?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In vitro germination tests were performed at 11 constant temperatures (range 2.5–35°C) to quantify the maximum germination percentage and germination rate of four subterranean clover cultivars (‘Antas’, ‘Denmark’, ‘Monti’ and ‘Narrikup’). These were used to estimate their cardinal temperatures, base (Tb), optimum (Topt) and maximum (Tmax) and the thermal time requirements for seed germination. Germination percentages were ≥ 88% in the 5–25°C temperature range for all cultivars. The time to reach 50% of final germination (T50) decreased from 12 to 2 days as the temperature increased from 2.5 to 17.5°C. The determined Topt were 16°C for ‘Monti’ and ‘Narrikup’ and 22°C for ‘Antas’ and ‘Denmark’. At a common Tb of 0°C the thermal time requirements for T50 were 32 ± 1°Cd for ‘Antas’ and ‘Denmark’ and 39 ± 1°Cd for ‘Narrikup’ and ‘Monti’. ‘Antas’ and ‘Denmark’ (both from Italy) germinated at the widest range of temperatures.
HANDLING EDITOR:
Introduction
The successful establishment and subsequent persistence of subterranean clover (Trifolium subterraneum L.) depends on germination of seeds which normally occurs during the autumn months. Provided moisture is available, temperature is the main factor which controls the percentage and germination rate of seeds. Seed germination can be quantified in relation to the accumulation of thermal time (∑°Cd). Simplified thermal time functions assume a linear heat accumulation above a constant specific base temperature (Tb). Improved functions attempt to incorporate the optimum (Topt) and maximum (Tmax) temperatures to reflect the parabolic enzymatic activity (Hay and Porter Citation2006; Elias et al. Citation2014) and tissue specific activation (Mittler et al. Citation2011) in response to temperature. Each plant phenological stage (germination, emergence and flowering) has a thermal time requirement which may differ among species and cultivars within a species (McMaster and Wilhelm Citation1997; Andreucci et al. Citation2012).
The quantification of cardinal temperatures and thermal time requirements for germination of five sub clover cultivars of the subterraneum subspecies (‘Dalkeith’, ‘Leura’, ‘Mt. Barker’, ‘Woogenellup’, ‘Tallarook’) has been published (Moot et al. Citation2000; Lonati et al. Citation2009; Monks et al. Citation2009). In these experiments the optimum germination temperature differed among cultivars and ranged from 13°C (‘Mt Barker’) to 20°C (‘Leura’). ‘Woogenellup’ had a higher optimum germination temperature (Topt 26°C) than ‘Mt Barker’ (Lonati et al. Citation2009). For ‘Mt. Barker’ the seed germination was only 54% at 24°C. The thermal time (Tt) requirement for germination of ‘Dalkeith’ was 27oCd while for ‘Woogenellup’ it was almost double (52°Cd) (Monks et al. Citation2009). In these experiments, the lowest temperature used was 5°C. As a winter annual, it is expected that development of sub clover could occur in temperatures ≤ 4°C (Mitchel and Lucanus Citation1960; Young et al. Citation1970; Baresel et al. Citation2018). These differences in temperature responses among cultivars have ecological and management implications. Once cardinal temperatures and accumulated Tt requirements have been defined, the information can be transferred across sites and seasons, matching the weather data, to support the selection of subspecies or cultivars most suitable for sowing (Boswell et al. Citation2003). For other annual clovers, it has been demonstrated that ‘Prima’ gland clover is suitable for temperate conditions or cooler condition in contrast to ‘Mihi’ Persian clover, which had the ability to germinate at temperature as high as 40°C (Nori et al. Citation2014). This study quantifies the cardinal temperatures and Tt requirements for germination of four sub clover cultivars that have been recently promoted for inclusion in New Zealand pastoral systems. One is from the yanninicum (i.e. ‘Monti’) and one is from the brachycalycinum subspecies (i.e. ‘Antas’), as well as two cultivars (‘Denmark’ and ‘Narrikup’) from the subterraneum subspecies.
Materials and methods
Seeds of four cultivars ‘Antas’, ‘Denmark’, ‘Monti’ and ‘Narrikup’ () were germinated at 11 constant temperatures (2.5, 5.0, 7.5, 10.0, 12.5, 15.0, 17.5, 20.0, 25.0, 30.0 and 35.0°C) in unlit incubators (Sanyo MIR 152, Sanyo Electric Co., Japan, at Field Research Centre, Lincoln University facilities). The cultivars, subspecies, seed lot and previously reported mean seed weight and year of commercialisation (Nichols et al. Citation2013) are presented in .
