ABSTRACT
Illthrift, defined as low body condition score (BCS), is a problem in adult ewes on many farms in New Zealand, and nematode parasites are often considered a significant contributing factor. Here we test the hypothesis that, over the lambing-lactation period, parasites are a significant causal factor in ewe illthrift, and that anthelmintic administration to low BCS ewes results in a greater improvement in condition than treatment of other ewes. Faecal nematode egg count (FEC) prior to lambing and ewe liveweight change over the study period were poorly correlated, and both were independent of pre-lambing BCS, as was ewe response to anthelmintic treatment. Some ewes lost, or gained, considerable amounts of weight independent of their BCS, the number and size of lambs reared, and anthelmintic treatment. We find no evidence that ewes of low BCS have higher FECs, or a larger response to anthelmintic treatment than ewes in better condition.
Introduction
Nematode parasites are ubiquitous in grazing livestock around the world and constitute one of the most important production limiting issues for farmers in New Zealand (Jackson et al. Citation2006; Lawrence et al. Citation2007). In most countries, control is achieved primarily through the routine use of broad spectrum anthelmintics (Molento Citation2009; Morgan et al. Citation2012). In New Zealand anthelmintic treatment programmes for sheep usually entail the routine treatment of lambs at approximately monthly intervals (Vlassoff et al. Citation2001; Lawrence et al. Citation2007) with the average number of annual treatments being about seven. Treating adult ewes around parturition is also a common practice, especially since the advent and marketing of persistent formulations such as moxidectin injection and controlled release capsules (Vlassoff et al. Citation2001; Lawrence et al. Citation2007). These products have been widely promoted on the basis of production benefits despite trial data suggesting that the net benefits are unreliable and often negative (Gogolowski et al. Citation1997; Garland and Leathwick Citation2015; Miller et al. Citation2015) and the increased selection for drug resistant parasite populations that result from their use (Leathwick et al. Citation1995, Citation2006, Citation2015; Lawrence et al. Citation2006). The rationale for treating ewes at this time includes the temporary and partial relaxation of anti-parasite immunity often associated with pregnancy and lactation (Brunsdon Citation1971), the associated rise in faecal nematode egg count (FEC) and potential for pasture contamination (Brunsdon Citation1970) and the liveweight benefits to the ewe often seen at weaning (Miller et al. Citation2015).
Illthrift, defined as ‘thin’ sheep with a body condition score (BCS) (Jeffries Citation1961; Kenyon et al. Citation2014) of 2 or less, is a common and serious problem in many ewe flocks in New Zealand, with these sheep being less productive than ewes which are in better condition (Mathias-Davis et al. Citation2013; Kenyon et al. Citation2014). The causes of illthrift are complex and often difficult to diagnose, but without an understanding of the causal factors practical solutions will be more difficult to find (West et al. Citation2001). Nematode parasitism is often considered to be a significant contributor to poor ewe health and several recent studies have focused on the production benefits of using long-acting anthelmintics in ewes with low BCS. These studies have invariably shown a benefit in ewe liveweight (West et al. Citation2009; Bingham et al. Citation2017). However, other studies have indicated that response to anthelmintic treatment is independent of condition score at the time of treatment (Miller et al. Citation2015). This latter finding is in contrast to the perception that skinny ewes have more worms, and that parasitism is a significant contributor to ewe illthrift. This, in turn, questions the conclusion that restricting anthelmintic treatments to ewes with low BCS will result in a greater production benefit to the farmer. The purpose of this study was to investigate the link between illthrift (low BCS) in ewes over the lambing/lactation period and parasitism. The hypothesis addressed was that nematode parasites are a significant causal factor in ewe illthrift over the lambing and lactation period and, consequently, that anthelmintic treatments administered to low BCS ewes will result in a greater improvement in ewe condition than treatment of other ewes.
Materials and methods
Study design
The data for this investigation were derived from two previously published studies (Leathwick et al. Citation2006; Miller et al. Citation2015). Collectively, in these two studies complete production data from treatment groups relevant to this investigation were measured in 2044 ewes, prior to lambing and at weaning. Details of the experimental designs and collection of data can be found in the published papers (Leathwick et al. Citation2006; Miller et al. Citation2015).
