ABSTRACT
Nitrogen (N) leaching losses from grazed pasture systems pose a risk to the environment with mitigation strategies urgently required to achieve regulatory limits. There has been increased interest in alternative forages to manipulate excretion of urinary-N of livestock. This review summarises research on key forage attributes which affect the pattern of urine-N excretion. The literature showed an opportunity to alter both N concentration and moisture concentration of forages to reduce urine patch N loading. Complementary mitigations of extending the grazing rotation and reducing fertiliser were tested in simulations in addition to a diuresis effect on nitrate leaching in pastoral dairy systems. Findings suggested that forage species alone could not substantially reduce (by more than 20%) nitrate leaching. Combining all forage, management and systems-based solutions led to the greatest reductions in nitrate leaching, 59 and 31% for Waikato and Canterbury. Nonetheless, reductions in N loss came at the expense of pasture productivity which will likely affect profitability and farmer adoption of this suite of solutions in the absence of other drivers. The role of plant secondary metabolites in microbial dynamics has implications on N recycling in soil and rumen environments and this was identified as an area for further research.
Introduction
In temperate grazed livestock production systems combining competitive, high quality, grass and legume pasture species is a well-established and profitable approach to feeding high-producing livestock. In New Zealand (NZ), this permanent grass-legume combination typically comprises of perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.) (Davies Citation2001; Chapman et al. Citation2018). However, the traditional ryegrass-white clover pastures are characterised by relatively high crude protein content throughout most of the year which normally exceeds the nutritional requirements of grazing livestock (including lactating dairy cows which have the highest demand for protein in their diet compared to other stock classes and types). This high crude protein of the pasture, when ingested by dairy cows, results in greater than 60% of the consumed nitrogen (N) being excreted in the urine (Vibart et al. Citation2009). The urine-N is then deposited onto pastoral soil in concentrated areas at high equivalent N application rates between 200-2000kg N ha−1 (Selbie et al. Citation2015). The urine-N that is not utilised by plants or immobilised into soil organic matter is vulnerable to N loss processes (e.g. leaching and gaseous N emissions) and forms a major contributor to fresh water pollution (Fraser et al. Citation1994; Castillo et al. Citation2000; McGechan and Topp Citation2004).
Future solutions to reduce environmental impacts of farming will likely be multi-faceted, with restrictions on farm N inputs and/or land use change strategies required to markedly reduce N leaching losses in N-sensitive catchments. Previous reviews have highlighted a range of strategies to reduce N losses from grazed pastoral systems by targeting different parts of the N cycle via the animal-plant-soil components, (Di and Cameron Citation2002; Barrett et al. Citation2007; Hoekstra et al. Citation2007; Cameron et al. Citation2013; Dijkstra et al. Citation2013; Beukes et al. Citation2014; Beeckman et al. Citation2018; Hartinger et al. Citation2018). More recently, there has been increased interest in the use of alternative forages (e.g. plantain; Plantago lanceolata) and use of plant breeding to target specific traits associated with greater plant N uptake or urine N dilution (Nicols and Crush Citation2007; Judson et al. Citation2018) as a strategy to mitigate N losses from grazed pastures. However, the move from well-known ryegrass-clover pasture systems to alternative multi-species pastures will need to be approached with caution to ensure successful on-farm implementation to deliver environmental benefits while meeting profitability targets or that there are not other unintended adverse consequences.
Identifying successful opportunities to reduce urinary-N excretion from animal feed manipulation has been the subject of extensive reviews (Castillo et al. Citation2000; Kebreab et al. Citation2001; Hoekstra et al. Citation2007; Dijkstra et al. Citation2013; Spek et al. Citation2013), which have largely shown three key animal-based pathways to reduce urine-N excretion. These include: matching animal N requirements with diet composition; improving synchrony of rumen energy and N; and shifting digestion of dietary protein to the small intestine. Based on these animal-based pathways the following list of attributes that are relevant for forages to reduce N losses are: 1. reduced herbage N concentration; 2. increased soluble carbohydrates; 3. reduced soluble protein. However, an additional pathway to manipulate the animal influence on soil N loading (g N/urination) from individual urine events includes forage characteristics which alter animal urination patterns. For instance, an animal may have the same daily N excretion but lower concentration of N in the urine, this in turn reduces N loading at the individual urine patch level, provided the urination area is not appreciably altered, and therefore subsequent nitrate leaching at the individual patch scale (Di and Cameron Citation2002). Forage characteristics capable of reducing urinary-N concentration may be due to lower protein solubility, driving lower blood urea levels, or greater plant water increasing water intake which may result in more dilute urine thereby lowering urinary N (UN) concentration (Spek et al. Citation2013; Ledgard et al. Citation2015). While the impacts of these plant attributes on N leaching can be quantified at the patch level (particularly using lysimeter techniques) it is more challenging to determine the impact of forage-based solutions to reduce N losses at the paddock and farm scale due to the complex nature of the farm system and the practical impossibility of measuring N leaching at large scales.
This review reports on the variation in forage attributes which may reduce the risk of N leaching from farm systems via animal dietary manipulation strategies. More specifically, the review will consider the role of key forage attributes for future use in temperate grazed dairy systems as this industry represents the greatest improvements required for reducing impact on fresh water quality. Finally, modelling will be used to demonstrate the potential of forage attributes to reduce nitrate leaching at the paddock scale in dairy farm systems.
Reduced herbage N concentration
The protein requirements of livestock fluctuate throughout the season, depending on their physiological state and protein requirements for growth, lactation and reproduction. Livestock feeding standards for energy and protein requirements in NZ are based on the metabolisable energy (ME) and metabolisable protein (MP) systems using equations derived in the UK and Australia (SCA Citation1990; AFRC Citation1993). The supply of MP is dictated by the rumen degradability of dietary protein, rumen microbial protein synthesis (MPS) and digestibility of microbial and rumen undegraded protein. Because N degradability is not a common laboratory measure, nutritionists make assumptions about the degradability characteristics of feeds when determining the minimum dietary crude protein content (CP). In total (TMR) or partial (PMR) mixed ration systems it is easier to balance diet CP and ME to meet livestock requirements, but in outdoor grazed pasture systems it is substantially more difficult to do this because of the inherent challenges in controlling plant N concentration while maintaining high growth rates and high forage quality.
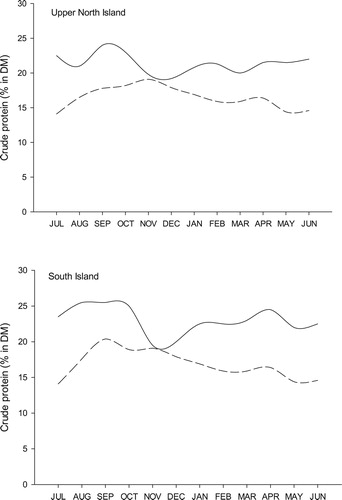
To demonstrate the N surplus typically encountered in pastoral dairy systems, the herbage crude protein concentration (CP%) requirements for a North and South Island dairy cows producing respective 340 or 400 kg MS/y has been plotted against average pasture CP% recorded for those areas (). Towards the end of lactation there is a large surplus of CP consumed in the diet. This physiological change coincides with reduced plant growth (and therefore N uptake) conditions, increased winter drainage and subsequently increased nitrate leaching risk. It is during this period that perhaps the greatest opportunity exists to identify forages and management strategies which can either reduce the amount of N voided in urine.
Figure 1. Seasonal fluctuation in the dietary N surplus of upper North Island and South Island ryegrass-clover based pastures. The solid line shows the average crude protein (% of DM) content of pastures (From Litherland and Lambert Citation2007) while the dashed line represents the minimum crude protein content (% of DM) required by a dairy cow in the upper North Island producing 340 kg milk solids per year or in the South Island producing 400 kg milk solids per year (LIC and DairyNZ Citation2018). Minimum dietary protein requirements were determined according to feeding standards for metabolisable protein (SCA Citation1990).
Recent research trials in warm (Waikato) and cool (Canterbury) regions of NZ were established to identify which forages, and management practises, resulted in lower herbage N (or crude protein concentration, which is assumed to be 16% N). Results regarding nutritive value and management responses have been published for the cool (Box et al. Citation2017a; Martin et al. Citation2017a; Martin et al. Citation2017b) and warm (Dodd et al. Citation2017) regions. In the cooler region, with long-term average air temperature differing by ∼4 degrees C compared to the warm region, grasses had the lowest herbage N concentration (mean of 2.7% for perennial ryegrass, Italian ryegrass, tall fescue, cocksfoot and prairie grass) followed by herbs (mean of 3.1% for chicory and plantain). Among the grasses, Italian ryegrass and prairie grass had lowest herbage N concentration, owing to the high DM yield. In the warm region, herbs had lower N concentration (2.6% N) compared with grasses (3.0% N), while legumes had the highest N concentration (>4.0%N). Grasses were most responsive to N fertiliser, and both grasses and herbs increased DM production with little effect on herbage N concentration up to application rates of 200 kg N/ha/year at the cool site (Martin et al. Citation2017b).
