ABSTRACT
Animal urine from grazing animals is responsible for the majority of New Zealand’s nitrous oxide (N2O) emissions. A field lysimeter study was conducted to determine the ability of the combination of the nitrification inhibitor dicyandiamide (DCD) and biochar to mitigate N2O emissions from a winter grazing forage crop soil. The results showed that over a 4 month period, applying cow urine at a rate of 700 kg N ha−1 increased N2O emissions from 0.7 kg N2O-N ha−1 to 14.7 kg N2O-N ha−1. The application of DCD at 20 kg ha−1 reduced total N2O emissions by 65%. The application of biochar at 5 t ha−1 had no significant effect on N2O emissions. The combination of DCD with biochar did not lead to greater N2O reductions than DCD alone. These results indicated that DCD was highly effective whereas biochar was ineffective in reducing N2O emissions in the winter forage soil.
Introduction
The agricultural sector produced about half of New Zealand’s greenhouse gas emissions in 2011, and nearly 30% of those emissions were nitrous oxide (N2O) (Ministry for the Environment Citation2013). Nitrous oxide is a powerful greenhouse gas, with a global warming potential about 298 times that of carbon dioxide (CO2) (IPCC Citation2007). Direct and indirect emissions from animal excreta deposited on to soil are responsible for the majority of New Zealand’s N2O emissions. The amount of N2O emitted in New Zealand increased by 29% between 1990 and 2011, primarily because of an increase in the number of dairy cows (Ministry for the Environment Citation2013). An increasing number of dairy herds are taken to graze high-yielding forage crops such as brassica during the winter (Drewry & Paton Citation2005). This can lead to soil compaction and a large number of urine patches in a small area due to the high stocking density. Both urine deposition and animal trampling favour denitrification and contribute to elevated N2O emissions (van Groenigen et al. Citation2005; Menneer et al. Citation2005). Therefore, mitigation options are sought to reduce N losses from winter grazed forage crops.
Nitrification inhibitors such dicyandiamide (DCD) have been shown to reduce agricultural N2O emissions from grazed pasture (e.g. Di & Cameron Citation2002; Di et al. Citation2010; de Klein et al. Citation2011). DCD has also been shown to reduce N2O emissions when applied to compacted soil (Ball et al. Citation2012) and to grazed winter feedlots (Smith et al. Citation2008). DCD inhibits the growth and activity of soil ammonia oxidising microorganisms, which inhibits the nitrification process. Thus there is a reduction in the amount of N2O emitted from nitrification. There is also a reduction in the amount of nitrate () available for denitrification. Furthermore, by reducing
leaching, DCD also reduces indirect N2O emissions (Clough et al. Citation2007).
Biochar is composed of the carbon-rich remains of organic matter heated in the absence of oxygen. Biochar not only has the potential to sequester atmospheric CO2 in soil (Clough & Condron Citation2010), but has also been used as an agricultural soil amendment to mitigate N2O emissions (e.g. Zhang et al. Citation2010; Taghizadeh-Toosi et al. Citation2011). The mechanisms by which biochar suppresses N2O emissions is the subject of considerable ongoing research. Biochar is thought to produce physical, chemical and biological changes within soil, leading to various alterations of the nitrogen cycle (Lehmann & Joseph Citation2009). Several authors (Ding et al. Citation2010; Hale et al. Citation2013; Hollister et al. Citation2013) have demonstrated the ability of biochar to remove ammonium () from solution, while Sarkhot et al. (Citation2013) found biochar removed up to 18% of
from dairy effluent. Moreover, N adsorbed by the addition of biochar to soil has been shown to be plant available, suggesting the potential for increased plant growth (Taghizadeh-Toosi et al. Citation2012; Zheng et al. Citation2013).
Both DCD and biochar independently have the potential to mitigate N2O emissions from urine-amended soil. The elevated soil concentrations that result from the inhibition of the nitrification process when DCD is used may have a synergistic effect with soil amended with biochar, which can remove
from soil.
The potential reduction in soil concentrations from the complementary action of biochar and DCD could reduce soil N2O emission; however, to date, no authors have described the combination of DCD and biochar to reduce soil N2O losses. The purpose of this research was to study the capacity of DCD and biochar together to reduce N2O emissions from a dairy winter forage grazing system.
