ABSTRACT
Triplet lambs have reduced survival and most deaths occur due to starvation and exposure, but there are a few simple and safe interventions. We hypothesised that buccal dextrose gel would increase blood glucose concentration, vigour, survival and early feeding in less vigorous triplet lambs. Triplet lambs meeting criteria for decreased vigour were randomised to 40% dextrose or placebo gel 1 mL/kg via the buccal mucosa at 1 hour of age. Primary outcome was survival at 3 hours. An additional study exploring the effect of gel on interstitial glucose concentrations was assessed by continuous glucose monitoring in twin lambs. Lambs randomised to dextrose gel did not have higher blood glucose concentrations or better survival than those randomised to placebo. Low temperature at 1 hour after birth, rather than birthweight or blood glucose concentrations, was associated with decreased survival. Interventions to address hypothermia rather than hypoglycaemia may be most effective in improving survival in less vigorous triplet lambs.
Introduction
Common agricultural practice is to maximise the number of lambs sold per ewe in order to maximise financial return. This can be achieved by increasing fecundity. In 2015, the ewe–lamb return reported for New Zealand was 120.9% (Beef and Lamb New Zealand Citation2015). In a 2015 survey of New Zealand sheep farmers, lamb survival was considered the most important area for future research (Greer et al. Citation2015). Improving lamb survival has the potential for significant economic benefit to New Zealand with the total flock of 29.1 million and lamb and mutton export value of NZ$2.8 billion for 2015 (Statistics New Zealand Citation2015).
Sheep have not evolved naturally to have multiple pregnancies, but have been selectively bred to increase fecundity and, with increasing litter size, the proportion of triplet conceptions increases (Amer et al. Citation1999). However, lamb survival rates in New Zealand are highest in singles (90%) and twins (88%) with only 54%–77% of triplets surviving (Kenyon et al. Citation2006; Muir & Thomson Citation2009). Lamb survival is affected by both maternal ewe factors including genotype, nutrition, stress, uterine environment, litter size, dystocia, maternal bonding and behaviour, and colostrum and milk production, and by lamb factors including birthweight and ability to withstand hypothermia (Dalton et al. Citation1980; Cloete & Scholtz Citation1998; Department for Environment Citation2004; Dwyer & Morgan Citation2006; Nowak & Poindron Citation2006; Morel et al. Citation2008; Kerslake et al. Citation2010a; Darwish & Ashmawy Citation2011).
Most lamb death occurs within the first 3 days after birth (Dalton et al. Citation1980). Starvation is the most frequent cause of death in multiples and increases with decreasing birthweight (Dalton et al. Citation1980). Lambs that are unable to stand and feed within the first few minutes have higher mortality (Cloete & Scholtz Citation1998). Since newborn lambs utilise their glycogen stores rapidly over the first few hours after birth (Shelley Citation1960), without adequate nutrition, vigour will decrease further, compounded by the deleterious effect of hypothermia and ultimately leading to the demise of the weakest lamb(s). Early feeding is advised for optimising survival (Nowak & Poindron Citation2006). We have observed previously that feeding of expressed colostrum to preterm or small lambs in the first few hours after birth appeared to increase vigour, udder seeking and successful spontaneous feeding (A. Jaquiery, unpubl. observations).
Intensive farming is associated with increased survival of multiple gestation lambs (Muir & Thomson Citation2009) but at financial and fatigue cost to the farmer (Fisher Citation2003) who must either house the ewes during parturition and monitor and intervene him/herself or pay farm labourers to do so. Intra-abdominal injection of 20% dextrose has been used elsewhere to revive starving and hypothermic lambs, but requires training in administration, is expensive and has potential risks (Department for Environment Citation2004; Martin Citation2010; Beef and Lamb New Zealand Citation2012). For farmers managing large flocks of sheep in the paddock, any method of improving survival must be cheap, quick and simple to administer and be safe in the food chain for those farming for meat. This is of particular relevance to New Zealand, where nearly 50% of livestock are managed on extensive hill or high country farms (Morris Citation2013).
We hypothesised that buccal dextrose gel, which is used to treat hypoglycaemic newborn babies (Harris et al. Citation2013), could also be useful in newborn lambs, potentially increasing energy in less vigorous lambs and thereby improving their ability to compete at the udder for nutrition, and hence improving survival.
We aimed to: (1) assess the effect of buccal administration of dextrose gel in the early postnatal period on triplet lamb survival, vigour and early feeding; and (2) determine whether buccal dextrose gel has any effect on the interstitial glucose concentration.
Methods
We studied lambs from triplet and twin pregnancies at a university research farm, central North Island, New Zealand. Ethical approval was obtained from the University of Auckland Animal Ethics Committee. All animal care was undertaken in accordance with the Animal Welfare Act Citation1999, (National Animal Ethics Advisory Committee Citation1999), the University of Auckland Code of Ethical Conduct and the National Animal Ethics Advisory Committee (NAEAC) Good Practice Guide for the Use of Animals in Research, Testing and Teaching (National Animal Ethics Advisory Committee Citation2010b).
