ABSTRACT
Nitrous oxide (N2O) emissions (EN2O) from drained peat soils used for pastoral agriculture have not been measured throughout the year in New Zealand. In response to this research gap, EN2O was measured fortnightly for 1 year in the Waikato region in a plot that was not grazed or nitrogen (N) fertilised. The time series was variable, the frequency distribution skewed and the fortnightly means correlated. To account for these factors, the data were loge transformed and an order 2 autoregressive model used to estimate a mean EN2O of 4.3 g N ha−1 d−1 and 95% confidence limits of 0.6–29.1 g N ha−1 d−1. There was a statistically significant, inverse relationship between EN2O and the depth to groundwater. In winter, when rainfall totalled 393 mm, EN2O and soil N content were significantly greater under a rain shelter designed to minimise N loss by leaching, than in an uncovered plot.
Introduction
Peat forms where plant growth exceeds decomposition under wet, anoxic conditions (Grover & Baldock Citation2013). In New Zealand, the peat used for agriculture has been drained. By draining peat, oxygen (O2) can diffuse more rapidly into the pore space, potentially increasing decomposition (Campbell et al. Citation2015). Decomposition consumes O2 and mineralises carbon (C) and nitrogen (N). Net N mineralisation and the transformation of N-containing compounds in soils can produce nitrous oxide (N2O), the third most important greenhouse gas (Davidson & Kanter Citation2014). Furthermore, N2O is the most important precursor to compounds that deplete stratospheric ozone (Ravishankara et al. Citation2009). Globally, agriculture is the largest source of anthropogenic N2O emissions, accounting for an estimated 56%–81% of the total (Davidson & Kanter Citation2014). In New Zealand, agriculture accounts for an estimated 94% of the anthropogenic N2O emissions (Ministry for the Environment Citation2016).
The transformation of N-containing compounds in soils to produce N2O emissions includes nitrification of ammonium ()—an aerobic, biochemical process. Nitrification yields nitrite (
), but when limited by O2 supply,
can be an electron acceptor and reduced to N2O. In soils, the wetter they become, the greater will be the rates of nitrification and N2O production (Smith et al. Citation2003; van der Weerden et al. Citation2012). When soils become anoxic—by another biochemical N transformation process called denitrification—nitrate (
) can be an electron acceptor and be sequentially reduced to
, nitric oxide (NO) and N2O and N2. This process will also be governed by wetness, N2O diffusion to the surface and emission into the atmosphere. In other words, for N2O produced in soils, wetness determines the likelihood of ‘entrapment’ or ‘escape’ and emission to the atmosphere (Clough et al. Citation1996; Smith et al. Citation2003). In soils, wetness varies throughout the year according to a balance of rainfall, evaporation, drainage and the depth to groundwater (e.g. Nieveen et al. Citation2005).
The N2O emissions (EN2O) from drained peat soils used for pastoral agriculture have been measured throughout the year in Europe. For example, on a dairy farm in the Netherlands, measurements were made at weekly to monthly intervals in fenced plots that were not grazed or fertilised and the mean EN2O was 11.5 g N ha−1 d−1 (Velthof et al. Citation1996; van Beek et al. Citation2011). In Denmark, measurements were made at 3 weekly intervals at three sites that were not grazed or fertilised and the mean EN2O was 14.1 g N ha−1 d−1 (Petersen et al. Citation2012). In New Zealand, the EN2O from drained peat soil used for pastoral agriculture has not been measured throughout the year. These drained peat soils were largely formed by the remains of endemic rush species (Wagstaff & Clarkson Citation2012). Around half of New Zealand’s peat land area is located in the Waikato region (Pronger et al. Citation2014). In this region, around 75,000 ha of former peat wetland has been drained, limed and fertilised for pastoral agriculture, mostly for grazing by dairy cattle (Campbell et al. Citation2015). When EN2O has not been measured from such soils, the Intergovernmental Panel on Climate Change has recommended an estimated mean of 22 g N ha−1 d−1 (de Klein et al. Citation2006). This estimate has been included in New Zealand’s inventory of N2O emissions from agricultural soils, reported annually in accordance with commitment to the United Nations Framework Convention on Climate Change (Ministry for the Environment Citation2016).
