ABSTRACT
Increasing the number of lambs with high-quality meat weaned per ewe, optimised for each farm system, is a key mechanism to improve the overall efficiency of sheep production. Lambing hoggets (yearlings) has long been identified as one way to improve efficiency, with the potential to maintain or increase production within the same environmental footprint. However the adoption of hogget lambing in New Zealand remains low due to both negative perception of the practice among farmers, and poor reproductive performance in hoggets. In this review we describe the processes contributing to reproductive success in hoggets, outline the research that underpins industry guidelines for lambing hoggets, and identify knowledge gaps and potential for improvement in hogget lambing efficiency.
The potential of hogget lambing
In New Zealand, while the majority of ewes (80%) attain puberty at approximately 8 months of age (i.e. as hoggets; Edwards et al. Citation2015), most (66–85%) are mated for the first time as two-tooths when they are approximately 18–20 months of age (Stevens Citation2010). A clear opportunity to increase farm profitability is through mating hoggets, so that they produce a lamb at 1 year of age (Kenyon et al. Citation2014). Simplistically, if ewes are mated five times during their lifetime, then producing an extra litter as a hogget has the potential to increase their lifetime production by 20% and increase their efficiency with little increase in environmental impact. However, there is the caveat that within a farm system the adoption of hogget lambing creates competition for feed resources, particularly during pregnancy and lactation. If reliable production of lambs from hoggets was achieved, at a farm system level the same number of lambs could be produced from a smaller capital stock, potentially reducing environmental impact.
Showing oestrus as a hogget (in systems that do not include hogget lambing) increases their lambing percentage as a two-tooth by approximately 15% (Edwards et al. Citation2015). Further, in addition to producing an additional lamb crop, it has been shown that ewes that produce a lamb as a hogget weaned an average of 3.24 lambs over the subsequent 3 production years compared with 2.4 lambs weaned by those which did not produce a lamb as a hogget (Levine et al. Citation1978). Hence, hogget lambing appears to improve reproductive efficiency beyond simply the production of an additional lamb crop.
Although the potential of hogget lambing is compelling, each hogget put to the ram will produce an average of only 0.6 lambs to weaning (Kenyon et al. Citation2004b), compared with 1.2 lambs in older ewes. This reduced performance is one of the reasons why the practice has not been more widely adopted in New Zealand and lambs from hoggets represent only 4% of lambs produced (Statistics New Zealand Citation2015). If the preferential feeding required to successfully grow hoggets to target mating weights (Kenyon Citation2012) is also considered, the potential economic gain can be quickly eroded.
One of the difficulties in understanding the cause of the relative inefficiency of hogget lambing is the way in which is it being measured. Results from a survey of farmers that lambed hoggets in 2002 (Kenyon et al. Citation2004b) reported the overall lambing rate calculated as the number of lambs present approximately 1 month after lambing/number of hoggets put to the ram. While this provides a general overview of production efficiency, it does not allow for ready assessment of where hogget lambing is failing.
Fertility of the ewe through her lifetime
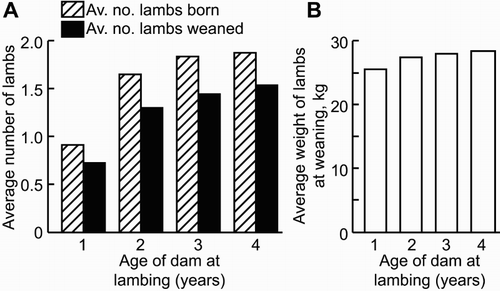
The fecundity of a ewe changes as she ages, with records from the Invermay research farm showing that reproductive performance increases sharply between lambing at one (i.e. hogget) and 2 years of age, with a further smaller increase as the ewe fully matures at 3 years of age (). This change in reproductive performance is primarily driven by the number of lambs born (NLB) as the pattern for number of lambs weaned mirrors that of NLB, indicating that, in an intensively monitored research farm system, lamb loss after birth is similar between ewes of different ages. Similarly, the weaning weight of the lamb, when adjusted for age of the lamb at weaning, is also relatively similar between ages, with just small increases between 1- and 2-year-old dams compared with older dams (). This pattern of differences in reproductive efficiency between hoggets and older ewes is observed across different breeds and multiple management systems although relative differences can vary (Boggess et al. Citation1991; Hanford et al. Citation2002, Citation2003).
Figure 1. Pattern of NLB and weaned, and average weaning weight of the lamb at different ewe ages (from Edwards et al. Citation2015, Citation2016). (A) The average NLB and weaned (NLW) for ewes at various ages. (B) The average weight of the lamb at weaning (adjusted for age at weaning) for ewes at various ages. Direct comparisons were available between ewes at one and 2 years of age (Edwards et al. Citation2016) and from ewes at 2, 3 and 4 years of age (Edwards et al. Citation2015). Measurements from 2-year-old dams were used to normalise data between data sets.
Reproductive processes that contribute to fertility of the young ewe
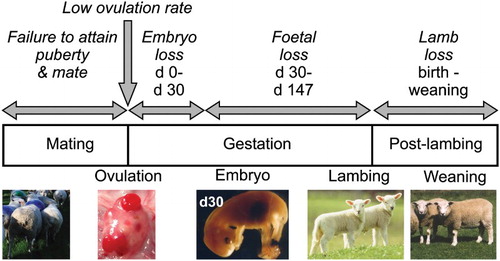
The ewe hogget needs to complete multiple reproductive processes in order to successfully raise a lamb to weaning (). The first hurdle is the initiation of reproductive cycles (i.e. attainment of puberty). She then needs to ovulate competent ova and be mated by a fertile ram. Her reproductive tract needs to be capable of supporting fertilisation and embryonic development, and nurturing a healthy foetus until birth. Finally, she needs to deliver a healthy live lamb, lactate, and raise the lamb to weaning. Failure to complete any of these steps will result in reproductive failure. In addition, negative modulation of ovulation rate (OR, i.e. the number of ova released at one cycle) as well as embryo, foetal, or lamb survival can limit the ability of the hogget to reach her reproductive potential.
Figure 2. Schematic diagram of factors influencing reproductive output of ewe hoggets. The hogget ewe must first attain puberty and be mated by the fertile ram. The upper limit of her reproductive potential is set by the number of ova she releases at mating. These ova must then be fertilised and undergo normal embryo and then foetal development. She must then deliver live lambs at birth and support them until weaning.
Measurement of each of these steps allows us to better understand which stages of the reproductive process are contributing to the poor reproductive performance in hoggets. In addition, by comparing the performance of the hogget with her performance as a two-tooth (i.e. 2 years of age), we can begin to identify the steps of the reproductive process in which the hogget is particularly vulnerable when compared with older ewes.
In a trial directly examining reproductive performance of hoggets, using industry best practices for hogget lambing (Kenyon Citation2012), key areas of reproductive loss were identified. These included failure of the hogget to attain puberty/be mated by the fertile ram, loss of the embryo during early gestation (prior to day 35), and loss of the lamb between birth and weaning (Edwards et al. Citation2016). In a direct comparison between reproductive performance as a hogget and as a two-tooth, key differences were observed in OR, failure to be mated by the fertile ram, and survival of the embryo until day 30, indicating that the hogget ewe is particularly vulnerable during these reproductive steps. Thus, targeting these specific reproductive steps in the hogget may allow an increase in her reproductive efficiency to levels approaching those seen in adult ewes.
Methods reported by farmers for selecting hoggets for mating (including weight or unspecified ‘other criteria’) did not significantly increase the lambing rate (Kenyon et al. Citation2004b). This finding suggests that the criteria (if any) that are currently being used to select hoggets for mating are not successful, indicating a need to develop new products/production systems to improve hogget reproductive performance in the identified key reproductive steps.
Known influences on reproductive performance
Development of the reproductive system
This section is intended to provide some basic information about the development of key organs/tissue involved in female fertility including the ovary, oviduct, uterus, brain, and pituitary (). When considering lamb survival and growth, the mammary tissue and lactation also need to be considered but are outside the scope of this review (see Rowson et al. Citation2012; Ferreira et al. Citation2013; Lerias et al. Citation2014; for current reviews).