Romney ewes underwent synchronisation of oestrous (CIDR G, Zoetis) (Wheaton et al. Citation1993) before mating with Dorset rams. Pregnant Romney ewes with triplet or twin conceptions detected by ultrasound scanning 49 and 70 days after ram exposure were fed a complete ration concentrate feed (10 MJ/kg) (UniC, Dunstan Nutrition) for 10 days during in-paddock acclimatisation as described in Oliver et al. (Citation2005) before being placed in the feedlot 1 week before their expected date of delivery (c. day 138) to acclimatise to the environment. On day 142, ewes were moved into individual pens to monitor for onset of labour. Ewes were continually in the presence of other ewes throughout the trial. Ewes and lambs were returned to the paddock 48 hours after birth of the last lamb and when the ewe and lambs were considered able to return outside (suckling established, adequate milk supply, ewe and lambs well bonded, weather not wet and windy [Department for Environment Citation2004]).
We observed all ewes with triplet pregnancies continually from onset of labour as intervention was likely to be required and data collection commenced from birth.
Ewes with twin pregnancies were monitored intermittently during daytime hours only as enrolment in the trial did not commence until 36 hours after birth.
Assistance was provided during delivery as required using standard University of Auckland Code of Ethical Conduct and AEC approved facility standard operating procedures which adhere to the Animal Welfare Act 1999 (National Animal Ethics Advisory Committee Citation1999) and conform to recommendations of the National Animal Welfare Advisory Committee (NAWAC) code issued under that Act (Sheep & Beef Code of Welfare 2008) (National Animal Ethics Advisory Committee Citation2010a) and included intervention for: (1) obvious malpresentation; (2) delayed progress of > 30 minutes after normally presenting part seen; and (3) no presenting part after 60 minutes of heavy contractions and correction of presentation if required. Birth assistance scores were allocated to each lamb: 0 = unassisted, easy uncomplicated delivery, short duration (<30 minutes from membrane rupture to lamb delivery; if time of membrane rupture not clear, <30 minutes from appearance of presenting part to lamb delivery); 1 = unassisted, easy uncomplicated delivery of long duration (>30 minutes from membrane rupture to lamb delivery; if time of membrane rupture not clear, >30 minutes from appearance of presenting part to lamb delivery); 2 = minor assistance required e.g. presentation corrected, little effort required to deliver lamb; 3 = major assistance required, difficult delivery requiring effort to deliver lamb (Matheson et al. Citation2011). Veterinary assistance ( birth assistance score 4) was not required for this study. After birth, lambs that were not breathing despite being licked and stimulated by the ewe over the first minute received simple resuscitation measures including clearing of membranes/other material from mouth and nose and stimulation by drying.
Trial design
Two randomised double-blind placebo controlled trials studying: (1) effect of buccal dextrose on triplet survival; and (2) effect of buccal dextrose on interstitial glucose concentrations.
Entry criteria
Effect of buccal dextrose on triplet survival trial
Lambs born from a triplet pregnancy that met at least one of the following inclusion criteria: third born lamb, birth assistance score = 3; lamb vigour ≥ 3 at 5 minutes; lamb weight ≤3.5 kg or rectal temperature ≤38 °C at 1 hour after birth. These criteria were intended to select lambs that were least vigorous and least likely to survive (Dalton et al. Citation1980; Stafford et al. Citation2007; Morel et al. Citation2008; Kerslake et al. Citation2010a). Vigour was scored using a validated scoring system of 0 to 4, where 0 = vigorous, active, has been standing on all four hooves and 4 = very weak lamb, little movement (Matheson et al. Citation2011). Lambs that did not meet the criteria for trial intervention were observed but received no buccal gel.
Effect of buccal dextrose on interstitial glucose trial
Twin lambs that were 36–48 hours old, spontaneously feeding and no health concerns for ewe or lambs. Twins were used for this exploratory study to minimise the total number of animals required by maximising the number of triplet lambs available for the triplet survival trial. We anticipated that dextrose gel would change the blood glucose concentration similarly in any fasted newborn lamb.
Randomisation
For both trials, intervention gel (40% dextrose) and identical appearing placebo (2% hydroxymethylcellulose) were manufactured by Biomed and provided in bottles labelled A or B. The allocation code was held by a member of the data team not involved in the trial procedures. Prior to onset of labour, ewes were randomly allocated to gel A or B by selection of a folded randomisation slip selected from a hat by a staff member not participating in trial interventions, data collection or analysis. Lambs eligible for trial buccal gel were subsequently given the allocated gel. All study investigators were masked to treatment group allocation throughout the study.
Interventions
Effect of buccal glucose on triplet survival trial
At 1 hour after birth, lambs were weighed and rectal temperature taken. Lambs that met the criteria for trial intervention had blood sampled from a jugular vein for measurement of blood glucose and lactate concentrations at 1 and 3 hours after birth using a Yellow Springs 2300 STAT Plus glucose and lactate analyser (Yellow Springs Instruments Inc.). Study gel 1 mL/kg (1 mL/kg of 40% dextrose = 400 mg/kg glucose) was massaged into the buccal mucosa immediately after blood glucose sampling at 1 hour and the lamb was returned to the pen.
