ABSTRACT
Seedlings of kale cultivars in New Zealand are highly susceptible to direct feeding by the wheat bug Nysius huttoni (Hemiptera: Lygaeidae), an endemic insect pest.
Two assays (choice and no-choice) were conducted to compare the relative susceptibility of seedlings of the six most popular kale cultivars in New Zealand (Kestrel, Gruner, Sovereign, Regal, Corka and Coleor). The earliest occurrence of feeding damage in the choice assay was on cv. Kestrel, significantly earlier than on Corka and Gruner. In the no-choice assay, significantly more wheat bugs were found on Kestrel than on Corka. Damage to Kestrel occurred significantly earlier than on all the other cultivars except Corka. Reduction in plant dry weight was significantly higher on Coleor and Kestrel. These results are important for developing integrated pest management protocols for kale pests.
Introduction
The wheat bug, Nysius huttoni White 1878 (Hemiptera: Lygaeidae), is an endemic New Zealand pest (Eyles Citation1960b) and is widely distributed in both the North and South Islands (Myers Citation1926; Eyles Citation1960a; Eyles and Ashlock Citation1969). Three other Nysius species belonging to the family Lygaeidae (Hemiptera) are recorded in New Zealand. Nysius convexus Usinger 1942 and N. liliputanus Eyles and Ashlock Citation1969 are endemic, whereas N. caledoniae Distant 1920 is an adventive species from Australia (Gurr Citation1957; Eyles and Ashlock Citation1969). Nysius huttoni has a wide host range comprising almost all brassicas, other cultivated crops and a wide variety of weeds, but N. convexus and N. liliputanus are recorded only in moss habitats.
Kale (Brassica oleracea L.), rape (Brassica napus L. var. napus), turnip (Brassica campestris L.) and swede (Brassica napus L. var. napo-brassica) are widely grown brassica crops for animal production systems in New Zealand (PGG Citation2009; Speciality Seeds Citation2016). Forage brassicas are normally drilled in summer (November to December) in New Zealand (PGG Citation2009) at which time the bug’s populations are at peak levels (Wei Citation2001). In New Zealand, about 400,000 ha of brassicas are grown annually (Horrocks et al. Citation2018). Forage brassicas have a high feeding value for ruminants, grow rapidly and have a high dry matter content (Speciality Seeds Citation2016). The wheat bug is a threat to 4- to 6-week-old brassica seedlings and plant populations can be reduced as a result of feeding damage at the base of the plants (Eyles Citation1965), causing a cankerous growth of tissue that can kill them or make them susceptible to breakage from wind and stock movement (AgPest Citation2016). There has been up to 90% damage in brassica crops in severe situations (AgPest Citation2016; Speciality Seeds Citation2016).
Forage brassicas may require several insecticide sprays/season to prevent damage (PGG Citation2009). Management of this pest in New Zealand relies on seed treatment with neonicotinoids and spraying with chlorpyrifos and permethrin insecticides (Goldson et al. Citation2015; Young Citation2018). All these pesticides are broad spectrum in nature (AgPest Citation2016; Horrocks et al. Citation2018). Neonicotinoids are systemic and traces of them have been found in pollen and nectar, which impact pollinators including wild bees (Goulson et al. Citation2008; Pook and Gritcan Citation2017), bumble bees (Whitehorn et al. Citation2012) and many other insect pollinators (Godfray et al. Citation2014). Hence, these pesticides are under increasing environmental pressure in Europe and many other countries (Cressey Citation2017; Woodcock et al. Citation2018). Furthermore, concern exists about non-target effects on insect natural enemies (Goulson et al. Citation2008; Goulson Citation2013), as well as birds and many fish species (Gibbons et al. Citation2015). Hence, these pesticides need to be replaed by environmentally friendly and an agro-ecological pest management strategies.
