. when sheep are the grazers), a lax or deferred pasture grazing regime (e.g. long rotational grazing or hay) is likely to be a more effective control strategy than an intensive grazing regime (e.g. set stocking or continuous grazing) particularly when C. arvense is a minor part of the sward such as in a newly established pasture. Under cattle grazing, a high-intensity/low-frequency rotational grazing system, where C. arvense is eaten and the neighbour species have time to regrow and compete between grazings, has proven effective (De Bruijn & Bork Citation2006). Logically, competitive exclusion of C. arvense will not be possible under any grazing system if the weed has already established dense clones that have excluded the potential competitor neighbour plant species. Under these conditions, resowing the pasture would be necessary before implementing a grazing regime to competitively exclude the thistle.
Acknowledgements
We thank the Ministry of Business, Innovation and Employment for funding this research and Carolyn Lusk, weed science technician, AgResearch, Lincoln, for assistance in the establishment, maintenance and harvesting of the two experiments.
References
- Ang BN, Kok LT, Holtzman GI, Wolf DD 1994a. Canada thistle (Cirsium arvense) response to simulated insect defoliation and plant competition. Weed Science 42: 403–410.
- Ang BN, Kok LT, Holtzman GI, Wolf DD 1994b. Competitive growth of Canada thistle, tall fescue, and crownvetch in the presence of a thistle defoliator, Cassida rubiginosa Muller (Coleoptera: Chrysomelidae). Biological Control 4: 277–284. 10.1006/bcon.1994.1035
- Bacher S, Schwab F 2000. Effect of herbivore density, timing of attack and plant community on performance of creeping thistle Cirsium arvense (L.) Scop. (Asteraceae). Biocontrol Science and Technology 10: 343–352. 10.1080/09583150050044619
- Bourdôt GW 1996. Interference between pasture plants and thistles – a review. Plant Protection Quarterly 11: 265–270.
- Bourdôt GW, Harvey IC, Hurrell GA, Saville DJ 1995. Demographic and biomass production consequences of inundative treatment of Cirsium arvense with Sclerotinia sclerotiorum. Biocontrol Science and Technology 5: 11–26. 10.1080/09583159550039981
- Bourdôt GW, Hurrell G, Saville D 2013. Frequency of occurrence and ground-cover of Cirsium arvense on pastoral farms in New Zealand – a farmer opinion survey. New Zealand Journal of Agricultural Research 57: 1–13.
- Bourdôt GW, Hurrell GA, Saville DJ, Leathwick DM 2006. Impacts of applied Sclerotinia sclerotiorum on the dynamics of a Cirsium arvense population. Weed Research 46: 61–72. 10.1111/j.1365-3180.2006.00481.x
- Cosgrove G, Edwards GR 2007. Control of grazing intake. In: Rattray PV, Brookes IM, Nicot A ed. Pasture and supplements for grazing animals. Hamilton, New Zealand Society of Animal Production Inc. Pp. 61–81.
- Cripps MG, Edwards GR, Bourdôt GW, Saville DJ, Hintz HL, Fowler SV 2010. Effects of pasture competition and specialist herbivory on the performance of Cirsium arvense. Biocontrol Science and Technology 20: 641–656. 10.1080/09583151003695407
- Cripps MG, Gassmann A, Fowler SV, Bourdôt GW, McClay AS, Edwards G 2011. Classical biological control of Cirsium arvense: lessons from the past. Biological Control 57: 165–174. 10.1016/j.biocontrol.2011.03.011
- De Bruijn SL, Bork EW 2006. Biological control of Canada thistle in temperate pastures using high density rotational cattle grazing. Biological Control 36: 305–315. 10.1016/j.biocontrol.2005.10.007
- De Bruijn SL, Bork EW, Grekul CW 2010. Neighbor defoliation regulates Canada thistle (Cirsium arvense) in pasture by mediating interspecific competition. Crop Protection 29: 1489–1495. 10.1016/j.cropro.2010.08.010
- Donald WW 1990. Management and control of Canada thistle (Cirsium arvense). Review of Weed Science 5: 193–250.
- Drlik T, Woo I, Swiadon L, Quarles W 2000. Integrated management of Canada thistle. The IPM Practitioner 22: 1–9.
- Edwards GR, Bourdôt GW, Crawley MJ 2000. Influence of herbivory, competition and soil fertility on the abundance of Cirsium arvense in acid grassland. Journal of Applied Ecology 37: 321–334. 10.1046/j.1365-2664.2000.00495.x
- Eerens JPJ, Seefeldt SS, Garry G, Armstrong Ml 2002. Controlling Californian thistle (Cirsium arvense) through pasture management. New Zealand Plant Protection 55: 111–115.
- Ferrero-Serrano Á, Collier TR, Hild AL, Mealor BA, Smith T 2008. Combined impacts of native grasss competition and introduced weevil herbivory on Canada thistle (Cirsium arvense). Rangeland Ecology & Management 61: 529–534. 10.2111/07-142R.1
- Grekul CW, Bork EW 2007. Fertilization augments Canada thistle (Cirsium arvense L. Scop) control in temperate pastures with herbicides. Crop Protection 26: 668–676. 10.1016/j.cropro.2006.06.005
- Hallgren E 1976. Development and competition in stands of ley plants and weeds. Swedish Journal of Agricultural Research 6: 255–261.
- Hartley MJ, Lyttle LA, Popay AI 1984. Control of Californian thistle by grazing management. In: Hartley MJ ed. Proceedings of the 37th New Zealand Weed and Pest Control Conference, 14–16 August, Christchurch. Pp. 24–27.
- Hartley MJ, Thomson NA 1982. Effect and control of Californian thistle in dairy pasture. Proceedings of the New Zealand Grassland Association 43: 104–107.
- Hurrell GA, Bourdôt GW 1996. Sclerotinia sclerotiorum and mowing independently reduce Californian thistle in a sheep pasture. In: O’Callaghan M ed. Proceedings of the 49th New Zealand Plant Protection Conference, 13–15 August, Nelson. Pp. 225–228.
- Knudson J, Meiman P, Brown C, Beck G, Paschke M, Redente E 2012. Canada thistle (Cirsium arvense) response to clipping and seeding of competitive grasses. American Journal of Plant Science 3: 1252–1259. 10.4236/ajps.2012.39151
- Leathwick DM, Bourdôt GW 2012. A conceptual model for the population dynamics of Cirsium arvense in a New Zealand pasture. New Zealand Journal of Agricultural Research 55: 371–384. 10.1080/00288233.2012.728532
- Moyo C 2008. Improving the efficiency of herbicide application to pasture weeds by weed-wiping and spot-spraying. Unpublished thesis, Massey, Palmerston North. 206 p.
- Rolston MP, Lambert MG, Clark DA, Devantier BP 1981. Control of rushes and thistles in pastures by goat and sheep grazing. In: Hartley MJ ed. Proceedings 34th New Zealand Weed and Pest Control Conference, 11–13 August, Blenheim. Pp. 117–121.
- Saville D 1990. Multiple comparison procedures: the practical solution. American Statistician 44: 174–180.
- Skinner K, Smith L, Rice P 2000. Using noxious weed lists to prioritize targets for developing weed management strategies. Weed Science 48: 640–644. 10.1614/0043-1745(2000)048[0640:UNWLTP]2.0.CO;2