ABSTRACT
Excretion of the human bacterial pathogen Campylobacter jejuni by naturally infected dairy cows was investigated by monitoring 18 and 17 cows on two different commercial farms fortnightly for up to 12 months. C. jejuni was enumerated in the collected faeces by a most probable number technique and genotyped by enterobacterial repetitive intergenic consensus (ERIC) sequences. On both farms, C. jejuni excretion was highly variable among the studied cows, with excretion patterns ranging from chronic to sporadic. Chronic excretion of C. jejuni was associated with long-term predominance of a given genotype, co-excretion of a few genotypes, or succession over time of dominant genotypes. Sporadic excretion was with re-excretion of the same C. jejuni genotype when there was less than 1.5 months between C. jejuni-positive samples. Overall, the results showed the complexity of C. jejuni excretion pattern by dairy cows with an animal and farm specificity.
Introduction
Campylobacter is a frequent bacterial cause of acute gastro-enteritis in humans, and can also cause severe post-infection neuropathies, including Guillain-Barré and Miller Fisher syndromes (Blaser and Engberg Citation2008). In the United States alone, Campylobacter infections cause US $1.7 billion in annual costs of illness (Batz et al. Citation2011). In New Zealand, a country with a higher rate of campylobacteriosis than other developed countries, Campylobacter infections and their sequelae have been estimated to annually cost $8 million in direct health care (Lake et al. Citation2010). The majority of the infections are caused by Campylobacter jejuni subsp. jejuni (here C. jejuni) (Lastovica and Allos Citation2008).
A wide range of host animals, including poultry, ruminants (cattle and sheep), or wild birds can carry C. jejuni asymptomatically as part of their gut microbiota and may contribute to Campylobacter infections in humans. Pathways for human infection include consumption of meat, dairy products or water contaminated by animal faeces, as well as direct contact with farm animals (Gilpin et al. Citation2013). In many industrialised countries, ruminants have been recognised as the second most important animal source of human campylobacteriosis, after poultry (Schildt et al. Citation2006; Gilpin, Thorrold et al. Citation2008a; Mullner et al. Citation2009; Longenberger et al. Citation2013). Significant relationships between high density of sheep or dairy cows and high prevalence of campylobacteriosis have been established (Spencer et al. Citation2012), and recent attribution studies have estimated that between 11–18% of human cases are related to cattle (Mullner et al. Citation2009). The zoonotic potential of dairy cows in particular has been highlighted by molecular similarity between C. jejuni isolates from raw cow’s milk and those isolated from humans (Hudson et al. Citation1999) or between cow faeces and infected people living or working on dairy farms in New Zealand (Gilpin, Scholes et al. Citation2008b).
Shedding of C. jejuni by cows, in various countries and with different husbandry practices, has largely been documented on a herd basis (Wesley et al. Citation2000; Nielsen Citation2002; Kwan et al. Citation2008; Häkkinen and Hänninen Citation2009). Despite differences in prevalence attributed to differences in methodology among studies, these studies have shown that the number of cows excreting C. jejuni varies widely temporally and among herds (Nielsen Citation2002; Häkkinen and Hänninen Citation2009; Grove-White et al. Citation2011). Comparatively few studies have focused on the shedding patterns of Campylobacter spp. by individual animals, and even fewer have reported on the shedding patterns of C. jejuni. Inglis et al. (Citation2004) and Minihan et al. (Citation2004), who monitored feedlot animals over periods of time ranging from four months to one year, reported that the excretion pattern of Campylobacter spp. was variable, although the majority of the studied animals were found to excrete Campylobacter spp. chronically. On the contrary, studies on dairy herds principally defined the excretion of Campylobacter as intermittent, with up to a half of the studied cows found positive for C. jejuni at more than one sampling occasion (Robinson Citation1982, Humphrey and Beckett Citation1987; Ross et al. Citation2008; Häkkinen and Hänninen Citation2009). Chronic excretion in dairy cows, which has recently been documented, has appeared to be rare with ∼10% of the cows found to excrete C. jejuni at every quarterly sampling (Ross et al. Citation2008; Häkkinen and Hänninen Citation2009).