The aim of this study was to measure EN2O throughout the year from drained peat soil used for pastoral agriculture in New Zealand. For this purpose, an experimental site was selected on a dairy farm in the Waikato region and EN2O measured at fortnightly intervals for 1 year. During the study, the measurement plot was not fertilised or grazed. We postulated that soil wetness and N availability would affect the temporal variability of EN2O. To test the hypothesis, additional measurements were made of rainfall, the depth to groundwater and soil water, and
contents, and temperature. We also postulated that rainfall would affect the temporal variability of soil N availability by
leaching (e.g. Boy-Roura et al. Citation2016). To test this hypothesis, we erected a rain shelter and during 100 days in winter, additional fortnightly measurements were made under the shelter of the soil’s water,
and
contents, and EN2O.
Materials and methods
Site
The site for measurements was a commercially managed dairy farm located about 15 km northeast of the Ruakura Research Centre near Hamilton, North Island (37.7°S, 175.4°E). Before the farm’s land was drained for dairying approximately 60 years ago, the former bog vegetation included wire rush (Empodisma robustum) and the endemic cane rush (Sporadanthus ferrugineus), their roots forming the bulk of the peat (Wagstaff & Clarkson Citation2012). Throughout the farm, there are approximately 2 m deep drains indicating the peat depth exceeded 1 m. The soil was a Kaipaki peat loam, which is a Mellow Mesic organic soil according to the New Zealand soil classification system (Hewitt Citation2010). A soil sample (composite of 10 cores to a depth of 75 mm taken on 11 December 2012) had an organic carbon (C) content of 317 g C kg−1, total N content of 17 g N kg−1 and pH of 6.6 (water).
The farm’s area of 165 ha was divided by fencing into paddocks for rotational grazing by dairy cattle. The permanent pasture was predominantly perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.). Following each grazing at approximately monthly intervals, urea (46% N) fertiliser was applied at a rate of 25 kg N ha−1 (approximately 200–250 kg N ha−1 yr−1). Before the measurements for this study began on 11 January 2013, urea fertiliser was last applied to the selected paddock on 31 August 2012 and the paddock was last grazed 1 month later. Afterwards, a 25 × 15 m area in the selected paddock was delineated by fencing. Within the fenced area, a 10 m long × 2 m wide clear plastic rain shelter was erected for measurements described below. A drain was located within 100 m of the fenced area. During the study, the fenced area’s sward was mown regularly to a height of 25 mm.
Measurements
To measure EN2O within the fenced area, two 10 m long transects were established at the beginning of the study. One transect was located in the uncovered plot and the other under the rain shelter. At 1 m intervals along each transect, a circular, steel ring was inserted to a depth of 100 mm (236 mm diameter, 20 mm protruding from the soil surface).
As stated, in the uncovered plot, the first set of EN2O measurements were made on 11 January 2013. This was 3 months after the final applications of urea fertiliser and grazing cattle excreta. This period was considered sufficient to eliminate any effects from the previous applications on EN2O (de Klein et al. Citation2014). Around the middle of the day, a 100 mm tall (static) chamber was fitted on to each base. Thereafter, EN2O measurements were made at fortnightly intervals for the rest of the year. The additional sets of 10 EN2O measurements under the rain shelter were made in winter on 10 and 23 May, 7 and 21 June, 19 July and 5, 20 and 30 August.
To measure EN2O, chambers were fitted on the bases and headspace air samples collected (de Klein et al. Citation2014). The samples were collected using a syringe via a tube fitted with a tap attached to the top of the chamber. The sample volume of around 10 mL was transferred through a 3 mm thick butyl rubber septa into an evacuated 6 mL vial (Exetainer), over-pressurised to prevent the incursion of ambient air during storage until analysis. From each chamber, three air samples were taken at 0.5 h intervals. EN2O was calculated as follows:where Δc is the change of N2O concentration in the chamber headspace during an enclosure period (µL L−1); Δt, the enclosure period; M, the molar mass of N in N2O (g/mol); Vm, the molar volume of gas at the sampling temperature and atmospheric pressure (L/mol); V, the headspace volume (m3); and A, the soil surface area (m2). The N2O concentrations for Δc were measured by a gas chromatography system described by de Klein et al. (Citation2014).
On the days when EN2O was measured, five soil samples were collected to a depth of 75 mm in the uncovered area and five under the rain shelter. The samples were weighed, dried at 105 °C for 24 h and weighed again to determine the gravimetric water content. To determine the volumetric water content, the bulk density was calculated from the dry weight and sample volume.