Materials and methods
Soil, lysimeter collection and climate
A Balmoral stony silt loam, classified as Pallic Orthic Brown Soil (Hewitt Citation2010), Udic Haplustept loamy skeletal (Soil Survey Staff Citation2010), typical of soils used for dairy winter forage grazing in the Canterbury region, South Island of New Zealand, was used in this study. Developed from gravelly glacial outwash alluvium and loess, the Balmoral soil consists of a thin, sandy silt loam topsoil containing about 30% stones. Below the topsoil, the stone and sand content increases substantially, making this soil very free draining. Prior to collecting the lysimeters, the soil at the site had key properties as follows: pH 5.9, Olsen P 24 mg kg−1, ,
,
,
,
, total C 42 g kg−1 and total N 4.1 g kg−1.
Lysimeters were collected during December 2010, using the method described by Cameron et al. (Citation1992). Briefly, steel casings (50 cm diameter × 70 cm depth) were pushed into the soil in small increments, while the surrounding soil was gently scraped away, leaving an undisturbed soil column within the casing. The edges of the soil column were sealed to the casing using molten petroleum jelly, to prevent edge flow effects. The lysimeters were then installed in a lysimeter facility, level with the surrounding soil. The outside of the lysimeters were back-filled to maintain the climatic conditions of the surrounding field.
The lysimeters were planted with the fodder crop kale (Brassica oleracea cv. Regal L.) in January 2011. After germination, the kale plants were thinned to a similar density to that found in a winter forage grazing system. Irrigation was supplied during the summer months at a rate of 15 mm every 3 days. At the time of sowing, a fertiliser mix containing phosphorus (5.5%), potassium (14.7%), sulphur (13.2%), calcium (12.4%), boron (0.37%) and molybdenum (0.02%) was applied to each lysimeter at a rate equivalent to 1600 kg ha−1. Lime, at a rate equivalent to 2500 kg ha−1, was also applied at the time of sowing. The kale was allowed to reach maturity and was harvested in June 2011, prior to applying the treatments to the lysimeters.
The average annual rainfall in the region is about 650 mm, and the average annual temperature about 12.1 °C. Water input throughout the duration of the experiment was maintained at the 75th percentile of the regional average using simulated rainfall (irrigation) from overhead sprinklers when required.
Treatments
Four treatments were randomly allocated to the lysimeters on 29 June 2011: urine (U); urine plus DCD (UD); urine plus biochar (UB); and urine plus DCD and biochar (UDB). A control (C) was used to provide background N2O emission data. All treatments, including the control, were replicated four times. Following harvest of the kale, the soil was wetted to field capacity by adding 10 mm of simulated rainfall. The UB and UDB treatment lysimeters were amended with biochar (Pacific Pyrolysis, ) at the rate of 5 t ha−1, which was incorporated into the top 5 cm of the soil using hand implements to minimise soil disturbance. The soil in all lysimeters was then trampled using cow hoof simulation equipment designed to provide approximately 200 kPa—similar to the pressure exerted by an adult cow hoof (Di et al. Citation2001). The surface of the soil was completely trampled twice, to simulate the heavy grazing typical under forage crops. The heavy trampling ensured the biochar was well mixed into the top 5 cm of soil, and an equal degree of soil disturbance between all treatments. DCD was dissolved in water and applied to the UD and UDB treatment lysimeters at a rate equivalent to 20 kg ha−1, a rate at which preliminary work had shown to be effective in the free-draining, urine-amended soil. Fresh urine was collected from non-lactating dairy cows that were feeding on kale, and analysed for total N concentration. The urine was standardised to 7.0 g N L−1 and applied to all lysimeters, except the controls, at a rate equivalent to 700 kg N ha−1. The controls received an equivalent volume of water.
Table 1. Characteristics of biochar used.
Nitrous oxide emissions
A closed chamber method, similar to that of Hutchison & Mosier (Citation1981), was used to determine N2O emissions. A water-filled trough sealed to the top of each lysimeter provided a gas-tight seal for the metal and polystyrene chamber used during sampling. At each sampling time, chambers 50 cm diameter × 10 cm high were placed on top of the lysimeters for a total of 40 min, with three samples taken 20 min apart. Samples were taken through a rubber septum located in the top of each chamber using a 60 mL syringe and hypodermic needle, and placed in evacuated 6 mL glass vials. Samples for N2O analysis were taken between 1200 and 1400 h at each sampling event. Nitrous oxide concentration was analysed using gas chromatography (SRI 8610GC, gas chromatograph, SRI Instruments).