Table 1. Subterranean clover cultivars, subspecies, seed lot and reported mean seed weight (mg) and commercialisation year of seeds used. Letters B, S and Y denote subspecies: B = brachycalycinum, S = subterraneum and Y = for yanninicum. (Nichols* et al. Citation2013).
Fifty scarified bare seeds were placed on filter paper, soaked with distilled water, in each of four Petri dishes per cultivar for incubation at each temperature. Temperatures were recorded every 30 minutes with a HOBO data logger (Onset Computer Corporation, Bourne, Massachusetts, USA). The mean temperature reached in each incubator at the 2.5–10°C range was 1.3°C lower than the set temperatures. Therefore, actual recorded temperatures were used for all calculations. The seeds were assessed daily and germinated seeds removed from the Petri dishes, which were then re-randomised within the incubator. Distilled water was added as required to ensure seed imbibition (ISTA Citation2017). Seeds were considered germinated when the radicle length exceeded the small diameter of the seed (Monks et al. Citation2009). The number of germinated seeds was used to estimate the cumulative germination over time (days), the percentage maximum germination and the number of days to 50% of the final germination (T50).
Cumulative germination
The cumulative percentage germination was plotted against days using a linear interpolation function between measurements adjacent to T50 (Approx) and a logistic curve function (Gompertz). The linear interpolation function used was ‘Approx.’ from R version 3.5.1, which estimates the number of days to reach T50 (Singh Citation2012; R Core team Citation2017). The generic linear function (Equation 1) Approx. uses the observed values and returns the components x and y containing n coordinates which interpolate the given data points (Soetaert et al. 2012). The rationale was to identify the reliability of using the simpler linear interpolation (Approx) compared to the traditional Gompertz curve used in previous germination studies (Black et al. Citation2002; Monks et al. Citation2009; Andreucci et al. Citation2012). The motivation to apply the Approx function was that R software is more accessible and faster when compared with other software.(1)
(1)
T50 = number of days to 50% of the final germination; GC50: the cumulative percentage of seeds germinated equals 50; ya: the observed cumulative percentage of seeds germinated <50; yb: the observed cumulative percentage of seeds germinated >50; xa: the number of days at ya < 50; xb: the number of days at yb > 50; T50 = number of days to 50% of the final germination.
The Gompertz function used for comparison with Approx three parameters (Andreucci et al. Citation2012; Nori et al. Citation2014; Equation 2 and Equation 3) and was fitted using the global curve fitting option in Sigmaplot 13th version (Gama-Arachchige et al. Citation2013).(2)
(2)
CG: the cumulative percentage of seeds germinated; t: time (number of days); C: is the final germination percentage; M: is a time scale (lag related) constant; B: is the rate of increase.
T50 was calculated using Equation 3 derived from the Gompertz function where CG = 50:(3)
(3)
M: is a time scale (lag related) constant; B: is the rate of increase.
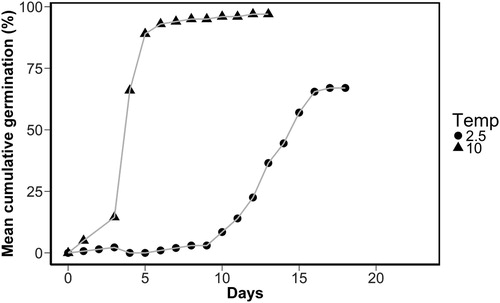
The equivalent of time to T50 is the germination or development rate (1/days to T50) (Moot et al. Citation2000) and the data for each cultivar were plotted against the temperature. displays an example of the cumulative response for seeds of ‘Narrikup’ incubated at 10°C and 2.5°C.
Figure 1. Cumulative germination percentage for ‘Narrikup’ subterranean clover seeds incubated at 10°C (▴) and 2.5°C (•).
There was a strong (r = 0.99) correlation between the estimated values obtained with Gompertz and Approx functions (). There was no difference (P = 0.40) between the parameter values produced by the two methods. Therefore, the Approx function from the R package was selected to calculate the number of days and the thermal time requirements (°Cd) for the sub clover germination. The germination rate (1/days to T50) and the maximum germination (%) were plotted against the mean actual temperature (°C).