Variation in forage N concentration is influenced by the availability of soil N for uptake, stage of growth, and the growth rate of the plant. The opportunity to select forages with naturally occurring low herbage N, while still maintaining herbage quality, is limited due to similar critical N thresholds across all C3 species (Gastal and Lemaire Citation2002). Therefore, setting expectations towards shifting management regimes to suit specific forages may realise greater and faster benefits than plant breeding in reducing herbage N concentration alone (Pontes et al. Citation2007). For example, Gastal and Lemaire (Citation2002) showed that as plants mature, their critical N requirements are reduced, and as a result N concentration in tissue declines as plants mature and accumulate carbohydrate (Fulkerson et al. Citation1998; Bryant et al. Citation2012; Martin et al. Citation2017b). There is a diurnal pattern in plant nutritive characteristics, whereby accumulation of water soluble carbohydrates during the daytime often dilutes and so reduces plant N concentration, and there is local evidence for this for a number of pasture species including legumes (Box et al. Citation2017a). Furthermore, a recent proof-of-concept grazing study (Bryant et al. Citation2014) using ryegrass-clover pastures, highlighted the use of extending the regrowth interval, and offering the daily pasture allocation in the afternoon, could reduce herbage N intake and urinary-N concentration compared with a short regrowth period and morning allocation. However, while the practices tested in that study achieved lower herbage N concentration and subsequent reduction in urinary-N concentration it was coupled with a decline in milk production. This finding was largely attributed to the higher pasture mass of the extended regrowth resulting in perennial ryegrass pastures rapidly losing quality and subsequently compromising animal production (Combellas and Hodgson Citation1979; Peyraud et al. Citation1996; Kay et al. Citation2018). Whether reductions in quality of other species occur to the same extent as with perennial ryegrass warrants further investigation.
Balancing soluble carbohydrate and protein
Improving microbial capture and utilisation of ammonia in the rumen is influenced by the quantity and solubility of both protein and carbohydrate sources. In diets with adequate crude protein concentration, increasing the solubility of protein has been shown to increase urinary-N losses (Wohlt et al. Citation1976; Giger-Reverdin et al. Citation2015). A review of high sugar ryegrass by Edwards et al. (Citation2007) revealed that increasing the balance between WSC and CP in perennial ryegrass was associated with a reduction in urinary-N excretion. Consequently, plant species or management factors which reduce protein solubility and improve the quantity and availability of an energy source may offer opportunities to reduce urinary-N losses.
Reduced protein solubility
The majority of the protein in plants is rumen degradable and following ingestion is converted in the rumen to polypeptides, amino acids (AA) and ammonia (McDonald et al. Citation2002). Rumen microbes utilise these N sources in conjunction with energy sources, primarily from the breakdown products of carbohydrates, to synthesise their own cell tissue. Along with any undigested protein, these microbes are voided from the rumen where 85% of the microbial protein is subsequently digested and absorbed from the small intestine. The efficiency of utilisation of proteins digested in the rumen is lower than protein digested in the small intestine (Black Citation1971) so shifting the site of digestion to the small intestine is often recommended to improve animal N use efficiency.
Losses of N can occur in the rumen when the rate and extent of degradation of dietary protein results in rapid fermentation to ammonia. Ammonia is absorbed through the rumen wall by passive diffusion across a concentration gradient and then converted to urea in the liver. Urea circulating in the blood is either recycled back into the rumen or excreted in urine via the kidneys. Consequently, reducing the source of degradable dietary protein may increase the proportion of absorbable amino acids entering the small intestines, ultimately decreasing N fermentation to ammonia and urea synthesis.
In forages, the solubility of protein is determined by fractionation analysis which segregate proteins into one of five solubility pools: A, B1, B2, B3 and C, whereby, A is soluble non-protein N and C is insoluble in acid detergent. The B pools are soluble but at increasingly slower rates from B1 to B3 (Licitra et al. Citation1996). In perennial ryegrass, the solubility of protein is very high with over 50% of total N being rapidly available as A and B1 fractions (Hoekstra et al. Citation2007; Bryant et al. Citation2012). These studies showed only limited options to manipulate N solubility of perennial ryegrass. Elizalde et al. (Citation1999) compared different forage species of lucerne (Medicago sativa), meadow brome (Bromus beibersteinii) and tall fescue (Festuca arundinacea) and showed high protein solubility in lucerne, intermediate for tall fescue and low for meadow brome. Among the legume groups, differences in solubility were observed by Krawutschke et al. (Citation2013) who recorded lower solubility in lotus (Lotus corniculatus L.) and red clover (Trifolium pratense L.) compared with lucerne, caucasian (Trifolium ambiguum) and white clovers. The results of Krawutschke et al. (Citation2013) were from a long-term study which demonstrated the strong influence of environmental and seasonal effects on protein fractions in legumes with soluble fractions A and B ranging between 14 and 60% of the total N. Future challenges will include identifying and using triggers to enhance naturally low protein solubility in plants while maintaining quality and other desirable traits.
Increased soluble carbohydrate
Sugar and starch are rapidly-available sources of energy for microbes in the rumen. In fresh forages such as perennial ryegrass, increasing water soluble carbohydrates has resulted in improved microbial N flow to the small intestine (Lee et al. Citation2002), maintained or improved milk yield (Miller et al. Citation2001; Moorby et al. Citation2006; Totty et al. Citation2013) and reduced urinary-N excretion (Edwards et al. Citation2007). Variation in WSC content between perennial ryegrass cultivars in NZ was reported by Easton et al. (Citation2009) who found larger variation between seasons (16–30% DM) than between cultivars (Canterbury: 21-28%, Manawatu 17-23%). For herb species, chicory and plantain, Minnee et al. (Citation2017) measured greater soluble carbohydrates (14%) compared with perennial ryegrass (7%). Conversely, Box et al. (Citation2017a) reported greater WSC for perennial ryegrass (23%) compared with chicory (19% WSC) or plantain (20% WSC). The discrepancy in ranking between herbs and grasses may be a product of the environmental conditions or analytical procedures as the two studies report carbohydrates in different terms. In standard feed tests, carbohydrates are regarded as being soluble or insoluble in detergents. The insoluble carbohydrates represent the crude fibre and the soluble carbohydrates are the remainder when all other primary nutrients have been accounted. In perennial ryegrass, the majority of these carbohydrates are extractable in water and are primarily fructan and sucrose (White Citation1973). Interestingly, although Box et al. (Citation2017a) measured more WSC in ryegrass compared with herbs, a greater proportion of the total DM for plantain was not accounted for. In plantain, for instance, L-arabinose accounts for 20–25% of the sugars (Brautigam and Franz Citation1985; Kardošová Citation1992) but it is unlikely to be detected in routine laboratory methods for WSC using anthrone reagents (Jiang et al. Citation2019). It is possible that the unrecorded sugars in plantain and chicory belong to other groups relating to sorbitol, mucilage and pectins which form part of the plant cell wall. The availability of these other carbohydrates as an energy source for rumen microbes is unclear but may play an important role in synchronising nutrient release in the rumen.
Synchronised release of nutrients
In grazing animals, the interaction between the physical presentation of the plants themselves and the animal has the potential to influence ingestive behaviour and disruption of plant cells, and thus availability of nutrients in the rumen. The opportunity to manipulate the release of intercellular components within ryegrass species was investigated by Boudon and Peyraud (Citation2001) who observed little effect of season or stage of maturity on the release of nutrients following ingestion. In a recent study comparing ingestive particle size reduction of chicory, lucerne and perennial ryegrass, Minnee et al. (Citation2018) reported lowest particle size reduction in chicory and that this resulted in a slower release of N from cell contents. Interestingly, in the same study, the release of carbohydrates did not appear to be impeded to the same extent as nitrogen. Quantitatively, the range in concentrations of the basic nutritive attributes is relatively small between forages grown under intensive production systems, yet the spatial arrangement of those compounds in fresh plant material has the potential to alter fermentation dynamics in the rumen and availability of absorbed nutrients.
Standard feed testing procedures are typically performed on forage samples which have been conserved by oven drying or lypholisation, making them easy to store without degradation. However, using dried ground tissue to predict rumen availability of protein and carbohydrates from plant cells of fresh forage may not adequately reflect the rumen interactions of freshly grazed forages. For instance, it is believed that plant-induced proteolysis contributes considerably to degradation and release of N in the rumen (Kingston-Smith et al. Citation2003). Plant-induced proteolysis is a stress response of ‘living’ plant tissue whereby peptidases are initiated within cell vacuoles upon entry to the rumen (Kingston-Smith et al. Citation2005; Pichard et al. Citation2006). There is considerable variation in proteolytic activity among and within forages (Pichard et al. Citation2006) and identification of plants with reduced proteolytic activity may offer an opportunity to improve rumen N utilisation (Kingston-Smith et al. Citation2002). Species with a high proteolysis activity index (PAI) such as phalaris, oats and lucerne may be expected to more rapidly degrade in the rumen than species with a low PAI such as brome, Italian ryegrass, lotus and red clover (Pichard et al. Citation2006). However, this area of research requires further exploration at the animal level to validate the impact of these hypotheses on N metabolism and N excretion in the ruminant.
Slowly degradable protein
A slower particle breakdown may result in slower release of ammonia or an increase outflow rate of undigested feed if daily DM intakes are high. The effect of altering dietary protein degradability has been demonstrated by Bohnert et al. (Citation1999) who observed improved N retention and lower urinary N excretion of wethers consuming diets with lower rumen degradability. Proof-of-concept for the hypothesis that reduced rumen degradability will reduce urinary N excretion has been inconsistent, particularly in diets which are already high in N (Castillo et al. Citation2000). Because digestibility of dietary and microbial protein is high, transfer of the site of digestion to the small intestine does not increase N retention if the MP of that animal is already being met. The absorbed amino acids are typically deaminated and excreted in the urine thereby increasing the N concentration of the urine. Increasing the proportion of total N which is indigestible does, however, increase partitioning of N from urine to dung (Barry Citation2011).