There is increasing pressure from consumers, media and governments to reduce pesticide use, but no practical alternatives are currently being offered to manage the wheat bug in New Zealand. It is therefore necessary to develop a cost-effective and sustainable method of pest management. Encouraging farmers to use integrated pest management (IPM) strategies that combine biological, cultural and chemical approaches in a compatible way could be one strategy to reduce pest damage and pesticide use in forage brassicas (Horrocks et al. Citation2018). Pest resistant/tolerant cultivars are vital component of many IPM programmes. The present study was undertaken to screen kale cultivars on the basis of susceptibility to wheat bugs and the results could guide the development of a future IPM programme for kale.
Materials and methods
Wheat bug collection and identification
Adult wheat bugs were collected from the weed shepherd’s purse Capsella bursa-pastoris (L.) Medik. (Brassicaceae) from the Iversen Field Plant Science Research Unit (43° 38′ 50.4″ S, 172° 27′ 29.9″ E) at Lincoln University in the spring of 2016. The collected bugs were preserved in 1.7 ml microtubes (MCT-175-C) that contained 70% ethanol and delivered to a Hemiptera taxonomist (Dr Marie-Claude Larivière, Landcare Research, Auckland, New Zealand) for confirmation and preserved in the Lincoln University Entomology Research Museum. After the confirmation of the species, N. huttoni were collected from the same field (see above) and used in laboratory rearing.
Wheat bug cultures
A wheat bug breeding colony was established following methods based on those of Burgess and Weegar (Citation1986) and He and Wang (Citation2000). Field-collected wheat bugs were released inside a transparent rectangular plastic container (29 × 19 × 10 cm) that contained a mesh-covered lid for air circulation. Fifty mating pairs were transferred to individual 50 ml polypropylene centrifuge tubes (11.0 cm length × 2.0 cm diameter) using a fine-hair brush. Food for Nysius consisted of fruiting twin cress (Lepidium didymum L.) (Brassicaceae) and hulled organic sunflower seeds (Helianthus annuus L.) (Asteraceae) (BioGro organic certified) which were replaced daily. Males were removed from the pair when the female began to lay eggs on the moistened cotton dental roll (10 mm × 38 mm) that was also included (Yang and Wang Citation2004). The tubes were checked daily and freshly laid eggs were removed using the brush and transferred to Petri dishes (5 cm diameter). The newly emerged nymphs along with the cotton dental rolls were transferred to another Petri dish (14 cm diameter) in which partially moistened filter papers had been placed. The colony was maintained in a controlled temperature (CT) room at the Bio-Protection Research Centre (http://bioprotection.org.nz), Lincoln University, New Zealand. The ambient temperature, humidity and photoperiod were 23°C with a 4°C range, 65% relative humidity and 16L: 8D.
Plant selection and cultivation
The uncoated seeds of the six most commonly used kale cultivars () were obtained from PGG Wrightson (http://www.pggwrightsonseeds.com) and Speciality Seeds (http://www.specseed.co.nz/). Their growth habits are described in .
Table 1. Phenological characteristics of the six most popular kale cultivars in New Zealand (data from www.pggwrightsonseeds.com and www.specseed.co.nz).
The seedlings were grown in a glasshouse with a mean temperature of 22°C and relative humidity of 40%. Seeds were direct-seeded into pots containing a potting mix made by mixing 400 L composted bark, 100 L pumice (1.0–7.0 mm), 1500 g Osmocote (slow, 3- to 4-month release plant food), 500 g horticultural lime and 500 g HydraFLO (wetting agent, www.solutions4earth.com). The seedlings were 9 days old and approximately 3.5 cm high when used in the bioassays. Those grown in the glasshouse were transferred to a CT room with a temperature of 21°C with a 4°C range and a day length of 16 h.