Figure 2. Number of days to T50 for ‘Antas’, ‘Denmark’, ‘Monti’ and ‘Narrikup’ subterranean clover seeds estimated by Gompertz and Approx functions (r = 0.99) for individual experimental units at 2.5 (□), 5 (○), 7.5(△), 10 (+) 12.5 (X), 17.5 (▽), 20 (![]()
 ) and 35°C (
) and 35°C (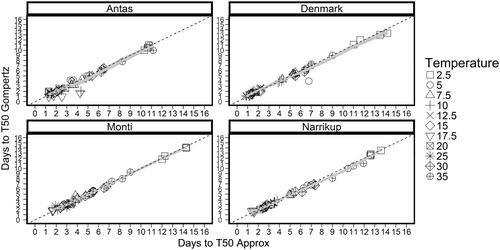
Estimates of cardinal temperatures
The calculations for the cardinal temperatures and thermal time requirements followed previously published the procedures (Angus et al. Citation1981; Moot et al. Citation2000; Lonati et al. Citation2009; Andreucci et al. Citation2012). Segmented linear regressions (ascending and descending) were used to determine the cardinal temperatures (°C): base (Tb), optimum (Topt) and maximum (Tmax). This bilinear-shaped response model of germination rate to temperature is considered reliable for determination of the cardinal temperatures when compared with non-linear functions (Nori et al. Citation2014; Parmoon et al. Citation2015; Andreucci et al. Citation2016). The germination rate data points which deviated from the linear model at high temperatures were excluded as these were outside the optimum cultivar range (Angus et al. Citation1981). The coefficients (slope and intercept), their standard errors and coefficient of determination (R2, %) for the linear regressions between germination rate and the various temperatures for subterranean clover cultivars are presented.
Thermal time for T50
For each cultivar the thermal time requirements for T50 at sub-optimal temperatures (Ttsub) and supra-optimal temperatures (Ttsup) were calculated using the equations previously applied for clover and other species. The estimated cardinal temperatures were used to calculate the daily Tt for T50 for each cultivar based on the bilinear functions (Angus et al. Citation1981; Lonati et al. Citation2009; Andreucci et al. Citation2012; Nori et al. Citation2014). Thermal time was also calculated with Tb = 0°C, by forcing the equation intercepts to zero, to allow comparisons among cultivars (Moot et al. Citation2000; Nori et al. Citation2014).
Treatment effects on final germination percentage were analysed using an analysis of variance (ANOVA) with means separated by least significant difference (α = 0.05) with R software version 3.5.1. (R Core team Citation2017). Standard error of the means (SE) is also reported.
Results
Cumulative and maximum seed germination
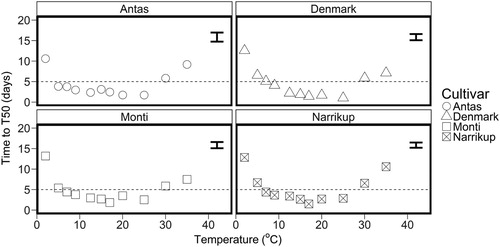
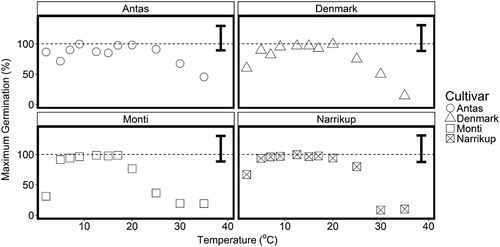
For all cultivars, the maximum final germination percentage was below 50% at 35°C.’Monti’ had a narrower germination temperature range than the other cultivars, as indicated by lower maximum germination % values for 2.5°C and 25°C ().
Figure 3. Mean maximum germination percentage for ‘Antas’ (○), ‘Denmark’ (△), ‘Monti’(□) and ‘Narrikup’ (![]()
The cumulative germination percentage over time (days) for each cultivar at various temperatures is shown in .
Figure 4. Mean cumulative germination percentage (%) for subterranean clover cultivars ‘Antas’, ‘Denmark’, ‘Monti’ and ‘Narrikup’ at different incubation temperatures 2.5 (□), 5 (○), 7.5(△), 10 (+) 12.5 (X), 17.5 (▽), 20 (![]()
 ) and 35°C (⊕). Error bars are the maximum standard error of the mean for final germination percentage.
) and 35°C (⊕). Error bars are the maximum standard error of the mean for final germination percentage.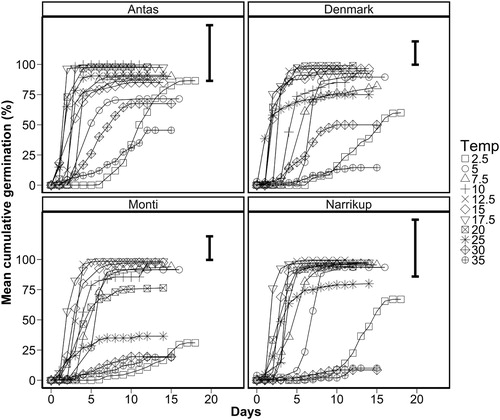
The data for mean maximum seed germination percentage showed a cultivar x temperature interaction (P < 0.02). The highest germination percentages were above 88% in the 5–25°C range for all cultivars (). At the lowest temperature tested (set as 2.5°C) the maximum germination was only 31% for ‘Monti’, which was lower (P < 0.01) than the 64% for ‘Denmark’ and ‘Narrikup’ and 87% for ‘Antas’. At 30°C and 35°C the overall mean maximum germination decreased to 29%. The mean maximum germination was reduced (P < 0.001) to 10% for ‘Narrikup’ while ‘Antas’ had ∼57% germination.
