ABSTRACT
In New Zealand, a genetically modified (GM) organism means any organism where genes or other genetic material have been modified by in vitro techniques. This includes New Breeding Technologies (NBT) such as gene editing. The aim here is to (a) examine the importance of consumer attitudes towards food produced from GM plants or from animals fed GM feed and (b) consider whether consumer attitudes would reduce the demand and acceptance of food produced by New Zealand pastoral farmers if GM forages were included in animal feed. Published surveys indicate that consumers were willing to purchase GM foods if they cost less than non-GM foods, although the magnitude of this discount varies across countries, the type of genetic modification and how it affects the food product. While there will always be a proportion of consumers against the use of GM in food production, the published evidence would suggest that the use of GM plants in New Zealand for food production will have no long-term deleterious effects in overseas markets. From a regulatory view point, the focus should be on regulating the benefit-risk issues associated with the end-product of genetic modification rather than the processes used in their development.
Introduction
The global use of genetic modification (GM) in improving yield, quality and environmental impacts (Mahaffey et al. Citation2016; Naranjo et al. Citation2020) of crops has grown consistently over the past 30 years and is now used on 191.7 million ha (ISAAA Citation2018), reaching farm-gate revenues of US$57 billion in 2016 (Scheitrum et al. Citation2020). GM crops have been adopted faster than most if not all crop technologies, particularly in the USA, Brazil, Argentina, Canada and India (Ichim Citation2021; Turnbull et al. Citation2021). Regulatory approaches vary across jurisdictions with perhaps the biggest difference being between the USA (business-like and promoting the profits of the industry) and Europe (restrictive and based on the precautionary principle) (Tsakok and Mengoub Citation2021). However, there has been considerable public concern over GM food and feed that is primarily focused on their impacts on human and animal health, environmental safety, labelling and consumer choice, intellectual property rights, ethics, food safety, poverty reduction and environmental conservation (Bawa and Anilakumar Citation2013; Blagoevska et al. Citation2021; Sanmugam et al. Citation2021). As new techniques for gene manipulation become mainstream it is appropriate that a systematic review is undertaken of consumer attitudes towards food resulting from genetically modified organisms and whether the regulatory processes in place are still fit for purpose.
While some consumers prefer non-GM products this is often based on conflicting or confused views possibly because consumer knowledge on the use of GM technologies in food production is low (reviewed by Wunderlich and Gatto Citation2015). In the USA, Canada, Belgium, France and Australia, a survey showed that 56%, 47%, 46%, 30% and 51% of respondents, respectively, indicated they would consume both GM and gene-edited food (Shew et al. Citation2018). While in other regions including New Zealand (McGuiness Institute Citation2013; Edwards Citation2017), there is continued scepticism and/or an inability to choose to consume GM food (Blagoevska et al. Citation2021; Gbashi et al. Citation2021; Muzhinji and Ntuli Citation2021; Purnhagen et al. Citation2021; Sanmugam et al. Citation2021). Factors influencing the release of GM foods would include – the need to transform the sustainability of agriculture, increasing costs of farm inputs, mitigating agricultural greenhouse gas emissions, adapting to climate change, e.g. more frequent and intense droughts, increasing animal welfare concerns and improving the precision of GM techniques (RSNZ Citation2010). Indeed, it is propositioned that GM cultivars are safer than those bred using traditional methods without the use of genetic modification because they are made in a very precise manner (Roberts Citation2018). This position is supported by peer-reviewed published work in respected journals and led to the Nobel Laureates Campaign demanding that non-scientific statements be withdrawn in the debate on the safety and value of using a genetic modification for crop development, particularly for the developing world. Some commentators have determined that banning GM crop use may prove detrimental to national and international economies (Lee and Giesbrecht Citation2021). They noted that using GM crops has increased farming efficiency, cost-effectiveness, a rise in income for developing countries, and diminish global economic inequality.
In New Zealand, genetic modification is regulated under the Hazardous Substances and New Organisms (HSNO) Act 1996 administered by the Ministry for the Environment. While research and development on genetic modification of plants is permitted indoors with the appropriate and approved level of containment, there have been few applications over the past 25 years for evaluation of genetically modified plants in outdoor containment trials. New Zealand’s image of being clean, green and ‘100% Pure’ has also provided impetus for those against the use and exploitation of GM plants to align this branding with a genetically modified organism (GMO)-free status (Edwards Citation2017). This connection between using GM technologies and losing the ‘clean, green and 100% Pure’ brand is vague and perception with no hard fact-based reality. The 100% Pure brand was launched by Tourism NZ to emphasise the purity of the New Zealand experience rather than the narrow notion of natural purity (Tourism NZ Citation2009). According to Kaefer (Citation2016):
the origin of the phrase “clean and green” is uncertain though its origins are believed to be relatively recent, linked to the Rainbow Warrior incident in 1985 and New Zealand’s ‘stance against nuclear energy and genetically modified organisms in the 1980s’ particularly the passing of the NZ Nuclear Free Zone, Disarmament, and Arms Control Act in 1987.