The degradability of forage protein in the rumen is influenced by the proportion of protein which is associated with cell wall, and therefore is more difficult to digest as a number of complex linkages need to be broken, and plant secondary metabolites which alter fermentation of both primary and secondary metabolites. The former component represents the plant protein bound into cell wall which is largely indigestible, measured as the acid detergent insoluble N fraction and referred to as ‘fraction C’ in fractionation studies. In perennial ryegrass this represents a small proportion of the total protein (<10%) with little variation between cultivars and N fertiliser levels (Hoekstra et al. Citation2007; Bryant et al. Citation2012). Increasing the regrowth interval can increase fraction C, but the overall effect is minimal compared with the decline in total plant N. In forages other than perennial ryegrass, the degradability of protein appears to be linked to plant secondary metabolites (PSM) which help to improve plant fitness in less productive species. For example, some forages produce PSM as a natural defence (photodamage, pathogens and herbivores) or an attractant (pollinators) to improve survival. Plant secondary metabolites are classified according to their chemical structure, composition and synthesis pathway and fall into one of three groups: terpenoids, alkaloids and phenolics (Poutaraud et al. Citation2017; Villalba et al. Citation2017) whose properties are discussed in the following section.
Plant secondary metabolites
The first of the PSM groups are terpenoids which tend to be associated with plant interaction and signalling with mycorrhiza. Well known examples of terpenoids occur in plantain as aucubin and catalpol which are iridoid glycosides (IG) due to the association of the monoterpene with sugar. Terpenes can act as plant defence chemicals and in the example of aucubin, which is found in plantain species, it has been shown to be associated with reduced growth of fungal pathogen Diaporthe adunca and reduced feeding and growth of beet armyworm (Spodoptera exigua) (Biere et al. Citation2004). The impact of aucubin-containing plantain has also been associated with N metabolism of microbia, reducing the rate of ammonia production in both soils (Carlton et al. Citation2019; de Klein et al. Citation2019) and rumen fluid (Navarrete et al. Citation2016). In P. lanceolata the aucubin levels are typically greater than catalpol (Box et al. Citation2019) though concentrations of the different metabolites vary greatly as they are dependent on season (Clemensen et al. Citation2017; Box et al. Citation2019), and level of herbivory and association with mychorriza (Wang et al. Citation2015; Clemensen et al. Citation2018). The extent to which terpernoids in forages such as plantain can reduce nutrient losses from animals still remains unclear, but there is increasing evidence that indicates some potential benefits and these are outlined in the following section. If aucubin delays nitrification rates then more N may be available for microbial protein synthesis. However, Wang et al. (Citation2015) noticed, in insect herbivores feeding on plantain, an increase in IG did not reduce intake rate but lowered growth rate, leading to the suggestion that PSM reduce the efficiency of nutrient utilisation. Interestingly, a similar effect has occasionally been observed in dairy cows grazing plantain or grass-clover pastures as a control, whereby greater apparent intake was measured for plantain but milk production was similar to the control (Box et al. Citation2017b; Mangwe et al. Citation2018). The theory that PSM may alter intake and nutrient use efficiency has been explored in greater detail for phenolics such as condensed tannins which will be addressed later in this review. Any direct link between aucubin and N metabolism requires further exploration.
The second PSM are alkaloids which are a group of PSM containing nitrogen, being biosynthesised from amino acids, and are structurally very diverse (Poutaraud et al. Citation2017). Bacteria, fungi, plants and even animals produce alkaloids, but in agriculture perhaps the most common alkaloids are those produced by endophyte fungi which have formed symbiotic associations with pasture species such as Lolium perenne and Festuca spp. Under deficient conditions (e.g. moisture stress and low soil fertility) the presence of alkaloid-producing endophyte improves host fitness in that environment by reducing herbivore pressure (Edwards et al. Citation1993; Fuchs et al. Citation2017) and improving competitive advantage (Takai et al. Citation2010; Thom et al. Citation2014). From an environmental perspective, the presence of endophyte in grasses has been associated with increased soil nitrification (Bowatte et al. Citation2011) which may confer negative environmental impacts. Endophyte alkaloids are also associated with animal health issues such as staggers and heat stress, though combining endophyte-infected tall fescue with phenolic-containing legumes has shown that the alkaloids bind with tannins (Clemensen et al. Citation2018) which may minimise the adverse effects of alkaloids on animal health. Plant breeding has also identified endophyte strains which produce fewer animal health issues (Barrett et al. Citation2015). There is little published information on the effect of endophyte on N metabolism and partitioning in ruminants. The effect of alkaloids on N metabolism in animals is more likely to be indirect, whereby the increased plant fitness may enable higher stocking rates which increase N losses. Alternatively the lower legume content, which is often associated with endophyte-containing pastures, will result in lower dietary N concentration, leading to lower N losses.
The third, and perhaps most studied of the PSM, are the phenolic compounds which are classified as containing one or more hydroxal-group in an aromatic ring. The key roles of phenolics are to act as a natural defence and to protect the plant from photodamage (Close and McArthur Citation2002). The most well-studied phenolics in ruminant systems are tannins and to a lesser extent saponins and polyphenol oxidase (PPO). Many of these studies reveal that the value of phenolics in N loss mitigation are through reducing the degradability of protein in the rumen. The modes of action of phenolics in the rumen differ both within and between types of phenolics and there is considerable variability in observed results. The undegraded protein passes from the rumen to the small intestine where it may be digested or excreted in dung undigested (Barry et al. Citation2001; Min et al. Citation2003). A wide range of plants contain tannins (Hoskin et al. Citation2006), although the concentrations are relatively low and are associated with structural tissue such as lignin which result in little effect in the rumen. On the other hand, plants containing high tannin content with bioactive characteristics include lotus spp. and sainfoin. In feeding trials with ruminants, these species have improved duodenal flow of essential amino acids resulting in greater liveweight gain or meat production (Barry Citation2011) with associated reductions in urinary N losses (Koenig and Beauchemin Citation2018).
Saponins on the other hand occur as a glycoside, and are found in plants such as lucerne (Clemensen et al. Citation2018). Saponins are also able to form complexes with protein in the rumen to reduce degradability, although several studies have demonstrated that saponins reduce rumen protozoa (Lu and Jorgensen Citation1987; Malik and Singhal Citation2008; Rajabi et al. Citation2017; Arvind et al. Citation2018). Reductions in protozoal numbers and their activity are implicated in lower rumen ammonia concentration as microbial populations increase to improve N capture in the absence of protozoa (Lu and Jorgensen Citation1987). Lower rumen ammonia concentration and improved N retention was demonstrated by Rajabi et al. (Citation2017) when supplementing sheep with pomegranate peel which contained both saponins and condensed tannins.
Polyphenol oxidase (PPO) is an enzyme present in many pasture species (Lee et al. Citation2006) and it aids the plant defence against pathogens and photodamage (Lee Citation2014). PPO is involved in reactions which deactivate proteases in plants, and which was initially proposed to improve N metabolism in the rumen by reducing rumen ammonia and improving N use efficiency (Pichard et al. Citation2006; Hart et al. Citation2016). Lee (Citation2014) later argued that, due to the requirement for oxygen in PPO reactions, the observed reductions in rumen protein degradability in red clover diets were likely attributed to other associated factors.
Using PSM to mitigate N losses is likely to pose considerable challenges due to the inherent variability in concentration of the multiple compounds (Darrow and Bowers Citation1997), and the threshold concentrations required in the diet to be effective. PSM in legume species may not reduce N losses because of their high N concentration. Because PSM can reduce availability of dietary proteins, there may be times of the year when the nutritional requirements of high producing livestock are not being met because PSM are near toxic or limiting levels. Close and McArthur (Citation2002) for example argued that because phenolics produced by plants enabled plants to deal with oxidative stress, phenolic concentrations would be greatest during periods of abiotic stress such as high sunlight, cold, drought or low nutrients. PSM in plantain (aucubin and catalpol) have shown to be highest in concentration in autumn, supporting the hypothesis that these terpenoids are responding to cooler temperatures or increased insect pressure late in the milk production season. Anecdotal evidence that the palatability of plantain by livestock is lower in autumn (Bryant et al. Citation2018) is likely to be linked to fluctuations in these compounds, and cow adaptation has been demonstrated to the overcome initial avoidance of plantain (Box et al. Citation2017b). The advantage of high PSM which reduce rumen degradability in autumn may reduce the risk of high urinary N loading during this high risk period () and suggests plantain as a potential forage for reduced N losses.
Urine dilution and N load distribution
While the composition of forages play an important role in determining nitrogen consumption and utilisation, forages also influence how those nutrients are excreted. Nitrate leaching from urine is influenced by the concentration of nitrogen in the urine and the area upon which urine is deposited (Li et al. Citation2012), both of which can be altered by the volume of urine. Although all mammals have relatively fixed circadian rhythms for urine excretion (Noh et al. Citation2011) there is considerable variation in the fluctuations in urination cycles (Betteridge et al. Citation2013; Shepherd et al. Citation2017a; Gregorini et al. Citation2018). Taking advantage of this variation may reduce the proportion of urine patches with high N leaching risk.
Evidence that altering urination volume and frequency had potential to reduce nitrate leaching was provided by Ledgard et al. (Citation2015) who used salt as a diuretic. Supplementing dairy cows with salt was shown to increase daily water intake and lead to higher total daily urine volumes at similar nitrogen intake. This resulted in cattle urinating more frequently (17% increase) under field grazing conditions. In associated soil lysimeter studies, decreasing the N rate in urine patches by 50% (from 600 to 300 kg N/ha) resulted in reduction in N leaching loss from the patches by 65% indicating a net gain even if total N excreted was unaffected by the salt. While this study demonstrated that administering salt to dairy cows had the potential to increase the area over which the urine-N was deposited, thereby probably reducing total nitrate leaching, the authors acknowledged potential animal side-effects due to sustained and large ingestions of salt. Alternatively, a number of grazing studies have been conducted that incorporate low DM forages, as a complete or partial diet, and these are summarised in . In those studies, which predominantly compared a control pasture consisting of perennial ryegrass and white clover with an alternative pasture containing one or both of chicory and plantain, milk yield is similar to the control when offered at a similar DM allocation ().