Time to T50
The number of days to reach T50 decreased from 12 ± 1 to 1.8 ± 0.3 as temperature increased from 2.5 to 17.5°C for all four cultivars (). Between 20 and 25°C the days to reach T50 remained constant (overall mean of 2.2 ± 0.4). For all cultivars at temperatures 30 and 35°C the number of days to T50 were 6 ± 0.4 and 9 ± 1, respectively.
Temperature and thermal time
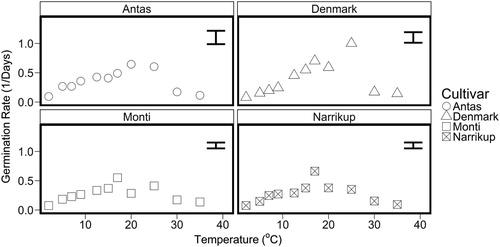
For all cultivars the germination rate increased from the minimum temperature up to the optimum temperature (Topt). There was then a decline in germination rate, indicated by the negative linear function until no germination occurred at an extrapolated maximum temperature (Tmax) ().
Figure 6. Germination rate (1/ days to T50) for ‘Antas’ (○), ‘Denmark’ (△), ‘Monti’ (□) and ‘Narrikup’(![]()
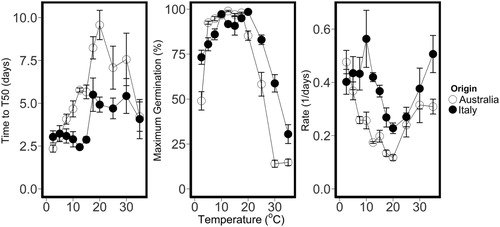
The number of days to reach T50 was affected (P = 0.0002) by the origin of the cultivars (). The seeds from ‘Antas’ and ‘Denmark’, cultivars that originated in Sardinia (Italy) reached T50 after 3.8 days while seeds from the Australian cultivars (‘Monti’ and ‘Narrikup’) required 5.7 days to reach T50. The seeds of Italian origin had a higher maximum germination percentage at 25, 30 and 35°C. At 2.5°C the Italian seeds had a maximum germination percentage of 73% while the Australian seeds had 50%. The rate of germination for the Sardinian lines was (0.39/day) was higher (P < 0.0001) than the germination rate (0.26/day) of seeds of Australian origin.
Figure 7. Mean number of days to T50, maximum germination percentage (%) and germination rate (1/days) for subterranean clover cultivars ‘Monti’ (yanninicum) ‘Narrikup’ (subterraneum) originated in Australia (○) and Antas’ (brachycalycinum) and ‘Denmark’ (subterraneum) originated in Italy (•) tested at Field Research Centre, Lincoln University, New Zealand. Time to T50: P = 0.0002, LSD = 0.9; maximum germination (%):P = 0.08, LSD = 9.0; Rate (1/days to T50): P < 0.001, LSD = 0.06. Error bars represent the standard error.
The estimated cardinal temperatures are shown in and the linear regressions between germination rate and temperatures displayed in . The estimated base temperature ranged from −4.5°C (‘Antas) to 1.6°C (‘Denmark’).
Table 2. Mean base (Tb), optimum (Topt) and maximum (Tmax) temperatures (°C) for 50% of germination estimated for sub clover seeds of ‘Antas’, ‘Denmark’, ‘Monti’ and ‘Narrikup’. SE = standard error. Ci = confidence interval.
Table 3. Coefficients and their standard errors and coefficient of determination (R2, %) for the linear regressions between germination rate (Y- axis, 1/ days to T50) and temperatures (X- axis, °C) for subterranean clover cultivars.
Topt was 16.0 ± 0.8°C for ‘Monti’ and ‘Narrikup’ in contrast to 22 ± 0.8°C for ‘Antas’ and ‘Denmark’ (). Cultivars differed (P < 0.001) in the thermal time requirements when they were estimated using the cultivar specific Tb (). ‘Monti’ and ‘Narrikup’ had a higher (P = 0.001) thermal time requirement in supra optimal temperatures (Ttsup, 48°Cd) than Antas’ and ‘Denmark’ (Ttsup, 35°Cd). The 95% confidence interval for Tb included 0°C (), thus it is possible to calculate the thermal time using a Tb of 0°C to allow cultivar comparison (Moot et al. Citation2000; Nori et al. Citation2014). When Tb = 0 was set the differences among cultivars were not significant (P = 0.06) and the mean thermal time required for T50 was 36°Cd.