Molecular typing studies have been used to investigate differences in the C. jejuni strains excreted amongst cows in the same herd. Results reported contrasting ecological dynamics in the C. jejuni population within cows over time. Häkkinen and Hänninen (Citation2009) used pulsed-field gel electrophoresis and were not able to show any difference in the C. jejuni genotypes excreted by individual cows over time. On the other hand, Ross et al. (Citation2008) who used enterobacterial repetitive intergenic consensus sequences (ERIC)-PCR found that the excreted strains changed over time for four of the seven dairy cows that were positive at more than one sampling occasion, while they were similar over time for the other three cows. Similar findings were reported by Kwan et al. (Citation2008) and Hänninen et al. (Citation1998). However, the small number of animals (2–30) and samplings (3–5) involved in these studies limited accurate determination of excretion patterns and the authors recommended further investigations into epidemiological patterns of C. jejuni in individual cows (Kwan et al. Citation2008; Ross et al. Citation2008). It is still not known, for example, whether cows are persistently colonised by the same strain with no disappearance from the gut population over time; whether cows are repeatedly exposed to and contaminated by different strains from their environment; or whether they carry several genotypes which they excrete intermittently or at a very low level. Such information is crucial for determining the potential drivers of C. jejuni excretion required for developing effective on-farm control measures.
The objective of this study was to investigate the pattern of C. jejuni excretion in naturally infected dairy cows by determining the temporal fluctuation of C. jejuni faecal concentration and the underlying changes in the bacterium genotypes.
Material and methods
Farm description
Two commercial dairy farms, located 50 km apart in the Waikato region, were chosen for the study and studied for a 12 month period in two consecutive years. Farms were selected on the basis of their management practices and on the willingness of the farmers to be involved in the study. Both farms were on flat land with no stream crossing them and cows drank untreated water sourced from a bore. On one farm (designated ‘Farm P’), the stocking rate was 3.2 cows/ha. The cows were on pasture all the time and received supplementary feed (to c.a. 20% of the food intake) in autumn and winter. The calving season was in July–August. On the other farm (designated ‘Farm H’), the stocking rate was 5.5 cows/ha and the cows used a herd home®-like housing facility every day for two to 18 h per day between June and September and for two to four hours between October and May. When the facility was not used, cows were kept on pasture. As well as grazing pasture, the cows received mixed rations of supplementary feedstuff including maize silage, potato, palm kernel, soya, straw, tapioca and kiwifruit to 50–80% of the food intake at all seasons. Cows feeding regime was decided by the farmer. The calving season was spread between February and May. Eighteen cows on Farm P and 17 cows on Farm H were chosen for sampling. The chosen cows were selected to represent the most frequent ages of the cows of the herds (4, 5 and 6 years old) and were Friesian–Jersey cross, Friesian or Ayrshire. All study cows were identified by their unique ear-tag number, were kept within the main herd on each farm and were subjected to normal husbandry practices.
Faecal sampling
Sampling was by direct retrieval in accordance with standard animal welfare practices (Part 6 of the Animal Welfare Act 1999-MAF). The animal ethic was granted by the Ruakura Animal Ethic committee (Approval number AgResearch 11464). Fresh faecal samples (50–200 g) from the rectum of each cow were collected every two weeks for up to 25 samplings on the pasture farm and up to 20 samplings on the housing farm. Sampling was in accordance with AgResearch Ruakura SOP 69 protocol (Faecal sampling from bovines and ovines) and was carried out by trained staff. Sampling took place either during morning milking or early in the morning during the non-lactating season. Gloves were changed between cows. Samples were analysed within 5 h of collection. Six of the 35 selected cows that were removed (for commercial reasons) from the farm during the course of the study were not replaced.
Enumeration of C. jejuni
C. jejuni was enumerated by a three-tube, three-dilution most probable number (MPN) technique, as recommended for animal faecal samples when concentration is required (Donnison, Citation2003). At least three 1 g aliquots for each homogenised faecal sample, or two or three dilutions of it, were inoculated into a series of three tubes containing selective Campylobacter modified Exeter broth and mCCDA agar as described in the NZ Reference method (Donnison, Citation2003). The presence of C. jejuni was confirmed from every mCCDA plate showing colonial growth by PCR with a primer pair specific for C. jejuni (Vandamme et al. Citation1997). The concentration of confirmed C. jejuni in the original sample was determined by reference to a three-tube MPN probability table (WHO Citation1984). When C. jejuni was not detected (i.e. concentration was below 0.3 C. jejuni (MPN) g−1 (fresh weight) faeces), a value of 0 was assigned to the MPN count. For each C. jejuni-positive sample, two distinct C. jejuni colonies were purified from two different dilutions within the MPN series. Obtention of pure colonies was using selective media and agar as above and as previously described (Rapp et al. Citation2014). The confirmation that the single colony isolates were C. jejuni was by PCR as described above.