The samples’ and
contents were also measured. The soil was extracted using 2 M KCl aqueous solution (1 mg soil: 10 mL KCl) then shaking for 1 h on an end-over-end shaker and filtering (Whatman 42) (Mulvaney Citation1996). The filtered soil extracts were analysed for
and
(mineral N) using a Skalar SAN++ segmented flow analyser (Skalar Analytical). The auto-analyser was assessed by measuring seven blank samples. Typically, the standard deviation was 0.1 mg L−1. The
content was assumed to be close to the detection limit in all samples, so the results reported as
content actually comprised a sum of the
and
contents.
On the days when EN2O was measured, the depth to groundwater was also measured. For this purpose, a 2 m long perforated pipe covered with fine-mesh gauze (Dipwell) was pushed into the soil. We constructed an electronic device that was lowered into the pipe and upon reaching the groundwater, an electronic circuit closed, an alarm sounded and the depth was recorded.
Rainfall and soil temperature at a depth of 100 mm were measured and recorded as hourly totals and means, respectively. As a result of data logger faults, the record was incomplete for the year 2013. However, a complete record of these data was available from measurements made at the Ruakura Research Centre. Comparison of the two data sets yielded satisfactory agreement, so the data from Ruakura were used for this study.
Statistical analyses
By repeating measurements, as done for this study, there can be correlated errors, which are included in the variability (e.g. Davis Citation2002). As stated, EN2O and the soil’s water, and
contents were measured on eight dates over 100 days in winter under the rain shelter (covered) and in the uncovered plot. These data were analysed using a linear mixed model which can accommodate serial correlation among a relatively small number of repeated measurements (Garrett et al. Citation2004). The fixed effects—levels of primary interest—were plot (covered or uncovered) and date as well as their interaction (plot × date). The random effect was the estimated correlation between the repeated measurements over the 100 day period. The analysis was carried out using SAS software, version 9.3 (SAS Institute).
In the uncovered plot, fortnightly measurements of EN2O were also done on 27 dates throughout 1 year. These data were analysed using an order 2 autoregressive (AR[2]) model which can accommodate autocorrelation among a larger number of repeated measurements than the linear mixed model (Shumway & Stoffer Citation2006). The purpose of this analysis was to estimate a grand mean and 95% confidence interval. Anticipating the results, firstly, the 27 fortnightly EN2O means were loge (base 2.718) transformed to remedy their skewness. These transformed data were then analysed using the AR(2) model. The AR(2) model incorporated the observed autocorrelation as a linear relationship between the mean at time t and the means at t–1 and t–2. Selection of the AR(2) model was based on the Akaike Information Criterion. This analysis was done using Minitab software, version 16.
Results
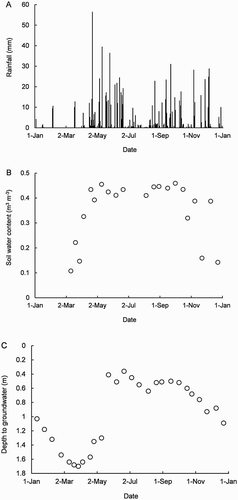
The study began in summer and during January–March, rainfall totalled 50 mm (). For context, the corresponding potential evaporation was 330 mm (Scotter & Heng Citation2003). Consequently, during January–March, the soil was dry and the depth to groundwater exceeded 1 m. During April–December, rainfall totalled 930 mm. For this period, on average, rain fell every other day, exceeding the estimated potential evaporation by 360 mm. For this reason, during April–December in the uncovered plot, soil water content (0–75 mm depth) was mostly near the maximum level (0.45 m3 m−3 or 58% water-filled pore space) and groundwater mostly near the surface (depth of 0.4–0.6 m).
Figure 1. Time courses of daily rainfall (A), soil water content (0–75 mm depth) (B) and depth to groundwater in the uncovered plot during the year 2013 (C).