Choice tests
Two seedlings of each kale cultivar were arranged in a circular fashion around the perimeter of a 23.0 cm diameter × 5.0 cm depth pot with cultivars arranged approximately 5.0 cm apart and 5.0 cm from the pot wall. Each kale cultivar with wheat bugs (treatment) being compared with a control with no bugs, so that two adjacent cylindrical sleeves (/pot) served as a block, with a total of ten blocks. Each cultivar was ‘marked’ on the outer wall of the choice pots. The pots were enclosed in cylindrical sleeves made of flexible transparent PVC sheets (1 mm thickness). The dimension (diameter x height) of the cylinders were 23.5 cm × 14 cm. The tops of the sleeves were covered with fine white mesh and Fluon® (BioQuip, fluoropolymer resin, PTFE-30) was used on the inner surface of the sleeves to prevent Nysius from climbing.
The study comprised a randomised complete block design (RCBD), with ten replicates. Twenty adult wheat bugs of the same age were starved for 12 h, then introduced into the centre of each cylinder. The times to first settlement (mins) and first obvious feeding damage on a seedling for each pot were recorded. Feeding damage was assessed by recording the presence or absence of girdling of the stem and/or discoloration of the leaf. These were the most common damage symptoms along with leaf distortion, twisted leaf veins and petiole, and finally collapse of the seedlings. Then the number of bugs on seedlings within each cylinder were counted at different time intervals following introduction (0.5, 1, 2, 4, 8 12, 24, 48, 72, 96, 120, 144, 168, 192 and 216 h). At the conclusion of the assay the survival rate of the bugs was assessed.
No-choice tests
Two seedlings of each cultivar were grown per 6.5 cm diameter × 5.0 cm depth pot, giving a total of six treatments. There were twenty replicates of each treatment, ten with wheat bugs and ten as controls. The pots were covered with 7 cm × 12 cm cylindrical sleeves constructed as above and seven bugs were introduced into the treatment cylinders. Treatments were randomised and assessed as above. At the completion of the assay, the dry weights of the seedlings including roots were measured and the percentage weight change calculated.
Statistical analysis
The mean numbers recorded in each cultivar at different time intervals were integrated over the 216 h period by the area under the curve (AUC) method (Hanley and McNeil Citation1983). Time data (mins) obtained from the experiments were first normalised by using the log10-transformation, and count data were normalised by using a square root transformation. The percentage reduction in plant dry weight (compared with the control) was not transformed. After normality checking, data were subjected to two-way (treatments and blocks) analysis of variance (ANOVA) and means were separated by unprotected least significance difference (LSD) at P < 0.05 (Saville Citation2015).
Results
Choice tests
For choice tests, settling time of the wheat bug on seedlings did not differ significantly between cultivars (). The time to first-feeding damage by the bugs across the kale cultivars varied significantly for the choice tests (). First-feeding damage occurred on Kestrel followed by Coleor, Sovereign and Regal, respectively, all of which were not significantly different from one another. However, feeding damage on Kestrel was significantly earlier than on Gruner and Corka.
Table 2. For the choice tests, mean time (Log10 transformed) required for settling and first-feeding damage on different kale cultivars (n = 10). Back-transformed means are given in brackets.
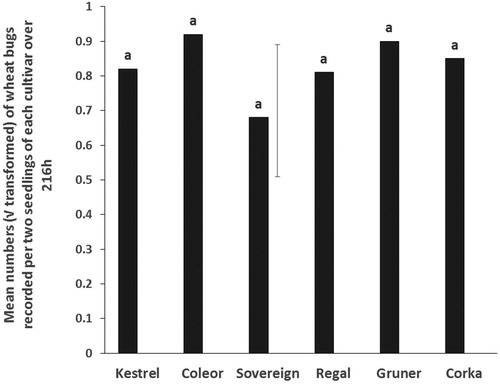
The number of wheat bugs on seedlings across kale cultivars over 216 h were not significantly different in choice tests (). However, the largest number of bugs was recorded on Coleor followed by Gruner with the lowest on Sovereign. The mean survival rate in the choice test was about 53%, averaging 10 wheat bugs/cylinder.