Genetic typing by ERIC-PCR
The DNA from each single colony confirmed as C. jejuni was subjected to PCR using the ERIC 2 primer (Versalovic et al. Citation1991) and the amplification protocol described previously (Weijtens et al. Citation1999). The banding patterns obtained were compared only for isolates that were processed in the same PCR run in order to ensure robust analysis (Meacham et al. Citation2003; Rasscharet et al. Citation2005). Three stages of comparison were followed. The DNA from isolates from each individual animal obtained over the 12-month sampling period was first compared to determine the similarities and differences in the C. jejuni isolates excreted over time by individual cows. The DNA of isolates from an individual cow was then successively compared to those of the other individuals on the same farm. Finally, representative isolates obtained from both farms were compared. Cluster analysis was used to determine relationships between isolates. The isolates obtained over time were considered the same type when they grouped at a greater than 90% similarity in their ERIC bands pattern. Different types were assigned a number (i.e. ERIC-type 1 to ERIC-type 36). Cluster analysis was performed with Quantity One Software version 4.6.6. (Bio-Rad Laboratories Inc., CA, USA) and based on the Dice similarity coefficient and the unweighted pair group method with arithmetic mean.
Microbiological data analysis
The number of successive samplings was used to quantify the periods of C. jejuni excretion and the length of time between excretion events. To aid interpretation, C. jejuni excretion patterns were considered as ‘sporadic’ when five or less C. jejuni-positive samples were repeatedly interspersed by one or more samplings in which C. jejuni was not detected in the faeces. Assuming no change in excretion status between two consecutive samplings, excretion patterns were classified as ‘chronic’ when >80% of a cow’s samples were positive for C. jejuni. Otherwise, the trends observed in the excretion patterns were defined as ‘not classified’.
Results
For individual cows on both farms there were periods during which C. jejuni was excreted continuously, periods of intermittent excretion and periods of no excretion. Overall, a continuum of C. jejuni excretion patterns ranging from chronic to sporadic was observed.
Sporadic excretion
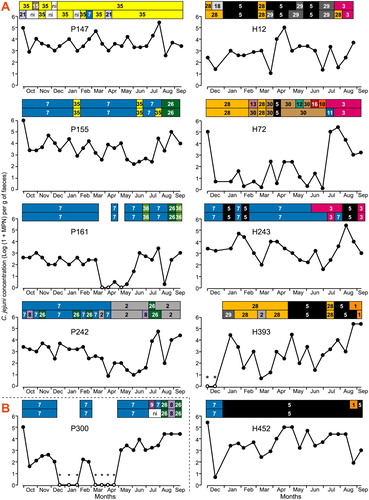
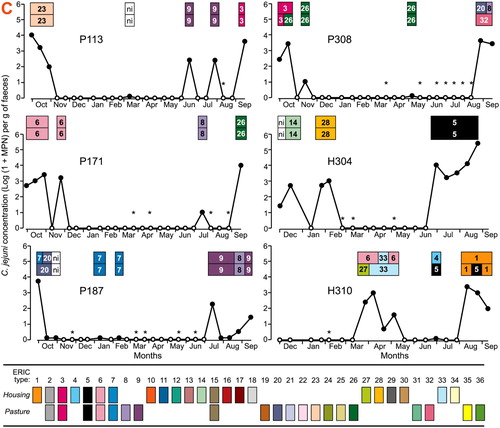
Short term excretion of C. jejuni that was interspersed with periods of no excretion was common on both farms. It was observed for seven (39%) cows on Farm P and four (24%) cows on Farm H (). Examples of sporadic excretion are shown in A (see Figure SA1 for other examples).
Figure 1. Examples of chronic (A), not classified (B) and sporadic (C) C. jejuni excretion patterns by dairy cows over 12 months on the pasture farm and 10 months on the housing farm. The alphanumeric labels for each cow denote its farm of origin (P for the pasture farm; H for the housing farm) and farm allocated cow ID number. Excretion over time (x-axis) is described in terms of faecal concentration (log10.g-1 fresh faeces) (y-axis) and ERIC types identified for the two colonies obtained for each C. jejuni-positive faecal sample (top line). Samples from which C. jejuni was not detected are represented by open circles (o); samples from which thermotolerant Campylobacter sp. other than C. jejuni was identified are highlighted by *. Each of the 36 ERIC types identified in the C. jejuni-positive samples is denoted using a specific colour and number; ni is for non-identified ERIC type.
Table 1. Proportion of chronic, sporadic and not classified excretion patterns of C. jejuni by dairy cows over 12 months on the pasture farm and 10 months on the housing farm. The farm allocated cow ID number is given in bracket.
The most common period during which C. jejuni was recovered was one or two consecutive fortnightly samplings; it was observed for five of the seven cows with sporadic excretion on Farm P. It occurred less frequently on Farm H, with one occurrence for cow H304 and one occurrence for cow H310. The observed excretion periods lasted for a maximum of five consecutive samplings. The length of time between observed C. jejuni excretions ranged from 1 to 15 consecutive samplings on Farm P and between one and nine consecutive samplings on Farm H.