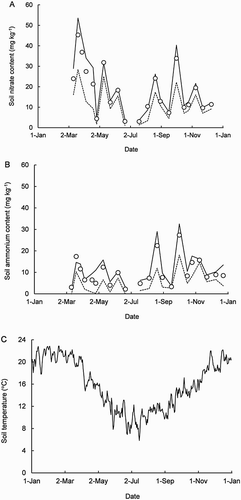
In the uncovered plot, time courses of the soil’s and
contents were variable (). Seasonal means were calculated to examine the temporal variability. For
content, beginning with January–March, the seasonal means were 35 ± 9, 17 ± 4, 12 ± 4 and 16 ± 4 mg kg−1, respectively (±95% confidence interval). For
content, the seasonal means were 9 ± 3, 10 ± 7, 10 ± 4 and 14 ± 3 mg kg−1, respectively. Thus, with the exception of a considerably higher
content in summer, there was little seasonal variability. For soil temperature, the seasonal means were 21.0 ± 0.3, 13.3 ± 0.7, 11.1 ± 0.4 and 17.9 ± 0.5 °C, respectively. Consolidating further, in spring and summer, the mean soil temperature was nearly 20 °C, decreasing by 8 °C in autumn and winter.
Figure 2. Time courses of soil nitrate (A) and ammonium (B) contents (0–75 mm depth) and temperature (C, at a depth of 100 mm) in the uncovered plot during the year 2013. The symbols are sample means of five replicates. The solid and dashed lines connect each sample’s 75th and 25th percentiles, respectively.
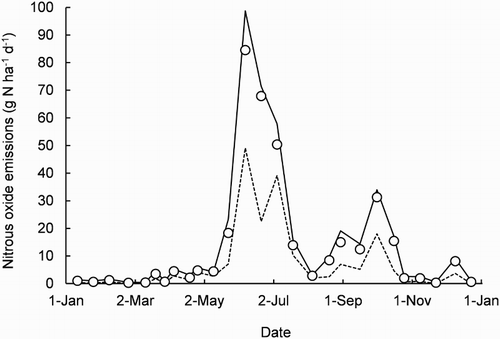
In the uncovered plot, the time course of EN2O was also variable, although not in the first 2 months of the year (). During 11 January–11 March, the mean was 0.7 ± 0.2 g N ha−1 d−1. For the next 2 months, the mean had increased to 3.3 ± 0.6 g N ha−1 d−1. This trend continued and on 23 May, the mean was 18.3 ± 9.1 g N ha−1 d−1 and the 25th, 50th and 75th percentiles were 7.1, 15.1 and 23.3 g N ha−1 d−1, respectively. The mean was greater than the 50th percentile value, indicating this sample’s distribution of 10 EN2O measurements was skewed. The next sample of measurements on 7 June had the largest mean of 84.5 ± 25.0 g N ha−1 d−1 and the mean was again greater than the 50th percentile value (74.5 g N ha−1 d−1). For 21 June, 5 July and 19 July, the means and variability had reduced to 67.9 ± 51.9, 50.4 ± 9.5 and 14.0 ± 2.6 g N ha−1 d−1, respectively, and for the rest of the year, the mean was 6.7 ± 1.8 g N ha−1 d−1, except for 31.3 ± 15.6 g N ha−1 d−1 on 1 October. For these 14 samples, the distribution of the 10 measurements was also skewed (). To determine if any of the variability of the EN2O measurements could be attributed to the proximity of one chamber base to another along the transect, correlation coefficients were calculated from the 27 fortnightly data sets, but the results showed no spatial pattern (data not shown).
Figure 3. Time course of nitrous oxide emissions (N2O-N, nitrogen) in the uncovered plot during the year 2013. The symbols are sample means of 10 repeatedly measured replicates. The solid and dashed lines connect each sample’s 75th and 25th percentiles, respectively.