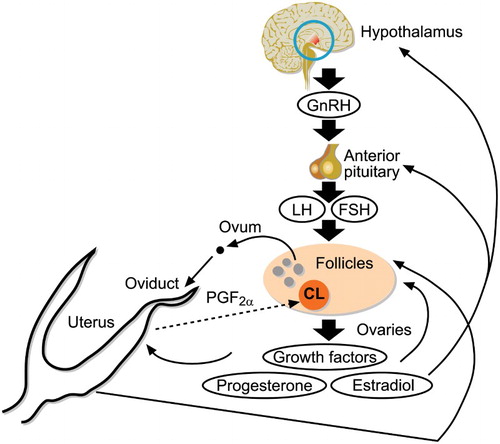
Figure 3. Schematic diagram of the organs and cells important for control of reproduction in sheep. The hypothalamus releases GnRH, which acts on the pituitary to control synthesis and release of FSH and LH. These two hormones act to stimulate the growth and maturation of the ovarian follicle. Hormones from the follicle also provide feedback to the hypothalamus and pituitary to regulate the release of FSH and LH in order to time the release of the oocyte. Oestradiol is a key hormone in coordinating the preovulatory LH surge, which causes release of the ovum from the follicle, and induces oestrus. The preovulatory LH surge also causes luteinisation of the remaining follicular cells to form the corpus luteum (CL). If pregnancy does not occur, the uterus releases prostaglandin F2α, which acts on the corpus luteum to cause regression of this structure, allowing another reproductive cycle to occur.
Development of the ovary
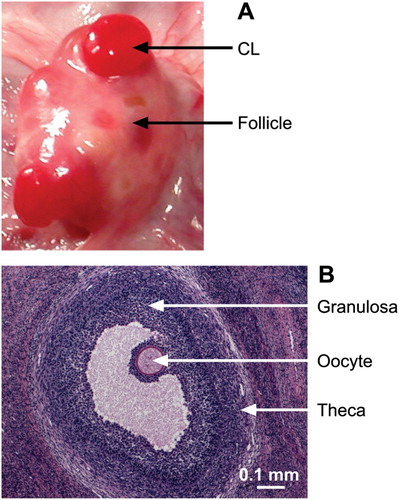
To better understand the development of the ovary, it is first necessary to understand the basic structures found in the ovary. In the adult ovary, the oocytes (i.e. the eggs) are contained within structures called the ovarian follicles (). The oocytes are surrounded by somatic cells termed granulosa and theca cells. These cells have multiple purposes, first to support the oocyte as it grows and matures prior to ovulation and, second, to be involved in the communication among the ovary, uterus, and pituitary. This communication is necessary to time the release of a mature ova (the ovulated oocyte) to coincide with the presence of sperm, and ensure that the ewe's reproductive tract (oviduct and uterus) is ready to support fertilisation and early embryo development. Following ovulation, which only occurs after the animal attains puberty, the remaining somatic cells of the ovarian follicle form the corpus luteum, which produces the hormone progesterone, which is essential for continued growth and development of the embryo and foetus.
Figure 4. Key ovarian structures. (A) An ovary with two corpora lutea (CL) and various follicles. (B) A haematoxylin- and eosin-stained section of the ovary showing an antral follicle with oocyte, granulosa cells and theca indicated.
In the sheep, the ovarian follicles form during foetal life, thus all the ova that the animal will ever have are present prior to birth (Sawyer et al. Citation2002). The growth and development of the ovarian follicle, and maturation of an oocyte for ovulation, takes approximately 6 months. Thus, the quality of the ova released today could have been influenced by an event that occurred months, or even years, ago.
The ovary begins to form around day 23 of gestation, with the first follicles formed at approximately day 75 and all follicles formed by day 110 of gestation (reviewed in Juengel & Smith Citation2014). At birth, follicles at various stages of development can be observed in the ovary. Only a few of these follicles will ever be ovulated, and ovulation does not occur until attainment of puberty. Thus, while a great majority of follicles begin to grow and mature, most of these (>99.9%) will not complete their development and will die by a process known as atresia, somewhere along the developmental pathway. However, the total number of ovarian follicles that an animal has at any point in time is related to reproductive function in both sheep and cattle, with increased numbers of follicles positively linked to improved fertility (Cushman et al. Citation2009; Lahoz et al. Citation2012; Mossa et al. Citation2012; Torres-Rovira et al. Citation2014). In cattle, the number of follicles that an animal is born with is controlled by both genetics and the environment (Evans et al. Citation2012; Walsh et al. Citation2014). Poor nutrition or mastitis in the mother during key steps of foetal ovarian development and follicle formation has been shown to decrease the number of follicles of her offspring (Evans et al. Citation2012). This is linked to changes in function of key reproductive structures such as the corpus luteum and the uterus, likely resulting in poorer reproductive function of the animal as an adult (Jimenez-Krassel et al. Citation2009).
These relationships have not been examined in depth in sheep and thus whether similar relationships exist, and how the effect of low follicle counts might change over the animal's lifetime, are not well understood. However, a few studies have linked antral follicle count (AFC) and anti-Mullerian hormone (AMH; an indirect measure of AFC), with fertility in young sheep (Lahoz et al. Citation2012; Torres-Rovira et al. Citation2014).
Development of the uterus
The oviduct and uterus also form during foetal life, but key structures in the uterus called uterine glands (which are critical for nourishment of the embryo during its early development) are formed in the first 8 weeks of life (reviewed in Spencer et al. Citation2005; Cooke et al. Citation2013; Vallet et al. Citation2013). If the development of these uterine glands is disrupted, the fertility of the animal throughout her lifetime will be compromised (Spencer & Gray Citation2006). It is known that hormones from the ovarian follicles are critical for the normal development of the uterine glands (Carpenter et al. Citation2003; Hayashi et al. Citation2008). However, the effects of environmental factors, such as nutrition, on uterine gland formation are not well understood. Furthermore, there is a paucity of information regarding the factors regulating the release of key hormones and growth factors from the ovarian follicles as well as the factors regulating the uterus's response to these hormones and growth factors. However, work using embryo transfer has shown that uterine function was not compromised in hoggets compared with adults (Quirke & Hanrahan Citation1977).
Establishment of communication among brain, ovary and uterus for initiation of reproductive cycles
A key developmental milestone for reproductive maturity is the establishment of the communication among the brain, ovary and uterus that leads to the initiation of reproductive cycles (). The establishment of this communication, and thus the advent of the first reproductive cycle, is often used as the marker for the attainment of puberty. The structures involved in this communication are all formed during birth but the feedback loops among them are refined postnatally. The hypothalamus in the brain produces gonadotropin releasing hormone (GnRH), which in turn causes the pituitary to release the gonadotropins: follicle-stimulating hormone (FSH) and luteinising hormone (LH). These hormones act upon the ovarian follicles to stimulate their growth and development, with the later stages of follicular development being dependent first on FSH, and then on both FSH and LH. The follicle produced hormones, including oestradiol, progesterone, and inhibin, feedback to the hypothalamus and pituitary to regulate production of GnRH, LH, and FSH (Scaramuzzi et al. Citation2011). Sometimes this feedback occurs directly to the cells producing these hormones, but sometimes the feedback is indirect through regulation of production of other neurohormones such as kisspeptin (reviewed in Smith & Clarke Citation2010; Valasi et al. Citation2012). A surge of LH causes ovulation of the mature follicle, and luteinisation of the somatic cells of the follicle, leading to formation of the corpus luteum (Niswender et al. Citation2000). Hormones from the ovarian follicle, particularly oestradiol and progesterone, cause changes in the oviduct and uterus to prepare it to receive the ova, facilitate fertilisation, and support embryonic development (Spencer et al. Citation2008; Hunter Citation2012). However, if a healthy embryo is not present, the uterus releases prostaglandin F2α to induce another reproductive cycle, giving the female another chance to become pregnant (Niswender et al. Citation2000).
As indicated earlier, ovarian follicles begin growing prior to birth and it is known that there is a burst of follicular activity during neonatal life. It is thought that hormones from the ovarian follicles feedback to the brain to help establish these communications links. These products from the ovary also influence development of the uterus. As the animal matures, continued communication occurs between the ovary and the brain until ovulation of a mature follicle at puberty. Often, this first ovulation is not accompanied by oestrous behaviour and thus is termed a silent heat. This may be followed by ovulation of another follicle 7–10 days later, which is a shorter interval than the normal intraovulatory period of 17 days in sheeps. This is accompanied by oestrous behaviour and thus mating and pregnancy can occur (Valasi et al. Citation2012).
Again, both the environment and genetics are known to regulate the timing of attainment of puberty. This timing is complicated by the seasonal nature of breeding in sheep. Assuming optimum conditions, the majority of ewe lambs will attain puberty during their first breeding season at approximately 8 months of age. However, if the ewe lamb is born late during the lambing season, or fails to achieve proper growth, she may not achieve sufficient maturity prior to the onset of seasonal anoestrus and thus her first reproductive cycle will not occur until her second breeding season almost a year later (reviewed in Valasi et al. Citation2012).