This study focused on the winter grazing period; consequently, N2O measurements were taken for 4 months, from June to October 2011, representing the approximate time a farmer may leave the paddock fallow before resowing with another crop.
Data analysis
Nitrous oxide emission rates were calculated from the increase in concentration of N2O between the first and second, and second and third gas samples taken at each sampling event (Hutchison & Mosier Citation1981). Daily N2O fluxes were then calculated on the assumption that the calculated hourly flux represented the average hourly flux for that day (de Klein et al. Citation2003). Total N2O emissions were calculated by integrating the daily emission fluxes.
Least significant differences (LSD) were calculated following analysis of variance using the GenStat software package (version 16, VSN International Ltd). Controls were omitted from LSD calculation due to unequal variance between treatments.
The emission factor (EF3), or proportion of applied N that was emitted as N2O, was calculated using Equation (1) (de Klein et al. Citation2003):(1 ) where EF(%) is the emission factor, ‘N2O-N total (treatment)’ is the cumulative total N2O emitted from a urine treatment, ‘N2O-N total (control)’ is the cumulative total N2O emitted from the comparative no-urine treatment, and ‘Urine N (applied)’ is the amount of N added as urine.
Results
Daily N2O emissions
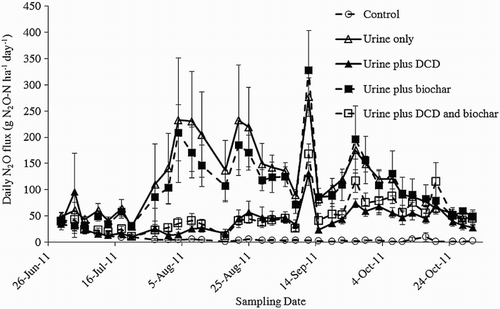
Dairy cow urine applied at 700 kg N ha−1 increased peak N2O emissions from 33 g N2O-N ha−1 day−1 in the control, to 277 g N2O-N ha−1 day−1 in the U only treatment () (P < 0.05). Applying DCD to the soil reduced peak N2O emissions by 53% to 130 g N2O-N ha−1 day−1 in the UD treatment (P < 0.05). Incorporating biochar into the soil had no significant effect on peak N2O, as peak emissions from the UB treatment reached 328 g N2O-N ha−1 day−1 (P > 0.05). The peak N2O emission from the UDB treatment of 168 g N2O-N ha−1 day−1 was not significantly different from the UD treatment (P > 0.05).
Total nitrous oxide emissions
The addition of dairy cow urine increased total N2O emissions from the trampled stony Balmoral soil from 0.7 kg N2O-N ha−1 to 14.7 kg N2O-N ha−1 () (P < 0.05). The application of DCD reduced total N2O emissions by 65% to 5.1 kg N2O-N ha−1 () (P < 0.05). Biochar did not significantly decrease N2O emissions, with N2O emissions from the UB treatment reaching a total of 13.3 kg N2O-N ha−1 (P > 0.05). The UDB treatment emitted a total of 6.1 kg N2O-N ha−1, showing that applying biochar with the DCD provided no improvement in N2O emission mitigation over DCD alone.
Table 2. Total N2O emissions, N2O emission factor and emission reductions from the lysimeters during study period.
Discussion
Results from this study show that treating winter grazed dairy forage soil with the nitrification inhibitor DCD was effective in reducing N2O emissions. DCD reduced total N2O emissions by 65% () and reduced peak N2O emissions by 53% in urine-affected areas of the soil (). The reduction in N2O emissions from the application of DCD presented in this study is similar to those reported elsewhere, from both pastoral and forage soil (e.g. Di et al. Citation2007; Smith et al. Citation2008; Di et al. Citation2010; van der Weerden et al. Citation2012).