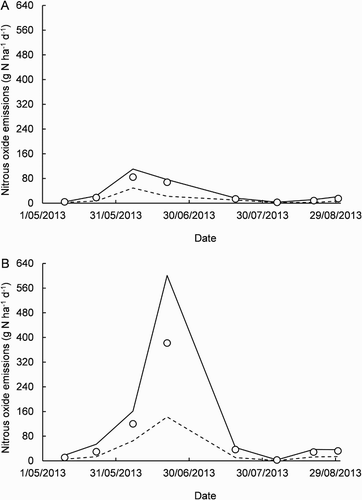
For 10 May–30 August, when EN2O was also measured fortnightly under the rain shelter (hereafter, covered plot), rainfall totalled 393 mm, the mean depth to groundwater was 0.6 m and the mean soil temperature 10.7 °C. In the uncovered and covered plots, the mean and
contents were 14.8 ± 3.4 and 9.1 ± 2.5 mg kg−1 and 66.0 ± 15.8 and 20.2 ± 10.0 mg kg−1, respectively (). These results were interpreted to suggest rainfall had induced greater N loss by leaching from the uncovered plot (Boy-Roura et al. Citation2016). For EN2O, results from the uncovered plot were most different from those from the covered plot on 21 June (). On this day, the mean
and
contents were 2.6 and 1.8, and 21.5 and 12.1 mg kg−1 in the uncovered and covered plots, respectively. Further, there was 19 mm of rain and there had been 117 mm in the previous 14 days. Moreover, the depth to groundwater was 0.36 m, the closest to the surface for the year. On 21 June, in the uncovered plot, the mean EN2O was 67.9 ± 51.9 g N ha−1 d−1 and the 25th, 50th and 75th percentiles were 21.6, 39.2 and 68.9 g N ha−1 d−1, respectively. In the covered plot, the corresponding mean was substantially greater at 382.1 ± 193.8 g N ha−1 d−1 and the 25th, 50th and 75th percentiles 126.4, 263.8 and 482.3 g N ha−1 d−1, respectively. For the seven other dates of measurement during 10 May–30 August, in the uncovered plot, the mean was 21.1 ± 7.2 g N ha−1 d−1 and the 25th, 50th and 75th percentiles 3.4, 9.1 and 21.4 g N ha−1 d−1, respectively. In the covered plot, the corresponding mean was again substantially greater at 37.4 ± 10.9 g N ha−1 d−1 and the 25th, 50th and 75th percentiles were 8.5, 18.6 and 43.5 g N ha−1 d−1, respectively. Using the linear mixed model, the mean EN2O was significantly larger in the covered plot than in the uncovered plot on 10 May, 21 June and 20 August (P = 0.002, P < 0.001 and P = 0.028, respectively).
Figure 4. Time courses of soil nitrate and ammonium contents during 10 May–30 August 2013 in the uncovered plot (A and C, respectively) and in a nearby plot covered by a rain shelter (B and D, respectively). The symbols are sample means of five replicates. The solid and dashed lines connect each sample’s 75th and 25th percentiles, respectively.
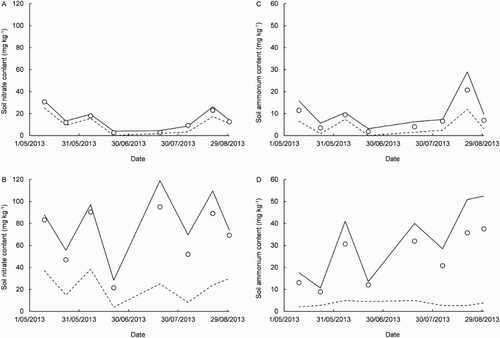
Figure 5. Time course of nitrous oxide emissions (N2O-N, nitrogen) during 10 May–30 August 2013 in the uncovered plot (A) and in a nearby plot covered by a rain shelter (B). The symbols are sample means of 10 repeatedly measured replicates. The solid and dashed lines connect each sample’s 75th and 25th percentiles, respectively.
Discussion
This has been the first study to measure EN2O from a drained peat pastoral soil throughout the year in New Zealand. During the study, the perennial ryegrass pasture was not grazed or fertilised. The results have shown the time course of EN2O in the uncovered plot was variable. The grand (raw) mean (mean of the 27 fortnightly means) was 13.2 g N ha−1 d−1. This was similar to the means from field studies in the Netherlands and Denmark at measurement sites that had also not been grazed or fertilised (van Beek et al. Citation2011; Petersen et al. Citation2012; Velthof et al. Citation1996).
For this study, the grand mean EN2O was not representative of the 27 fortnightly means. There were only eight fortnightly means larger than 13.2 g N ha−1 d−1, while 16 fortnightly means were less than 5.0 g N ha−1 d−1. Moreover, the actual middle value (50th percentile, also known as median) was 4.4 g N ha−1 d−1. The much larger grand mean was attributed mainly to three exceptionally large fortnightly means of 50.4–84.5 g N ha−1 d−1 during 7 June–5 July (). Thus, the distribution of the 27 fortnightly means was skewed. As indicated, for each of the 27 sampling dates, the distribution of the 10 EN2O measurements across the uncovered plot was also skewed. Consequently, the temporal variability of EN2O was skewed as well as the spatial variability.