Table 4. Mean sub (Ttsub, from Tb to Topt) and supra (Ttsup, Topt to Tmax) optimal thermal time requirements (°Cd) for T50 estimated for sub clover seeds of ‘Antas’, ‘Denmark’, ‘Monti’ and ‘Narrikup’. Values in brackets (± SE) are the mean standard error.
Discussion
Germination of sub clover seeds was maximised (>80%) over a broad (5–20°C) temperature range which is aligned with previous work with other cool season annual legumes (Butler et al. Citation2014; Sharifiamina et al. Citation2017). The data suggest that 90% of maximum metabolic rate is reached by 12.5°C and little further rate increase occurs from 12.5°C to 25°C. The maximum germination and germination rate declined above 25°C. ‘Monti’ had ∼ 53% of maximum germination at this supra optimal temperature (25°C) similar to results obtained with ‘Mt. Barker’ (Lonati et al. Citation2009). Among the cultivars, ‘Antas’ had its maximum germination (>80%) across the widest range of temperatures. This brachychalycinum line originated from Sardinia (Nichols et al. Citation2013) where temperatures can fluctuate between a high of 26°C and a low of 9°C in autumn (SardegnaARPA Citation2018). ‘Antas’ seeds had >80% germination at temperatures near 2°C which indicates this cultivar could be more adapted to cool environments, south facing slopes and a wide spread emergence period. In contrast, ‘Narrikup’ and ‘Denmark’ (subterraneum) germination was >65% at 2°C, while germination of ‘Monti’ was < 50%.
Adaptability is a desirable feature for commercial cultivars to maximise germination over a range of temperatures and ensure successful establishment compared with a subspecies with a narrower optimum temperature range. The high germination of ‘Antas’ seeds at 2°C indicates that in their population, there was a proportion of seeds able to germinate outside the optimum temperature range. Ecologically, this represents an adaptive strategy for the survival of this annual species under non optimum soil temperature conditions (Butler et al. Citation2014). For ‘Antas’ this advantage could be related to the large seed mass (>9 mg/seed) () associated with its geographic origin. For legumes, a positive relationship between seed size and the ability to germinate has been reported. Schaal (Citation1980) showed that the percentage germination was 42% for light lupin (Lupinus texensis) seeds weighing 17 mg, while heavy seeds (>40 mg) had 98% germination. Berger et al. (Citation2017) considered the large seeds of Lupinus albus as a strategic trait which allowed this legume to adapt to a range of environments and increased its geographical distribution compared with other lupins. Maranon and Grubb (Citation1993) stated that large seeded species/cultivars are associated with success under resource-rich conditions, which this germination experiment represents. However, it is not possible to generalise that subterranean clover seeds with larger size always have higher percentages of germination, since the species of Fabaceae do not always fit this pattern (Valencia-Díaz et al. Citation2015). As observed in this experiment, the large seeded ‘Monti’ (9.8 mg) did not have a higher maximum germination and faster rate than the seeds of the other three cultivars.
‘Denmark’ (also originating from Sardinia, Italy) had the second broadest optimum range with reduced germination (∼60%) only at 2.5 and 30°C. Ghamkar et al. (Citation2014) previously reported that subterraneum and brachycalycinum subspecies are found in a more extensive range of temperatures (3.7 − 27°C) than the yannicums. In the current in vitro experiment, ‘Monti’ and ‘Narrikup’ had their germination percentage and germination rates reduced to <40% and 0.3 (1/days) respectively at 30 and 35°C, consistent with other annual legumes (Butler et al. Citation2014; Nori et al. Citation2014). In contrast, Sharifiamina et al. (Citation2017) observed that at 35°C the germination percentage was >80% for Australian bred ‘Napier’-yanninicum seeds (Nichols et al. Citation2013). These contrasting findings may occur because some subterranean clover seeds can avert germination when temperatures are beyond their physiological optimum (>30°C) through thermo-inhibition (Baskin and Baskin Citation2004). This could be the case for ‘Denmark’, ‘Monti’ and ‘Narrikup’. High temperatures denaturate enzymes (Elias et al. Citation2014) and hinder synthesis of gibberellic acid (GA) which is necessary for germination inducing the biosynthesis of abscisic acid (ABA), a germination inhibitor (Bahuguna and Jagadish Citation2015).
The cardinal temperatures estimated for ‘Denmark’ and ‘Narrikup’ agreed with the values previously reported for other subterraneum cultivars ().