In contrast to DCD, the incorporation of biochar into this soil had no significant effect on N2O emissions from dairy cow urine areas. However, other authors have reported that biochar can reduce N2O emissions. For example, in fertilised rice paddy field soil, Zhang et al. (Citation2010) reported a decrease in N2O emissions of 21%–28% when biochar was added at rates of 10–40 t ha−1 and Wang et al. (Citation2011) found biochar reduced N2O emission from rice paddy soil by 61%. Similarly, when applied at a rate equivalent to 15 t ha−1, biochar reduced N2O emissions from soil amended with an N-rich slurry by 47% (Bruun et al. Citation2011). Importantly, Bruun et al. (Citation2011) found no significant reduction in N2O emissions when biochar was applied at a lower rate equivalent to 5 t ha−1 (similar to this study), suggesting that N2O emission reductions from biochar may be rate dependent. In this study, biochar was applied at 5 t ha−1 because this was considered a practical amount for farmers to apply and work into the soil using conventional methods.
Results reported in the literature vary, as some authors have been unable to detect N2O emission reductions when biochar was applied at rates higher than 5 t ha−1. For example, at 10 t ha−1, biochar did not significantly alter N2O emissions from subtropical pasture (Scheer et al. Citation2011), and Clough et al. (Citation2010) found no change in N2O emission from urine-amended soil when biochar was added at a rate of 30 t ha−1. Conversely, Singh et al. (Citation2010) also found that biochar at 10 t ha−1 was effective in reducing N2O emissions from soil. Other factors may have influenced the sorption capacity of the biochar used in this study.
Singh et al. (Citation2010) concluded that biochar feedstock, pyrolysis temperature and biochar porosity can have marked effects on the N2O mitigation potential of biochar when added to soil. Clough et al. (Citation2010) postulated that inhibitory substances in the Pinus feedstock used in their study may have affected nitrifying organisms, and therefore affected the N2O mitigating capacity of the biochar. They also suggested that the biochar was inadequately weathered and therefore the potential cation exchange capacity of the biochar was insufficient to deplete soil .
Some authors (e.g. Clough et al. Citation2010; Singh et al. Citation2010; Wang et al. Citation2011) have shown a temporal change in N2O emissions relative to control treatments when biochar was added to soil. However, N2O emissions from the biochar treatments in this study were not significantly different from the other urine treatments indicating that the biochar did not have a significant effect on emissions. In the long term, biochar may lead to differences in N2O emissions between the biochar and non-biochar treatments. Further research is needed to determine the long-term effects on N2O emissions when DCD and biochar are combined.
The emission factors—or proportion of applied N released by the soil as N2O—reported in this study were within the range of values presented in the review by Cameron et al. (Citation2013), including a similar study on a stony soil in Canterbury (Di & Cameron Citation2006). Furthermore, the 65% reduction in N2O emissions due to DCD in a forage system, as presented here, is very similar to the rate of 67% adopted for pastoral grazing included in reporting to the IPCC by the New Zealand Government (Ministry for the Environment Citation2013). The DCD reduction rate presented here is also close to the average reduction rate of 54% calculated in a review of published results by de Klein et al. (Citation2011).
The rate of biochar application used in this study may have been insufficient to provide any N2O emission mitigation. Further research using higher rates of biochar is required to determine if a rate effect was inhibiting the N2O emission mitigation capacity of the biochar in this study, or whether other factors such as the age of the biochar, the biochar feed stock, or the pyrolysis conditions were not optimal for use in winter grazing systems. Incorporating the biochar into the soil at the time of cultivation (as opposed to the time of grazing) may allow for a greater amount of weathering, and therefore improve the efficacy of biochar in mitigating N2O emissions.
Conclusions
The application of a nitrification inhibitor, DCD, reduced N2O emissions from urine-affected areas of winter grazed dairy forage soil by 65%, while incorporating biochar into the soil had no significant effect on emissions. Combining biochar with DCD did not provide any advantage in N2O emission reductions over applying DCD alone.
Acknowledgements
We would like to thank Steve Moore, Neil Smith, Trevor Hendry, Roger Atkinson and Manjula Premarante of Lincoln University for technical support. DCD has been withdrawn from use on farms in New Zealand and its use is now restricted to research. Protocols to ensure that DCD use in research does not enter the food chain are supported by soil and plant testing for DCD residues.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
We would like to thank the New Zealand Agricultural Greenhouse Gas Research Centre for funding the research.