No-choice tests
Settling time of the wheat bug did not differ significantly between cultivars (). In no-choice tests, feeding damage was detected earliest on Kestrel followed by Corka, both of which were significantly more susceptible than Gruner, Sovereign and Regal. Coleor was the third earliest for feeding damage and differed significantly only from Kestrel ().
Table 3. For the no-choice tests, mean time (Log10 transformed) required for settling and first feeding damage on different kale cultivars (n = 10). Back-transformed means are given in brackets.
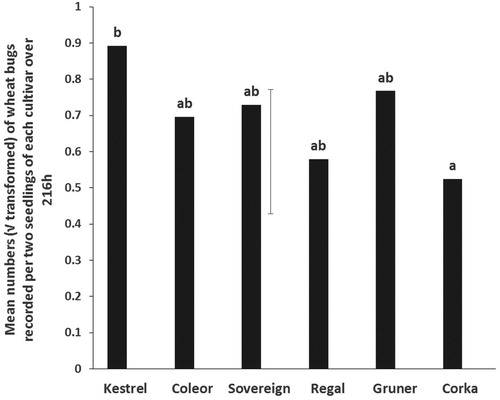
In the no-choice tests, the number of wheat bugs observed on Kestrel seedlings was significantly higher than on Corka but not significantly different from Gruner, Sovereign, Coleor or Regal (). Kestrel, Gruner, Sovereign, Regal and Coleor were not significantly different from each other.
Figure 2. No-choice tests. Mean numbers (√ transformed) of adult wheat bugs recorded in each of six kale cultivars over 216 h. Means with no letters in common are significantly different (Unprotected LSD; P < 0.05). The vertical bar is the least significant difference, LSD (5%) (n = 10).
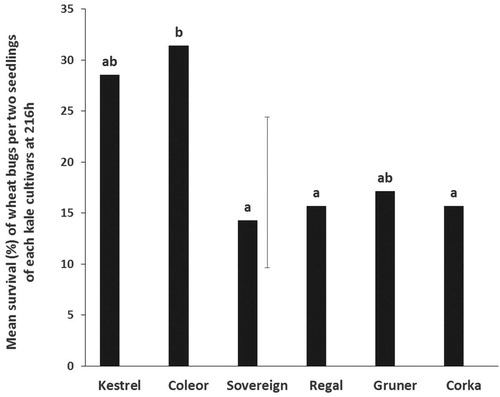
Survival rate was low, averaging between one and two bugs/cylinder. The highest survival rate occurred on Coleor, followed by Kestrel and Gruner, all of which were not significantly different from one another. Also, the survival on Kestrel, Gruner, Sovereign, Regal and Corka did not differ significantly. Furthermore, the survival rate on the latter three cultivars was significantly lower than on Coleor ().
Figure 3. No-choice tests. Mean survival (%) of N. huttoni adults on six kale cultivars at 216 h. Means with no letters in common are significantly different (Unprotected LSD; P < 0.05). The vertical bar is the least significant difference, LSD (5%) (n = 10).
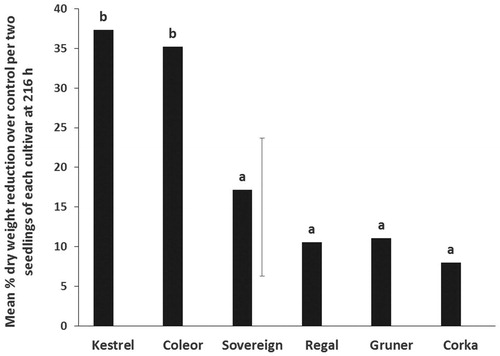
Seedling dry weight reduction by the bug, compared with controls was significantly higher in Kestrel and Coleor than on the other four cultivars. The lowest reduction was recorded on Corka which was not significantly different from that on Sovereign, Gruner and Regal, respectively ().