The role of body weight/body mass index in attainment of puberty is well established for multiple species (Davies Citation2006; Valasi et al. Citation2012; Diskin & Kenny Citation2014). An Iranian study found that date of birth had a significant effect on age and body weight at puberty with ewe lambs born late in the season being lighter and younger at onset of puberty (Bathaei & Leroy Citation1997). These animals may have been affected by seasonal grass growth and it was suggested that puberty in animals born earlier is delayed until there is sufficient feed available to allow them to reach the necessary body weight. However, an alternative interpretation is that day length at birth affects onset of puberty. In addition, this study found that ewe lambs which grew faster pre-weaning tended to reach puberty at a younger age and heavier body weight. Ewe lambs that grew most rapidly after weaning tended to be older and heavier at puberty. These findings suggest that growth rates over a long period influence the timing of onset of puberty.
In a 5-year study, the percentage of ewes failing to achieve puberty during their first breeding season averaged approximately 20%, but it ranged from <10% to over 35% for the different years. Animal weight was clearly linked to failure to attain puberty during the first breeding season, as the average weight of those failing to attain puberty were always less than those attaining puberty. However, weight differences could not explain the differences between years in the proportion attaining puberty (Edwards et al. Citation2015).
Ovulation rate
Regulation of the number of ova released at a reproductive cycle (OR) is complex, involving endocrine hormones such as follicle-stimulating hormone as well as local growth factors such as growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15; Scaramuzzi et al. Citation2011; Juengel et al. Citation2013). The majority of the ovarian follicles present in the ovary are primordial/transitory, or type 1/1a follicles. These follicles are considered to be quiescent and remain in the ovary until they are stimulated to grow. The factors controlling the recruitment of follicles into the growing pool are currently not well defined but these factors are produced within the ovary itself. Some hormones that appear to regulate the number of follicles recruited into the growing pool include AMH and steroids such as oestrogens. The growth of the ovarian follicle from the type 1 stage of development until ovulation takes approximately 6 months. The initial stages of development of the follicle are thought to be controlled primarily by factors produced by the ovary, with growth factors produced by the oocyte being key regulators of early follicular development. Members of the transforming growth factor beta superfamily, which include the oocyte-produced GDF9 and BMP15, are key regulators of these stages of ovarian follicular development.
The pituitary gonadotropins, FSH and LH, are essential for the last few stages of follicular growth and maturation. Key feedback loops exist between the ovary and the pituitary whereby FSH and LH drive production of ovarian steroids and protein hormones such as inhibin. These, in turn, feedback to the hypothalamus and pituitary to initially decrease concentrations of FSH in particular. As the large growing follicles become increasingly dependent on FSH for survival, those that have not matured sufficiently to survive with the decreasing FSH concentrations cease to develop and undergo a process of atresia. It is only those follicles that have matured sufficiently to express LH receptors in granulosa cells that can survive during this period of reduced FSH. These follicles continue to grow and produce oestrogens, which, upon reaching a sufficient concentration, cause the release of GnRH and thus the preovulatory LH surge, resulting in ovulation of the fully mature ovulatory follicles. Local ovarian growth factors, such as those belonging to the insulin-like growth factor family, or BMP15 and GDF9, play a role in determining how many follicles ovulate through regulating the production of the ovarian steroids and inhibin. Additionally, they regulate expression of the receptors for FSH and particularly LH to allow more follicles to survive in the periods of low FSH concentrations, thus increasing OR (reviewed in Scaramuzzi et al. Citation2011; Juengel et al. Citation2013).
Fertilisation and embryo survival
Assuming the ewe is mated by a fertile ram, fertilisation failure is thought to be low at 1–2% (Quinlivan et al. Citation1966). The majority of this work has been done in older ewes so fertilisation failure could be higher in hoggets. In studies which directly compare cleavage rates (a measure of fertilisation) between hoggets and adults, cleavage rate was not lower in oocytes collected from prepubertal ewes than was observed in oocytes from adult ewes (O’Brien et al. Citation1997; Reader et al. Citation2015). Thus, fertilisation failure does not appear to be a major contributor to poor reproductive performance of hoggets but, to our knowledge, this has not been thoroughly tested in vivo.
Hoggets do, however, have lower embryo survival, with approximately twice as many embryos lost during the first 35 days of pregnancy as older ewes (Edwards et al. Citation2016). Based on studies using embryos collected from hormonally stimulated juvenile and adult animals, this reduced embryo survival has been linked to factors affecting quality of the ova and/or the ability of the oviduct to nurture the first few cells divisions of the embryo (Quirke & Hanrahan Citation1977). In addition, the low success rate of producing embryos following in vitro maturation and fertilisation of prepubertal oocytes compared with oocytes from adults (O’Brien et al. Citation1997; Reader et al. Citation2015) points to an issue with development of the oocyte. In naturally mated adult ewes, the majority of the embryo loss occurs prior to day 14 of pregnancy, with a significant loss observed prior to day 4 (O’Connell et al. Citation2016). This is consistent with quality of the ova and/or oviduct being significant contributors to reproductive success in both hoggets and adult ewes.
The understanding of factors affecting survival of the early embryo in vivo is difficult to address experimentally and thus is still in its infancy. The relative contribution of environment and genetic effects on embryo/foetal survival in adult ewes have been examined by comparing the number of ova ovulated, to the NLB. Given that the majority of loss occurs prior to day 35 of pregnancy (Quinlivan et al. Citation1966; O’Connell et al. Citation2016), these outcomes are more likely driven by embryo versus foetal loss. The ability of the ewe to successfully nurture an embryo was lowly heritable at 4.4% (Shorten et al. Citation2013). Direct heritability (i.e. the effects of the embryo's genes) for embryo survival was not significant although it should be noted that this does not rule out the potential for recessive lethal genes being present in the population as has been observed in dairy cattle (VanRaden et al. Citation2011; Fritz et al. Citation2013). In sheep, the permanent maternal environment accounted for 8.5% of the variation observed in embryo survival (Shorten et al. Citation2013), suggesting that environmental events, potentially during development/maturation of the reproductive organs during gestation or early life as discussed above, are more important than genetic effects. However, it is import to note that these studies were undertaken with animals 2 years or older at lambing.
Foetal survival
There is anecdotal evidence that foetal loss occurs more frequently in hoggets than in older ewes (Ridler et al. Citation2015). In vaccinated hoggets (see below) this loss is largely as a result of low pre-mating weight and/or low weight gain in early pregnancy. However, when environmental conditions and nutrition are optimal, loss of the foetus in the later stages of pregnancy is relatively low (<5%) and not different between young adult and hogget ewes (Edwards et al. Citation2016). Hence provision of sufficient nutrition as well as control of diseases that can cause foetal death is important and discussed further below.
Parturition and lamb survival and performance
Difficulty with parturition and subsequent failure of the lamb to survive, particularly during the first week of life, accounts for a significant loss of lambs across dam age groups (Everett-Hincks et al. Citation2014). In closely shepherded research flocks, lamb loss from birth to weaning was similar between hoggets and two-tooths, suggesting that hoggets do not differ from two-tooths in terms of their ability to successfully birth and raise lambs (Edwards et al. Citation2016). However, surveys of commercial flocks provide some evidence of higher lamb loss in hoggets than adult ewes, raising the possibility that the hogget, and her offspring, are more susceptible to adverse environmental conditions including nutritional restriction (Morel et al. Citation2010; Young et al. Citation2010). Key causes of lamb deaths from hogget mothers include dystocia and starvation/exposure (Young et al. Citation2010). The mortality rate to weaning of lambs born to hoggets is approximately 28% (McMillan Citation1983), with 42% of these being due to dystocia, accounting for approximately 12 deaths per 100 lambs born. Birth weight was found to be important with both light and heavy lambs being more likely to die.
Growth rates of lambs raised by hoggets did not differ from those raised by two-tooth mothers (Edwards et al. Citation2016). Differences in weaning weight were due to lambs born to hoggets being younger at the time of weaning than those born to two-tooths. However, in commercial flocks in the United States, the adjusted weaning weight at 60 days of lambs raised by hoggets (<19 months when lambs born) was lower than those raised by older mothers. The magnitude of the difference ranges from approximately 1–3.5 kg depending on breed (Boggess et al. Citation1991).