References
- Ball BC, Cameron KC, Di HJ, Moore S. 2012. Effects of trampling of a wet dairy pasture soil on soil porosity and on mitigation of nitrous oxide emissions by a nitrification inhibitor, dicyandiamide. Soil Use Manage. 28:194–201. doi: 10.1111/j.1475-2743.2012.00389.x
- Bruun EW, Muller-Stover D, Ambus P, Hauggaard-Nielsen H. 2011. Application of biochar to soil and N2O emissions: potential effects of blending fast-pyrolysis biochar with anaerobically digested slurry. Eur J Soil Sci. 62:581–589. doi: 10.1111/j.1365-2389.2011.01377.x
- Cameron KC, Di HJ, Moir JL. 2013. Nitrogen losses from the soil/plant system: a review. Ann App Biol. 162:145–173. doi: 10.1111/aab.12014
- Cameron KC, Smith NP, McLay CDA, Fraser PM, McPherson RJ, Harrison DF, Harbottle P. 1992. Lysimeters without edge flow—an improved design and sampling procedure. Soil Sci Soc Am J. 56:1625–1628. doi: 10.2136/sssaj1992.03615995005600050048x
- Clough TJ, Bertram JE, Ray JL, Condron LM, O’Callaghan M. Sherlock RR, Wells NS. 2010. Unweathered wood biochar impact on nitrous oxide emissions from a bovine-urine-amended pasture soil. Soil Sci Soc Am J. 74:852–860. doi: 10.2136/sssaj2009.0185
- Clough TJ, Condron LM. 2010. Biochar and the nitrogen cycle: introduction. J Environ Qual. 39:1218–1223. doi: 10.2134/jeq2010.0204
- Clough TJ, Di HJ, Cameron KC, Sherlock RR, Metherell AK, Clark H, Rys G. 2007. Accounting for the utilization of a N2O mitigation tool in the IPCC inventory methodology for agricultural soils. Nutr Cycl Agroecosys. 78:1–14. doi: 10.1007/s10705-006-9069-z
- Di HJ, Cameron KC. 2002. The use of a nitrification inhibitor, dicyandiamide (DCD), to decrease nitrate leaching and nitrous oxide emissions in a simulated grazed and irrigated grassland. Soil Use Manage. 18:395–403. doi: 10.1111/j.1475-2743.2002.tb00258.x
- Di HJ, Cameron KC. 2006. Nitrous oxide emissions from two dairy pasture soils affected by different rates of a fine particle suspension nitrification inhibitor, dycyandiamide. Biol Fert Soils. 42:472–480. doi: 10.1007/s00374-005-0038-5
- Di HJ, Cameron KC, Milne J, Drewry JJ, Smith NP, Hendry T, Moore S, Reijnen B. 2001. A mechanical hoof for simulating animal treading under controlled conditions. New Zeal J Agr Res. 44:111–116. doi: 10.1080/00288233.2001.9513465
- Di HJ, Cameron KC, Sherlock RR. 2007. Comparison of the effectiveness of a nitrification inhibitor, dicyandiamide, in reducing nitrous oxide emissions in four different soils under different climatic and management conditions. Soil Use Manage. 23:1–9. doi: 10.1111/j.1475-2743.2006.00057.x
- Di HJ, Cameron KC, Sherlock RR, Shen JP, He JZ, Winefield CS. 2010. Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. J Soil Sediment. 10:943–954. doi: 10.1007/s11368-009-0174-x
- Ding Y, Liu YX, Wu WX, Shi DZ, Yang M, Zhong ZK. 2010. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Poll. 213:47–55. doi: 10.1007/s11270-010-0366-4
- Drewry JJ, Paton RJ. 2005. Soil physical quality under cattle grazing of a winter-fed brassica crop. Aust J Soil Res. 43:525–531. doi: 10.1071/SR04122
- van Groenigen JW, Velthof GL, Bolt FJEvd, Vos A, Kuikman PJ. 2005. Seasonal variation in N2O emissions from urine patches: Effects of urine concentration, soil compaction and dung. Plant Soil. 273:15–27. doi: 10.1007/s11104-004-6261-2
- Hale SE, Alling V, Martinsen V, Mulder J, Breedveld GD, Cornelissen G. 2013. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere. 91:1612–1619. doi: 10.1016/j.chemosphere.2012.12.057
- Hewitt AE. 2010. New Zealand soil classification. Landcare Research Science Series(1). Lincoln: Manaaki Whenua Press.