Discussion
Kale is an important forage crop for ruminants (cattle and sheep), being drilled during the summer for winter feeding in New Zealand (Speciality Seeds Citation2016). Damage from wheat bugs is obvious during the seedling stage of that crop (PGG Citation2009; AgPest Citation2016). The aim of this work was to examine the susceptibility of this bug on a range of commercial kale cultivars. The results confirmed that, in order of preference, wheat bugs favoured the kale cultivars Kestrel, Coleor and Gruner over Sovereign, Corka and Regal ( and , ). Significantly higher survival of the bug was recorded on Coleor and Kestrel than on Regal, Corka and Sovereign, respectively. The bug’s preference for Kestrel and Coleor could be partly caused by various cultivar characteristics such as digestibility, palatability, leaf to stem ratio, growth vigour, and concentrations of S-methyl cysteine sulphoxide (SMCO) compared with the other cultivars (PGG Citation2009). Damage to the bugs’ favoured cultivars can later lead to the death of the seedlings and further reduce their seedling number per unit area in brassica fields. Hence, this pest sometimes called a crop establishment pest (AgPest Citation2016). The cultivars growth rate in both choice and no-choice tests were similar. However, the first obvious damage was noticed on Kestrel in both choice and no-choice tests. This could be the result of the more prompt settling and higher numbers of N. huttoni on Kestrel. Damage was slowest to appear on Corka in choice tests, and on Regal in no-choice tests. However, high mortality (70%–80%) of the bugs was recorded on all the cultivars, perhaps due to the limited availability of food in these experiments (Wei Citation2001). The greatest reduction in plant dry weight occurred on Kestrel and Coleor. Higher numbers of bugs settled over time with a high survival rate on these cultivars (). Gruner was the medium category of cultivar in terms of preference by the bugs ( and ). Although these cultivar rankings imply that Kestrel and Coleor could be avoided by growers, other more important agronomic factors such as yield and diseases resistance can be the main criteria for cultivar selection. For example, past studies on forage brassicas have mostly focused on varietal screening for resistance to clubroot disease and other aspects of varietal improvements (Asrat et al. Citation2010; Bradshaw and Wilson Citation2012) but not resistance to the wheat bug. However, there is evidence that disease resistance in brassicas may be negatively correlated with insect resistance (Rostás and Hilker Citation2002).
The bugs’ preference for some cultivars may be affected by cues comprising volatile plant chemicals or by visual cues (Finch and Collier Citation2000). Among plant chemicals, glucosinolates have been widely studied in crucifers and they can have feeding deterrent or stimulatory properties on generalist or specialist insects, respectively (Renwick Citation2002). Further, the variation in glucosinolate profile between cultivars can also affect the host-plant preference (Poelman et al. Citation2009). However, the chemical basis of resistance to wheat bugs on forage brassicas is not known.
Global agriculture is beginning to adopt ‘sustainable intensification’ approaches (Pretty et al. Citation2018). Reasons for this include insecticide resistance, along with a decline in the rate at which new insecticide molecules are developed (Nauen and Denholm Citation2005; Hawkins et al. Citation2018). There is also increasing consumer resistance to pesticides in some markets (Wollaeger et al. Citation2015).
An IPM strategy developed with farmer input has been suggested to reduce reliance on insecticides (Warner Citation2007; Horrocks et al. Citation2018). While the results in this study show cultivar differences in wheat bug susceptibility, further research is needed to investigate if the best cultivars in this study are also less susceptible to other potential insect pests of kale crops such as aphids, beetles and caterpillars. In the future, insecticide use in kale crops, and the potential environmental impacts, could be minimised by incorporating less susceptible cultivars, such as Corka or Regal, into an integrated pest management programme with other management tools such as biological control and the use of ‘soft’ chemicals (Dent Citation2000; Horrocks et al. Citation2018).