Table 5. Mean base (Tb), optimum (Topt) and maximum (Tmax) temperatures (oC) estimated for germination for sub clover cultivars in New Zealand.
In previous publications the lowest temperature used for germination tests was 5°C. The rationale to use 5°C was that at this low temperature the enzymatic activity would be insufficient to begin and complete the germination process. The seeds would take a long time to germinate or eventually rot and die (Nori et al. Citation2014). In the present study the maximum germination of subterranean clover seeds was 86% and the rate was ∼ 0.3 1/days T50 at 5°C. The capacity of sub clovers to withstand lower temperatures could be associated with the subspecies or a cultivar effect. A study with Arabidopsis thaliana, for example, demonstrated that low temperatures (4°C) during the imbibition phase stimulated germination due to an increase of seed bioactive GA levels in mutant lines with the gene AtGA3ox1. The same bioactivity was not observed in wild types without gene AtGA3ox1 (Yamauchi et al. Citation2004). For pea seeds Macherel et al. (Citation2007) reported that germination at 0°C was possible, as seed cell mitochondria were activate at temperatures as extreme as −3°C to 40°C. In this case the authors stated that the lipid composition and the accumulation of stress proteins conferred the rare temperature tolerance during the germination process. It is probable that for sub clover cultivars similar mechanisms would regulate the germination, especially at low temperatures (i.e. ‘Antas’). These inferences require further investigation using a larger range of cultivars from the three subspecies.
Differences among the cultivars were found for Topt and Tmax. For ‘Denmark’ a Topt of 24°C was determined, which is similar to the reported Topt for ‘Woogenellup’ (26°C, ). The Topt for ‘Narrikup’ however, was almost 10°C lower (16°C), and similar to the Topt found for ‘Mount Barker’ (Lonati et al. Citation2009) and gland clover ‘Prima’(T. glanduliferum Boiss) (Nori et al. Citation2014). Knowledge of these values can assist in the selection of cultivar and species compatibility in a multi-species pasture seed mix (Lonati et al. Citation2009) because not all temperatures will contribute equally to the rate of germination (Boswell et al. Citation2003).
For example, if sowing a seed mix of ‘Denmark’ and ‘Narrikup’ at a mean soil temperature of 16°C, ‘Narrikup’ would have a germination advantage over ‘Denmark’ which could impact on the final plant stand. Subterranean clover cultivar mixtures have been recommended based on other genotype traits (Lucas et al. Citation2015; Teixeira et al. Citation2017) but the extent to which differences in cardinal temperatures and thermal time requirements would impact the field establishment of different cultivars in a mixture needs further investigation.
The cardinal temperatures also permit estimates of a transferable coefficient for thermal time accumulation in sub and supra optimal temperatures. We observed that for the Italian lines ‘Antas’ and ‘Denmark’ the mean Ttsup was 17°Cd, which contrasted with the Ttsup of 43°Cd for the Australian cultivars ‘Monti’ and ‘Narrikup’. These differences in cardinal temperature parameters and thermal time requirements for T50 reflect the diverse climatic and ecogeographic regions of origin, more than the phylogenetic association among the sub clover subspecies (Kigel et al. Citation2015).
The use of the bilinear regressions with Tb set at 0°C (Nori et al. Citation2014; Andreucci et al. Citation2016), Topt set to 18°C and Tmax set to 36°C permits direct comparison of the Tt requirements among the different sub clover cultivars and subspecies. With the regression analysis that had Tb fixed to zero, the mean thermal time to T50 was 36°Cd, slightly above that of 31°Cd found for ‘Mount Barker’ and for ‘Tallarook’ (Moot et al. Citation2000) and 28°Cd reported by Monks et al. (Citation2009) for ‘Dalkeith’.
In New Zealand the common sowing time and voluntary re-seedling of sub clover occur in late summer and autumn following sufficient rain (Moot et al. Citation2003). During this period (February – June in New Zealand) mean soil temperatures oscillate between 5 and 22°C and rarely surpass 30°C (NIWA Citation2017), corresponding to the optimum range for sub clover seed germination of most of these studied cultivars. Our results showed that ‘Antas’ and ‘Denmark’, which originated in Sardinia (Italy), germinated at the widest range of temperatures. The adaptive strategy of these cultivars maximises the germination over a range of temperatures experienced in the field, and would also, increase the survival of these cultivars under non-optimum soil temperature conditions.