Manipulation of key processes and their effect on hogget reproductive performance
Puberty onset
It is generally accepted that a female will not attain puberty until she reaches approximately 60–65% of her mature body weight (reviewed in Valasi et al. Citation2012). A key element of this is achieving sufficient body fat/metabolic fuel (Valasi et al. Citation2012) although relationships with muscle mass have also been observed (Rosales Nieto et al. Citation2015). Given the relationship between body fat and attainment of puberty, a key hormone likely to be involved in regulating timing of attainment of puberty is leptin (LEP). LEP is produced by fat cells and is known to be involved in modulating the amount of body fat by regulating appetite (Roh et al. Citation2016). However, it is also know to provide feedback on nutritional status to the reproductive system (Scaramuzzi et al. Citation2011). LEP concentrations have been shown to be positively correlated with earlier attainment of puberty in ewe lambs (Rosales Nieto et al. Citation2013a). Supporting a role for LEP in regulation of puberty attainment is the recently identified association between mutations in the LEP receptor (LEPR) and the age at attainment of puberty, as well as the percentage of ewes that attain puberty during their first breeding season (Haldar et al. Citation2014). Ewe lambs that were homozygous for the mutations in LEPR were over 2 weeks older when they attained puberty than their wild-type contemporaries. Additionally, <70% of those homozygous for the mutations attained puberty during their first breeding season, compared with > 90% of their wild-type contemporaries. This affect was not related to poorer growth of the lamb, but is hypothesised to be related to reduced signalling by LEP to tissues controlling reproduction.
Age at attainment of puberty is likely a multigenic trait, being affected by a large number of genes, potentially some with larger effects such as the LEPR mutations. Age at attainment of puberty is moderately heritable (Bathaei & Leroy Citation1997; Toe et al. Citation2000) with heritabilities for age and body weight at puberty estimated to be 0.14 and 0.37, respectively. Therefore genetic selection to reduce the age at attainment of puberty should improve hogget fertility given that failure to attain puberty is one cause of reproductive inefficiency in hoggets (Edwards et al. Citation2016). This improvement would be achieved through increasing the number of animals mated by the fertile ram as, in well-grown hoggets, 97% of those that had attained puberty prior to fertile ram introduction were mated by the fertile ram in the first 17 days (one reproductive cycle), compared with 85% of those that had not attained puberty prior to fertile ram introduction. One risk of this approach would be the potential that ewe lambs that reach puberty earlier would have a smaller mature body size. However, we have demonstrated that those ewe lambs that attain puberty in their first year are larger as adults than those that do not (Edwards et al. Citation2015). Further, it has been shown that onset of puberty is advance, and reproductive performance in improved in hoggets selected for superior growth (Rosales Nieto et al. Citation2013a; Citation2013b).
Initiation of puberty can also be influenced by social-sexual cues (reviewed by Ungerfeld Citation2007; Valasi et al. Citation2012). For example, exposing hoggets to rams can induce puberty in animals that are peripubertal. This is likely induced by pheromones that interact with regulatory pathways in the brain to hasten the attainment of puberty. The use of ram exposure can improve hogget lambing outcome and is discussed below.
A second, potentially less-recognised issue with hoggets, is the failure to be bred by the fertile ram even when reproductive cycles have been initiated. Of those ewes that failed to be bred by the fertile ram, over 80% had actually ovulated (Edwards et al. Citation2016). While for some of these hoggets this may have been their first ovulation, and thus a silent heat as discussed above, this was not always the case as others had attained puberty (as measured by receptiveness to a vasectomised ram) prior to introduction of the fertile ram. Oestrous behaviour in the ewe is complex. Oestrogens are key hormones in governing oestrous behaviour with higher levels of oestrogens linked to more active seeking of the ram by the ewe (Allison & Davis Citation1976). In maiden ewes being mated in order to lamb for the first time as a two-tooth, it was noted that their ram seeking behaviour was less than for older ewes (Allison & Davis Citation1976). It is also known that rams differ in their mating drive and the number of ewes they will breed during the breeding system in a group mating situation (Stellflug et al. Citation2006). While it is known that the ratio of rams to hoggets is important for successful hogget mating (discussed below), whether the success of hogget mating could be improved by selecting rams that more actively seek ewes in oestrous remains to be explored.
Control of OR
It is also known that there are strong positive relationships between body weight and OR in mature ewes. In addition, provision of extra nutrition just prior to mating (nutritional flushing) increases OR, a practice which is commonly used in commercial sheep production. However, whether these relationships hold true in hoggets is less certain. When comparing hoggets fed to maintain body weight during the mating period, with those gaining approximately 180 g/d during this same period, the percentage of ewes that became pregnant and the NLB per ewe lamb exposed to the ram were all increased in those ewe lambs fed to gain weight. However, overall pregnancy rate in the trial was low (21%) and OR was not measured (Rosales Nieto et al. Citation2015). In ewe hoggets that had been treated in accordance with an oestrus synchronisation programme, twinning rate was reduced in those that were fed to maintain their weight in the 2 weeks prior to mating, compared with those that were fed to gain weight (ad-lib). Thus, restricting growth during pre-breeding/early pregnancy negatively effects lambing rate, probably through suppression of OR (Mulvaney et al. Citation2010).
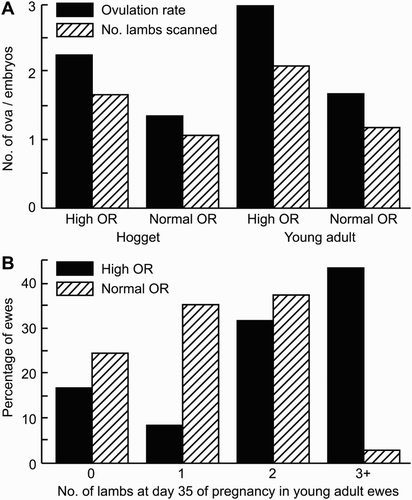
Regulation of OR is also genetically controlled, being lowly to moderately heritable (Notter Citation2008). Indeed, many different mutations that have a major effect on OR in sheep have been identified (Juengel et al. Citation2013). Hoggets carrying one of these mutations will have increased OR compared with non-carrier controls, however the relative differences between OR as a hogget and as an adult ewe are still maintained (). To date, we are not aware of successful selection of animals which have a consistent OR during their lifetime. Therefore, while animals with genetics for a high OR will have higher ORs as hoggets than those without, they will also have higher ORs as adults, likely leading to increases in triplet and even quadruplet litters later in life, which can be difficult to manage on-farm.
Figure 5. The effect of OR of reproductive performance (from Edwards & Juengel Citation2016). (A) Average OR and number of lambs scanned at approximately day 35 of gestation for hoggets and young adult ewes (approximately 20 months of age) from a flock with a normal OR and a flock with a high OR (heterozygous carriers of the Inverdale gene). (B) The respective pattern of ewes carrying zero, one, two, or three or more embryos for the high and normal OR ewes at 2 years of age. Data from ewes that were bred by the fertile ram during a 17-day period (i.e. one reproductive cycle).
Regulation of hormones normally involved in control of ovulation is also known to be able to increase OR in ewes. For example, administration of exogenous FSH during the later stages of follicular growth is known to increase the number of follicles ovulated and forms the basis for multiple ovulation embryo transfer methods (Menchaca et al. Citation2010). Immunisation against BMP15, GDF9, androstenedione or inhibin can all lead to increased ORs (Anderson et al. Citation1998; Juengel et al. Citation2011, Citation2004). Currently, there are commercially available products for increasing OR in adult ewes based on immunisation against androstenedione. Early studies have also shown that these vaccines can increase ovulate rate in hoggets (Ronayne et al. Citation1991). However, whether this leads to increased lamb production has not been well studied. It is known that immunisation against androstenedione delays early embryo growth (Boland et al. Citation1986; Scaramuzzi et al. Citation1993; O’Connell et al. Citation2016) and given that embryo development in hoggets is more vulnerable than in adult ewes (Edwards et al. Citation2016), further research in this area is warranted.
Control of embryo survival
There is some evidence that there are mutations in single genes that may have a major effect on the mother's ability to support an embryo/foetus to term. Preliminary evidence from lines of adult sheep have supported this concept, with daughters of some sires having an enhanced embryo survival phenotype with potential to increase lambing rate by an average of 0.2 lambs (Juengel et al. Citation2012). However, the identity of specific gene mutations underlying this phenotype has not been determined, nor is their effect in hoggets known. Deleterious mutations that decrease a ewe's ability to nurture an embryo are also likely to exist. For example, mutations in the LEPR gene have been linked to, on average, 0.2 fewer lambs born to heterozygote adult ewes which ovulate three ova, compared with wild-type triplet ovulating ewes (Juengel et al. Citation2016). Whether this mutation would affect embryo survival in hoggets is uncertain as ewes with lower ORs are less severely affected or may not be affected at all.
When examining the relationship between body weight and embryo survival in adult ewes, a quadratic relationship was observed, with poor embryo survival in very light and very heavy ewes (Shorten et al. Citation2013). This is indicative of a nutritional interaction with embryo survival, with either too little or too much nutrition compromising survival of the embryo. Restricting nutrition around the time of conception so that the animals are not gaining weight does appear to reduce the percentage of ewe hoggets that become pregnant (Mulvaney et al. Citation2010; Rosales Nieto et al. Citation2015).