On both farms, marked changes in C. jejuni faecal concentration occurred regardless of the length of recovery and non-recovery periods. Three log10 to four log10 differences in C. jejuni faecal concentration between two successive samplings were observed once (cows P113 and P187; H261 and H310), twice (P308; H304) or four times (P171).
Chronic excretion
Chronic excretion was observed for 7 (39%) of the 18 cows on Farm P and for 12 (71%) of the 17 cows on Farm H () and the higher incidence on Farm H was statistically significant (p = 0.06; two-sample binomial test). C. jejuni was detected in all the collected faeces for four and seven of these cows on farm P (P47, P147, P155, P242) and farm H (H12, H72, H89, H224, H239, H243 and H452), respectively (C and S1C). Shorter periods of C. jejuni excretion were observed for one cow on Farm P (12 and 9 consecutive samplings, P161), and two cows on Farm H (8 and 10 consecutive samplings; six and 11 consecutive samplings; H268 and H213).
Chronic excretion was associated with a relatively constant concentration of C. jejuni for three of the seven (43%) chronic excreters on Farm P and for eight of the 12 (67%) chronic excreters on Farm H. For the remaining chronic shedders, concentration varied by up to 4 log between consecutive samplings.
Not classified C. jejuni excretion patterns
The remaining four cows on Farm P and one cow on Farm H had no discernable C. jejuni excretion pattern (B and S1B).
Genetic changes of identified C. jejuni ERIC types over time
For the majority (7/10) of the cows with sporadic excretion, a succession of the identified ERIC types was observed when the recovery periods were separated by at least three consecutive samplings. The succession was either for a single ERIC type (for example, types 23, 9 and 3 in cow P113; types 14, 28 and 5 in cow H304), or for a combination of ERIC types (for example, types 6, 27 and 33 followed by types 1, 4 and 5 in cow P310; types 3 and 26 followed by types 8, 20 and 32 in cow H308). There was less frequent re-occurrence of the same ERIC type in periods of recovery separated by more than three consecutive samplings. When the recovery periods were interrupted by one or two samplings with no C. jejuni recovery, the detected ERIC types were similar (for example, type 9 in cow P113; type 8 in cow P132; type 6 in cow P171, type 7 in cow P187; type 26 in cow P71).
For the cows that had chronic excretion, more than one type of change in the excreted C. jejuni genotypes was identified. Chronic C. jejuni excretion could be associated with long-term detection of a given ERIC type (for example, type 35 identified for 19 consecutive samplings in cow P147, type 7 for 14 and 15 consecutive samplings in cows P242 and P164, respectively; type 5 for 18 consecutive samplings in cow H452). The recovery of a given ERIC type was in cases interrupted by one to two sampling-periods during which either no C. jejuni was detected (cows P161 and H109) or another ERIC type was detected (for example, type 35 and type 7 in cow P155; types 7 and 36 in cow P161; types 7 and 5 in cows H239 and H243; types 33 and 28 in cow H89).
Chronic C. jejuni excretion could alternatively be associated with changes in the detected ERIC types. These changes include (i) the detection of different ERIC types over successive samplings (illustrated by P242, H72, H213, H268, H107, H393, H239) and (ii) repetitive succession of three to four ERIC types (examples include types 19, 22 and 24 in cow P47; types 5 and 24 in cow P62; types 3, 5, 28, 29 in cow H12; types 1, 3, 7 and 5 in cow H224). Overall, the long-term detection of a given ERIC type was more frequently observed in the chronic shedder cows on Farm P (3/7 cows) than on Farm H (1/12 cows), and there were more changes in the excreted ERIC types on Farm H compared to Farm P. In the chronic shedder cows, abrupt changes in C. jejuni concentration over two consecutive samplings were not statistically associated with changes in the identified ERIC types.
Discussion
Each farm was monitored fortnightly over a 12-month period. On both farms, the observed excretion patterns were cow-specific and ranged from chronic to sporadic excretion. However, the proportions of each shedding pattern differed between farms, with chronic excretion predominating on Farm H and sporadic excretion on Farm P. There was also more changes in the excreted C. jejuni genotypes observed on Farm H than on Farm P.
Chronic excretion of C. jejuni was associated with three different ERIC patterns, these were: long-term shedding of a single dominant genotype; shedding of up to three genotypes in random succession; or succession over time of different genotypes.