Conclusions
In New Zealand, late summer and autumn temperatures (range of 5−22°C) appear ideal for germination of sub clover seeds. Germination of sub clover seeds was between 80 and 100% at temperatures between 5 and 20°C. The use of a linear regressions allowed calculations of the cardinal temperatures and thermal time requirements for germination of subterranean clovers ‘Antas’, ‘Denmark’, ‘Narrikup’ and ‘Monti’. The thermal time requirement for 50% germination was ∼36°Cd, with optimum temperature estimated to be 20°C. The effect of temperature on germination showed that ‘Antas’ seeds can germinate under low temperature 2.5°C. ‘Antas’ and ‘Denmark’, both introductions from Italy, had higher germination over a wider temperature range than ‘Monti’ and ‘Narrikup’ (both Australian lines).
Acknowledgements
The authors are grateful to the Dryland Pasture and Field Research Centre students, staff who assisted with data collection and analysis and the reviewers for their time improving the manuscript. The authors acknowledge the statistical advice of Mr. David Saville and technical assistance from Dr. Keith Pollock and Malcolm Smith.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andreucci M, Black AD, Moot DJ. 2012. Cardinal temperatures and thermal time requirements for germination of forage brassicas. Agronomy New Zealand. 42:181–191.
- Andreucci MP, Moot DJ, Black AD, Sedcole R. 2016. A comparison of cardinal temperatures estimated by linear and nonlinear models for germination and bulb growth of forage brassicas. European Journal of Agronomy. 81:52–63. doi: 10.1016/j.eja.2016.08.010
- Angus JF, Machenzie DH, Morton R, Schafer C A. 1981. Physic development in field crops II. Thermal and photoperiodic responses of spring wheat. Field Crops Research. 4:269–283. doi: 10.1016/0378-4290(81)90078-2
- Bahuguna RN, Jagadish KS V. 2015. Temperature regulation of plant phenological development. Environmental and Experimental Botany. 111:83–90. doi: 10.1016/j.envexpbot.2014.10.007
- Baresel JP, Nichols P, Charrois A, Schmidhalter U. 2018. Adaptation of ecotypes and cultivars of subterranean clover (Trifolium subterraneum L.) to German environmental conditions and its suitability as living mulch. Genetic Resources and Crop Evolution. 65:2057–2068. doi:10.1007/s10722-018-0672-z.
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research. 14:1–16. doi: 10.1079/SSR2003150
- Berger JD, Shrestha D, Ludwig C. 2017. Reproductive strategies in Mediterranean legumes: trade-offs between phenology, seed size and vigor within and between wild and domesticated Lupinus species collected along aridity gradients. Frontiers in Plant Science. 8:1–16. doi: 10.3389/fpls.2017.00548
- Black A, Moot D, Lucas R. 2002. Seedling development and growth of white clover, caucasian clover and perennial ryegrass grown in field and controlled environments. Proceedings of the New Zealand Grassland Association. 64:197–204. doi: 10.33584/jnzg.2002.64.2463
- Boswell CC, Lucas RJ, Lonati M, Fletcher A, Moot DJ. 2003. The ecology of four annual clovers adventive in New Zealand grasslands. Legumes for Dryland Pastures. 11:175–184.
- Butler TJ, Celen AE, Webb SL, Krstic D, Interrante SM. 2014. Temperature affects the germination of forage legume seeds. Crop Science. 54:2846–2853. doi: 10.2135/cropsci2014.01.0063
- Elias M, Wieczorek G, Rosenne S, Tawfik DS. 2014. The universality of enzymatic rate-temperature dependency. Trends in Biochemical Sciences. 39:1–7. doi: 10.1016/j.tibs.2013.11.001
- Gama-Arachchige NS, Baskin JM, Geneve RL, Baskin CC. 2013. Quantitative analysis of the thermal requirements for stepwise physical dormancy-break in seeds of the winter annual Geranium carolinianum (Geraniaceae). Annals of Botany. 111:849–858. doi: 10.1093/aob/mct046
- Ghamkhar K, Nichols PGH, Erskine W, Snowball R, Murillo M, Appels R, Ryan MH. 2014. Hotspots and gaps in the world collection of subterranean clover (Trifolium subterraneum L. The Journal of Agricultural Science. 1:1–15.
- Hay JR, Porter KM. 2006. Development and Phenology. In The physiology of crop yield. 2nd ed. Oxford: Blackwell Publishing. 7–34.
- ISTA. 2017. International rules for seed testing. Basserdorf: International Seed Testing Association.
- Kigel J, Rosental L, Fait A. 2015. Seed physiology and germination of grain legumes. In: Handbook of plant breeding – grain legumes. 10th ed. New York: Springer; p. 327–364.