The calculation of the grand (raw) mean assumed each of the 27 fortnightly means was independent. However, for this study, the EN2O measurements were repeated at the same locations. Thus, the 27 fortnightly means seemed unlikely to be independent. Prior to a second analysis of these data using an AR(2) model which accounted for autocorrelation, the 27 fortnightly means were loge (base 2.718) transformed to remedy their skewness. The appropriateness of the AR(2) model was confirmed by the calculated residuals no longer exhibiting autocorrelation. The AR(2) model estimated a loge grand mean of 1.47 and accompanying loge standard error of 0.95. Taking twice the standard error for 95% confidence and back-transforming the results, the estimated grand mean EN2O was 4.3 g N ha−1 d−1 and the 95% confidence limits were 0.6–29.1 g N ha−1 d−1. This estimated grand mean was substantially smaller than the grand (raw) mean of 13.2 g N ha−1 d−1, but only slightly smaller than the median of 4.4 g N ha−1 d−1. Because the estimated grand mean was nearly equal to the median, loge transforming the 27 fortnightly means was considered to have been effective and the effect of autocorrelation minor. In addition, as stated, a mean of 22 g N ha−1 d−1 for drained peat pastoral agriculture soils has been used for New Zealand’s EN2O inventory (Ministry for the Environment Citation2016). From this study, the estimated grand mean and median suggest the mean of 22 g N ha−1 d−1 should be reduced to improve the accuracy of New Zealand’s EN2O inventory.
As stated, in the uncovered plot, the time course of EN2O was variable. To try to better understand the variability, by regression analysis, loge transformed EN2O values were related to the depth to groundwater (depth, m). Using an asymptotic exponential function, the regression assumptions of constant variance and normality of the residuals were met. The resultant relationship was:where parameters a, b and c (± standard error) were 0.14 ± 0.37, −16.60 ± 9.49 and 3.65 ± 1.36, respectively (). The standard error of a loge EN2O estimate was 1.0 or 20% of the mean. The relationship had an inflection at a depth to groundwater of 0.6 m. For drained peat soils beneath pasture in Sweden, 0.5 m deep monoliths were collected, subjected to a controlled depth to groundwater of either 0.4 or 0.8 m and, like this study, EN2O was greater for the lesser depth (Berglund & Berglund Citation2011).
Figure 6. Relationship between fortnightly mean nitrous oxide (N2O-N, nitrogen) emissions in the uncovered plot and the corresponding depth to groundwater. The curve was fitted by regression as described in the text.
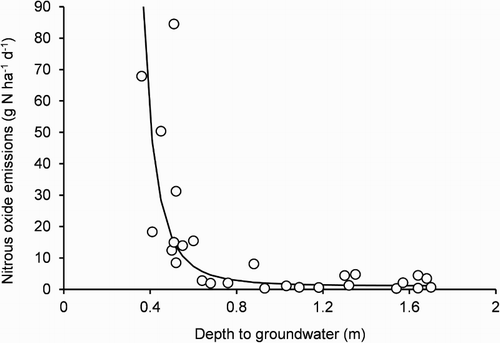
Moreover, for drained peat soils beneath pasture or cultivated for crop production in Germany, there was an inverse relationship between annual means of EN2O and the depth to groundwater (Flessa et al. Citation1998). For drained peat soil cultivated for vegetable production in Canada, there was a negative linear relationship between annual means of EN2O and the depth to groundwater (Rochette et al. Citation2010). For their study, the depth varied from 0.8 m up to 0.6 m and by inserting these values into their regression equation, the estimated EN2O was 11.0 and 76.7 g N ha−1 d−1, respectively. By inserting 0.8 and 0.6 m into the regression equation from this study, the estimated EN2O was 2.8 and 7.3 g N ha−1 d−1, respectively. Alternatively, by inserting 0.547 and 0.376 m into the regression equation from this study, the estimated EN2O was 10.9 and 76.8 g N ha−1 d−1, respectively.