Excretion of a single dominant genotype for up to 12 consecutive months suggests successful establishment of this genotype in the cow’s gut. Long-term dominance of a single genotype appeared to be more frequent on Farm P than on Farm H, and was observed in a previous study involving less intensive monitoring under dairy practices similar to those on Farm P (Ross et al. Citation2008). This pattern of shedding was also reported by On et al. (Citation1999). The limited number of genotypes associated with a particular cow may be due to an adaptation of one or more genotypes to that cow. Such an adaptation may exclude or at least limit the establishment of other genotypes (Sheppard et al. Citation2014). Interestingly, the duration of excretion of a given genotype was variable among the cows on both farms, possibly reflecting contrasting gut factors. The commensal microbiota in the gut has been found to be cow-specific (Dowd et al. Citation2008) and variable in time (Rudi et al. Citation2012), hence the extent to which the competition by the natural bovine intestinal microbiota mediates the long-term persistence of C. jejuni in the bovine host warrants further investigation. Relationship between cows feeding regime, which has been linked to specific microbiota (De Menezes et al. Citation2011) and the duration of C. jejuni excretion should also be further investigated.
Chronic shedding of multiple genotypes, such as in cows H224 or H12, could reflect the successful establishment of several C. jejuni genotypes in the gut at the same time. This was observed on Farm H in particular, on which chronic shedding was the major pattern. It has been shown that different niches for C. jejuni colonization exist within the gastrointestinal tract of beef cattle (Stanley et al. Citation1998; Inglis et al. Citation2005). As bacterial cells from different genotypes occupying the same niche would have increased opportunity to undergo genetic modification through the exchange of genetic material (Sheppard et al. Citation2014), the co-excretion of genetically stable genotypes observed in our study supports the theory of successful establishment of different genotypes in different cryptic niches.
Chronic excretion on Farm H was also frequently associated with the succession over time of a series of ERIC genotypes, which were dominant for a period. This was possibly due to successive infections leading to the gut niche replacement of an established genotype by a new type. It has been suggested that there may be an upper limit to the number of C. jejuni cells that can be present in the gut of chicken (Dowd et al. Citation2008). If this is true in dairy cattle, when a sequential infection is observed, one strain may need to be partially displaced for a second to establish colonization.
On both farms, sporadic excretion was associated with re-excretion of the same C. jejuni genotype when the length of time observed between C. jejuni-positive samples was short (<1.5 months). Re-excretion of the same genotype at short intervals is suggestive of colonization by C. jejuni without measurable excretion. Another explanation for intermittent excretion of C. jejuni may be an intake of new genotypes from environmental sources (Hänninen et al. Citation1998; Ross et al. Citation2008; Häkkinen and Hänninen Citation2009). Sporadic excretion due to new infection from the farm environment could particularly apply to the patterns associated with the longer (>1.5 month) length of time observed between C. jejuni-positive samples, for which excretion of different C. jejuni genotypes was observed.
The two studied farms had different herd management strategies, including feeding and calving management, hence determining the impact of a particular management on C. jejuni excretion by comparing the two farms is not straightforward. However, a possible reason for the frequent change in the C. jejuni genotypes excreted by cows on Farm H is the greater use of stored supplementary feed. We observed this feed attracted birds and rats throughout the year. Wildlife living in close proximity to livestock could be a source of Campylobacter infection. Previous studies have found that the C. jejuni strains isolated from birds and rats were similar to those found in the dairy cows on the same farm (Adhikari et al. Citation2004; Devane et al. Citation2005; Meerburg and Kijlstra Citation2007). Other authors have concluded that the direction of infection was predominantly from livestock to wild birds (Hald et al. Citation2001; Hughes et al. Citation2009). Further epidemiological studies are required to better define the nature and effect of on-farm sources on the C. jejuni excretion patterns by dairy cows. As observed for the chronic excretion patterns, the genotypes excreted with a sporadic pattern seemed to be cow-specific, showing that the diversity of genotypes present in a cow environment is likely high. Another difference between the two farms is the calving season, as the cows on Farm P and on Farm H calved in winter and in autumn, respectively. Calving has been associated with metabolic requirement changes and decrease in immune protection (Hammon et al. Citation2006), however in our study no discernible changes in C. jejuni excretion patterns were noticed at calving or early lactation stage (data not shown). This suggests that, if any, the effect of calving on C. jejuni excretion might not as important as the effect of on-farm sources for cow contamination.
The study approach was to monitor a limited number of cows relatively intensively. Previous overseas studies that have reported chronic and sporadic excretions of C. jejuni in dairy cows (Humphrey and Beckett, Citation1987; Hänninen et al. Citation1998; Häkkinen and Hänninen Citation2009) were based on faecal samplings of dairy cows that were separated by two to three months, and were limited to the presence/absence or semi-quantitative analysis of C. jejuni. Our more intensive strategy of sampling the same cows over time, combined with measuring concentrations and genotyping analysis, provides a firmer basis to establish the underlying changes in the excreted C. jejuni genotypes. The large range of excretion patterns was unexpected and hampered statistical analysis. Larger scale studies would be valuable in confirming the excretion patterns and associated underlying reasons found in the current study.