- Lonati M, Moot DJ, Aceto P, Cavallero A, Lucas RJ. 2009. Thermal time requirements for germination, emergence and seedling development of adventive legume and grass species. New Zealand Journal of Agricultural Research. 52:17–29. doi: 10.1080/00288230909510485
- Lucas RJ, Mills A, Wright S, Black AD, Moot DJ. 2015. Selection of sub clover cultivars for New Zealand dryland pastures. Journal of New Zealand Grasslands. 77:203–210. doi: 10.33584/jnzg.2015.77.459
- Macherel D, Benamar A, Avelange-Macherel MH, Tolleter D. 2007. Function and stress tolerance of seed mitochondria. Physiologia Plantarum. 129:233–241. doi: 10.1111/j.1399-3054.2006.00807.x
- Maranon T, Grubb PJ. 1993. Physiological basis and ccological significance of the seed size and relative growth rate relationship in Mediterranean annuals. Functional Ecology. 7:591–599. doi: 10.2307/2390136
- McMaster GS, Wilhelm WW. 1997. Growing degree-days: one equation, two interpretations. Agricultural and Forest Meteorology. 87:291–300. doi: 10.1016/S0168-1923(97)00027-0
- Mitchel KJ, Lucanus R. 1960. Growth of pasture species in controlled environment: II. Growth at low temperatures. New Zealand Journal of Agricultural Research. 3:647–655. doi: 10.1080/00288233.1960.10427144
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. 2011. ROS signaling: the new wave? Trends in Plant Science. 16:300–309. doi: 10.1016/j.tplants.2011.03.007
- Monks DP, SadatAsilan K, Moot DJ. 2009. Cardinal temperatures and thermal time requirements for germination of forage brassicas. Agronomy New Zealand. 39:95–109.
- Moot DJ, Black AD, Scott WR, Richardon J. 2003. Leaf development and dry matter production of subterranean clover cultivars in relation to autumn sward management. Legumes for Dryland Pastures. 11:193–200.
- Moot DJ, Scott WR, Roy AM, Nicholls AC. 2000. Base temperature and thermal time requirements for germination and emergence of temperate pasture species. New Zealand Journal of Agricultural Research. 43:15–25. doi: 10.1080/00288233.2000.9513404
- Nichols PGH, Foster KJ, Piano E, Pecetti L, Kaur P, Ghamkhar K, Collins WJ. 2013. Genetic improvement of subterranean clover (Trifolium subterraneum L.). 1. Germplasm, traits and future prospects. Crop and Pasture Science. 64:312–346. doi: 10.1071/CP13118
- NIWA. 2017. New Zealand national institute of water and atmosphere research. Auckland: National Institute of Water and Atmosphere Research-Science-Climate. [Accessed 2017 November]. https://www.niwa.co.nz/our-science/climate.
- Nori H, Moot DJ, Black AD. 2014. Thermal time requirements for germination of four annual clover species. New Zealand Journal of Agricultural Research. 57:30–37. doi: 10.1080/00288233.2013.863786
- Parmoon G, Moosavi SA, Akbari H, Ebadi A. 2015. Quantifying cardinal temperatures and thermal time required for germination of Silybum marianum seed. Crop Journa. l3:145–151.
- R Core team. 2017. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- SardegnaARPA. 2018. Dipartimento Specialistico Regionale MeteoClimatico. Sassari [Accessed 2018 February]. http://www.sar.sardegna.it/.
- Schaal BA. 1980. Reproductive capacity and seed size in Lupinus texensis. American Journal of Botany. 67:703–709. doi: 10.1002/j.1537-2197.1980.tb07700.x
- Sharifiamina S, Shayanfar A, Moot DJ, Bloomberg M. 2017. Subterranean clover (Trifolium subterraneum L.) seed germination responses to temperature and water potential. Proceedings of the 18th Australian Society of Agronomy Conference. 18–21.
- Singh A. 2012. Generalized linear model approach to analyzing proportional data. Proceedings of the 7th International Postharvest Symposium. 1167–1172.
- Soetart K, Cash J, Mazzia F. 2012. Solving ordinary differential equations in R. In Solving Differential Equations R. 41–80. Springer: Berlin, Heidelberg.
- Teixeira C, Lucas RJ, Lewis T, Moot DJ. 2017. From establishment to re-establishment: a field evaluation of sub clover cultivars. Proceedings of the 18th Australian Society of Agronomy Conference. 3–6.
- Valencia-Díaz S, Flores-Morales A, Flores-Palacios A, Perea-Arango I. 2015. How does the presence of endosperm affect seed size and germination? Botanical Sciences. 93:783–789. doi: 10.17129/botsci.251
- Yamauchi Y, Ogawa MA, Kuwahara Y, Hanada A, Kamiya Y, Yamaguchi S. 2004. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. The Plant Cell. 16:367–378. doi: 10.1105/tpc.018143
- Young JA, Evans RA, Kay BL. 1970. Germination characteristics of range legumes. Journal of Range Management. 23:98–103. doi: 10.2307/3896108