The results of many experiments with mineral soils have indicated a positive effect of temperature on EN2O, reflecting a positive effect of temperature on the net N mineralisation rate (e.g. Smith Citation1997). However, for the drained peat pastoral soils in Denmark, there was not a statistically significant relationship between EN2O and soil temperature (Petersen et al. Citation2012). Alternatively, for monoliths from a peaty forest soil in Scotland, a statistically significant positive linear relationship between 3 hourly values of EN2O and soil temperature accounted for 51% of the variability on days when the water content was constant (Smith et al. Citation1998). For this study, in the uncovered plot, there was a statistically significant correlation of 0.71 between soil temperature and the depth to groundwater (P < 0.001). Thus, as shown by the time courses of these variables ( and ), the warmer the soil, the greater was the depth to groundwater. As also shown, the greater the depth to groundwater, the smaller was EN2O. While regression analysis indicated a negative effect of temperature on EN2O (data not shown), this result was discarded.
Conclusions
During the study, while the drained peat soil under pasture had no N inputs from fertiliser or excreta returns, the time course of EN2O was variable. Using loge transformed values to remedy their skewed distribution and an order 2 autoregressive model to account for correlation between the 27 fortnightly means, the estimated grand mean EN2O was 4.3 g N ha−1 d−1 and 95% confidence interval was 0.6–29.1 g N ha−1 d−1. The grand mean was almost equal to the median, indicating loge transformation was effective and the effect of autocorrelation minor. There was a statistically significant curvilinear relationship between EN2O and the depth to groundwater with an inflection around 0.6 m. During winter, EN2O was greatest, yet additional measurements under a rain shelter indicated the mean EN2O and soil mineral N content were significantly larger. While the rain shelter had no effect on the depth to groundwater, minimising soil N loss by leaching evidently had a positive effect on EN2O. Further studies are needed under different conditions to develop better understanding of the temporal variability of EN2O from drained peat soil used for pastoral agriculture in New Zealand.
Acknowledgements
We are grateful to Brendon van Vugt for allowing us to work on his farm, Teresa Parayil-Symon and Manjula Premartne for the gas chromatography measurements, Jiafa Luo and Mike Rollo for valuable discussions and Cecile de Klein, Diana Selbie and three anonymous reviewers for helpful comments on the manuscript. Daily rainfall and hourly soil temperature data from the CliFlo web site were provided free of charge by the National Institute of Water and Atmosphere.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Berglund Ö, Berglund K. 2011. Influence of water table level and soil properties on emissions of greenhouse emissions from cultivated peat soil. Soil Biol Biochem. 43:923–931. doi: 10.1016/j.soilbio.2011.01.002
- Boy-Roura M, Cameron KC, Di HJ. 2016. Identification of nitrate loss indicators through regression methods based on a meta-analysis of lysimeter studies. Environ Sci Pollut R. 23:3671–3680. doi: 10.1007/s11356-015-5529-9
- Campbell DI, Wall AM, Nieveen JP, Schipper LA. 2015. Variations in CO2 exchange for dairy farms with year-round rotational grazing on drained peatlands. Agric Ecosyst Environ. 202:68–78. doi: 10.1016/j.agee.2014.12.019
- Clough TJ, Sherlock RR, Cameron KC, Ledgard SF. 1996. Fate of urine nitrogen on mineral and peat soils in New Zealand. Plant Soil. 178:141–152. doi: 10.1007/BF00011172
- Davidson EA, Kanter D. 2014. Inventories and scenarios of nitrous oxide emissions. Environ Res Lett. 9:105012. doi:10.1088/1748-9326/9/10/105012
- Davis CS. 2002. Statistical methods for the analysis of repeated measurements. New York: Springer. 416 p.
- Flessa H, Wild U, Klemisch M, Pfadenhauer J. 1998. Nitrous oxide and methane fluxes from organic soils under agriculture. Eur J Soil Sci. 49:327–335. doi: 10.1046/j.1365-2389.1998.00156.x
- Garrett M, Fitzmaurice GM, Laird NM, Ware JH. 2004. Applied longitudinal analysis. Hoboken, NJ: John Wiley and Sons. 740 p.
- Grover SPP, Baldock JA. 2013. The link between peat hydrology and decomposition: beyond von post. J Hydrol. 479:130–138. doi: 10.1016/j.jhydrol.2012.11.049
- Hewitt AE. 2010. New Zealand soil classification. Landcare Research Science Series No. 1. 3rd ed. Lincoln, New Zealand: Manaaki Whenua Press. 136 p.