Figure 2 Effect of feeding control (CON) perennial ryegrass white clover pastures (RGWC) or alternative (Alt) forages which either consist of more or less than 50% RGWC, on milk production of dairy cows.
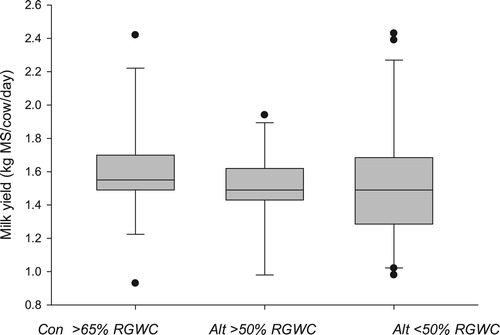
Table 1. Summary of sources of information and forage effects when fed to lactating dairy cows during grazing or indoor feeding studies
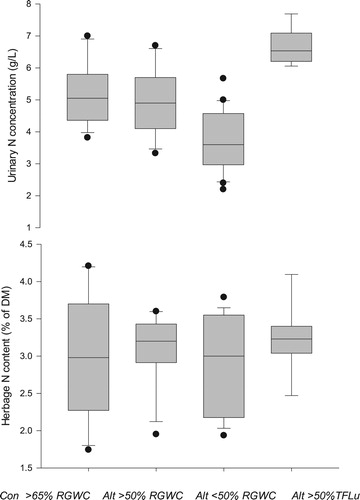
The effect of forage type on herbage N concentration was variable and was related to the time of year, growing conditions, and regrowth interval. The majority of grazing trials were conducted in Canterbury where management ensured that fertiliser and moisture were not limiting, and herbage N concentrations were relatively high with an average of >3% N. In spite of only small differences in herbage N concentration, there was consistently lower urinary N concentration of spot urine samples when alternative forages accounted for more than half of the diet (). The inclusion of low DM forages altered at least the distribution of N excretion, potentially reducing N load from urinations occurring during the day. This outcome could be explained using dynamic, mechanistic modelling which also predicted variation in the distribution of urinary N excretion when MINDY was offered plantain under different allocation regimes (Gregorini et al. Citation2018). The forages which predominantly feature in these studies where lower urinary N occurs were plantain and, to a lesser extent, chicory. The reason for the reduction in urinary N concentration on plantain-based pastures could be driven by a number of factors but one of the initial theories is water diuresis caused by low DM in plantain. Diuretics are substances which cause an increase in the production of urine and whose presence interferes with homeostatic mechanisms (i.e. anti-diuretic hormone (ADH) for maintaining body water balance (Merrill Citation2008)). Two common forms of diuresis are water diuresis which is caused by excessive consumption of water (inhibiting ADH), and osmotic diuresis which is caused by the presence of high concentration of solutes restricting the reabsorption of water in the convoluted tubules of the renal system (Mathisen et al. Citation1981).
Figure 3 Effect of feeding control (CON) perennial ryegrass white clover pastures (RGWC) or alternative (Alt) forages which either consist of more or less than 50% RGWC or more than 50% Tall fescue and lucerne (TFLu) on urinary N concentration of spot samples and herbage N concentration.
Using sheep in an indoor feeding study, O’Connell et al. (Citation2016) investigated the effect of plantain on diuresis and showed that plantain causes a water diuresis in sheep compared with ryegrass control forages. In those studies, water intake was restricted, the studies were short duration and the diuresis effect diminished between day 1 and subsequent days. Those researchers proposed that consumption of plantain interfered with the vasopressin receptors: further research is required to confirm this hypothesis. While animal species do vary in their urination patterns (Domingue et al. Citation1991), we have also observed examples of diuresis occurring in dairy cows grazing plantain. Box et al. (Citation2017b) reported increased urine volumes with 50 or 100% plantain diets. Similarly Mangwe et al. (Citation2019) also showed increased urine volumes from cows grazing plantain compared with ryegrass clover. Those authors also included chicory in the comparison and observed higher urination than either ryegrass or plantain, and they attributed that to low feed DM content and large feed water intakes. Typically grazing cows will urinate an average 2 L /urination roughly 13 times per day (Shepherd et al. Citation2017a). On high moisture forages increased urine volumes were generally displayed through increased frequency of urination, with a small (non-statistical) increase in mean urine volume per urination (Box et al. Citation2017b; Mangwe et al. Citation2019). There is considerable diurnal variability in urination event size, cows will urinate smaller events more frequently during the day, while at night glomerular filtration rate (within the kidney) slows and the bladder is able to store more urine (Noh et al. Citation2011) so there are fewer larger volume events during the night (Shepherd et al. Citation2017a; Mangwe et al. Citation2019).
The inclusion of plantain or chicory does not always result in increased urine volumes (Edwards et al. Citation2015; Nkomboni Citation2017; Bryant et al. Citation2018). Normally animals will adjust their source of water to meet their requirements and increased trough water intakes have been observed by Dodd et al. (Citation2018) and Bryant et al. (Citation2018) in comparisons between diverse pastures including forbs and standard ryegrass-white clover pastures. Bryant et al. (Citation2018) using urine sensors attached to grazing dairy cows (Shepherd et al. Citation2017a), showed that variation in forage water intake is likely to have contributed to changes in diurnal N urination. Those results demonstrated that there is 2–3 fold diurnal variation in urinary N concentrations and therefore conclusions from spot urine samples obtained at one or two times during the day may be misleading. Indoor feeding trials have demonstrated that, irrespective of forage type, N intake is still the leading driver of total urinary N excretion.
Impact of forage attributes at the paddock level – modelling approach
Leaching, and other effects of forages on soil-plant-animal systems, are only practicably measurable on the scale of the animal (e.g. urine sensors) or patch (e.g. lysimeters) but is only important at the scale of the paddock or above. The purpose of whole-paddock simulations is to bridge this gap in scale and so to help translate measurements of difference at the scale of the animal or patch to whole-paddock outcomes.
The review above summarised key nutritional factors which could be used to reduce the risk of nitrate leaching and the ability of a range of common pastoral forages to meet these criteria are summarised in . The obvious candidate is lower herbage N concentration. The review has identified there is large variation in plant N concentration within species, so management for lower herbage N concentration seems more promising. Increasing the soluble C:N ratio of forages has also shown reduced urinary N loss, but this operates largely through the same mechanisms as total herbage N. In theory, reducing protein degradability is expected to reduce urinary N excretion by partitioning more N to dung, but the evidence that this can be achieved with forages is inconsistent. Increasing moisture content of forages to elicit a diuresis effect does appear to reduce urine patch N loading. Consequently, the following modelling simulations investigated the additive effects of adopting a forage attribute (high moisture content), a management attribute (increased regrowth to achieve low N and elevated C:N ratio), and a farm systems consideration (lower N fertiliser rate).
Table 2. Ranking of common pasture forages for attributes which can be managed to reduce animal N excretion and urine patch loading
Methods
The simulation model used for this work was the Agricultural Production Systems Simulator (APSIM) v7.10 r4191 (Holzworth et al. Citation2014) particularly including a new technology for explicitly accounting for the effects of urine patches on N leaching (Snow et al. Citation2017). Details of the model configuration, soil and climate and animal assumptions are provided in Snow and Meenken (Citation2018). Simulations were run under two climates, Waikato and Canterbury, representing two contrasting dairying environments in NZ. Daily weather data from 1972 to 2016 for the locations was obtained from the NIWA Virtual Climate Station Network (Tait and Turner Citation2005; Cichota et al. Citation2008).
Only a single paddock of a farm was simulated. A physical summary of the baseline farms and scenarios are presented in . Stocking density (cows /ha) during the grazing was calculated by the combination of the assumed stocking rate of the whole farm, an assumption that there were 25 paddocks on the farm, and the number of days for the full grazing event. The latter factor was treated as increasing the stocking density during the grazing event rather than increasing the duration of the event so that the simulation is more accurately a representation of a part of a back-fenced strip-grazed paddock. The timing of grazing was controlled by a combination of the paddock reaching a set pre-grazing standing dry matter (DM) and a maximum duration since the last grazing event. The pasture was grazed to a set post-grazing DM in one simulation-day.
Table 3. Production and environmental indicators from the baseline (S0) simulation along with the diuretic effect (S1), extended regrowth period (S2) and reduced fertiliser (S3) scenarios in Canterbury and Waikato. Normalised N leached is the raw value divided by the effective stocking rate (ESR; see definition in the text).
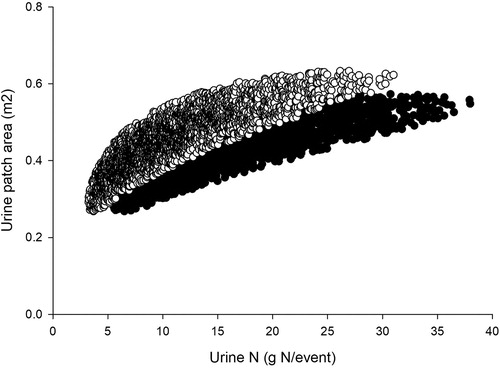
The amount of urine N excreted per cow was calculated using the N consumed by the cow from the combination of fresh pasture and supplementary silage, and the cow N partitioning Snow and Meenken (Citation2018). The total number of urine patches was determined for each grazing event from the scenario assumption of selecting the number of urinations per cow per day (11 for the baseline and 15 for simulations including a diuresis effect) and the stocking density during the grazing event. We had available data from cow urination sensors giving >3000 pairs of urine patch area and N amount () for individual urinations from cows fed a diet without or with plantain and lucerne as reported in Dodd et al. (Citation2018). To convert the urination sensor data to N load within the urine-affected patch area we used data from Beatson (Citation2017) to convert from urination volume to wetted area and assumed an edge effect where plants within 0.1 m of the wetted area could access urinary N (Shepherd et al. Citation2017b; Cichota et al. Citation2018). The required number of volume-amount pairs were drawn from the appropriate sample with replacement.