Acknowledgements
We are grateful to the New Zealand Ministry of Foreign Affairs and Trade (MFAT), Lincoln University, the Bio-Protection Research Centre and the Agriculture and Forestry University (AFU), Nepal, for their financial, technical and logistical help with this PhD study. Special thanks to Janine Johnson for editorial assistance and Dr Marie-Claude Larivière for help with taxonomic identification of N. huttoni. We also thank Specialty Seeds, and PGG Wrightson (Kimihia Research Centre) for providing seeds for the experiments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AgPest. 2016. Nysius-wheat bug. New Zealand: AgResearch; [accessed 2016 Mar 5]. http://agpest.co.nz/?pesttypes=nysius-wheat-bug.
- Asrat S, Yesuf M, Carlsson F, Wale E. 2010. Farmers’ preferences for crop variety traits: lessons for on-farm conservation and technology adoption. Ecological Economics. 69(12):2394–2401. doi:10.1016/j.ecolecon.2010.07.006.
- Bradshaw JE, Wilson RN. 2012. Kale population improvement and cultivar production. Euphytica. 184(2):275–288. doi:10.1007/s10681-011-0612-x.
- Burgess L, Weegar HH. 1986. A method for rearing Nysius ericae (Hemiptera: Lygaeidae), the false chinch bug. The Canadian Entomologist. 118(10):1059–1061. doi:10.4039/Ent1181059-10.
- Cressey D. 2017. The bitter battle over the world’s most popular insecticides. Nature. 551(7679):156–158.
- Dent D. 2000. Insect pest management. Wallingford, UK: CABI Publishing; p. 299.
- Eyles AC. 1960a. Insects associated with the major fodder crops in the North Island. New Zealand Journal of Agricultural Research. 3(6):994–1008. doi:10.1080/00288233.1960.10419310.
- Eyles AC. 1960b. Variation in the adult and immature stages of Nysius huttoni White (Hemiptera: Lygaeidae) with a note on the validity of the genus Brachynysius Usinger. Transactions of the Royal Entomological Society of London. 112(4):53–72. doi:10.1111/j.1365-2311.1960.tb00494.x.
- Eyles AC. 1965. Damage to cultivated cruciferae by Nysius huttoni white (Heteroptera: Lygaeidae). New Zealand Journal of Agricultural Research. 8(2):363–366. doi:10.1080/00288233.1965.10422367.
- Eyles AC, Ashlock PD. 1969. The genus Nysius in New Zealand (Heteroptera: Lygaeidae). New Zealand Journal of Science. 12(4):713–727.
- Finch S, Collier RH. 2000. Host-plant selection by insects: a theory based on ‘appropriate/inappropriate landings’ by pest insects of cruciferous plants. Entomologia Experimentalis et Applicata. 96(2):91–102. doi:10.1046/j.1570-7458.2000.00684.x.
- Gibbons D, Morrissey C, Mineau P. 2015. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environmental Science and Pollution Research. 22(1):103–118. doi:10.1007/s11356-014-3180-5.
- Godfray HCJ, Blacquière T, Field LM, Hails RS, Petrokofsky G, Potts SG, Raine NE, Vanbergen AJ, McLean AR. 2014. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proceedings of the Royal Society B: Biological Sciences. 281(1786).
- Goldson SL, Bourdôt GW, Brockerhoff EG, Byrom AE, Clout MN, McGlone MS, Nelson WA, Popay AJ, Suckling DM, Templeton MD. 2015. New Zealand pest management: current and future challenges. Journal of the Royal Society of New Zealand. 45(1):31–58. doi:10.1080/03036758.2014.1000343.
- Goulson D. 2013. An overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology. 50(4):977–987.
- Goulson D, Lye GC, Darvill B. 2008. Decline and conservation of bumble bees. Annual Review of Entomology. 53:191–208.
- Gurr L. 1957. Observations on the distribution, life history, and economic importance of Nysius huttoni (Lygaeidae: Hemiptera). New Zealand Journal of Scienceand Technology. 38:710–714.
- Hanley JA, McNeil BJ. 1983. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 148(3):839–843.
- Hawkins NJ, Bass C, Dixon A, Neve P. 2018. The evolutionary origins of pesticide resistance. Biological Reviews. 1–21.