Effect of buccal glucose on interstitial glucose trial
On the second day after birth, twin born lambs were separated from the ewe immediately following an observed feed and placed in an adjacent pen to undergo fasting. Only one twin was separated from the ewe at a time. Forty-five minutes after fasting commenced, wool was clipped from a small area on the lamb’s back, the area was disinfected with hibitane in alcohol, and a continuous glucose monitor sensor (Medtronic ‘Sof-Sensor’) was inserted subcutaneously. The monitor (CGMS Gold, Medtronic, MiniMed) was attached after 15 minutes of sensor ‘wetting time’ and secured with veterinary tape. Blood was sampled from a jugular vein for measurement of blood glucose and lactate concentrations at 2, 3, 4 and 6 hours after fasting commenced. Study gel 1 mL/kg was massaged into the buccal mucosa at 2 hours after the baseline blood sampling.
Observations
Effect of buccal glucose on triplet survival trial
Lamb vigour was scored at 5 minutes after birth. Additional measures of lamb activity included time from birth to first stand (defined as up on all four hooves for at least 5 seconds) and time from birth to first successful feed (defined as teat in mouth and appeared to be sucking for at least 5 seconds).
Feeding behaviour was observed from birth to 3 hours and documented as: (1) number of suck attempts (in position with head under ewe in udder area, but prevented from achieving sucking by ewe movement or leaves region within 5 seconds); (2) number of successful sucks (teat in mouth and appeared to be sucking for at least 5 seconds) (Matheson et al. Citation2011).
All lambs were weighed at 1 hour after birth.
Effect of buccal glucose on interstitial glucose trial
No additional observations were made.
Outcomes
Effect of buccal glucose on triplet survival trial
The primary outcome was lamb survival at 3 hours after birth. Lambs that did not feed successfully by 3 hours were considered likely to have died in an outdoor environment without monitoring and intervention (Cloete & Scholtz Citation1998), and were therefore allocated to non-survival as the primary outcome (proxy death). These lambs were removed from the study, given simple cares (e.g. a coat if cold) and artificially fed. Survivors subsequently were rehomed.
Additional exploratory analyses included: (1) effect of rectal temperature on survival; (2) effect of birth rank on survival; (3) effect of time to feed on blood glucose concentration; and (4) effect of time to stand on survival.
Effect of buccal glucose on interstitial glucose trial
The primary outcome of this exploratory study was to determine if administration of buccal dextrose was associated with an increment in interstitial glucose concentration. Additional exploratory analyses included: (1) time of onset of increment in interstitial glucose after buccal gel administration; and (2) duration of increment in interstitial glucose.
Statistical analysis
Data for both trials were analysed using JMP version 10.0 (SAS Institute Inc.). Interstitial glucose data were analysed using SAS (v9.3 SAS Institute Inc.). Categorical data were compared using contingency analysis with Pearson’s test for large samples and Fisher’s exact 2 tail if n < 5. Continuous data were compared using analysis of variance or Wilcoxon (Mann–Whitney) tests as appropriate. Primary and secondary outcomes were compared between groups using logistic regression. Continuous glucose monitoring data were recalibrated using retrospective calibration algorithms (Signal et al. Citation2012) and compared between groups using a mixed model analysis to account for repeated measures and time from gel administration. Data are presented as median (range), number (%), β coefficient (standard error) and regression coefficient or odds ratio and 95% confidence intervals.
Power and sample size
We estimated that to show a reduction of triplet lamb loss from 25% to 20%, with 80% power, we would need 32 lambs allocated to placebo and 32 lambs allocated to dextrose gel. No power or sample size was calculated for the exploratory study on the effect of buccal glucose on interstitial glucose. Number of lambs studied was determined by available resources within the study time period.
Results of effect of buccal glucose on triplet survival trial
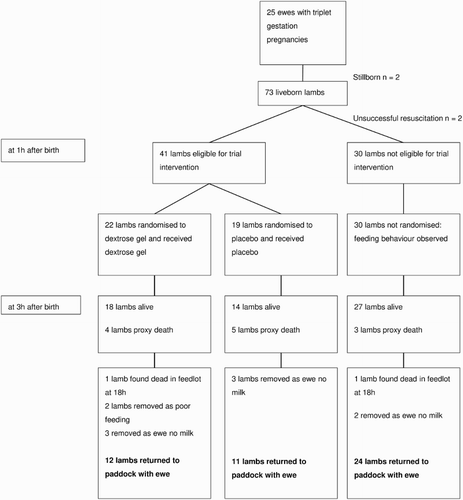
A total of 75 lambs were born to 25 ewes with triplet pregnancies who delivered at term gestation during six lambing groups between October 2013 and October 2014 (). Thirty of the 75 (40%) lambs presented normally and 45/75 (60%) presented abnormally. Overall, 39/75 (52%) lambs required birth assistance and 36/45 (80%) of those with an abnormal presentation required birth assistance. Two lambs were stillborn and two lambs with weak cardiac impulse and not breathing were unable to be resuscitated. Twenty-four of the 75 lambs needed resuscitation at birth. Forty-one lambs met the criteria for trial intervention and 30 lambs that did not meet the criteria remained under observation only.