Figure 4. Data for individual urine event amount (horizontal axis) and patch area (vertical axis) for a diet without (closed circles) or with (open circles) diuretic components of lucerne and plantain. The data was taken from the trial described by Dodd et al. (Citation2018).
Scenarios
Baseline (S0) simulations in the Canterbury environment were conducted with assumptions of 11 urinations/cow/day with urine volume-N amount sampled from the without plantain and lucerne data, a normal regrowth period (to achieve two and a half to three leaf appearance of ryegrass) and a stocking rate of 3.25 cows/ha. In the Canterbury simulation, 200 kg N/ha/y was applied in even monthly applications to a light soil (75 mm to 0.6 m deep and 88 mm plant-available water to 0.9 m deep), excluding May-July, centre-pivot irrigation and no grazing from 15-May to 30-July. Baseline simulations in the Waikato environment were a stocking rate of 2.25 cows/ha, 100 kg N/ha/y was applied in even monthly applications excluding May-July, to a relatively heavy soil (96 mm plant-available water to 0.6 m deep and 137 mm plant-available water to 0.9 m deep), no irrigation and the cows were grazed on-farm while dry in winter. For each location, three variations were simulated:
S1 – forage only effect, as for S0 except diuresis increases urination frequency from 11 to 15 events/cow/day and the urine event data were sampled from the with plantain and lucerne data set;
S2 – forage + management effect, as for S1 except the extended regrowth parameters (pre-grazing DM of 3200 kg DM /ha and post-grazing residual 1650 kg DM /ha during the milking season) were used to control grazing;
S3 – forage + management + systems effect, as for S2 except the amount of fertiliser was reduced by 50 kg N /ha /year.
Results and discussion
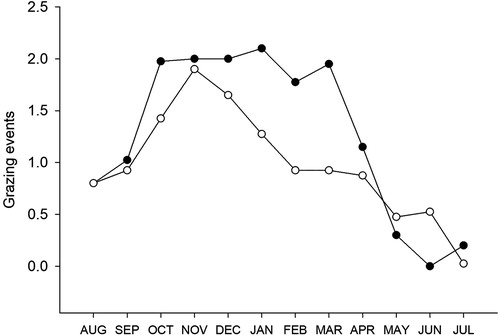
On average, the simulations produced 15.3 grazing events /year in Canterbury and 11.7 in Waikato for the S0 (baseline) scenarios (). These events were distributed over the year as shown in , and ranged from no grazing in some months, to a maximum of 2.1 events per month in the irrigated Canterbury environment. The results show some limitations of the single-paddock grazing rules. While there was at least one winter grazing event per in year while the cows were dry in Waikato, the events were skewed towards May and June with only one year (of 40) having a grazing event in July. In Canterbury the maximum number of grazing events in any month was three. These occurred in months under highly favourable growing conditions with grazing events in both the first and last few days of the month. The leaching for Canterbury under S0 might seem high but is well within the range reported by Robson et al. (Citation2015; page 129) for similar dairy farms managed to industry-agreed good management practice.
Figure 5. Average number of grazing events by month for the baseline scenarios for Canterbury (closed circles) and Waikato (open circles).
One effect of the single-paddock rules was that the stocking rate varied between locations (as intended) but also with scenario as summarised in . The simulation effective stocking rate (ESR), calculated from the cow-grazing-days in each year divided by the days per year, varied following the number of grazing events and the amount of imported silage. Baseline ESR for Canterbury was 3.6 and for Waikato was 2.1 (effective cows /ha). Compared to the baseline S0 scenario, ESR increased slightly for S1 (diuretic effect) and then decreased for S2 (extended regrowth period) and S3 (reduced fertiliser).
shows several other outputs from the simulations. For the amount of herbage grazed or ensiled and compared to S0; S1 increased by 2% in both Canterbury and Waikato; S2 decreased by 5% in Canterbury and 7% in Waikato; and S3 decreased by 8% in Canterbury and 16% in Waikato. This last result indicates that the decrease from 100 to 50 kg N /ha /year in Waikato had a substantial effect on pasture growth.
The diuretic effect (comparison of S1 to S0) reduced leaching in the Canterbury environment (9 kg N /ha /year; 3 kg N /ha /year /ESR) slightly more than that likely in Waikato (6 kg N /ha /year; 3 kg N /ha /year /ESR) but the greatest percentage reduction was in Waikato with a 21% reduction compared to 6% in Canterbury. The diuretic effect reduced mean urine patch load by 31% which amounted to reductions of 136 kg N /ha in Canterbury and 129 kg N /ha in Waikato. However, this was at the cost of a greater coverage of the paddock area in urine patches. The addition of the diuretic effect to the simulation increases the annual total coverage of urine patches by 11% in Canterbury and 9% in Waikato. The area of the paddock affected by urine more than once in a calendar year more than doubled – from 6 to 13% in Canterbury and from 3 to 7% in Waikato. The increases in the area affected counteracted somewhat the reduced urine patch load so that the reductions in whole-paddock leaching was less than the reduction in urine patch load.
The S2 scenario investigated the additional effect of increasing the regrowth period by increasing the pre- and post-grazing herbage biomass values such that the interval between grazings was increased. This scenario resulted in decreased ESR (), a reduction in the number of grazing events, and less net importation of silage in both locations. Leaching was reduced by a further 16 kg N /ha /year in Canterbury and by 6 kg N /ha /year in Waikato to give leaching values that were reductions of 17% in Canterbury and 41% in Waikato when comparing to S0. In the S3 scenario, a reduction in annual fertiliser, further reduced stocking and production variables and that resulted in additional reductions in N leaching of 31% in Canterbury and 59% in Waikato compared to their S0 values.
While there were some limitations of the single-paddock representation (c.f. whole-farm management), the results here are likely to be broadly representative particularly when comparing relative changes across scenarios. The scenarios here have intentionally confounded the location with soil type, irrigation, and other farm management practices such as fertiliser regime and winter grazing to make them broadly applicable for the prevalent dairy farms in each region. It may well be that a diuretic effect will be more effective in the Canterbury climate on deeper soils or with a lower stocking rate.
Summary
For farmer adoption and regulation reasons, the target of these simulations was to assess if a 20% reduction in leaching might be achieved through a plant-induced diuresis effect on cows. We further considered the sequential additive effects of an extended regrowth period and a reduction in fertiliser inputs. We took an optimistic stance of adopting the experimental results of the effects of forage on diuresis that increased the average number of urinations per cow per day from 11 to 15 and an accompanying increase in the volume and decrease in N amount of individual urination events. We assumed that such effects could be achieved year-round, which remains to experimentally validated.
Simulation results showed that the diuresis effect (S1) might reduce leaching in the Waikato location by more than 20% but that only a very minor (6%) reduction was likely in Canterbury. Part of the minor reduction in Canterbury was likely to have been affected by an increase in effective stocking rate (ESR) so a leaching rate normalised by ESR was also calculated. The normalised reduction in leaching in Canterbury was still only minor (−7%). The results of the S2 scenarios (extending the regrowth period) showed strong reductions in leaching in both locations (an additional 11% reduction in Canterbury and 20% in Waikato) and reducing fertiliser further reduced leaching (14% in Canterbury and 14% in Waikato) in both locations.
The modelling simulations suggested that if the diuresis effect can be established consistently, then based on a diuresis effect alone, a high moisture forage such as plantain or chicory will likely be moderately to quite effective in reducing leaching in Waikato, but are not likely to be effective in Canterbury on light soils. It seems likely that for farm systems, soils and climates like those in the Canterbury simulations will require a combination of approaches (diuresis, lengthened regrowth and reduced fertiliser) to achieve large (> 20%) reductions in leaching. In our modelling scenarios, the greatest reductions in nitrate leaching arose from reduced N fertiliser and lower stocking rates and the subsequent drop in pasture yield. The implications of these outcomes point directly to reductions in profitability, which small reductions in fertiliser expenses are unlikely to offset.
Conclusions
The purpose of this review was to investigate opportunities, across a range of pastoral forage species, for their potential to reduce N losses from grazing livestock systems. As many of the issues farmers face in nutrient management are due to dietary N surplus there is little dispute that forages with lower herbage N concentration could play a significant role in reducing N loss. Among perennial grass and forb species there is limited variation in herbage N concentration as most plants have a similar critical N at optimal growth. However, there is considerable temporal and spatial variation within species in the distribution of N as well as variation between N concentration in plant parts which highlights the potential of management of forages to manipulate N ingested. Across species, the variation in plant chemistry alters the form and availability of proteins following ingestion, resulting in some forages containing lower proportions of soluble protein. The synthesis of secondary compounds, which are produced to improve plant fitness, have implications on the digestion and metabolism of the ruminants that consume them. There was evidence of opportunity to alter partitioning of N through these pathways to reduce excretion of N in the urine.