In brief, the first trial was carried out on an 18 ha area of the Flock House Research Farm in the Manawatu region of the North Island, New Zealand. The area had been subdivided into 11 farmlets, which functioned as self-contained miniature farms, in three incomplete replicate blocks. For the purpose of this analysis only nine of these farmlets were considered, three replicates of three anthelmintic treatment options. In Treatment 1, ewes were administered a controlled release capsule (CRC) pre-lambing, which released 32.5 mg albendazole per day for 100 days (Extender 100; Nufarm Animal Health, Auckland, NZ). Ewes that exceeded 65 kg liveweight were given two capsules simultaneously, as per the manufacturer’s recommendations. Treatment 2 ewes were given a single oral dose of albendazole (Albendazole Sheep; Ancare NZ Ltd, Auckland, NZ) at docking (tail-docking of lambs, 2–4 weeks after lambing) at a dose rate of 4.75 mg/kg liveweight, based on the weight of the heaviest animal in each mob. Treatment 3 ewes remained untreated.
Within each farmlet, ewes and their offspring were maintained for most of the year rotating around the paddocks to minimise potential paddock effects. However, for the period of interest in this analysis (i.e. from pre-lambing until weaning) the ewes were set-stocked over all the paddocks within their respective farmlets. Initially, approximately 320 ewes were divided among each of the four treatment groups (in the original design) balanced for liveweight, date of birth and number of lambs at scanning. From then on, the ewes grazed on the same farmlet and were monitored for 5 years, unless they died or were culled. Each year approximately 25% of the ewes were replaced with younger animals (<2 years old) which had been reared as lambs on the trial. Any ewes which died were replaced from a worm-free pool of spare ewes kept for this purpose. Complete data, useful for this analysis, was only available for the last four years of the study.
The second study was carried out on commercial sheep and beef farms in the Wairarapa region of the North Island of New Zealand in 2011 and 2012 (Miller et al. Citation2015). For the purposes of this analysis, data from seven on-farm trials was available from the first year and from five on-farm trials in the second year. Only two treatment groups were included in this analysis, an untreated control and a group treated with a long acting CRC releasing 160 mg of abamectin, 4.62 g albendazole, 24 mg selenium and 120 mg cobalt over approximately 100 days (Bionic, Merial N.Z. Ltd, Auckland, N.Z.). Like the first (Flock House) trial, all the ewes were set-stocked for lambing and remained grazing the same pastures until weaning, however, unlike the first trial all the treatment groups were run together.
Measurements and calculations
In both trials, a series of measurements were made at strategic time points. For the purpose of this analysis the data of interest were the liveweight (LWT) and BCS of ewes 2–4 weeks pre-lambing and at lamb weaning (approximately 12 weeks after lambing) and the number and liveweight of all lambs present at weaning. Lambs were tagged at birth to ensure correct allocation of lambs to the ewe. Sheep were weighed using automatic walk-on scales while the BCS were measured on a scale of 1–5 by manually estimating the depth of fat covering the lumbar region of the spine (Shands et al. Citation2009; Kenyon et al. Citation2014). Within each trial, all BCS estimates were made by the same observer to avoid any observer bias.
Faecal samples were collected from all ewes pre-lambing, coincident with administration of CRCs, for the estimation of FEC. The number of eggs in a 2 g subsample of wet faeces was counted using a modified McMaster technique in which each egg counted equates to 50 eggs per g (epg).
The raw data were used to calculate a number of variables for inclusion in the analysis. Ewe LWT prelambing was subtracted from the LWT at weaning to calculate the change in liveweight over the period of interest (denoted by LWG). Similarly, change in BCS was calculated by subtracting prelambing BCS from weaning BCS. These values were then categorised as positive (an increase in BCS), no change (BCS remained the same) and negative (BCS decreased).
The kg lamb reared by each ewe was calculated from the number of lambs present at weaning and their liveweights.
Statistical analysis
The main response of interest in this study was the ewe LWG, and its association with prelambing BCS and change in BCS. The individual animal data were initially graphed to explore patterns, followed by analysis of covariance (ANCOVA) to gauge the evidence provided by the data for significant treatment and other effects.
For the Wairarapa study, different flocks of ewes were used in each of the farms in each year, so this factor (Trial) was considered as a high level experimental factor. Since treatments were randomly allocated to animals within each of these trials, this experimental factor was regarded as nested within trials. There was no intent to extrapolate to other farms or treatments and so these were regarded as fixed-effect experimental factors, with only animal variation treated as a random effect.