- Hollister CC, Bisogni JJ, Lehmann J. 2013. Ammonium, nitrate, and phosphate sorption to and solute leaching from biochars prepared from corn stover (Zea mays L.) and oak wood (Quercus spp.). J Environ Qual. 42:137–144. doi: 10.2134/jeq2012.0033
- Hutchison GL, Mosier AR. 1981. Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci Soc Am J. 45:311–316. doi: 10.2136/sssaj1981.03615995004500020017x
- IPCC. 2007. Climate change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge, UK: Cambridge University Press.
- de Klein CAM, Barton L, Sherlock RR, Li Z, Littlejohn RP. 2003. Estimating a nitrous oxide emission factor for animal urine from some New Zealand pastoral soils. Aust J Soil Res. 41(3):381–399. doi: 10.1071/SR02128
- de Klein CAM, Cameron KC, Di HJ, Rys G, Monaghan RM, Sherlock RR. 2011. Repeated annual use of the nitrification inhibitor dicyandiamide (DCD) does not alter its effectiveness in reducing N2O emissions from cow urine. Anim Feed Sci Tech. 166-167:480–491. doi: 10.1016/j.anifeedsci.2011.04.076
- Lehmann J, Joseph S. 2009. Biochar for environmental management: an introduction. In: Lehmann J, Joseph S, editors. Biochar for environmental management, science and technology. London: Earthscan; p. 1–12.
- Menneer J, Ledgard S, McLay C, Silvester W. 2005. Animal treading stimulates denitrification in soil under pasture. Soil Biol Biochem. 37:1625–1629. doi: 10.1016/j.soilbio.2005.01.023
- Ministry for the Environment. 2013. New Zealand’s greenhouse gas inventory 1990–2011 (ME 1113). Wellington: Ministry for the Environment.
- Sarkhot DV, Ghezzehei TA, Berhe AA. 2013. Effectiveness of biochar for sorption of ammonium and phosphate from dairy effluent. J Environ Qual. 42:1545–1554. doi: 10.2134/jeq2012.0482
- Scheer C, Grace PR, Rowlings DW, Kimber S, Van Zwieten L. 2011. Effect of biochar amendment on the soil-atmospere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant Soil. 345:47–58. doi: 10.1007/s11104-011-0759-1
- Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A. 2010. Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J Environ Qual. 39:1224–1235. doi: 10.2134/jeq2009.0138
- Smith LC, de Klein CAM, Monaghan RM, Catto WD. 2008. The effectiveness of dicyandiamide in reducing nitrous oxide emissions from a cattle-grazed, winter forage crop in Southland, New Zealand. Aust J Exp Agr. 48:160–164. doi: 10.1071/EA07262
- Soil Survey Staff. 2010. Keys to soil taxonomy. Washington, DC: USDA.
- Taghizadeh-Toosi A, Clough TJ, Condron LM, Sherlock RR, Anderson CR, Craigie RA. 2011. Biochar incorporation into pasture soil suppresses in situ nitrous oxide emissions from ruminant urine patches. J Environ Qual. 40:468–476. doi: 10.2134/jeq2010.0419
- Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM. 2012. Biochar adsorbed ammonia is bioavailable. Plant Soil. 350:57–69. doi: 10.1007/s11104-011-0870-3
- Wang JY, Zhang M, Xiong ZQ, Liu PL, Pan GX. 2011. Effects of biochar addition on N2O and CO2 emissions from two paddy soils. Biol Fert Soil. 47:887–896. doi: 10.1007/s00374-011-0595-8
- van der Weerden TJ, Styles TM. 2012. Reducing nitrous oxide emissions from grazed winter forage crops. Proc New Zeal Grassland Ass. 74:57–62.
- Zhang AF, Cui LQ, Pan GX, Li LQ, Hussain Q, Zhang XH, Zheng JW, Crowley D. 2010. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Ag Ecosyst Environ. 139:469–475. doi: 10.1016/j.agee.2010.09.003
- Zheng H, Wang ZY, Deng X, Herbert S, Xing BS. 2013. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma. 206:32–39. doi: 10.1016/j.geoderma.2013.04.018