Conclusion
This longitudinal study of C. jejuni in individual dairy cattle that used a combination of microbiological and molecular methods has contributed to an increase in understanding of C. jejuni epidemiology within the bovine host. Striking features were that individual excretion patterns of C. jejuni can vary widely among cows subjected to the same general husbandry practices, and that farm practices or environment seem to impact on the introduction and establishment of a given genotype in a given cow. Further studies on factors that favour colonization of the cow gut, survival in the environment and transmission to cows are needed before practical recommendations can be made to reduce C. jejuni carriage and shedding by dairy cows.
Figure S1. Additional examples of chronic (A), not classified (B) and sporadic (C) excretion patterns of C. jejuni by the studied cows. Legend is as described in Figure 1.
Download TIFF Image (1.5 MB)Figure S1. Additional examples of chronic (A), not classified (B) and sporadic (C) excretion patterns of C. jejuni by the studied cows. Legend is as described in Figure 1.
Download TIFF Image (1.3 MB)Acknowledgements
The authors are grateful for the co-operation and advice of the farmers involved in this study; to B. Wise and A. McGowan (AgResearch), E. Blythe and P. Martin (Agriscience Consulting) who helped with sampling; to P. Hunt for graphic design of Figure and Appendix; and to Drs A. Donnison, A. Cookson and R. Munday (AgResearch) for their valuable comments and helpful discussions. The animal ethic was granted by the Ruakura Animal Ethic committee.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adhikari B, Connolly JH, Madie P, Davies PR. 2004. Prevalence and clonal diversity of Campylobacter jejuni from dairy farms and urban sources. New Zealand Veterinary Journal. 52:378–383. doi: 10.1080/00480169.2004.36455
- Batz MB, Hoffmann S, Morris Jr JG. 2011. Ranking the risks: the 10 pathogen-food combinations with the greatest burden on public health. Florida: Emerging Pathogens Institute at University of Florida.
- Blaser MJ, Engberg J. 2008. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3rd ed. Washington (DC): ASM Press; p. 99–121.
- De Menezes D, Lewis E, O’Donovan M, O’Neill BF, Clipson N, Doyle EM. 2011. Microbiome analysis of dairy cows fed pasture or total mixed ration diets. FEMS Microbiology Ecology. 78:256–265. doi: 10.1111/j.1574-6941.2011.01151.x
- Devane M, Nicol C, Ball A, Klena JD, Scholes P, Hudson JA, Baker MG, Gilpin BJ, Garrett N, Savill MG. 2005. The occurrence of Campylobacter subtypes in environmental reservoirs and potential transmission routes. Journal of Applied Microbiology. 98(40):980–990. doi: 10.1111/j.1365-2672.2005.02541.x
- Donnison A. 2003. Isolation of thermotolerant Campylobacter – review and methods for New Zealand laboratories. Report prepared for the Ministry of Health, Wellington, New Zealand. [accessed 2010 Nov 10]. http://www.moh.govt.nz/notebook/nbbooks.nsf/0/73166EB251837F95CC257834000271DB/$file/IsolationOfThermotolerantCampylobacter.pdf.
- Dowd SE, Callaway TR, Wolcott RD, Sun Y, McKeehan T, Hagevoort RG, Edrington TS. 2008. Evaluation of the bacterial diversity in the faeces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiology. 8:125. doi:10.1186/1471-2180-8-125.
- Gilpin BJ, Scholes P, Robson B, Savill MG. 2008. The transmission of thermotolerant Campylobacter spp. to people living or working on dairy farms in New Zealand. Zoonoses and Public Health. 55(7):352–360. doi:10.1111/j.1863-2378.2008.01142.x.
- Gilpin BJ, Thorrold B, Scholes P, Longhurst RD, Devane M, Nicol C, Walker S, Robson B. 2008. Comparison of Campylobacter jejuni genotypes from dairy cattle and human sources from the Matamata-Piako district of New Zealand. Journal of Applied Microbiology. 105:1354–1360. doi:10.1111/j.1365-2672.2008.03863.x.
- Gilpin BJ, Walsh G, On SL, Smith D, Marshall JC, French NP. 2013. Application of molecular epidemiology to understand campylobacteriosis in the Canterbury region of New Zealand. Epidemiology Infection. 141:1253–1266. doi: 10.1017/S0950268812001719
- Grove-White DH, Leatherbarrow AJ, Cripps PJ, Diggle PJ, French NP. 2011. Molecular epidemiology and genetic diversity of Campylobacter jejuni in ruminants. Epidemiology and Infection. 139:1661–1671. doi:10.1017/S0950268810002736.
- Häkkinen M, Hänninen ML. 2009. Shedding of Campylobacter spp. in Finnish cattle on dairy farms. Journal of Applied Microbiology. 107:898–905. doi:10.1111/j.1365-2672.2009.04269.x.