For the Flock house study, the animals were first randomly allocated to the three treatments followed by animals within treatment groups being further split into the three pasture blocks which were considered to be representing different soil/pasture types. Hence, besides the treatments, ‘pasture-block’ also has been considered as an experimental factor. The aim is to explore the treatment and other effects adjusted for this pasture-block effect. Since, approximately 25% of the ewes were replaced in each year within each treatment and pasture-block, and that the measurements of interest in this study could be regarded as independent across the years (i.e. there was approximately 9 months between periods of observation), the ‘year’ factor was also considered as an experimental factor. As in the Wairarapa trial, the experimental factors were regarded as fixed-effects, and only animal variation was regarded as a random effect.
Ewe BCS and FEC measured prelambing, and the kg of lamb weaned by each ewe were included as covariates in the modelling process. Besides the Treatment and Trial effects for the Wairarapa study and Treatment, Pasture block and Year effects for the Flock House study, the model also included change in BCS as a fixed effect and the various interaction and nested effects were investigated.
Least squares means of treatments and relevant interactions were estimated and pairwise comparisons of these were carried out by Fisher’s LSD test, only if there was sufficient evidence for the significance of the corresponding effects. For the purposes of interpretation we have considered a p-value between 0.05 and 0.10 as providing weak evidence of an effect (indicating a trend) (Ganesh and Cave Citation2017) and the actual p-values are provided, along with the 95% confidence interval (CI), for the effect of interest.
Variation in LWG between treatment groups was investigated using Barlett’s test for homogeneity of variance. Also, the relationship between LWG and prelambing FEC was further investigated by linear regression.
The effect of prelambing BCS on prelambing FEC was examined using an ANOVA. Here, prelambing BCS was considered as a fixed effect and as before the associated interaction and nested effects were included for the Flock house and Wairarapa studies.
The response LWG met assumptions for ANCOVA satisfactorily. However, a cuberoot transformation of prelambing FEC was required for the ANOVA process. As the latter resulted in only approximately satisfactory assumptions, we evaluated appropriate permutation tests (Anderson Citation2001), and the resulting significance of the various effects were similar to those of the base ANOVA model, thus the results of the latter have been retained.
All analyses were carried out using the R software version 3.4.1 (R Core Team 2017).
Results
From the Flock House study, complete data records were available from 819 ewes over the 4 years of data collection. Averaged across the years there was a significant difference between the anthelmintic treatments in ewe LWG from pre-lambing to weaning (p = .011), but there were also significant differences between years in this response (p = .004) i.e. in some years there was a treatment effect and in other years there was not. The average benefit over the four years, compared to the untreated animals, was 1.20 kg (95%CI: −0.10 to 2.51 kg) in the CRC-treated animals, while the orally treated ewes were on average 0.34 kg (95%CI: −1.64 to 2.50 kg) lighter than the untreated i.e. there was no liveweight benefit to an oral treatment at tail-docking. While there was a trend (p = .070) towards a difference between the CRC-treated and untreated animals, the largest difference was between the CRC and oral drench treatments (p = .018).
The influence of BCS pre-lambing on LWG was not significant (p = .260) indicating that weight gain was independent of BSC at the time of treatment. There was, however, a significant relationship between change in BCS (from pre-lambing to weaning) and LWG over the same period (p < .001) in that ewes which showed a negative response in BCS also tended to exhibit a lower LWG, and vice versa (A). The measured pattern of BCS change was independent of anthelmintic treatment (p = .551) and was not affected by the year of measurement (p = .178) (A). Investigation of the homogeneity of variance in LWG indicated that there was no difference between the treatments i.e. ewes treated with anthelmintic showed similar variation in LWG to untreated ewes. Hence, there was a pattern where some ewes gained condition, some lost condition and the remainder stayed the same (A, ) and this was not affected by year or by anthelmintic treatment.
Figure 1. Liveweight gain and change in body condition score (▾ = decrease, ▴ = increase and • = no change) over the period from 2–4 weeks pre-lambing to weaning for individual ewes which were either left untreated, treated with an oral anthelmintic at tail-docking, or administered a controlled release capsule pre-lambing in the Flock House study (A) or either left untreated or administered a controlled release capsule pre-lambing in the Wairarapa studies (B) (A–H are the farms; 1 = 2011, 2 = 2012).