By design, lambs that met the criteria for study intervention were less likely to survive than those that did not, as indicated by lambs that met the criteria, having a higher birth assistance score, being more likely to be third born, more likely to require resuscitation, having a higher (worse) vigour score, being slower to feed and having fewer successful feeds in the first hour after birth (). However, lambs randomised to dextrose gel were also less likely to survive than those randomised to placebo as indicated by being more likely to require birth assistance, having a higher birth assistance score, being of lower birthweight and having a lower rectal temperature at 1 hour after birth ().
Table 1. Baseline characteristics of triplet lambs.
Lambs that survived to 3 hours after birth had a higher rectal temperature at 1 hour than those that died (median [range] 39.7 [35.6, 41.1] °C vs 39.3 [33.6, 40.9] °C, P = 0.02). Birthweight was similar for lambs that survived and those that died (mean ± SD 4256 ± 761 g vs 4025 ± 720 g, P = 0.34), but was higher in lambs that did not require birth assistance than in those that did (4551 ± 750 g vs 3873 ± 592 g, P < 0.0001) and higher birthweight was associated with higher rectal temperature at 1 hour (β = 0.34 ± 0.16 °C/kg, r2 = 0.06, P = 0.04). There was no association between requirement for birth assistance and rectal temperature (median [range] temperature for lambs that needed birth assistance 39.7 [35.6, 40.9] °C vs did not need birth assistance 39.5 [33.6, 41.1] °C, P = 0.35). We therefore adjusted primary and secondary outcomes for rectal temperature at 1 hour and requirement for birth assistance.
Outcomes
The majority of lambs met the primary outcome of lamb survival at 3 hours after birth (59/71, 83%). Of the 12 lambs (17%) that met the criteria for proxy death, four were removed from the study before 3 hours as they appeared near death and eight had not fed by 3 hours. An additional six lambs died in the paddock and 11 lambs required formula feeds beyond the 3 hour intensive monitoring trial period. Therefore, overall 65/75 (87%) scanned triplet fetuses were alive at 6 weeks after birth, with 23/65 survivors (35%) having needed intervention including artificial feeding. Hence, only 42/75 (56%) survived to 6 weeks without active intervention. As expected, lambs that met the criteria for study intervention were more likely to die before 6 weeks than lambs that did not meet the criteria (adjusted odds ratio [OR ] = 3.3, 95% CI 1.1, 10.5, P = 0.03).
For those lambs that met the criteria for intervention, blood glucose concentration at 1 hour was not associated with survival at 3 hours (median [range] survived 2.37 [0.82, 7.17] mM vs died 2.66 [0.32, 4.41] mM, P = 0.65) or at 6 weeks (2.44 [0.87, 6.77] mM vs 2.47 [0.32, 7.17] mM, P = 0.91). Blood lactate concentration at 1 hour was also not associated with survival at 3 hours (survived 3.22 [1.28, 13.60] mM vs died 3.70 [2.36, 12.30] mM, P = 0.25) or at 6 weeks (2.10 [1.28, 13.60] mM vs 3.14 [1.28, 12.30] mM, P = 0.92).
There was no difference in the primary outcome of survival to 3 hours after birth between the lambs who received dextrose or placebo gel (adjusted OR 2.0, 95% CI 0.3, 16.5, P = 0.50). When compared with placebo gel, dextrose gel did not significantly increase the number of suck attempts after gel, the number of successful feeds, or change blood glucose or lactate concentrations.
Additional exploratory analyses
There was no significant effect of birth order on survival at 3 hours for all 75 lambs born, with 20/25, 80% of firstborn, 19/25, 76% of second born and 20/25, 80% of third born lambs surviving (overall P = 0.92).
The positive relationship between time to first feed and blood glucose concentration at 1 hour did not reach statistical significance (β = 0.012 ± 0.006 mM/hour, r2 = 0.11, P = 0.06).
For analysis of the effect of time to stand on survival, four lambs that were stillborn or did not respond to resuscitation were excluded and lambs that never stood (n = 3) were allocated a time to first stand of 180 minutes. Lambs that took longer to stand were less likely to survive at 3 hours (median [range] time to stand survived 16 [4–46] minutes vs died 27 [7–180] minutes, P = 0.02).
Results of effect of buccal glucose on interstitial glucose
Ten lambs were born at term to five ewes with twin pregnancies in June 2014. Three ewes and therefore six lambs (three male) were randomised to dextrose gel and two ewes and therefore four lambs (one male) to placebo gel. All lambs completed 6 hours of fasting and 4 hours of interstitial glucose monitoring. Baseline characteristics were similar in lambs randomised to dextrose and placebo gel. Median (range) birthweight was similar for both groups (dextrose 4900 g [3500, 6400] vs placebo 4500 g [600, 5300], P = 0.67). Complete interstitial glucose data were available for nine lambs (the sensor failed at 2 hours in one lamb).