- de Klein CAM, Luo J, Woodward KB, Styles T, Wise B, Lindsey S, Cox N. 2014. The effect of nitrogen concentration in synthetic cattle urine on nitrous oxide emissions. Agr Ecosyst Environ. 188:85–92. doi: 10.1016/j.agee.2014.02.020
- de Klein CAM, Novoa RSA, Ogle S, Smith KA, Rochette P, Wirth T, McConkey B, Mosier A, Rypdal K, Walsh M, Williams SA. 2006. N2O emissions from managed soils, and CO2 emissions from lime and urea application. Chapter 11, 2006 Intergovernmental Panel on Climate Change guidelines for national greenhouse gas inventories. http://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_11_Ch11_N2O&CO2.pdf
- Ministry for the Environment. 2016. New Zealand’s greenhouse gas inventory 1990–2014 http://www.mfe.govt.nz/publications/climate-change/new-zealands-greenhouse-gas-inventory-1990-2014
- Mulvaney RL. 1996. Nitrogen—inorganic forms. In: Sparks DL, editor. Methods of soil analysis: part 3. Madison, WI: Soil Science Society of America; p. 1123–1184.
- Nieveen JP, Campbell DI, Schipper LA, Blair IJ. 2005. Carbon exchange of grazed pasture on a drained peat soil. Global Change Biology. 11:607–618. doi: 10.1111/j.1365-2486.2005.00929.x
- Petersen SO, Hoffmann CC, Schäfer C-M, Blicher-Mathiesen G, Elsgaard L, Kristensen K, Larsen SE, Torp SB, Greve MH. 2012. Annual emissions of CH4 and N2O, and ecosystem respiration, from eight organic soils in Western Denmark managed by agriculture. Biogeosciences. 9:403–422. doi: 10.5194/bg-9-403-2012
- Pronger J, Schipper LA, Hill RB, Campbell DI, McLeod M. 2014. Subsidence rates of drained agricultural peatlands in New Zealand and the relationship with time since drainage. J Environ Qual. 43:1442–1449. doi: 10.2134/jeq2013.12.0505
- Ravishankara AR, Daniel JS, Portmann RW. 2009. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science. 326:123–125. doi: 10.1126/science.1176985
- Rochette P, Tremblay N, Fallon E, Angers DA, Chantigny MH. 2010. N2O emissions from an irrigated and non-irrigated organic soil in Easter Canada as influenced by N fertilizer addition. Eur J Soil Sci. 61:186–196. doi: 10.1111/j.1365-2389.2009.01222.x
- Scotter DS, Heng L. 2003. Estimating reference crop evaporation in New Zealand. J Hydrol (NZ). 42:1–10.
- Shumway RH, Stoffer DS. 2006. Times series analysis and its applications with R examples. 2th ed. New York: Springer. 575 p.
- Smith KA. 1997. The potential for feedback effects induced by global warming on emissions of nitrous oxide emissions by soils. Glob Change Biol. 3:327–338. doi: 10.1046/j.1365-2486.1997.00100.x
- Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A. 2003. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eu J Soil Sci. 54:779–791. doi: 10.1046/j.1351-0754.2003.0567.x
- Smith KA, Thomson PE, Clayton H, McTaggart IP, Conen F. 1998. Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmos Environ. 32:3301–3309. doi: 10.1016/S1352-2310(97)00492-5
- van Beek CL, Pleitjer M, Kuikman PJ. 2011. Nitrous oxide emissions from fertilized and unfertilized grassland on peat soil. Nutr Cycl Agroecosys. 89:453–461. doi: 10.1007/s10705-010-9408-y
- van der Weerden TJ, Kelliher FM, de Klein CAM. 2012. Influence of pore size distribution and soil water content on nitrous oxide emissions. Soil Res. 50:125–135.
- Velthof GL, Brader AB, Oenema O. 1996. Seasonal variations in nitrous oxide losses from managed grasslands in The Netherlands. Plant Soil. 181:263–274. doi: 10.1007/BF00012061
- Wagstaff SJ, Clarkson BR. 2012. Systematics and ecology of the Australasian genus Empodisma (Restionaceae) and description of a new species from peatlands in northern New Zealand. PhytoKeys. 13:39–79. doi: 10.3897/phytokeys.13.3259