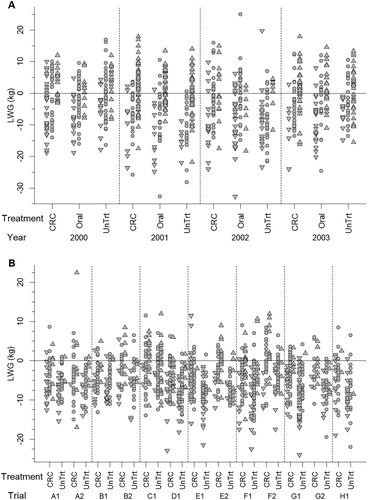
Table 1. The number (percentage) of ewes which had a negative, zero or positive change in body condition score when they were treated pre-lambing with a 100 day controlled release anthelmintic capsule, administered an oral anthelmintic at tail-docking or remained untreated.
When included as a covariate in the analysis the weight (kg) of lamb produced by each ewe at weaning was a significant variable influencing ewe LWG (p < .001) (Miller et al. Citation2015) and indicated that ewes which produced more and/or heavier lambs tended to have lower LWG.
From the Wairarapa trials, complete data records were available for 1225 ewes. Overall, ewes treated with a CRC pre-lambing had higher liveweight gains over the lambing-lactation period than untreated ewes (p < .001), although differences were not significant in all trials. Treated ewes were on average 2.40 kg (95%CI: 1.60 to 3.20 kg) heavier at weaning. As above, LWG was independent of BCS pre-lambing (p = .456) but was significantly correlated with change in BCS (p < .001), i.e. while LWG was similar regardless of ewe BCS pre-lambing, those ewes which lost condition also tended to have lower LWG and vice versa (B). As in the Flock House study, this pattern of change in BCS was not affected by anthelmintic treatment (p = .875) with some ewes gaining condition, some losing condition and some remaining the same, irrespective of anthelmintic treatment (). The test for homogeneity of variance indicated differences in variance in some trials, however, in all cases these differences reflected the CRC-treated ewes being more variable in LWG than the untreated. Averaged across all the trials there was no difference in variance between the two treatment groups.
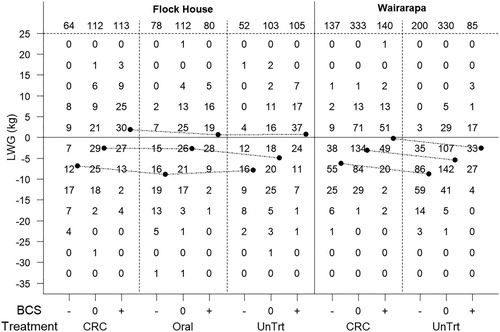
In both data sets the scale of the response to anthelmintic treatment was small relative to the variation in LWG shown by individual ewes (). For example, in the Flock House study average LWG was negative being −2.92 kg (95% CI: −3.84 to −8.64 kg), −4.46 kg (95% CI: −3.84 to −8.64 kg) and −4.12 kg (95% CI: −3.84 to −8.64 kg) for the CRC, oral treatment and untreated groups, respectively. The response to treatment with a CRC was therefore a +1.2 kg liveweight advantage compared to the untreated (this difference was not significant). However, approximately 39% of untreated ewes, and 40% of CRC-treated ewes, showed a liveweight change of more than 10 kg either side of the mean (the mean being a −4.12 kg LWG in the untreated ewes). These means were adjusted for BCS pre-lambing and kg lamb weaned so these changes in body weight could not be attributed to ewe BCS or to the number and size of lambs reared.
Figure 2. Number of animals falling within 5 kg intervals of liveweight gain (LWG) over the period from 2–4 weeks pre-lambing to weaning for individual ewes which were either left untreated (UnTrt), treated with an oral anthelmintic at tail-docking (Oral), or administered a controlled release capsule (CRC) pre-lambing, and grouped into change in body condition score (negative (−), no change (0), positive(+)). Symbols (•) denote the overall mean LWG. Total number of animals are shown at the top of graph.