Other effects of weight
The effect of weight on hogget lambing more generally has also been investigated. In one study (Moore et al. Citation1983), a weaning rate (lambs weaned/ewes mated) of over 50% was achieved from Coopworth and Perendale hoggets with pre-mating weights of at least 30 kg, while Romneys with a pre-mating weight of 35 kg gave a weaning rate of 47% which dropped to 13% with a 29 kg pre-mating weight. This indicates that breed is an important consideration in targeting mating weights and that hoggets with a relatively low body weight for their breed are less likely to lamb successfully. Again, however, it is not clear from this study where the losses were occurring. Lambing ewes as hoggets significantly reduced their weight at weaning but did not affect their two-tooth pre-mating weight and did not consistently affect their reproductive performance as two-tooths (Moore et al. Citation1983). However, a more recent study in which two-tooth mating weight was lower in ewes that had lambed as a hogget showed a small negative effect of hogget lambing on two-tooth reproductive performance (Kenyon et al. Citation2008c). These findings are important as a perceived detrimental effect on two-tooth performance is cited as a reason why farmers do not breed from their hoggets (Kenyon et al. Citation2004a) and support the importance of managing mated hoggets to optimise weight gain post-lambing to ensure good condition at two-tooth mating.
Weight of hoggets at mating has been shown to affect pregnancy rate at scanning and scanning percentage (Corner-Thomas et al. Citation2015), as well as lambing percentage (Kenyon et al. Citation2004b). Both pregnancy rate and scanning percentage increased as live weight increased to maximum at 52.5 kg (Corner-Thomas et al. Citation2015). Similarly, lambing percentage increased with weight such that hoggets weighing greater than 49 kg at mating had a lambing rate 25.5% higher than those of less than 36 kg (Kenyon et al. Citation2004b). Kenyon et al. (Citation2005) showed that hoggets that were mated by the ram in the first cycle were significantly heavier than those mated in the second cycle although the average difference in weight was less than 1.5 kg. Similar results were also reported by Kenyon et al. (Citation2006b). Weight of hoggets has also been shown to affect their lambs, with pre-mating weight of hoggets having a small but consistent positive effect on the birth weight and growth rate of singleton lambs to weaning (Kenyon et al. Citation2006b).
Nutrition
Kenyon et al. (Citation2008a) investigated how nutrition during pregnancy affects pregnancy outcome. Synchronised hoggets were assigned to either medium, medium/high or high feeding groups following the breeding period. Ewe hoggets in the ‘high’ group were less likely to hold to the first service (54.5%) than the medium (78.2%) or medium/high (83.6%) groups. However, the overall pregnancy rate of all feeding groups was the same (44.5–50.9%), as were the lambing rates (41.2–48.3%). Additionally, in this study, twin lambs born to hoggets in the medium group were significantly lighter than their single-born counterparts but this was not seen in the other feeding groups.
A related study also showed that significantly fewer ewe hoggets on ‘high’ or ‘low’ feeding regimes produced any lambs compared with those on a ‘medium’ feeding regime (Mulvaney et al. Citation2008). Lambs born to ‘low’ nutrition hoggets were also lighter at birth and had lower lamb survival rates than the other treatment groups.
Management practices to improve hogget reproductive performance
Vasectomised rams
The use of vasectomised (teaser) rams in the management of breeding hoggets has been investigated. The advantages of using teasers with hoggets is twofold: to induce first oestrus in ewes that have not yet reached puberty, and to induce oestrus early to produce earlier born lambs. Exposure to vasectomised rams for 17 days prior to intact ram introduction has been shown to increase the number of hoggets that conceive in the first mating cycle and increase the scanning percentage (Kenyon et al. Citation2006a). Weaning weights of singleton lambs born to teased hoggets were higher than for those born to unteased hoggets but that this was entirely due to an earlier birth date (Kenyon et al. Citation2006b). They also showed that the use of the teaser led to 70.7% of hoggets having lambed within the first 17 days of lambing compared with 42.3% in unteased hoggets.
Kenyon et al. (Citation2007a) used vasectomised rams at differing ram:hogget ratios for 17 days, prior to introducing intact rams for two cycles (34 days). The effect of teasing at all ratios tested (1:32 to 1:197) increased the probability that the ewe hogget would mate in the first cycle by at least 16.9%, although over two cycles there was no difference in the proportion of hoggets not mated. Teasing did not affect the litter size at scanning. Hoggets teased at a ratio of 1:197 tended to have lower pregnancy rates than those teased at a ratio of 1:32. Over two cycles, significantly fewer unteased hoggets were pregnant compared with those teased at 1:32 and this was also observed (but not significant) at the other ratios tested. These data suggest that exposure of hoggets to teaser rams, even at low ratios, is beneficial in increasing both the proportion of hoggets mated in the first cycle as well as overall pregnancy rates.
Although exposure to vasectomised rams is useful in increasing the efficiency of hogget lambing, such rams are expensive to produce and maintain. As an alternative, Kenyon et al. (Citation2008b) investigated the possibility of using rams with a shortened scrotum for teasing hogget ewes. These rams are often found on New Zealand farms as they have reduced fertility while maintaining male growth characteristics. However, the results of this trial showed that ram hoggets with a shortened scrotum did not have the same effect as vasectomised rams and therefore would not provide a cost-effective alternative.
Timing of mating
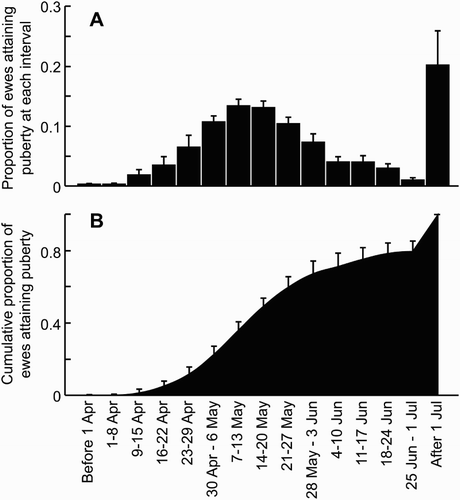
When considering the length of the breeding season, lambing percentage was found to increase with the length of time hoggets were exposed to the ram, up to a maximum at 40 days, and also with the percentage of rams to hoggets up to a maximum of 4% (Kenyon et al. Citation2004b). The timing of mating is also important and the usual practice is to mate hoggets approximately two to 4 weeks later than older ewes. In the South Island of New Zealand, hoggets are usually mated in late April to mid-May, which corresponds with the timing of onset of puberty (). However, the delay in mating, driven by both later introduction of the fertile rams as well as extending the length of the breeding season, leads to an extended lambing season with hoggets lambing later than the adult ewes. Later born lambs will either be lighter at weaning if weaned at a similar time as the lambs of the older ewes, or need to be weaned later to achieve similar weaning weights. Delayed weaning can limit the time available to ensure the hogget has reached the optimal mating weight for her two-tooth breeding season with implications for her two-tooth performance. The extended lambing season can also affect the timing of feed requirements on the farm with pressure on feed availability leading to slower growth and delaying achievement of slaughter weight.
Figure 6. The timing of attainment of puberty in ewe hoggets (from Edwards & Juengel Citation2016). (A) The average (+SEM) proportion of hoggets that attain puberty in a given week. (B) The cumulative proportion (+SEM) of ewes that have attained puberty by a given week.
Ram age
The age of the ram has also been shown to affect the success of hogget lambing (Kenyon et al. Citation2007b). Teased hoggets were more likely to be mated in the first cycle if mature rams were used than if either ram hoggets or two-tooth rams were used; however fewer of the ewe hoggets mated to mature rams in the first cycle held to service compared with the other two groups. Hence there was no overall difference in pregnancy rates after the first cycle. They concluded that previously unused two-tooth rams and experienced mature rams are likely to give the best results.
Other factors
Animal health treatments have been found to be important for successful hogget lambing. Vaccination for toxoplasmosis and/or campylobacter, pre-mate shearing and managing single and multiple bearing ewes separately during lactation have been shown to increase lambing percentage of hoggets (calculated as the number of lambs present approximately 1 month after lambing/number of hoggets put to the ram (Kenyon et al. Citation2004b). Vaccination for toxoplasmosis and/or campylobacter reduces the incidence of late pregnancy abortion and now forms part of industry best practice for hogget lambing (Kenyon Citation2012). Vaccination for leptospirosis has also been shown to reduce late pregnancy loss in hogget dams and should also be considered by farmers (Ridler et al. Citation2015).