- Hald B, Madsen JJ, Rahbek C, Chriel M, Nielsen EM, Bang DD, Lodal J, Jespersen JB, Wainø M, Dietz HH, et al. 2001. Campylobacter spp. carriage by wild birds, rodents, insects and other animals in the immediate environment of cattle, pigs and poultry farms in Denmark. Abstract: Twelfth International Workshop on Campylobacter, Helicobacter and Related Organisms. Aarhus, Denmark, 6-10 September 2003, International Journal of Medical Microbiology 2g3, pp.140 (Suppl. no.35).
- Hammon DS, Evjen IM, Dhiman TR, Goff JP, Walters JL. 2006. Neutrophil function and energy status in Holstein cows with uterine health disorders. Veterinary Immunology and Immunopathology. 113:21–29. doi:10.1016/j.vetimm.2006.03.022.
- Hänninen ML, Niskanen M, Korhonen L. 1998. Water as reservoir for Campylobacter jejuni infection in cows studied by serotyping and pulsed-field gel electrophoresis (PFGE). J Vet Med B. 45:37–42. doi:10.1111/j.1439-0450.1998.tb00764.x.
- Hudson JA, Nicol C, Wright J, Whyte R, Hasell SK. 1999. Seasonal variation of Campylobacter types from human cases, veterinary cases, raw chicken, milk and water. Journal of Applied Microbiology. 87(1):115–124. doi:10.1046/j.1365-2672.1999.00806.x.
- Hughes LA, Bennett M, Coffey P, Elliott J, Jones TR, Jones RC, Lahuerta-Marin A, Leatherbarrow AH, McNiffe K, Norman D, et al. 2009. Molecular epidemiology and characterisation of Campylobacter spp. isolated from wild bird populations in northern England. Applied and Environmental Microbiology. 75(10):3007–3015. doi: 10.1128/AEM.02458-08
- Humphrey TJ, Beckett P. 1987. Campylobacter jejuni in dairy cows and raw milk. Epidemiology and Infection. 98:263–269. doi: 10.1017/S0950268800062014
- Inglis GD, Kalischuk LD, Busz HW. 2004. Chronic shedding of Campylobacter species in beef cattle. Journal of Applied Microbiology. 97:410–420. doi:10.1111/j.1365-2672.2004.02313.x.
- Inglis GD, Kalischuk LD, Busz HW, Kastelic JP. 2005. Colonization of cattle intestines by Campylobacter jejuni and Campylobacter lanienae. Applied and Environmental Microbiology. 71(9):5145–5153. doi:10.1128/AEM.71.9.5145–5153.2005 doi: 10.1128/AEM.71.9.5145-5153.2005
- Kwan PLS, Birtles A, Bolton FJ, French NP, Robinson SE, Newbold LS, Upton M, Fox AJ. 2008. Longitudinal study of the molecular epidemiology of Campylobacter jejuni in cattle on dairy farms. Applied and Environmental Microbiology. 74:3626–3633. doi:10.1128/AEM.01669-07.
- Lake RJ, Cressey PJ, Campbell DM, Oakley E. 2010. Risk ranking for foodborne microbial hazards in New Zealand: burden of disease estimates. Risk Analysis. 30:743–752. doi:10.1111/j.1539-6924.2009.01269.x.
- Lastovica A, Allos BM. 2008. Clinical significance of Campylobacter and related species other than Campylobacter jejuni and Campylobacter coli. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3rd ed. Washington (DC): ASM Press; p. 123–149.
- Longenberger AH, Palumbo AJ, Chu AK, Moll ME, Weltman A, Ostroff SM. 2013. Campylobacter jejuni infections associated with unpasteurized milk-multiple states. Clinical Infectious Diseases. 57:263–266. doi:10.1093/cid/cit231.
- Meacham KJ, Zhang L, Foxman B, Bauer RJ, Marrs CF. 2003. Evaluation of genotyping large numbers of Escherichia coli isolates by enterobacterial repetitive intergenic consencus-PCR. Journal of Clinical Microbiology. 41:5224–5226. doi:10.1128/JCM.41.11.5224-5226.2003.
- Meerburg BG, Kijlstra A. 2007. Role of rodents in transmission of Salmonella and Campylobacter. Journal of the Science of Food and Agriculture. 87:2774–2781. doi: 10.1002/jsfa.3004
- Minihan D, Whyte P, O’Mahony M, Fanning S, McGill K, Collins JD. 2004. Campylobacter spp. in Irish feedlot cattle: a longitudinal study involving pre-harvest and harvest phases of the food chain. Journal of Veterinary Medicine. B, Infectious Diseases and Veterinary Public Health. 51(1):28–33. doi:10.1046/j.1439-0450.2003.00722.x.