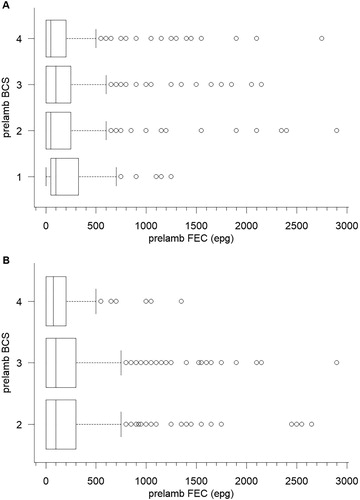
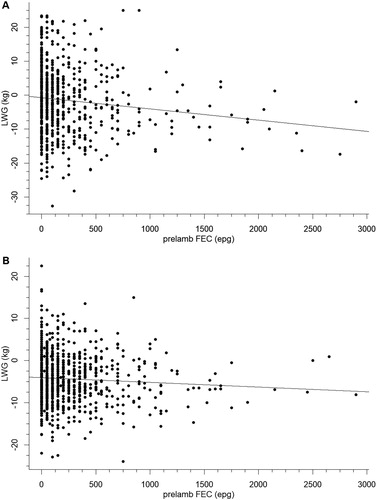
The role of nematode infection was also investigated using the FECs prior to lambing and administration of the anthelmintics (A,B). When included as a covariate in the ANCOVA, pre-lambing FEC was significant (p = .005) in the Flock House study but not in the Wairarapa study (p = .269). However, simple linear regression analysis of LWG against FEC indicated that in the fitted models, FEC accounted for ≤1% of the variation in LWG i.e. 99% of the variation in LWG was associated with factors other than pre-lambing FEC. ANOVA of pre-lambing FEC against ewe BCS showed the FECs were similar in ewes irrespective of their BCS in the Flock house study (p = .124, A), but in the Wairarapa study there was a significant negative relationship between BCS and FEC (p = .005, B). However, as with the relationship between prelambing FEC and LWG (above), the R2-values were very low (1.2% and 1.7% for the Flock House and Wairarapa studies, respectively), indicating that BCS was a very poor predictor of FEC. This can be seen in the overlaps in the data () where for each BCS category 50% of the FECs are low (below the median) and the median values are consistently low and similar (for the Flock House study median FEC was 100, 50, 100 and 50 for BCS 1, 2, 3 and 4, while for the Wairarapa study median FEC was 100, 100 and 75 for BCS 2, 3 and 4, respectively, ). Therefore, the significant difference between mean FEC in the different BCS categories in the Wairarapa study appears to be due to a small number of individuals with high FEC values ().
Figure 3. Relationship between faecal nematode egg count (FEC) pre-lambing and liveweight gain (LWG) over the peri-parturient period in ewes that were either untreated, treated pre-lambing with a 100-day controlled release capsule or treated with an oral anthelmintic after lambing at tail-docking in a 4-year study at Flock House (A) or a series of 12 trials over 2 years in the Wairarapa (B). Fitted equations are LWG = −2.695–0.002 × FEC (R2 = 0.92%) for A and LWG = −4.313–0.001 × FEC (R2 = 0.34%) for B.
Discussion
The purpose of this study was to investigate the link between illthrift (low BCS) in adult ewes over the lambing/lactation period and infection with nematode parasites. The hypothesis under investigation was that nematode parasites are a significant causal factor in ewe illthrift (low BCS) and, consequently, that anthelmintic treatments administered to low BCS ewes result in a greater improvement in ewe condition than treatment of other ewes. Analysis of these two data sets failed to find any evidence to support this hypothesis.
Ewe FEC prior to lambing showed poor correlation with both pre-lambing BCS and LWG over the pre-lambing-lactation period (i.e. the R2-values were ≤1%). While the ANOVA showed higher mean FEC in ewes of low BCS in the Wairarapa study, but not the Flock House study, these relationships were again very weak (R2-values < 2%). Therefore, BCS was a very poor indicator of FEC, with the majority of FECs being low (or zero) irrespective of BCS. Liveweight gain of ewes from pre-lambing to weaning was correlated with change in BCS over the same period i.e. not surprisingly, ewes which tended to gain weight also tended to gain condition and vice versa. However, LWG was independent of ewe BCS measured before lambing (as found by Cook Citation2009) and also the pre-lambing FEC.