Future directions
While considerable progress has been made in understanding factors that limit hogget fertility, hoggets still only achieve, on average approximately 50% of their reproductive potential, indicating room for improvement. The importance of proper nutrition, vaccination against disease, and following management guidelines regarding the appropriate use of rams for both hastening the attainment of puberty and achieving high pregnancy rates, have been well documented. However, even when industry best practice is followed, key limits to reproductive potential are observed in attainment of puberty/mating to the fertile ram, OR, survival of the embryo and survival of the lamb. Thus, development of new approaches to overcome these limitations, specifically targeted to hoggets, are needed to improve hogget fertility.
The observation that the proportion of hoggets that fail to attain puberty during the first year of life varies considerably, from 10% to 35% (Edwards et al. Citation2015), suggests that this is a key mechanism underlying the variability of hogget reproductive performance from year to year. While it is clear that proper growth of the hogget is essential for attainment of puberty, this year-to-year variability could not be explained by variation in growth alone. Our interpretation is that there are other environmental factors that are affecting attainment of puberty; however, the identity of these factors is currently unknown. One possibility is the presence of factors such as zearalenone (Zinedine et al. Citation2007) in the pastures that could be interfering with expression of oestrous; however, this remains to be tested. Clearly, understanding and preventing this variability would help improve the consistency of hogget lambing performance from year to year.
Increasing OR of well-grown hoggets would also be likely to improve hogget fertility. Products using immunisation against androstenedione are available for use in adult ewes; however, their effect in hoggets has not been well characterised (Ronayne et al. Citation1991). Potential issues include the possibility of increasing the number of hoggets having triplets, or failure to capitalise on the increased OR if embryo survival is poor. Understanding how well-grown hoggets perform when bearing multiple lambs will be key to determining if these products offer a robust method to improve hogget fertility. Alternatively, OR could be increased through genetics, but the key to this approach would be determining whether there are genetic pathways that can provide a consistent OR across the animal's lifetime.
Improving embryo survival and, ultimately, pregnancy rates as well as numbers of lambs born to each pregnant ewe is another key pathway to improving hogget fertility. Embryo loss in hoggets is approximately twice that observed in adult ewes, representing a point in the reproductive process that is particularly sensitive in hoggets. While a lack of adequate nutrition is clearly detrimental to embryo survival, providing excess nutrition does not improve this trait, and over-nutrition appears to be detrimental. To improve this process in hoggets requires a better understanding of why hoggets have higher embryo loss.
An additional factor that influences final pregnancy rate is some ewes failing to be mated, even though they are ovulating. This may be related to poor expression of oestrous, as a result of lower production of oestradiol by hoggets. The mating drive of rams also seems to differ and whether rams with a high mating drive would more proactively seek oestrus hoggets is not clear. If so, selection of rams capable of breeding a large number of ewes may improve pregnancy rates in hoggets. Interestingly, lack of oestrogen signalling has also been linked to poor embryo survival in the female (Winuthayanon et al. Citation2015). As with rams, some ewes have a higher mating drive than others in that they will mate with more than a single ram when in a group mating situation, with oestradiol likely to be a key regulator of this trait. Whether this trait is repeatable and heritable, and how this may link with embryo survival, particularly in hoggets, is not well understood.
Loss of lambs perinatally also represents a significant loss of reproduction potential in all ewes. However, it is not clear if this loss differs between hoggets and adult ewes. There is some evidence that well-grown hoggets may be better mothers than mature ewes, particularly if they are left alone at lambing. Lamb losses perinatally could be improved by using a sire that produces lower birth weight lambs with consequently lower incidence of dystocia.
It is also important to recognise that component solutions designed to improve hogget lambing need to be placed in a whole farm systems context. It is clear that if adequate nutrition is not available for the hogget both before, during and after pregnancy, she will not reach her potential. Additionally, her performance as a two-tooth may be compromised. If the ewe achieves her adult reproductive potential as a hogget, this demand for additional feed for the pregnant/lactating hogget will be even greater than that required for the current level of hogget fertility. Thus, it is essential that any system designed to improve hogget lambing considers the whole farm system over multiple years.
Acknowledgements
The authors would like to thank current and former members of the AgResearch Reproduction team, and Invermay farm staff for their contributions to this work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allison AJ, Davis GH. 1976. II. Effects of age of ewe, live weight, and paddock size on duration of oestrus and ram-seeking activity. N Z J Exp Agric. 4:269–274.
- Anderson ST, Bindon BM, Hillard MA, O’Shea T. 1998. Increased ovulation rate in Merino ewes immunized against small synthetic peptide fragments of the inhibin alpha subunit. Reprod Fertil Dev. 10:421–431. doi: 10.1071/RD98094
- Bathaei SS, Leroy PL. 1997. Note on age and body weight at puberty in Mehraban Iranian fat-tailed ewe lambs. Trop Anim Health Prod. 29:55–59. doi: 10.1007/BF02632349
- Boggess MV, Wilson DE, Rothschild MF, Morrical DG. 1991. National sheep improvement program: age adjustment of weaning weight. J Anim Sci. 69:3190–3201. doi: 10.2527/1991.6983190x
- Boland MP, Nancarrow CD, Murray JD, Scaramuzzi RJ, Sutton R, Hoskinson RM, Hazelton IG. 1986. Fertilization and early embryonic development in androstenedione-immunized Merino ewes. J Reprod Fert. 78:423–431. doi: 10.1530/jrf.0.0780423
- Carpenter KD, Hayashi K, Spencer TE. 2003. Ovarian regulation of endometrial gland morphogenesis and activin-follistatin system in the neonatal ovine uterus. Biol Reprod. 69:851–860. doi: 10.1095/biolreprod.103.016337
- Cooke PS, Spencer TE, Bartol FF, Hayashi K. 2013. Uterine glands: development, function and experimental model systems. Mol Hum Reprod. 19:547–558. doi: 10.1093/molehr/gat031
- Corner-Thomas RA, Ridler AL, Morris ST, Kenyon PR. 2015. Ewe lamb live weight and body condition scores affect reproductive rates in commercial flocks. N Z J Agric Res. 58:26–34. doi: 10.1080/00288233.2014.974766
- Cushman RA, Allan MF, Kuehn LA, Snelling WM, Cupp AS, Freetly HC. 2009. Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: investigation of influence of stage of the estrous cycle, age, and birth weight. J Anim Sci. 87:1971–1980. doi: 10.2527/jas.2008-1728
- Davies MJ. 2006. Evidence for effects of weight on reproduction in women. Reprod Biomed Online. 12:552–561. doi: 10.1016/S1472-6483(10)61180-7
- Diskin MG, Kenny DA. 2014. Optimising reproductive performance of beef cows and replacement heifers. Animal. 8(Suppl 1):27–39. doi: 10.1017/S175173111400086X
- Edwards SJ, Juengel JL. 2016. Key considerations when lambing hoggets (ewe lambs). Proceedings of the 59th ESA, 47th SRB and 26th ANZBMS Annual Scientific Meeting; Gold Coast, Queensland.