- He XZ, Wang Q. 2000. Oviposition and feeding behaviour of Nysius huttoni White (Heteroptera: Lygaeidae). New Zealand Entomologist. 23(1):71–76. doi:10.1080/00779962.2000.9722070.
- Horrocks A, Horne PA, Davidson MM. 2018. Demonstrating an integrated pest management strategy in forage-and seed-brassica crops using a collaborative approach. New Zealand Plant Protection. 71:112–120.
- Myers JG. 1926. Biological notes on New Zealand Heteroptera. Proceedings of The Royal Society of New Zealand. 56:449–511.
- Nauen R, Denholm I. 2005. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Archives of Insect Biochemistry and Physiology. 58(4):200–215. doi:10.1002/arch.20043.
- PGG. 2009. Forage focus; [accessed 2017 August 22]. https://www.seedtreatment.co.nz/insect-pests-and-diseases/wheat-bug.html.
- Poelman EH, Dam NM, Loon JJA, Vet LEM, Dicke M. 2009. Chemical diversity in Brassica oleracea affects the biodiversity of insect herbivores. Ecology. 90(7):1863–1877. doi:10.1890/08-0977.1.
- Pook C, Gritcan I. 2017. Neonicotinoid insecticide residues in New Zealand maize paddock soil. PeerJ Preprints. 5:e2919v2911.
- Pretty J, Benton TG, Bharucha ZP, Dicks LV, Flora CB, Godfray HCJ, Goulson D, Hartley S, Lampkin N, Morris C, et al. 2018. Global assessment of agricultural system redesign for sustainable intensification. Nature Sustainability. 1(8):441–446. doi:10.1038/s41893-018-0114-0.
- Renwick JAA. 2002. The chemical world of crucivores: lures, treats and traps. Entomologia Experimentalis et Applicata. 104(1):35–42.
- Rostás M, Hilker M. 2002. Asymmetric plant-mediated cross-effects between a herbivorous insect and a phytopathogenic fungus. Agricultural and Forest Entomology. 4(3):223–231. doi:10.1046/j.1461-9563.2002.00147.x.
- Saville DJ. 2015. Multiple comparison procedures-cutting the Gordian knot. Agronomy Journal. 107(2):730–735. doi:10.2134/agronj2012.0394.
- Speciality Seeds. 2016. Pasture pests: wheat bug (Nysius huttoni). New Zealand: Specialty Seeds; [accessed 2016 May 5]. http://specseed.co.nz/downloads/Nysius-PasturePests-SpecialtySeedsNZ.pdf.
- Warner KD. 2007. Agroecology in action. Extending alternative agriculture through social networks. Cambridge: The MIT Press, Massachussetts Institute of Technology; p. 273.
- Wei YJ. 2001. Nysius huttoni (Hemiptera: Lygaeidae): life history and some aspects of its biology and ecology in relation to wing development and flight [PhD thesis]. Christchurch: University of Canterbury.
- Whitehorn PR, O’Connor S, Wackers FL, Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science. 336(6079):351–352. doi:10.1126/science.1215025.
- Wollaeger HM, Getter KL, Behe BK. 2015. Consumer preferences for traditional, neonicotinoid-free, bee-friendly, or biological control pest management practices on floriculture crops. HortScience. 50(5):721–732.
- Woodcock BA, Ridding L, Freeman SN, Pereira MG, Sleep D, Redhead J, Aston D, Carreck NL, Shore RF, Bullock JM, et al. 2018. Neonicotinoid residues in UK honey despite European Union moratorium. PLOS ONE. 13(1):e0189681. doi:10.1371/journal.pone.0189681.
- Yang L, Wang Q. 2004. Precopulation sexual selection in Nysius huttoni White (Heteroptera: Lygaeidae) in relation to morphometric traits. Journal of Insect Behavior. 17(5):695–707.
- Young S. 2018. New Zealand Novachem agrichemical manual. Christchurch, New Zealand: Agrimedia Ltd; p. 912.