Outcomes
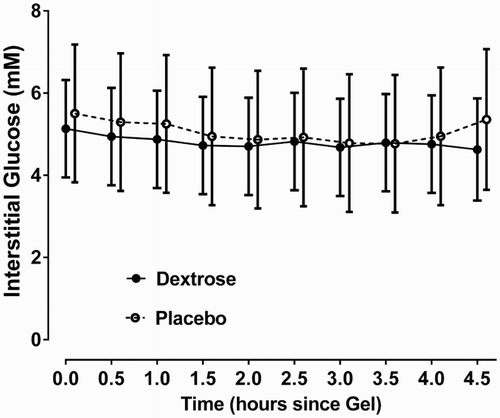
Mean interstitial glucose concentrations were similar in lambs who received dextrose and placebo gel (P = 0.78) and decreased slightly over the study period in both groups, (mean [SD]) 5.32 [0.46] mM to 4.99 [0.48] mM, P < 0.0001). This decrease was similar in both treatment groups (P time*treatment = 0.066). ()
Figure 2. Interstitial glucose concentrations in twin lambs given dextrose or placebo gel. Mean (95% CI) interstitial glucose concentrations for each 30 minute period from time of gel administration to completion of continuous glucose monitoring (total duration 4.5 hours) for lambs given dextrose (n = 6) and placebo (n = 4) gel. Overall mean interstitial glucose concentrations were similar in both groups (P = 0.78). Concentrations decreased slightly over the study period in both groups, (P < 0.0001), and this decrease was also similar in both groups (P time*treatment = 0.066).
Discussion
Recent clinical research in newborn babies, including preterm and small babies, has demonstrated that buccal administration of dextrose gel reverses hypoglycaemia in the first few hours after birth (Harris et al. Citation2013). We had previously observed an increase in vigour in small weak lambs given colostrum and hypothesised that buccal dextrose gel may have a similar effect and reduce the need for artificial feeding and separation of the ewe and lamb. We therefore developed this trial to explore the effect of buccal dextrose gel on lamb survival on lambs that were least vigorous and therefore less likely to be able to compete with siblings to obtain adequate nutrition immediately after birth.
Primary outcome
Contrary to our hypothesis, dextrose gel had no effect on survival at 3 hours after birth. There are several possible reasons for this finding. First, although all the lambs that met the criteria for intervention had some element of impaired vigour, lambs that were randomised to the dextrose gel group were significantly less vigorous than those randomised to placebo. Despite adjustment for this imbalance between groups in the analysis, the difference in baseline characteristics that could affect the primary outcome of survival at 3 hours after birth may have limited our ability to detect a difference between dextrose gel and placebo.
Second, hypoglycaemia may not be a major contributor to lamb death. Indeed, on secondary analysis we found no association between blood glucose at 1 hour after birth and survival at 3 hours or at 6 weeks. Although colostrum improved lamb vigour in previous trials, it may have been another substrate, rather than glucose, in the colostrum that was important. Intra-abdominal injection of 20% dextrose has been used elsewhere (Department for Environment Citation2004; Martin Citation2010; Beef and Lamb New Zealand Citation2012) to revive starving and hypothermic lambs, but has not been tested in a randomised controlled trial.
Third, the dose of dextrose gel we used may not have been large enough to have any effect on survival. This dose was chosen based on a combination of the dose used for treatment of hypoglycaemia in humans (Lilien et al. Citation1980; Harris et al. Citation2013) (0.5 mL/kg 40% dextrose or 200 mg/kg) and the doses being investigated for prevention of hypoglycaemia in humans (Hegarty et al. Citation2015) which included a larger single dose of 1 mL/kg 40% dextrose gel. We wished to separate the lamb and ewe only once, to administer gel to minimise disruption of ewe–lamb bonding, and so selected a single dose regime. We selected a dose of 1 mL/kg as we considered it more likely to increase the glucose concentration than the 0.5 mL/kg dose, but anticipated that an even larger dose volume would increase the time to administer and therefore increase the separation time for ewe and lamb and increase the distress to both. However, the dose previously used for intra-abdominal injection was 10 mL/kg of 20% dextrose (2000 mg/kg), which is five times the dose that we investigated. Indeed, in our second exploratory study of interstitial glucose concentrations, dextrose gel did not change blood glucose concentrations. If there was a transient increase in blood glucose concentration that we missed due to timing of blood sampling, we should have detected this on the interstitial monitoring. Moderate agreement between interstitial and blood glucose concentrations has been demonstrated previously during continuous glucose monitoring in newborn lambs (mean age 6 days, range 3 to 11 days) during insulin-induced hypoglycaemia (Harris et al. Citation2009). Therefore, it seems unlikely the dose of gel that we investigated actually changed circulating glucose concentrations.
It is possible that 400 mg/kg glucose may have been absorbed and metabolised without any detectable increase in circulating glucose concentrations, particularly in the twin lambs where 2 hours of fasting may not have been enough to deplete hepatic glycogen stores, cause hypoglycaemia and inhibit insulin production. Furthermore, handling for interventions including buccal gel administration may still have been stressful to the lambs, leading to increased catecholamine production and therefore inducing glucagon release of glucose from glycogen stores for both dextrose and placebo gel groups.
Finally, the dextrose gel may not have been absorbed via the buccal mucosa. There are no published data on the efficacy of buccal dextrose absorption in lambs (or humans). Sheep buccal mucosa is used for in-vitro testing of human medications (Hoogstraate & Wertz Citation1998) and buccal massage of dextrose gel is effective in treatment of hypoglycaemia in adult humans (American Diabetes Association Citation2013), children (Clarke et al. Citation2009) and newborn babies (Harris et al. Citation2013). However, sheep buccal mucosa is keratinised and thicker than human buccal mucosa with more similarity to the human palatal mucosal than the non-keratinised human buccal mucosa (Sa et al. Citation2016). Therefore, it is possible that a 400 mg/kg buccal mucosal dose of glucose is less well absorbed in lambs than in humans.