Ewe LWG was improved by treatment with a 100-day anthelmintic CRC in most of the Wairarapa trials, and in some years in the Flock House study (although in the latter, overall, CRC treatment was not significantly better than the untreated). However, response to treatment was independent of ewe BCS and did not affect change in BCS. Hence, low BCS ewes did not show a greater response to anthelmintic treatment than ewes in better condition. Also, the average response to treatment with a CRC was modest compared to the LWG changes occurring in many of the animals. A proportion of ewes lost, or gained, considerable amounts of weight (; ) which was negatively correlated with the number and size of lambs reared and positively correlated with change in BCS. By comparison, anthelmintic treatment resulted in only a small improvement in LWG () and did not influence the proportion of ewes which gained or lost condition (). Further, anthelmintic treatment did not result in reduced variation in LWG between the groups, which might be expected if parasites were effecting LWG in some animals more than others.
Collectively, these findings show no compelling evidence that ewes of low BCS have more parasites than ewes with higher BCS (as indicated by FEC) and no evidence of a larger response to anthelmintic treatment by low BCS ewes. Although some of the trial farms tested positive for the presence of anthelmintic resistant parasites, only one farm recorded a mean FEC > 30 epg in CRC-treated ewes (compared to 250–720 epg in untreated ewes), suggesting that for the most part the CRCs controlled parasite infection (data not shown). Individual ewes exhibited sizable changes (positive, neutral or negative) in both LWG and BCS which were independent of BCS prior to lambing, and unaffected by treatment with a 100-day anthelmintic treatment. Importantly, the popular perception that low BCS ewes treated with anthelmintic will all show an improvement in condition appears not to be true. We, therefore, conclude that there is no evidence that illthrift (low BCS) in ewes is to any significant degree associated with nematode parasites.
The impact of internal parasite infection on adult ewes has received considerable attention in New Zealand over many years (Brunsdon and Adam Citation1975; Milligan Citation1982; Familton et al. Citation1995; Sumner et al. Citation1995; Gogolewski et al. Citation1997; West et al. Citation2009; Miller et al. Citation2015), with results overall being inconsistent with respect to the benefits and cost-effectiveness of treating ewes with anthelmintic over the parturition / lactation period. More recently, in response to the escalating issue of anthelmintic resistance, attention has focused on the benefits of treatments administered only to those ewes in poorer condition as a form of targeted selective treatment (Cook Citation2009; West et al. Citation2009; Leathwick and Besier Citation2014; Bingham et al. Citation2017). This approach is largely based on the perception that the benefits of treatment will be greater in animals of low BCS (Cook Citation2009). An implicit, but often unstated, assumption in taking this approach is that ewes with low BCS are skinny because they have more worms and so the response and benefit from treating these animals will be greater than treating animals in better condition. However, with the exception of a study by Cook (Citation2009), no attempt has been made to verify this assumption.
An unexpected finding from this current study was the variability and size of the LWGs, and in the changes in BCS, of the ewes over the lambing/lactation period. Based on the initial hypothesis, there was an a priori expectation that an anthelmintic treatment administered to low BCS ewes would result in a positive response (i.e. increase in LWG and BCS) across all the treated animals, relative to the untreated control. However, a consistent positive response was not observed, in fact LWGs varied considerably both in direction and in size, with a proportion of ewes gaining more than 5 kg (despite losing the weight of their lambs) while some lost more than 15 kg (). Further, this pattern occurred irrespective of ewe BCS at the time of treatment and whether the animals were administered an anthelmintic, even when the administered anthelmintic was a 100-day CRC which should have eliminated parasite infection for almost the entire period of measurement. This pattern of positive, neutral and negative BCS change is similar to that described by Mathias-Davis et al. (Citation2013) from four Southland flocks, suggesting that this may, in fact, be a normal occurrence amongst lactating ewe flocks. Unfortunately, this earlier work gave no indication of whether anthelmintic treatments were given to the ewes (Mathias-Davis et al. Citation2013).