- Mullner P, Spencer SE, Wilson DJ, Jones G, Noble AD, Midwinter AC, Collins-Emerson JM, Carter P, Hathaway S, French NP. 2009. Assigning the source of human campylobacteriosis in New Zealand: a comparative genetic and epidemiological approach. Infection, Genetics and Evolution. 9(6):1311–1319. doi:10.1016/j.meegid.2009.09.003.
- Nielsen EM. 2002. Occurrence and strain diversity of thermophilic campylobacters in cattle of different age groups in dairy herds. Letters in Applied Microbiology. 35:85–89. doi:10.1046/j.1472-765X.2002.01143.x.
- On SLW, Atabay HI, Corry JEL. 1999. Clonality of Campylobacter sputorum bv. paraureolyticus determined by macrorestriction profiling and biotyping, and evidence for long term persistent infection in cattle. Epidemiology and Infection. 122:175–182. doi: 10.1017/S0950268898001824
- Rapp D, Ross CM, Cave V, Muirhead RW. 2014. Prevalence, concentration and genotypes of Campylobacter jejuni in faeces from dairy herds managed in farm systems with or without housing. Journal of Applied Microbiology. 116(4):1035–1043. doi:10.1111/jam.12425.
- Rasscharet G, Houf K, Imberechts H, Grijspeerdt K, De Zutter L, Heyndricks M. 2005. Comparison of five repetitive-sequence-based PCR typing methods for molecular discrimination of Salmonella enterica isolates. Journal of Clinical Microbiology. 43:3615–3623. doi:10.1128/JCM.43.8.3615-3623.2005.
- Robinson DA. 1982. Campylobacter infection in milking herds. In: Newell DG, editor. Campylobacter: epidemiology, pathogenesis, and biochemistry. Lancaster: MTP Press; p. 274.
- Ross CM, Donnison AM, Clark DA. 2008. Effect of using a stand-off pad on Campylobacter jejuni strain diversity in a herd of dairy cows. Letters in Applied Microbiology. 47:192–196. doi:10.1111/j.1472-765X.2008.02397.x.
- Rudi K, Moen B, Sekelja M, Frisli T, Lee MRF. 2012. An eight-year investigation of bovine livestock faecal microbiota. Veterinary Microbiology. 160(3–4):369–377. doi:10.1016/j.vetmic.2012.06.003.
- Schildt M, Savolainen S, Hänninen ML. 2006. Long-lasting Campylobacter jejuni contamination of milk associated with gastrointestinal illness in a farming family. Epidemiology and Infection. 134:401–405. doi:10.1017/S0950268805005029.
- Sheppard SK, Cheng L, Méric G, de Haan CPA, Llarena A-K, Marttinen P, Vidal A, Ridley A, Clifton-Hadley F, Connor TR, et al. 2014. Cryptic ecology among host generalist Campylobacter jejuni in domestic animals. Molecular Ecology. 23:2442–2451. doi:10.1111/mec.12742.
- Spencer SE, Marshall J, Pirie R, Campbell D, Baker MG, French NP. 2012. The spatial and temporal determinants of campylobacteriosis notifications in New Zealand, 2001-2007. Epidemiology and Infection. 140(9):1663–1677. doi:10.1017/S0950268811002159.
- Stanley KN, Wallace J, Currie JE, Diggle PJ, Jones K. 1998. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. Journal of Applied Microbiology. 85:472–480. doi:10.1046/j.1365-2672.1998 doi: 10.1046/j.1365-2672.1998.853511.x
- Vandamme P, Van Doorn LJ, Al Rashid ST, Quint WGV, Van Der Plas J, Chan VL, On SLW. 1997. Campylobacter hyoilei Alderton et al. 1995 and Campylobacter coli Véron and Chatelain 1973 are subjective synonyms. International Journal of Systematic Bacteriology 47:1055–1060. doi: 10.1099/00207713-47-4-1055
- Versalovic J, Koeuth T, Lupski R. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Research. 19:6823–6831. doi:10.1093/nar/19.24.6823.
- Weijtens MJBM, Reinders RD, Urlings HAP, Van der Plas J. 1999. Campylobacter infections in fattening pigs; excretion pattern and genetic diversity. Journal of Applied Microbiology. 86:63–70. doi:10.1046/j.1365-2672.1999.00636.x.
- Wesley IV, Wells SJ, Harmon KM, Green A, Schroeder-Tucker L, Glover M, Siddique I. 2000. Fecal shedding of Campylobacter and Arcobacter spp. in dairy cattle. Applied and Environmental Microbiology. 66(5):1994–2000. doi: 10.1128/AEM.66.5.1994-2000.2000
- World Health Organization. 1984. Guidelines for drinking-water quality, Vol. 1: recommendations. Geneva: World Health Organization.