Overall survival
The overall survival of the triplet cohort of lambs studied was higher than that reported previously (Dalton et al. Citation1980; Kenyon et al. Citation2006; Muir & Thomson Citation2009), with 87% of scanned triplet fetuses alive at 6 weeks after birth. This might have been due to the high level of intervention among our cohort. We housed the ewes indoors during lambing, thereby providing shelter and a higher environmental temperature than ewes and lambs would have been exposed to in the paddock. It is notable that only 56% of our cohort survived to 6 weeks without active intervention. This active intervention included birth assistance to lambs that were malpresented, which may have reduced the stillbirth and early deaths. We also resuscitated 37% of lambs following birth and artificially fed all those that met the criteria for proxy death; some of these lambs may not have survived without these interventions.
Lambs that met criteria for intervention were more likely to die before 6 weeks than those that were observed. This confirms that we selected the most vulnerable lambs for this randomised trial and reinforces the importance of targeting the less vigorous lambs for potential interventions that might increase survival.
Lambs with a lower birthweight had a lower rectal temperature and lambs with a lower rectal temperature were more likely to die. Smaller lambs have a larger body surface area, which leads to increased heat loss, and this was apparent even for our cohort of lambs managed indoors. Previous studies have also reported lower survival rates in smaller lambs (Dalton et al. Citation1980) and lower rectal temperatures in smallest triplet lambs (Stafford et al. Citation2007; Kerslake et al. Citation2010a). However, it is interesting that neither birthweight nor blood glucose concentration predicted survival for our cohort, suggesting that temperature rather than other aspects of physiology related to size at birth is a major opportunity for intervention to improve survival in triplets.
Thermoregulation is influenced by body surface area, environmental temperature, body composition and intrinsic hormonal control i.e. thyroid and adrenergic hormones. Triplet lambs, especially lightest-birthweight triplets, have lower thyroid hormone concentrations than twins and heaviest triplets (Kerslake et al. Citation2010a) and higher tri-iodothryronine concentrations are associated with increased heat production in both twin and triplet lambs (Kerslake et al. Citation2010b). However, supplementation of maternal ewe diet with iodine has not been shown to increase lamb plasma thyroid concentrations (Kerslake et al. Citation2010b).
Feeding
By design, less vigorous lambs that met the criteria for intervention took longer to feed than those that were only observed. The overall median time to first feed was 67 minutes. Median time to feed varies by breed (Cloete & Scholtz Citation1998) and may take longer for subsequent multiples than first born and singleton lambs, but has been reported as between 30 and 60 minutes (Romney/Romney × Southdown) (Wallace Citation1949), 51 minutes (in Romney/Merino × Romney) (Asofi Citation1984) and 90 minutes (Scottish Blackface and Suffolk) (Matheson et al. Citation2011). We are unable to comment on the relationship between time to first feed and survival because all lambs that had not fed by 3 hours after birth were allocated to proxy death and had artificial feeds for ethical reasons. However, the duration from birth to first feed had no effect on blood glucose concentration. This may be because the normal physiology of glycaemic control is already stable after 1 hour; the time at which we first measured blood glucose concentration.
We did not demonstrate any increase in attempted or successful feeding following dextrose gel. However, the physiology of feeding is complex and it is possible that hypoglycaemia is one of the normal physiological processes following birth that increase the lambs’ desire to feed. Furthermore, the simple effect of separating the lamb and ewe may have either a positive or negative effect on desire to feed, as may the stimulus of buccal mucosa massage. Maternal behaviour also affects the lambs’ ability to successfully feed, and varies with breed and parity, but we did not document maternal behaviour in our cohort (Cloete & Scholtz Citation1998; Nowak & Poindron Citation2006).
Limitations
Limitations to the trial included not achieving our calculated sample size, which was not possible for logistic reasons. This may have reduced the potential to detect any difference in survival or other exploratory analyses. Additionally, for the lambs that underwent continuous glucose monitoring, it is possible that the fasting period was too short, and that we therefore missed the maximal potential to demonstrate an increase in interstitial glucose in response to dextrose gel. However, it is also possible that the buccal dextrose or buccal stimulation effected release of insulin, which would lower blood glucose concentrations, or that adrenaline was secreted in response to the stress of separation from ewe and handling, which would raise blood glucose concentrations. We did not monitor serum insulin or alternative substrates other than lactate.
Conclusion
In less vigorous triplet lambs, 400 mg/kg buccal dextrose gel given at 1 hour of age does not increase survival. Lower temperature at 1 hour after birth, rather than birthweight or blood glucose concentration, was associated with decreased survival in this cohort. Our findings may suggest that interventions to address hypothermia rather than just hypoglycaemia may be most effective in improving survival in less vigorous triplet lambs.