The current data, along with that of earlier studies (Gogolewski et al. Citation1997; Miller et al. Citation2015), supports the view that LWG of ewes over lactation is often, but not always, improved by anthelmintic treatment. However, this study was not undertaken to evaluate the cost-benefit of administering anthelmintic treatment to ewes pre- or post-lambing, rather, the purpose here was to better understand the relationship between parasite infection and illthrift in individual adult ewes. While nematode infections are an important production limiting variable on many New Zealand sheep farms (Vlassoff et al. Citation2001) the analysis indicates that whatever detrimental effect parasites are having on ewe LWG and BCS they are having it uniformly across all ewes which are unprotected by anthelmintic, irrespective of their BCS. Further, there are large changes in ewe LWG and BCS occurring which cannot be linked to parasitism i.e. they occur irrespective of anthelmintic treatment. Low BCS in ewes is likely to have multiple causes with earlier studies suggesting that feed quality and quantity, the residual effects of facial eczema and chronic pneumonia are likely to be important contributing factors (West et al. Citation2001). The key finding from this study is the indication that in many cases nematode parasites are unlikely to be a significant factor contributing to low BCS in ewes. This is an important finding because, as pointed out by West et al. (Citation2001) without a proper understanding of the causes of illthrift practical solutions will be difficult to achieve. We conclude, based on these data, that attempting to solve an illthrift problem in ewes through administration of anthelmintics is likely to fail and farmers would be better off looking at causes other than parasitism.
Acknowledgements
We thank Chris Garland and the farmers involved in the Wairarapa Anthelmintic Trials who motived us to keep asking questions from the data. Christian Sauermann, Ian Sutherland and two anonymous referees made helpful comments on an earlier manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Dave M. Leathwick http://orcid.org/0000-0002-4247-6416
Additional information
Funding
References
- Anderson MJ. 2001. Permutation tests for univariate or multivariate analysis of variance and regression. Canadian Journal of Fisheries and Aquatic Sciences. 58:626–639. doi: 10.1139/f01-004
- Bingham C, Hodge A, Mariadass B. 2017. Comparison of two long acting pre-lambing anthelmintic treatments on the productivity of ewes in low body condition. New Zealand Veterinary Journal. 65:152–155. doi: 10.1080/00480169.2016.1249528
- Brunsdon RV. 1970. The spring-rise phenomenon: seasonal changes in the worm burdens of breeding ewes and in the availability of pasture infection. New Zealand Veterinary Journal. 18:47–54. doi: 10.1080/00480169.1970.33861
- Brunsdon RV. 1971. The post-parturient rise in the faecal nematode egg count of ewes: some host-parasite relationships. New Zealand Veterinary Journal. 19:100–107. doi: 10.1080/00480169.1971.33942
- Brunsdon RV, Adam JL. 1975. Internal parasites and animal production. New Zealand Society of Animal Production, Occasional Publication No. 4.
- Cook T. 2009. Pre-Lamb drenching of ewes – what result do you expect? Proceedings of the Sheep and Beef Cattle Veterinarians of the New Zealand Veterinary Association. 39:125–127.
- Familton AS, McAnulty RW, Thompson KF, Sedcole JR. 1995. The effect of anthelmintic treatment of ewes during pregnancy. Proceedings of the New Zealand Society of Animal Production. 55:211–213.
- Ganesh S, Cave V. 2017. P-values, p-values everywhere!. New Zealand Veterinary Journal. 66(2):55–56. doi: 10.1080/00480169.2018.1415604
- Garland CB, Leathwick DM. 2015. A cost-benefit analysis of pre- and post-lambing anthelmintic treatments to twin-bearing ewes on commercial farms in the southern North Island of New Zealand. New Zealand Veterinary Journal. 63:220–226. doi: 10.1080/00480169.2015.1012133
- Gogolewski RP, Rugg D, Allerton GR, Kawhia D, Barrick RA, Eagleson JS. 1997. Demonstration of the sustained anthelmintic efficacy of a controlled-release capsule formulation of ivermectin in ewes under field conditions in New Zealand. New Zealand Veterinary Journal. 45:163–166. doi: 10.1080/00480169.1997.36017
- Jackson R, Rhodes AP, Pomroy WE, Leathwick DM, West DM, Waghorn TS, Moffat JR. 2006. Anthelmintic resistance and management of nematode parasites on beef cattle-rearing farms in the North Island of New Zealand. New Zealand Veterinary Journal. 54:289–296. doi: 10.1080/00480169.2006.36713
- Jeffries BC. 1961. Body condition scoring and its use in management. Tasmanian Journal of Agriculture. 32:19–21.