- Edwards SJ, Juengel JL, O’Connell AR, Johnstone PD, Farquhar PA, Davis GH. 2015. Attainment of puberty by ewes in the first year of life is associated with improved reproductive performance at 2 years of age. Small Rumin Res. 123:118–123. doi: 10.1016/j.smallrumres.2014.11.006
- Edwards SJ, Smaill B, O’Connell AR, Johnstone PD, Stevens DR, Quirke LD, Farquhar PA, Juengel JL. 2016. Reduced ovulation rate, failure to be mated and fertilization failure/embryo loss are the underlying causes of poor reproductive performance in juvenile ewes. Anim Reprod Sci. 167:125–132. doi: 10.1016/j.anireprosci.2016.02.017
- Evans AC, Mossa F, Walsh SW, Scheetz D, Jimenez-Krassel F, Ireland JL, Smith GW, Ireland JJ. 2012. Effects of maternal environment during gestation on ovarian folliculogenesis and consequences for fertility in bovine offspring. Reprod Domest Anim. 47:31–37. doi: 10.1111/j.1439-0531.2012.02052.x
- Everett-Hincks JM, Mathias-Davis HC, Greer GJ, Auvray BA, Dodds KG. 2014. Genetic parameters for lamb birth weight, survival and death risk traits. J Anim Sci. 92:2885–2895. doi: 10.2527/jas.2013-7176
- Ferreira AM, Bislev SL, Bendixen E, Almeida AM. 2013. The mammary gland in domestic ruminants: a systems biology perspective. J Proteomics. 94:110–123. doi: 10.1016/j.jprot.2013.09.012
- Fritz S, Capitan A, Djari A, Rodriguez SC, Barbat A, Baur A, Grohs C, Weiss B, Boussaha M, Esquerre D, et al. 2013. Detection of haplotypes associated with prenatal death in dairy cattle and identification of deleterious mutations in GART, SHBG and SLC37A2. PLoS One. 8:e65550. doi: 10.1371/journal.pone.0065550
- Haldar A, French MC, Brauning R, Edwards SJ, O’Connell AR, Farquhar PA, Davis GH, Johnstone PD, Juengel JL. 2014. Single-nucleotide polymorphisms in the LEPR gene are associated with divergent phenotypes for age at onset of puberty in Davisdale ewes. Biol Reprod. 90:1–7. doi: 10.1095/biolreprod.113.115923
- Hanford KJ, Van Vleck LD, Snowder GD. 2002. Estimates of genetic parameters and genetic change for reproduction, weight, and wool characteristics of Columbia sheep. J Anim Sci. 80:3086–3098. doi: 10.2527/2002.80123086x
- Hanford KJ, Van Vleck LD, Snowder GD. 2003. Estimates of genetic parameters and genetic change for reproduction, weight, and wool characteristics of Targhee sheep. J Anim Sci. 81:630–640. doi: 10.2527/2003.813630x
- Hayashi K, O’Connell AR, Juengel JL, McNatty KP, Davis GH, Bazer FW, Spencer TE. 2008. Postnatal uterine development in Inverdale ewe lambs. Reproduction. 135:357–365. doi: 10.1530/REP-07-0323
- Hunter RH. 2012. Components of oviduct physiology in eutherian mammals. Biol Rev Camb Philos Soc. 87:244–255. doi: 10.1111/j.1469-185X.2011.00196.x
- Jimenez-Krassel F, Folger JK, Ireland JL, Smith GW, Hou X, Davis JS, Lonergan P, Evans AC, Ireland JJ. 2009. Evidence that high variation in ovarian reserves of healthy young adults has a negative impact on the corpus luteum and endometrium during estrous cycles in cattle. Biol Reprod. 80:1272–1281. doi: 10.1095/biolreprod.108.075093
- Juengel JL, Davis GH, McNatty KP. 2013. Using sheep lines with mutations in single genes to better understand ovarian function. Reproduction. 146:R111–R123. doi: 10.1530/REP-12-0509
- Juengel JL, French MC, O’Connell AR, Edwards SJ, Haldar A, Brauning R, Farquhar PA, Dodds KG, Galloway SM, Johnstone PD, Davis GH. 2016. Mutations in the leptin receptor gene associated with delayed onset of puberty are also associated with decreased ovulation and lambing rates in prolific Davisdale sheep. Reprod Fertil Dev. 28:1318–1325. doi: 10.1071/RD14382
- Juengel JL, Hudson NL, Whiting L, McNatty KP. 2004. Effects of immunization against bone morphogenetic protein 15 and growth differentiation factor 9 on ovulation rate, fertilization, and pregnancy in ewes. Biol Reprod. 70:557–561. doi: 10.1095/biolreprod.103.023333
- Juengel JL, O’Connell AR, Farquhar PA, Johnstone PD, Davis GH. 2012. Evidence for a single gene affecting embryo/fetal survival in the davisdale flock. Proceedings of the 17th International Congress on Animal Reproduction; Vancouver, Canada.
- Juengel JL, Proctor L, Wearne K, Olliver D, Hudson N, Jensen D, Davis GH, Johnstone P, McNatty K. 2011. Effects of immunization against androstenedione or bone morphogenetic protein 15 (BMP15) on reproductive performance in sheep. Reprod Domest Anim. 46:115 (Abstract P130).
- Juengel JL, Smith P. 2014. Formation of ovarian follicles in ruminants. In: Juengel JL, Miyamoto A, Price C, Reynolds LP, Smith MF, Webb R, editors. Reproduction in domestic ruminants VII. Leicestershire: Context Products; p. 277–293.
- Kenyon PR, editor. 2012. Hogget performance: unlocking the potential. Wellington: Beef + Lamb New Zealand.
- Kenyon PR, Morel PC, Morris ST, Burnham DL, West DM. 2006a. The effect of length of use of teaser rams prior to mating and individual liveweight on the reproductive performance of ewe hoggets. N Z Vet J. 54:91–95. doi: 10.1080/00480169.2006.36618
- Kenyon PR, Morel PC, Morris ST, Burnham DL, West DM. 2007a. Effect of the ratio of teaser rams used prior to breeding on the reproductive performance of ewe hoggets. N Z Vet J. 55:342–345. doi: 10.1080/00480169.2007.36791
- Kenyon PR, Morel PC, Morris ST, West DM. 2005. The effect of individual liveweight and use of teaser rams prior to mating on the reproductive performance of ewe hoggets. N Z Vet J. 53:340–343. doi: 10.1080/00480169.2005.36571
- Kenyon PR, Morel PC, Morris ST, West DM. 2007b. Effect of the age of rams on reproductive performance of ewe hoggets. N Z Vet J. 55:184–187. doi: 10.1080/00480169.2007.36765
- Kenyon PR, Morel PCH, West DM, Morris ST. 2006b. Effect of liveweight and teasing of ewe hoggets prior to breeding on lambing pattern and weight of singleton lambs. N Z J Agric Res. 49:341–347. doi: 10.1080/00288233.2006.9513724
- Kenyon PR, Morris ST, Burnham DL, West DM. 2008a. Effect of nutrition during pregnancy on hogget pregnancy outcome and birthweight and liveweight of lambs. N Z J Agric Res. 51:77–83. doi: 10.1080/00288230809510437
- Kenyon PR, Morris ST, Perkins NR, West DM. 2004a. Hogget mating in New Zealand – a survey. Proc N Z Soc Anim Prod. 64:217–222.
- Kenyon PR, Morris ST, West DM. 2008b. Can Romney ram lambs whose scrotums had been shortened by the use of a rubber ring be used as an alternative to vasectomised Perendale rams for inducing early breeding activity in Romney ewe lambs? N Z Vet J. 56:326–329. doi: 10.1080/00480169.2008.36854
- Kenyon PR, Pinchbeck GL, Perkins NR, Morris ST, West DM. 2004b. Identifying factors which maximise the lambing performance of hoggets: a cross sectional study. N Z Vet J. 52:371–377. doi: 10.1080/00480169.2004.36454
- Kenyon PR, Proctor L, Morel PC, Morris ST, West DM. 2008c. The effect of breeding ewe lambs on subsequent two-year-old ewe performance. Livest Sci. 115:206–210. doi: 10.1016/j.livsci.2007.07.008
- Kenyon PR, Thompson AN, Morris ST. 2014. Breeding ewe lambs successfully to improve lifetime performance. Small Rumin Res. 118:2–15. doi: 10.1016/j.smallrumres.2013.12.022
- Lahoz B, Alabart JL, Monniaux D, Mermillod P, Folch J. 2012. Anti-Mullerian hormone plasma concentration in prepubertal ewe lambs as a predictor of their fertility at a young age. BMC Vet Res. 8:118. doi: 10.1186/1746-6148-8-118
- Lerias JR, Hernandez-Castellano LE, Suarez-Trujillo A, Castro N, Pourlis A, Almeida AM. 2014. The mammary gland in small ruminants: major morphological and functional events underlying milk production – a review. J Dairy Res. 81:304–318. doi: 10.1017/S0022029914000235
- Levine JM, Vavra M, Phillips R, Hohenboken W. 1978. Ewe lamb conception as an indicator of future production in farm flock Columbia and Targhee ewes. J Anim Sci. 46:19–25. doi: 10.2527/jas1978.46119x
- McMillan WH. 1983. Hogget lamb mortality. Proc N Z Soc Anim Prod. 43:33–36.
- Menchaca A, Vilariño M, Crispo M, de Castro T, Rubianes E. 2010. New approaches to superovulation and embryo transfer in small ruminants. Reprod Fertil Dev. 22:113–118. doi: 10.1071/RD09222
- Moore RW, Sumner RMW, Bass JJ, Hockey HUP. 1983. Hogget lambing and its effect on the subsequent two-tooth performance of three breeds. Proc N Z Soc Anim Prod. 43:21–24.
- Morel PC, Wickham JL, Morel JP, Wickham GA. 2010. Effects of birth rank and yearling lambing on long-term ewe reproductive performance. Proc N Z Soc Anim Prod. 70:88–90.
- Mossa F, Walsh SW, Butler ST, Berry DP, Carter F, Lonergan P, Smith GW, Ireland JJ, Evans AC. 2012. Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J Dairy Sci. 95:2355–2361. doi: 10.3168/jds.2011-4325
- Mulvaney FJ, Kenyon PR, Morris ST, West DM. 2008. Ewe lamb nutrition during pregnancy affects pregnancy outcome. Aust J Exp Agric. 48:1085–1089. doi: 10.1071/EA08078
- Mulvaney FJ, Morris ST, Kenyon PR, Morel PCH, West DM. 2010. Effect of nutrition pre-breeding and during pregnancy on breeding performance of ewe lambs. Anim Prod Sci. 50: 953–960. doi: 10.1071/AN10040
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. 2000. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 80:1–29.