Acknowledgements
We thank the staff at Liggins Research Farm for their assistance with monitoring labour, undertaking observations and animal husbandry: Gregg Pardoe, Maggie Worthington and Travis Gunn; all those who participated in the 24-hour watches of ewes in labour and of lamb feeding behaviour during the trial: Emma Buckels, Nataliia Burakevych, Karen Mumme, Sarah Sharp, Tanya Poppe, Logan Williams, Catherine and Jason Mokrzecki; Safayet Hossain for randomisation of treatment allocation; Jess Wilson for data management; and Biomed Ltd (Auckland, New Zealand) for providing the study gels.
J. E. Hegarty participated in all trial interventions, data collection, analysis, interpretation and wrote and coordinated all drafts of the manuscript; J. E. Harding contributed to data analysis, interpretation and edited all drafts of the manuscript. J. D. and G. C. recalibrated the raw interstitial glucose data. G. G. undertook analysis, and contributed to interpretation of, the interstitial glucose data. M. H. O. assisted with study design and advised on animal welfare issues. A. L. J. conceived the study, participated in trial interventions and data collection and contributed to data analysis and interpretation, reviewed all versions of the manuscript and has overall responsibility for the study. All authors have read and contributed to the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCiD
J. E. Hegarty http://orcid.org/0000-0001-8563-6590
J. E. Harding http://orcid.org/0000-0003-2697-1422
Additional information
Funding
References
- Amer PR, McEwan JC, Dodds KG, Davis GH. 1999. Economic values for ewe prolificacy and lamb survival in New Zealand sheep. Livest Prod Sci. 58:75–90. doi: 10.1016/S0301-6226(98)00192-4
- American Diabetes Association. 2013. Standards of medical care in diabetes-2013. Diabetes Care. 36(Suppl 1): S11–S66.
- Asofi MK. 1984. A study on the breeding performance of Romney and Border Leicester cross Romney ewe hoggets and two-year-old ewes [Unpublished thesis]. Massey University, Massey Research Online. Available from: http://mro.massey.ac.nz/handle/10179/3598
- Beef and Lamb New Zealand. 2012. Reviving newborn lambs. [cited 2016 Jan 4]. Available from: http://beeflambnz.com/farm/farm-facts-resources/animal-breeding-and-growth/?dk = reviving&ds=&dt=
- Beef and Lamb New Zealand. 2015. Lamb Crop 2015. [cited 2016 Jan 4]. updated December 2015. Available from: http://www.beeflambnz.com/news-events/media-releases/2015/december/new-zealand-lamb-crop-2015/
- Clarke W, Jones T, Rewers A, Dunger D, Klingensmith GJ. 2009. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 10(Suppl 12):134–145. doi: 10.1111/j.1399-5448.2009.00583.x
- Cloete SWP, Scholtz AJ. 1998. Lamb survival in relation to lambing and neonatal behaviour in medium wool Merino lines divergently selected for multiple rearing ability. Aust J Exp Agr. 38(8):801–811. doi: 10.1071/EA98095
- Dalton DC, Knight TW, Johnson DL. 1980. Lamb survival in sheep breeds on New Zealand hill country. New Zeal J Agr Res. 23:167–173. doi: 10.1080/00288233.1980.10430783
- Darwish RA, Ashmawy TA. 2011. The impact of lambing stress on post-parturient behaviour of sheep with consequences on neonatal homeothermy and survival. Theriogenology. 76(6):999–1005. doi: 10.1016/j.theriogenology.2011.04.028
- Department for Environment, Food and Rural Affairs. 2004. Improving lamb survival. [cited 2016 Jan 4]. Available from: www.defra.gov.uk
- Dwyer CM, Morgan CA. 2006. Maintenance of body temperature in the neonatal lamb: effects of breed, birth weight, and litter size. J Anim Sci. 84(5):1093–1101. doi: 10.2527/2006.8451093x
- Fisher M. 2003. New Zealand farmer narratives of the benefits of reduced human intervention during lambing in extensive farming systems. J Agric Environ Ethics. 16(1):77–90. doi: 10.1023/A:1021758427469
- Greer AW, Corner-Thomas RA, Logan CM, Kenyon PR, Morris ST, Ridler AL, Hickson RE, Blair HT. 2015. Perceived importance of areas of future research: results from a survey of sheep farmers. New Zeal J Agr Res. 58(4):359–370. doi: 10.1080/00288233.2015.1037461
- Harris DL, Battin MR, Williams CE, Weston PJ, Harding JE. 2009. Cot-side electro-encephalography and interstitial glucose monitoring during insulin-induced hypoglycaemia in newborn lambs. Neonatol. 95(4):271–278. doi: 10.1159/000166847
- Harris DL, Weston PJ, Signal M, Chase JG, Harding JE. 2013. Dextrose gel for treating neonatal hypoglycaemia: a randomised placebo-controlled trial (The Sugar Babies Study). Lancet. 382(9910):2077–2083. doi: 10.1016/S0140-6736(13)61645-1
- Hegarty JE, Harding JE, Gamble GD, Crowther C, Edlin R, Alsweiler JM. 2015. Preventing neonatal hypoglycaemia with oral dextrose gel: a randomised controlled trial. J Paediatr Child H. 51(Supplement S1: Abstracts of the 19th Annual Meeting of The Perinatal Society of Australia and New Zealand (PSANZ), 19–22 April 2015): 68.