- Kenyon PR, Maloney SK, Blache D. 2014. Review of sheep body condition score in relation to production characteristics. New Zealand Journal of Agricultural Research. 57:38–64. doi: 10.1080/00288233.2013.857698
- Lawrence KE, Leathwick DM, Rhodes AP, Jackson R, Heuer C, Pomroy WE, West DM, Waghorn TS, Moffat JR. 2007. Management of gastrointestinal nematode parasites on sheep farms in New Zealand. New Zealand Veterinary Journal. 55:228–234. doi: 10.1080/00480169.2007.36773
- Lawrence KE, Rhodes AP, Jackson R, Leathwick DM, Heuer C, Pomroy WE, West DM, Waghorn TS, Moffat JR. 2006. Farm management practices associated with macrocyclic lactone resistance on sheep farms in New Zealand. New Zealand Veterinary Journal. 54:283–288. doi: 10.1080/00480169.2006.36712
- Leathwick DM, Besier RB. 2014. The management of anthelmintic resistance in Australasia – strategies and experiences. Veterinary Parasitology. 204:44–54. doi: 10.1016/j.vetpar.2013.12.022
- Leathwick DM, Miller CM, Atkinson DS, Haack NA, Alexander RA, Oliver A-M, Waghorn TS, Potter JF, Sutherland IA. 2006. Drenching adult ewes: implications of anthelmintic treatments pre- and post-lambing on the development of anthelmintic resistance. New Zealand Veterinary Journal. 54:297–304. doi: 10.1080/00480169.2006.36714
- Leathwick DM, Miller CM, Fraser K. 2015. Selection for anthelmintic resistant Teladorsagia circumcincta in pre-weaned lambs by treating their dams with long-acting moxidectin injection. International Journal for Parasitology: Drugs and Drug Resistance. 5:209–214.
- Leathwick DM, Vlassoff A, Barlow ND. 1995. A model for nematodiasis in New Zealand lambs: the influence of drenching and grazing management on the development of anthelmintic resistance. International Journal for Parasitology. 25:1479–1490. doi: 10.1016/0020-7519(95)00059-3
- Mathias-Davis HC, Shackell GH, Greer GJ, Bryant AI, Everett-Hincks JM. 2013. Ewe body condition score and the effect on lamb growth rate. Proceedings of the New Zealand Society of Animal Production. 73:131–135.
- Miller CM, Ganesh S, Garland CB, Leathwick DM. 2015. Production benefits from pre- and post-lambing anthelmintic treatments of ewes on commercial farms in the southern North Island of New Zealand. New Zealand Veterinary Journal. 63:211–219. doi: 10.1080/00480169.2015.1007108
- Milligan K. 1982. Drenching adult sheep: is it worthwhile? New Zealand Journal of Agriculture. 144:18.
- Molento MB. 2009. Parasite control in the age of drug resistance and changing agricultural practices. Veterinary Parasitology. 163:229–234. doi: 10.1016/j.vetpar.2009.06.007
- Morgan ER, Hosking BC, Burston S, Carder KM, Hyslop AC, Pritchard LJ, Whitmarsh AK, Coles GC. 2012. A survey of helminth control practices on sheep farms in Great Britain and Ireland. The Veterinary Journal. 192:390–397. doi: 10.1016/j.tvjl.2011.08.004
- Shands CG, McLeod B, Lollback ML, Duddy G, Hatcher S, O’Halloran WJO. 2009. Comparison of manual assessments of ewe fat reserves for on-farm use. Animal Production Science. 49:630–636. doi: 10.1071/AN09031
- Sumner RMW, Watson TC, Hosking BC. 1995. Effect of controlling internal parasitism on productivity of Merino breeding ewes. Proceedings of the New Zealand Society of Animal Production. 55:205–208.
- Vlassoff A, Leathwick DM, Heath ACG. 2001. The epidemiology of nematode infections of sheep. New Zealand Veterinary Journal. 49:213–221. doi: 10.1080/00480169.2001.36235
- West DM, Pomroy WE, Kenyon PR, Morris ST, Smith SL, Burnham DL. 2009. Estimating the cost of subclinical parasitism in grazing ewes. Small Ruminant Research. 86:84–86. doi: 10.1016/j.smallrumres.2009.09.024
- West DM, Thompson KG, Holloway PM. 2001. Illthrift in ewes-a necropsy survey. Proceedings of the Society of Sheep and Beef Cattle Veterinarians of the New Zealand Veterinary Association. 31:189–193.