Animals, plants, and soils are inextricably linked in grazed systems, so achieving successful environmental outcomes at the farm level requires recognition of these interactions. The review revealed that forages differing in moisture content can influence urination patterns of N loading with subsequent reductions in N leaching at the patch scale. The impact of a diuretic effect of forage on nitrate leaching, at the farm scale depends on the environment and the interaction between those linkages. The paddock-scale modelling in this review demonstrated that by adopting a suite of plant and management approaches to limit N losses, reductions in nitrate leaching of over 50% could be achieved in principle. However, the greatest reductions in N leaching did come at the cost of pasture production, stocking rate and ultimately a net reduction in pastoral feed supply which is likely to lead to reductions in profitability. The loss in milk yield per hectare with reduced stocking rate is a probable result of dedicated efforts to reduce N loss.
Acknowledgements
Research was completed as part of the Forages for Reduced Nitrate Leaching programme with principal funding from the New Zealand Ministry of Business, Innovation and Employment and co-funding from research partners DairyNZ, AgResearch, Plant & Food Research, Lincoln University, the Foundation for Arable Research and Manaaki Whenua – Landcare Research. Acknowledgment is made to the APSIM Initiative which takes responsibility for quality assurance and a structured innovation programme for APSIM's software, which is provided free for research and development use (see www.apsim.info for details). The authors declare no conflict of interest in these results.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AFRC. 1993. Energy and Protein Requirement of Ruminants: An Advisory Manual Prepared by the AFRC technical Committee on Responses to Nutrients. Walling-ford, UK: CAB International.
- Arvind K, Chaturvedi VB, Singh AK, Anamika SK. 2018. Effect of different levels of lucerne (Medicago sativa) in diet on rumen fermentation and energy metabolism of ewes. Indian Journal of Small Ruminants. 24:51–56.
- Barrett BA, Faville MJ, Nichols SN, Simpson WR, Bryan GT, Conner AJ. 2015. Breaking through the feed barrier: options for improving forage genetics. Animal Production Science. 55(7):883–892.
- Barrett BA, Pacheco D, McNabb WC, Easton HS. 2007. Opportunities to improve nitrogen utilisation in the rumen by reduction in plant-mediated proteolysis. Proceedings of the New Zealand Grassland Association. 69:187–192.
- Barry TN. 2011. Legend session: forage secondary compounds; past, present and future. Proceedings of the New Zealand Society of Animal Production. 71:314–321.
- Barry TN, McNeill DM, McNabb WC. 2001. Plant secondary compounds; their impact on forage nutritive value and upon animal production. 19th International Grassland Congress; Sao Pedro, Brazil. Eds Gomide JA, Mattos WRS, DaSilva SC. Fundacao Estudos Agrarios Luiz Queiroz (Fealq). pp. 445–452.
- Beatson C. 2017. Urine N excretion and urine N loading of lactating dairy cows fed ryegrass and plantain as forage and silage. A Dissertation submitted in partial fulfilment of the requirements for the Degree of Bachelor of Agricultural Science with Honours. Lincoln University.
- Beeckman F, Motte H, Beeckman T. 2018. Nitrification in agricultural soils: impact, actors and mitigation. Current Opinion in Biotechnology. 50:166–173.
- Betteridge K, Costall DA, Li FY, Luo D, Ganesh S. 2013. Why we need to know what and where cows are urinating - a urine sensor to improve nitrogen models. Proceedings of the New Zealand Grassland Association. 75:119–124.
- Beukes PC, Gregorini P, Romera AJ, Woodward SL, Khaembah EN, Chapman DF, Nobilly F, Bryant RH, Edwards GR, Clark DA. 2014. The potential of diverse pastures to reduce nitrogen leaching on New Zealand dairy farms. Animal Production Science. 54:1971–1979.
- Biere A, Marak HB, Damme J. 2004. Plant chemical defense against herbivores and pathogens: generalized defense or trade-offs? Oecologia. 140:430–441.
- Black JL. 1971. A theoretical consideration of the effect of preventing rumen fermentation on the efficiency of utilization of dietary energy and protein in lambs [article]. British Journal of Nutrition. 25:31–55.
- Bohnert DW, Larson BT, Lewis SJ, Richards KC, Swanson KC, Harmon DL, Mitchell GE. 1999. Net nutrient flux in visceral tissues of lambs fed diets differing in supplemental nitrogen source. Journal of Animal Science. 77:2545–2553.
- Boudon A, Peyraud JL. 2001. The release of intracellular constituents from fresh ryegrass (Lolium perenne L.) during ingestive mastication in dairy cows: effect of intracellular constituent, season and stage of maturity. Animal Feed Science and Technology. 93:229–245.
- Bowatte S, Barrett B, Luscombe C, Hume DE, Luo DW, Theobald P, Newton PCD. 2011. Effect of grass species and fungal endophyte on soil nitrification potential. New Zealand Journal of Agricultural Research. 54:275–284.
- Box LA, Edwards GR, Bryant RH. 2016. Milk production from late lactation cows grazing temporally and spatially separated monocultures of plantain and pasture. 7th Australasian Dairy Science Symposium; 16–18 November; Sydney, Australia.
- Box LA, Edwards GR, Bryant RH. 2017a. Diurnal changes in the nutritive composition of four forage species at high and low N fertiliser. Journal of New Zealand Grasslands. 79:111–118.
- Box LA, Edwards GR, Bryant RH. 2017b. Milk production and urinary nitrogen excretion of dairy cows grazing plantain in early and late lactation. New Zealand Journal of Agricultural Research. 60:470–482.
- Box LA, Edwards GR, Bryant RH. 2019. Seasonal and diurnal changes in aucubin, catalpol and acteoside concentration of plantain herbage grown at high and low N fertiliser inputs. New Zealand Journal of Agricultural Research. 62:343–353.
- Brautigam M, Franz G. 1985. Structural features of Plantago lanceolata mucilage. Planta Medica. 4:293–297.
- Bryant RH, Dalley DE, Gibbs J, Edwards GR. 2014. Effect of grazing management on herbage protein concentration, milk production and nitrogen excretion of dairy cows in mid-lactation. Grass and Forage Science. 69:644–654.
- Bryant RH, Gregorini P, Edwards GR. 2012. Effects of N fertilisation, leaf appearance and time of day on N fractionation and chemical composition of Lolium perenne cultivars in spring. Animal Feed Science and Technology. 173:210–219.
- Bryant RH, Miller ME, Greenwood SL, Edwards GR. 2017. Milk yield and nitrogen excretion of dairy cows grazing binary and multispecies pastures. Grass and Forage Science. 72:806–817.
- Bryant RH, Welten BG, Costall D, Shorten PR, Edwards GR. 2018. Milk yield and urinary-nitrogen excretion of dairy cows grazing forb pasture mixtures designed to reduce nitrogen leaching. Livestock Science. 209:46–53.
- Cameron KC, Di HJ, Moir JL. 2013. Nitrogen losses from the soil/plant system: a review. Annals of Applied Biology. 162:145–173.
- Carlton AJ, Cameron KC, Di HJ, Edwards GR, Clough TJ. 2019. Nitrate leaching losses are lower from ryegrass/white clover forages containing plantain than from ryegrass/white clover forages under different irrigation. New Zealand Journal of Agricultural Research. 62:150–172.
- Castillo AR, Kebreab E, Beever DE, France J. 2000. A review of efficiency of nitrogen utilisation in lactating dairy cows and its relationship with environmental pollution. Journal of Animal and Feed Sciences. 9:1–32.
- Chapman DF, Lee JM, Rossi L, Cosgrove GP, Stevens DR, Crush JR, King WM, Edwards GR, Popay AJ. 2018. Implications of grass-clover interactions in dairy pastures for forage value indexing systems. 1. context and rationale. New Zealand Journal of Agricultural Research. 61:119–146.
- Cichota R, Snow VO, Tait AB. 2008. A functional evaluation of virtual climate station rainfall data. New Zealand Journal of Agricultural Research. 51:317–329.
- Cichota R, Vogeler I, Snow V, Shepherd M, McAuliffe R, Welten B. 2018. Lateral spread affects nitrogen leaching from urine patches. Science of the Total Environment. 635:1392–1404.
- Clemensen AK, Provenza FD, Lee ST, Gardner DR, Rottinghaus GE, Villalba JJ. 2017. Plant secondary metabolites in alfalfa, birdsfoot trefoil, reed canarygrass, and tall fescue unaffected by two different nitrogen sources. Crop Science. 57:964–970.
- Clemensen AK, Rottinghaus GE, Lee ST, Provenza FD, Villalba JJ. 2018. How planting configuration influences plant secondary metabolites and total N in tall fescue (Festuca arundinacea Schreb.), alfalfa (Medicago sativa L.) and birdsfoot trefoil (Lotus corniculatus L.): implications for grazing management. Grass and Forage Science. 73:94–100.
- Close DC, McArthur C. 2002. Rethinking the role of many plant phenolics - protection from photodamage not herbivores? Oikos. 99:166–172.
- Combellas J, Hodgson J. 1979. Herbage intake and milk production by grazing dairy cows 1. The effects of variation in herbage mass and daily herbage allowance in a short-term trial. Grass and Forage Science. 34:209–214.
- Darrow K, Bowers MD. 1997. Phenological and population variation in iridoid glycodsides of Plantago lanceolata (Plantaginaceae). Biochemical Systematics and Ecology. 25:1–11.
- Davies A. 2001. Competition between grasses and legumes in established pastures. In: Tow PG, Lazenby A, editor. Competition and succession in pastures. Wallingford, UK: Cabi; p. 63–83.
- Di HJ, Cameron KC. 2002. Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutrient Cycling in Agroecosystems. 64:237–256.
- Dijkstra J, Oenema O, Groenigen JWV, Spek JW, Vuuren AMV, Bannink A. 2013. Diet effects on urine composition of cattle and N2O emissions. Animal. 7:292–302.