- Hoogstraate JAJ, Wertz PW. 1998. Drug delivery via the buccal mucosa. Pharm Sci Technol Today. 1(7):309–316. doi: 10.1016/S1461-5347(98)00076-5
- Kenyon PR, Revell DK, Morris ST. 2006. Mid-pregnancy shearing can increase birthweight and survival to weaning of multiple-born lambs under commercial conditions. Aust J Exp Agr. 46(7):821–825. doi: 10.1071/EA05329
- Kerslake JI, Kenyon PR, Stafford KJ, Morris ST, Morel PCH. 2010a. Does the physiological status of lambs within a twin- and triplet-born litter differ during the first 12 hours of life?. Anim Prod Sci. 50(6):522–527. doi: 10.1071/AN09179
- Kerslake JI, Kenyon PR, Stafford KJ, Morris ST, Morel PCH. 2010b. Can maternal iodine supplementation improve twin-and triplet-born lamb plasma thyroid hormone concentrations and thermoregulation capabilities in the first 24-36 h of life?. J Agr Sci. 148(4):453–463. doi: 10.1017/S0021859610000286
- Lilien LD, Pildes RS, Srinivasan G, Voora S, Yeh TF. 1980. Treatment of neonatal hypoglycemia with minibolus and intraveous glucose infusion. J Pediatr. 97(2):295–298. doi: 10.1016/S0022-3476(80)80499-9
- Martin SJ. 2010. Hypothermia in newborn lambs. [cited 2016 Jan 6]. Available from: http://www.omafra.gov.on.ca/english/livestock/sheep/facts/98-089.htm
- Matheson SM, Rooke JA, McIlvaney K, Jack M, Ison S, Bunger L, Dwyer CM. 2011. Development and validation of on-farm behavioural scoring systems to assess birth assistance and lamb vigour. Animal. 5(5):776–783. doi: 10.1017/S1751731110002430
- Morel PCH, Morris ST, Kenyon PR. 2008. Effect of birthweight on survival in triplet-born lambs. Aust J Exp Agr. 48(7):984–987. doi: 10.1071/EA07401
- Morris ST. 2013. Sheep and beef cattle production systems. In: Dymond JR, editor. Ecosystem services in New Zealand – conditions and trends. Lincoln, New Zealand: Manaaki Whenua Press; p. 79–84.
- Muir PD, Thomson BC. 2009. Survival and performance of multiple lambs (03OFR01). [cited 2016 Jan 4]. Available from: www.meatandwoolnz.com
- National Animal Ethics Advisory Committee. 1999. Animal welfare act 1999. Wellington, New Zealand: New Zealand Government.
- National Animal Ethics Advisory Committee. 2010a. Sheep & beef cattle code of welfare. Wellington, New Zealand: New Zealand Government.
- National Animal Ethics Advisory Committee. 2010b. Good practice guide for the use of animals in research, testing and teaching. Wellington, New Zealand: New Zealand Government.
- Nowak R, Poindron P. 2006. From birth to colostrum: early steps leading to lamb survival. Reprod Nutr Dev. 46(4):431–446. doi: 10.1051/rnd:2006023
- Oliver MH, Hawkins P, Harding JE. 2005. Periconceptional undernutrition alters growth trajectory and metabolic and endocrine responses to fasting in late-gestation fetal sheep. Pediatr Res. 57(4):591–598. doi: 10.1203/01.PDR.0000155942.18096.9C
- Sa G, Xiong X, Wu T, Yang J, He S, Zhao Y. 2016. Histological features of oral epithelium in seven animal species: as a reference for selecting animal models. Eur J Pharm Sci. 81:10–17. doi: 10.1016/j.ejps.2015.09.019
- Shelley HJ. 1960. Blood sugars and tissue carbohydrate in foetal and infant lambs and rhesus monkeys. J Physiol. 153(3):527–552. doi: 10.1113/jphysiol.1960.sp006553
- Signal M, Le Compte A, Harris DL, Weston PJ, Harding JE, Chase JG, Chyld Study G. 2012. Impact of retrospective calibration algorithms on hypoglycemia detection in newborn infants using continuous glucose monitoring. Diabetes Technol Therapeut. 14(10):883–890. doi: 10.1089/dia.2012.0111
- Stafford KJ, Kenyon PR, Morris ST, West DM. 2007. The physical state and metabolic status of lambs of different birth rank soon after birth. Livest Sci. 111(1–2):10–15. doi: 10.1016/j.livsci.2006.10.018
- Statistics New Zealand. 2015. Agricultural Production Statistics: June 2015 (final). [cited 2016 Aug 30]. Availabe from: http://www.stats.govt.nz/c./media/Statistics/Browse%20for%20stats/AgriculturalProduction_final/HOTPJun15final/AgriculturalProduction_finalJun15finalHOTP.pdf
- Wallace LR. 1949. Observations of lambing behaviour in ewes. Proc New Zeal Soc Anim Prod. 9:85–96.
- Wheaton JE, Carlson KM, Windels HF, Johnston LJ. 1993. Cidr - a new progesterone-releasing intravaginal device for induction of estrus and cycle control in sheep and goats. Anim Reprod Sci. 33(1–4):127–141. doi: 10.1016/0378-4320(93)90111-4