- Notter DR. 2008. Genetic aspects of reproduction in sheep. Reprod Domest Anim. 43(Suppl 2):122–128. doi: 10.1111/j.1439-0531.2008.01151.x
- O’Brien JK, Catt SL, Ireland KA, Maxwell WMC, Evans G. 1997. In vitro and in vivo developmental capacity of oocytes from prepubertal and adult sheep. Theriogenology. 47:1433–1443. doi: 10.1016/S0093-691X(97)00134-9
- O’Connell AR, Demmers KJ, Smaill B, Reader KL, Juengel JL. 2016. Early embryo loss, morphology, and effect of previous immunization against androstenedione in the ewe. Theriogenology. 86:1285–1293. doi: 10.1016/j.theriogenology.2016.04.069
- Quinlivan TD, Martin CA, Taylor WB, Cairney IM. 1966. Estimates of pre- and perinatal mortality in the New Zealand Romney Marsh ewe. I. Pre- and perinatal mortality in those ewes that conceived to one service. J Reprod Fert. 11:379–390. doi: 10.1530/jrf.0.0110379
- Quirke JF, Hanrahan JP. 1977. Comparison of the survival in the uteri of adult ewes of cleaved ova from adult ewes and ewe lambs. J Reprod Fert. 51:487–489. doi: 10.1530/jrf.0.0510487
- Reader KL, Cox NR, Stanton JAL, Juengel JL. 2015. Mitochondria and vesicles differ between adult and prepubertal sheep oocytes during IVM. Reprod Fertil Dev. 27:513–522. doi: 10.1071/RD13359
- Ridler AL, Vallee E, Corner RA, Kenyon PR, Heuer C. 2015. Factors associated with fetal losses in ewe lambs on a New Zealand sheep farm. N Z Vet J. 63:330–334. doi: 10.1080/00480169.2015.1037813
- Roh SG, Suzuki Y, Gotoh T, Tatsumi R, Katoh K. 2016. Physiological roles of adipokines, hepatokines, and myokines in ruminants. Asian-Australas J Anim Sci. 29:1–15. doi: 10.5713/ajas.16.0001R
- Ronayne E, Fitzsimons J, Hanrahan JP, Quirke JF, Roche JF. 1991. Effects of active immunisation of female lambs against androstenedione on LH secretion, onset of puberty and ovulation rate. Anim Reprod Sci. 24:283–292. doi: 10.1016/S0378-4320(05)80011-9
- Rosales Nieto CA, Ferguson MB, Macleay CA, Briegel JR, Martin GB, Thompson AN. 2013a. Selection for superior growth advances the onset of puberty and increases reproductive performance in ewe lambs. Animal. 7:990–997. doi: 10.1017/S1751731113000074
- Rosales Nieto CA, Ferguson MB, Macleay CA, Briegel JR, Wood DA, Martin GB, Thompson AN. 2013b. Ewe lambs with higher breeding values for growth achieve higher reproductive performance when mated at age 8 months. Theriogenology. 80:427–435. doi: 10.1016/j.theriogenology.2013.05.004
- Rosales Nieto CA, Ferguson MB, Thompson H, Briegel JR, Macleay CA, Martin GB, Thompson AN. 2015. Relationships among puberty, muscle and fat, and liveweight gain during mating in young female sheep. Reprod Domest Anim. 50:637–642. doi: 10.1111/rda.12542
- Rowson AR, Daniels KM, Ellis SE, Hovey RC. 2012. Growth and development of the mammary glands of livestock: a veritable barnyard of opportunities. Semin Cell Dev Biol. 23:557–566. doi: 10.1016/j.semcdb.2012.03.018
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. 2002. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 66:1134–1150. doi: 10.1095/biolreprod66.4.1134
- Scaramuzzi RJ, Baird DT, Campbell BK, Driancourt MA, Dupont J, Fortune JE, Gilchrist RB, Martin GB, McNatty KP, McNeilly AS, et al. 2011. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev. 23:444–467. doi: 10.1071/RD09161
- Scaramuzzi RJ, Nancarrow CD, Murray JD, Walton JR. 1993. Reproductive wastage in the androstenedione-immune ewe. J Reprod Fert. 98:617–624. doi: 10.1530/jrf.0.0980617
- Shorten PR, O’Connell AR, Demmers KJ, Edwards SJ, Cullen NG, Juengel JL. 2013. Effect of age, weight, and sire on embryo and fetal survival in sheep. J Anim Sci. 91:4641–4653. doi: 10.2527/jas.2013-6415
- Smith JT, Clarke IJ. 2010. Seasonal breeding as a neuroendocrine model for puberty in sheep. Mol Cell Endocrinol. 324:102–109. doi: 10.1016/j.mce.2010.03.007
- Spencer TE, Gray CA. 2006. Sheep uterine gland knockout (UGKO) model. Methods Mol Med. 121:85–94.
- Spencer TE, Hayashi K, Hu J, Carpenter KD. 2005. Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol. 68:85–122. doi: 10.1016/S0070-2153(05)68004-0
- Spencer TE, Sandra O, Wolf E. 2008. Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction. 135:165–179. doi: 10.1530/REP-07-0327
- Statistics New Zealand. 2015. Agricultural production statistics: June 2014 (final). Wellington (New Zealand).
- Stellflug JN, Cockett NE, Lewis GS. 2006. Relationship between sexual behavior classifications of rams and lambs sired in a competitive breeding environment. J Anim Sci. 84:463–468. doi: 10.2527/2006.842463x
- Stevens DR. 2010. On-farm yearling lambing outcomes from feeding practices before mating and during pregnancy. Proc N Z Soc Anim Prod. 70:113–117.
- Toe F, Rege JE, Mukasa-Mugerwa E, Tembely S, Anindo D, Baker RL, Lahlou-Kassi A. 2000. Reproductive characteristics of Ethiopian highland sheep. I. Genetic parameters of testicular measurements in ram lambs and relationship with age at puberty in ewe lambs. Small Rumin Res. 36:227–240. doi: 10.1016/S0921-4488(99)00117-0
- Torres-Rovira L, Gonzalez-Bulnes A, Succu S, Spezzigu A, Manca ME, Leoni GG, Sanna M, Pirino S, Gallus M, Naitana S, Berlinguer F. 2014. Predictive value of antral follicle count and anti-Mullerian hormone for follicle and oocyte developmental competence during the early prepubertal period in a sheep model. Reprod Fertil Dev. 26:1094–1106. doi: 10.1071/RD13190
- Ungerfeld R. 2007. Socio-sexual signalling and gonadal function: opportunities for reproductive management in domestic ruminants. Soc Reprod Fertil Suppl. 64:207–221.
- Valasi I, Chadio S, Fthenakis GC, Amiridis GS. 2012. Management of pre-pubertal small ruminants: physiological basis and clinical approach. Anim Reprod Sci. 130:126–134. doi: 10.1016/j.anireprosci.2012.01.005
- Vallet JL, McNeel AK, Johnson G, Bazer FW. 2013. Triennial reproduction symposium: limitations in uterine and conceptus physiology that lead to fetal losses. J Anim Sci. 91:3030–3040. doi: 10.2527/jas.2012-6138
- VanRaden PM, Olson KM, Null DJ, Hutchison JL. 2011. Harmful recessive effects on fertility detected by absence of homozygous haplotypes. J Dairy Sci. 94:6153–6161. doi: 10.3168/jds.2011-4624
- Walsh SW, Mossa F, Butler ST, Berry DP, Scheetz D, Jimenez-Krassel F, Tempelman RJ, Carter F, Lonergan P, Evans AC, Ireland JJ. 2014. Heritability and impact of environmental effects during pregnancy on antral follicle count in cattle. J Dairy Sci. 97:4503–4511. doi: 10.3168/jds.2013-7758
- Winuthayanon W, Bernhardt ML, Padilla-Banks E, Myers PH, Edin ML, Lih FB, Hewitt SC, Korach KS, Williams CJ. 2015. Oviductal estrogen receptor α signaling prevents protease-mediated embryo death. eLife. 4:e10453. doi: 10.7554/eLife.10453
- Young EA, Yuan JV, Everett-Hincks JM. 2010. Yearling lambing performance and primary cause of lamb death. Proc N Z Soc Anim Prod. 70:96–100.
- Zinedine A, Soriano JM, Molto JC, Manes J. 2007. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol. 45:1–18. doi: 10.1016/j.fct.2006.07.030