- Dodd MB, Dalley DE, Elliot WC. 2017. Establishment year productivity, botanical composition and nutritive value of grass/lucerne/plantain dairy pasture mixtures. Journal of New Zealand Grasslands. 79:223–228.
- Dodd MB, Dalley DE, Wims CM, Elliott D, Griffen A. 2018. A comparison of temperate pasture species mixtures selected to increase dairy cow production and reduce urinary nitrogen excretion. New Zealand Journal of Agricultural Research. doi:10.1080/00288233.2018.1518246.
- Domingue BMF, Dellow DW, Wilson PR, Barry TN. 1991. Comparative digestion in deer, goats, and sheep. New Zealand Journal of Agricultural Research. 34:45–53.
- Easton HS, Stewart AV, Lyons TB, Parris M, Charrier S. 2009. Soluble carbohydrate content of ryegrass cultivars. Proceedings of the New Zealand Grassland Association. 71:161–166.
- Edwards GR, Bryant RH, Smith N, Hague H, Taylor S, Ferris A, Farrell L. 2015. Milk production and urination behaviour of dairy cows grazing diverse and simple pastures. Proceedings of the New Zealand Society of Animal Production. 75:79–83.
- Edwards GR, Lucas RJ, Johnson MR. 1993. Grazing preference for pasture species by sheep is affected by endophyte and nitrogen fertility. Proceedings of the New Zealand Grassland Association. 55:137–141.
- Edwards GR, Parsons AJ, Rasmussen S, Bryant RH. 2007. High sugar ryegrasses for livestock systems in New Zealand. Proceedings of the New Zealand Grassland Association. 69:161–171.
- Elizalde JC, Merchen NR, Faulkner DB. 1999. Fractionation of fiber and crude protein in fresh forages during the spring growth. Journal of Animal Science. 77:476–484.
- Fraser PM, Cameron KC, Sherlock RR. 1994. Lysimeter study of the fate of nitrogen in animal urine returns to irrigated pasture. European Journal of Soil Science. 45:439–447.
- Fuchs B, Krischke M, Mueller MJ, Krauss J. 2017. Herbivore-specific induction of defence metabolites in a grass-endophyte association. Functional Ecology. 31:318–324.
- Fulkerson WJ, Slack K, Hennessy DW, Hough GM. 1998. Nutrients in ryegrass (Lolium spp.), white clover (Trifolium repens) and kikuyu (Pennisetum clandestinum) pastures in relation to season and stage of regrowth in a subtropical environment. Australian Journal of Experimental Agriculture. 38:227–240.
- Gastal F, Lemaire G. 2002. N uptake and distribution in crops: an agronomical and ecophysiological perspective. Journal of Experimental Botany. 53:789–799.
- Giger-Reverdin S, Maaroufi C, Peyronnet C, Sauvant D. 2015. Effects of particle size and dietary nitrogen content on the nutritive value of pea-based diets in mid-lactation goats. Animal Feed Science and Technology. 210:56–65.
- Gregorini P, Bryant RH, Beck MR, Edwards GR. 2018. Plantain: It is not only the dietary content, but how we graze it. New Zealand Journal of Animal Science and Production. 78:151–156.
- Gregorini P, Provenza FD, Villalba JJ, Beukes PC, Forbes MJ. 2018. Diurnal patterns of urination and drinking by grazing ruminants: a development in a mechanistic model of a grazing ruminant, MINDY. Journal of Agricultural Science. 156:71–81.
- Hart EH, Onime LA, Davies TE, Morphew RM, Kingston-Smith AH. 2016. The effects of PPO activity on the proteome of ingested red clover and implications for improving the nutrition of grazing cattle. Journal of Proteomics. 141:67–76.
- Hartinger T, Gresner N, Sudekum KH. 2018. Does intra-ruminal nitrogen recycling waste valuable resources? A review of major players and their manipulation. Journal of Animal Science and Biotechnology. doi:10.1186/s40104-018-0249-x.
- Hoekstra NJ, Schulte RPO, Struik PC, Lantinga EA. 2007. Pathways to improving the N efficiency of grazing bovines. European Journal of Agronomy. 26:363–374.
- Hoekstra NJ, Struik PC, Lantinga EA, Amburgh MEV, Schulte RPO. 2007. Can herbage nitrogen fractionation in Lolium perenne be improved by herbage management? NJAS - Wageningen Journal of Life Sciences. 55:167–180.
- Holzworth DP, Huth NI, Devoil PG, Zurcher EJ, Herrmann NI, McLean G, Chenu K, van Oosterom EJ, Snow V, Murphy C, et al. 2014. APSIM - Evolution towards a new generation of agricultural systems simulation. Environmental Modelling and Software. 62:327–350.
- Hoskin SO, Wilson PR, Ondris M, Bunod AH. 2006. The feeding value of forage herbs: studies with red deer. Proceedings of the New Zealand Grassland Association. 68:199–204.
- Jiang S, Bryant RH, Jiao J, Tung R. 2019. Brief communication: investigation of the water-soluble carbohydrates content of Plantain (Plantago Lanceolata L.). New Zealand Journal of Animal Science and Production. 79:174–176.
- Judson HG, Moorhead AJE, Fraser PM, Peterson M, Kemp PD, Edwards GR. 2018. Reducing nitrate leaching from the urine patch – a plant based approach. Proceedings of the 8th Australasian Dairy Science Symposium pp. 46–53. Palmerston North.
- Kardošová A. 1992. Polysaccharides from the leaves of Plantago lanceolata L., var. LIBOR: an a-D-Glucan. Chemical Papers. 46:127–130.
- Kay J, Edwards P, Clement AR, Bryant R. 2018. Effect of pre-graze mowing at different pre-graze masses on cow and pasture performance Sustainable meat and milk production from grasslands. In: Proceedings of the 27th General Meeting of the European Grassland Federation, Cork, Ireland. Teagasc. pp 268–270.
- Kebreab E, France J, Beever DE, Castillo AR. 2001. Nitrogen pollution by dairy cows and its mitigation by dietary manipulation. Nutrient Cycling in Agroecosystems. 60:275–285.
- Kingston-Smith AH, Bollard AL, Armstead IP, Thomas BJ, Theodorou MK. 2003. Proteolysis and cell death in clover leaves is induced by grazing. Protoplasma. 220:119–129.
- Kingston-Smith AH, Bollard AL, Thomas HM, Theodorou MK. 2002. The potential to decrease nitrogen losses from pasture by manipulating plant metabolism. Proceedings of the 19th General Meeting of the European Grassland Federation, La Rochelle, France, 27-30 May 2002. Pp. 136–137.
- Kingston-Smith AH, Merry RJ, Leemans DK, Thomas H, Theodorou MK. 2005. Evidence in support of a role for plant-mediated proteolysis in the rumens of grazing animals. British Journal of Nutrition. 93:73–79.
- de Klein CAM, van der Weerden TJ, Luo J, Cameron KC, Di HJ. 2019. A review of plant options for mitigating nitrous oxide emissions from pasture-based systems. New Zealand Journal of Agricultural Research. doi:10.1080/00288233.2019.1614073.
- Koenig KM, Beauchemin KA. 2018. Effect of feeding condensed tannins in high protein finidhing diets containing corn and distillers grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. Journal of Animal Science. 96:4398–4413.
- Krawutschke M, Kleen J, Weiher N, Loges R, Taube F, Gierus M. 2013. Changes in crude protein fractions of forage legumes during the spring growth and summer regrowth period. Journal of Agricultural Science. 151:72–90.
- Ledgard SF, Welton B, Betteridge K. 2015. Salt as a mitigation option for decreasing nitrogen leaching losses from grazed pastures. Journal of the Science of Food and Agriculture. 95:3033–3040.
- Lee MRF. 2014. Forage polyphenol oxidase and ruminant livestock nutrition. Frontiers in Plant Science. 5:694.
- Lee MRF, Colmenero JDJO, Winters AL, Scollan ND, Minchin FR. 2006. Polyphenol oxidase activity in grass and its effect on plant-mediated lipolysis and proteolysis of Dactylis glomerata (cocksfoot) in a simulated rumen environment. Journal of the Science of Food and Agriculture. 86:1503–1511.
- Lee MRF, Harris LJ, Moorby JM, Humphreys MO, Theodorou MK, MacRae JC, Scollan ND. 2002. Rumen metabolism and nitrogen flow to the small intestine in steers offered Lolium perenne containing different levels of water-soluble carbohydrate. Animal Science. 74:587–596.
- LIC and DairyNZ. 2018. New Zealand Dairy Statistics 2017–18. Hamilton (New Zealand): Livestock Improvement Corporation Limited & DairyNZ Limited.
- Li FY, Betteridge K, Cichota R, Hoogendorn CJ, Jolly BH. 2012. Effects of nitrogen load variation in animal urination events on nitrogen leaching from grazed pasture. Agriculture, Ecosystems & Environment. 159:81–89.
- Licitra G, Hernandez TM, Van Soest PJ. 1996. Standardization of procedures for nitrogen fractionation of ruminant feeds. Animal Feed Science and Technology. 57:347–358.
- Litherland AJ, Lambert MG. 2007. Chapter 6. factors affecting the quality of pastures and supplements produced on farms. In: PV Rattray, IM Brookes, AM Nicol, editor. Pastures and Supplements for Grazing Animals. Hamilton (New Zealand): New Zealand Society of Animal Production Occasional Publication No. 14; p. 81–96.
- Lu CD, Jorgensen NA. 1987. Alfalfa saponins affect site and extent of nutrient digestion in ruminants. Journal of Nutrition. 117:919–927.
- Malik PK, Singhal KK. 2008. Saponin content of lucerne fodder and its effect on rumen fermentation and microbial population in crossbred bulls. Indian Journal of Animal Sciences. 78:298–301.
- Mangwe MC, Bryant RH, Beck MR, Beale N, Bunt C, Gregorini P. 2019. Forage herbs as an alternative to ryegrass-white clover to alter urination patterns in grazing dairy systems. Animal Feed Science and Technology. 252:11–22.
- Mangwe MC, Bryant RH, Beck MR, Fleming AE, Gregorini P. 2018. Grazed chicory, plantain or ryegrass–white clover alters milk yield and fatty acid composition of late-lactating dairy cows. Animal Production Science. doi 10.1071/AN18537.
- Martin K, Bryant RH, Hodge S, Edwards GR. 2017a. Effect of autumn regrowth interval and nitrogen fertiliser on dry matter yield and plant characteristics of six forage species. Journal of New Zealand Grasslands. 79:61–66.
- Martin K, Edwards G, Bryant R, Hodge M, Moir J, Chapman D, Cameron K. 2017b. Herbage dry-matter yield and nitrogen concentration of grass, legume and herb species grown at different nitrogen-fertiliser rates under irrigation. Animal Production Science. 57(7):1283–1288.
- Mathisen O, Raeder M, Kill F. 1981. Mechanisms of osmotic diuresis. Kidney International. 19:431–437.
- McDonald P, Edwards RA, Greenhalgh JFD, Morgan CA. 2002. Animal Nutrition. 6th ed. Harlow (UK): Prentice Hall.
- McGechan MB, Topp CFE. 2004. Modelling environmental impacts of deposition of excreted nitrogen by grazing dairy cows. Agriculture, Ecosystems & Environment. 103:149–164.
- Merrill ME. 2008. Chapter 6, Kidneys and Renal Physiology. In: Our marvellous bodies: An Introduction to the Physiology of Human Health. U.S.A: Rutgers University Press; p. 94–108.
- Miller LA, Moorby JM, Davies DR, Humphreys MO, Scollan ND, MacRae JC, Theodorou MK. 2001. Increased concentration of water-soluble carbohydrate in perennial ryegrass (Lolium perenne): milk production from late-lactation dairy cows. Grass and Forage Science. 56:383–394.
- Min BR, Barry TN, Attwood GT, McNabb WC. 2003. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Animal Feed Science and Technology. 106:3–19.
- Minnee EMK, Waghorn GC, Gregorini P, Bryant RH, Chapman DF. 2018. Characterisics of boli formed by dairy cows upon ingestion of fresh ryegrass, lucerne or chicory. Animal. 15:1–10.
- Minnee EMK, Waghorn GC, Lee JM, Clark CEF. 2017. Including chicory or plantain in a perennial ryegrass/white clover-based diet of dairy cattle in late lactation: feed intake, milk production and rumen digestion. Animal Feed Science and Technology. 227:52–61.
- Moorby JM, Evans RT, Scollan ND, MacRae JC, Theodorou MK. 2006. Increased concentration of water-soluble carbohydrate in perennial ryegrass (Lolium perenne L.). evaluation in dairy cows in early lactation. Grass and Forage Science. 61:52–59.
- Navarrete S, Kemp PD, Pain SJ, Back PJ. 2016. Bioactive compounds, aucubin and acteoside, in plantain (Plantago lanceolata L.) and their effect on in vitro rumen fermentation. Animal Feed Science and Technology. 222:158–167.
- Nicols SN, Crush JR. 2007. Selecting forage grasses for improved nitrate retention - a progress report. Proceedings of the New Zealand Grassland Association. 69:207–211.
- Nkomboni D. 2017. Effect of plantain (Plantago lanceolata L.) proportion in the diet on nitrogen use, milk production and behaviour of lactating dairy cows. Masters Dissertation. Canterbury, New Zealand: Lincoln University.
- Noh JY, Han DH, Yoon JA, Kim MH, Ko IG, Kim EJ, Kim KH, Kim CJ, Cho S. 2011. Circadian ryhthms in urinary functions: possible roles of circadian clocks? International Neurourology Journal. 15:64–73.
- O’Connell CA, Judson HG, Barrell GK. 2016. Sustained diuretic effect of plantain when ingested by sheep. Proceedings of the New Zealand Society of Animal Production. 76:14–17.
- Peyraud JL, Comeron EA, Wade MH, Lemaire G. 1996. The effect of daily herbage allowance, herbage mass and animal factors upon herbage intake by grazing dairy cows. Annales de Zootechnie. 45:201–217.
- Pichard GR, Tesser BR, Vives C, Solari C, Hott A, Larrain RE. 2006. Proteolysis and characterization of peptidases in forage plants. Agronomy Journal. 98:1392–1399.
- Pontes LS, Carrere P, Andueza D, Louault F, Soussana JF. 2007. Seasonal productivity and nutritive value of temperate grasses found in semi-natural pastures in Europe: responses to cutting frequency and N supply. Grass and Forage Science. 62:485–496.
- Poutaraud A, Michelot-Antalik A, Plantureux S. 2017. Grasslands: a source of secondary metabolites for livestock health. Journal of Agricultural and Food Chemistry. 65:6535–6553.
- Rajabi M, Rouzbehan Y, Rezaei J. 2017. A strategy to improve nitrogen utilization, reduce environmental impact, and increase performance and antioxidant capacity of fattening lambs using pomegranate peel extract. Journal of Animal Science. 95(1):499–510.
- Robson MC, Brown HE, Hume E, Lilburne L, Pinxterhuis IJB, Snow VO, Williams RH, B + LNZ, Development Matters, DINZ, ECan, HortNZ, NZPork. 2015. Overview Report – Canterbury Matrix of Good Management Project. Report No. R15/104. (https://www.ecan.govt.nz/document/download?uri=2362429)
- SCA. 1990. Australian Agricultural Council. Ruminants Subcomittee - Feeding Standards for Australian Livestock: Ruminants. CSIRO Australia.
- Selbie DR, Buckthought LE, Shepherd MA. 2015. Chapter four – The challenge of the urine patch for managing nitrogen in grazed pasture systems. Advances in Agronomy. 129:229–292.
- Shepherd M, Shorten PR, Costall D, MacDonald KA. 2017a. Evaluation of urine excretion from dairy cows under two farm systems using urine sensors. Agriculture, Ecosystems & Environment. 236:285–294.
- Shepherd MA, Carlson B, Shorten PR. 2017b. The contribution of the urine patch edge to urine-N uptake: comparison of pasture species. December 2017. Report for AgResearch through SSIF funding. Client Report Number: RE450/2018/002, p. 48.
- Snow VO, Cichota R, McAuliffe RJ, Hutchings NJ, Vejlin J. 2017. Increasing the spatial scale of process-based agricultural systems models by representing heterogeneity: The case of urine patches in grazed pastures. Environmental Modelling & Software. 90:89–106.
- Snow VO, Meenken ED. 2018. Modelling the Likely Diuretic Effects of Plantain on N Leaching. Report for Forages for Reduced Nitrate Leaching Programme Management Committee (FRNL PMC) RE450/2018/026. 46pp.
- Spek JW, Dijkstra J, Duinkerken GV, Bannink A. 2013. A review of factors influencing milk urea concentration and its relationship with urinary urea excretion in lactating dairy cattle. Journal of Agricultural Science. 151:407–423.
- Tait A, Turner R. 2005. Generating multiyear gridded daily rainfall over New Zealand. Journal of Applied Meteorology. 44:1315–1323.
- Takai T, Sanada Y, Yamada T. 2010. Influence of the fungal endophyte Neotyphodium uncinatum on the persistency and competitive ability of meadow fescue (Festuca pratensis Huds.). Grassland Science. 56:59–64.
- Thom ER, Popay AJ, Waugh CD, Minnee EMK. 2014. Impact of novel endophytes in perennial ryegrass on herbage production and insect pests from pastures under dairy cow grazing in northern New Zealand. Grass and Forage Science. 69:191–204.
- Totty VK, Greenwood SL, Bryant RH, Edwards GR. 2013. Nitrogen partitioning and milk production of dairy cows grazing simple and diverse pastures. Journal of Dairy Science. 96:141–149.
- Vibart RE, Koolaard J, Barrett BA, Pacheco D. 2009. Exploring the relationships between plant chemical composition and nitrogen partitioning in lactating dairy cows fed ryegrass-based diets. Proceedings of the New Zealand Society of Animal Production. 69:188–195.
- Villalba JJ, Costes-Thire M, Ginane C. 2017. Phytochemicals in animal health: diet selection and trade-offs between costs and benefits. Proceedings of the Nutrition Society. 76:113–121.
- Waghorn GC, Griffen A, Bryant M, Dalley DE. 2018. Digestion and nitrogen excretion by Holstein/Friesian cows fed grasses with lucerne or lucerne and plantain. Animal Production Science. doi:10.1071/AN18105.
- Wang M, Bezemer TM, Putten WHVD, Biere A. 2015. Effects of the timing of herbivory on plant defense induction and insect performance in ribwort plantain (Plantago lanceolata L.) depend on plant mycorrhizal status. Journal of Chemical Ecology. 41:1006–1017.
- White LM. 1973. Carbohydrate reserves of grasses: a review. Journal of Range Management. 26:13–18.
- Wohlt JE, Sniffen CJ, Hoover WH, Johnson LL, Walker CK. 1976. Nitrogen metabolism in wethers as affected by dietary protein solubility and amino acid profile. Journal of Animal Science. 42(5):1280–1289.
- Woodward SL, Waghorn GC, Bryant M, Benton A. 2012. Can diverse pasture mixtures reduce nitrogen losses? Proceedings of the 5th Australasian Dairy Science Symposium; Melbourne, Australia. pp. 463–464.