Interestingly, the first consumer GMO product developed through genetic engineering was to produce human insulin to treat diabetes was approved by the USA Food and Drug Agency in 1982. But the first GMO product created through genetic engineering was the Flavr Savr tomato which became available for sale in 1994 after studies evaluated by federal agencies in the USA proved it to be as safe as traditionally bred tomatoes (Food and Drug Administration Citation2022). Coyle and Fairweather (Citation2005) considered the timing of the ‘clean green image’ of New Zealand being adopted by an anti-biotechnology lobby coincided with the government trying to co-create New Zealand as an innovator in biotechnology. It could be argued that the use of GM technologies to combat pest and disease challenges would lead to a reduction in synthetic chemical applications in the form of pesticides and fungicides, and therefore become a part of environmental beneficial integrated pest management systems (Naranjo et al. Citation2020).
While not necessarily opposed to GM technologies, many exporting companies in New Zealand view the GMO-free brand as a marketing opportunity. Yet despite this the New Zealand government has continued to fund research on genetically modified organisms (Caradus Citation2008). The regulatory framework established from the HSNO Act to provide the Environmental Protection Authority (EPA) a process for decision making uses a case-by-case approach for assessing the risk-cost–benefit of each genetic modification event (Dinica Citation2021). This process has been labelled as technocratic and inconsistent with the precautionary principle of regulation (McGuiness Institute Citation2013) and needs to be changed (Rolleston Citation2010). The McGuiness Institute report also concluded that ‘New Zealand is no further ahead on public policy regarding outdoor use of GMOs than it was when the Commissioners reported their findings in 2001’. In the 9 years since the report from the McGuiness Institute was published nothing has changed and indeed New Zealand continues to be poorly equipped to make a strategic decision on the release GMOs to the outdoors (Howard Citation2022). A further report published by the New Zealand Productivity Commission Citation2021 entitled ‘New Zealand firms: Reaching for the frontier’ concluded that New Zealand’s approach to regulating genetic modification techniques under the Hazardous Substances and New Organisms Act 1996 was last reviewed in 2001 and does not reflect technological advances since that time. It boldly recommended that the Government should undertake a full review of the regulation of genetic modification, to ensure it is fit for purpose and supports domestic innovation. On this topic, the report proposed 10 recommendations of which six were:
consider the emerging regulatory approaches in other jurisdictions, particularly New Zealand’s key product destination and competitor markets;
consider the trade and regulatory enforcement impacts from different treatment of GM technologies in different markets;
assess consumer attitudes in New Zealand and internationally;
consider the potential impacts on New Zealand firms that wish to retain GM-free status, and on New Zealand’s reputation and brand more generally;
assess the fitness for purpose of the current regulatory oversight and enforcement arrangements;
consider the merits of separate legislation and/or a standalone regulator for genetic technologies.
The Royal Commission on Genetic Modification (Eichelbaum et al. Citation2001) stated that – ‘Genetic modification has been used freely in New Zealand for more than a decade as a research tool, for medical purposes, and in food ingredients’. Indeed, Food Standards Australia and New Zealand (FSANZ), which is governed by separate legislation, lists on its website (FSANZ Citation2021) 93 foods of plant origin across 10 species, and 4 foods of microbial origin produced using gene technologies. The intended use of the microbial GM food ingredients is as analogues of meat and infant formula. So, while the New Zealand public can purchase food with GM ingredients, appropriately labelled, it is currently not possible for New Zealand farmers to use GM technologies in food production.
The Royal Commission on Genetic Modification (Eichelbaum et al. Citation2001) further contended that ‘genetic modification holds exciting promise, not only for conquering diseases, eliminating pests and contributing to the knowledge economy, but for enhancing the international competitiveness of the primary industries so important to our country’s economic well-being’. And –
Technology is integral to the advancement of the world. Fire, the wheel, steam power, electricity, radio transmission, air and space travel, nuclear power, the microchip, DNA: the human race has ever been on the cusp of innovation. Currently, biotechnology is the new frontier. Continuation of research is critical to New Zealand’s future. As in the past, we should go forward but with care.
However, while genetic modification has continued to be used as a research tool and for medical purposes (Kannan and Najjar Citation2020), where it has been shown to provide benefits and be safe, it has gained no traction, momentum or impact in improving New Zealand’s economic wellbeing. The option to ‘go forward but with care’ has in conjunction with the HSNO Act stopped attempts to bring genetic modification technologies out of containment, so that demonstrated benefits in controlled environments can be extended to real-world field trials.
Reasons for lack of applications to EPA for release of GMOs from containment include:
Perceived difficulty in obtaining a positive outcome
Reactions from the anti-GMO lobby
Time and cost
A reluctance by Governments of either persuasion to review and change the HSNO Act despite extensive lobbying; and perhaps most importantly
A reluctance from agricultural and horticultural exporters to publicly support this technology, support that would inevitably be required for a successful EPA application hearing.
As others have stated ‘the future of New Zealand’s bioeconomy requires theoretical concepts and analytical frameworks able to capture national public debates on the implications of genetic-engineering, relative to other biotechnologies and bioresources’ (Dinica Citation2021). Some have argued that New Zealand's regulatory framework warrants review in light of the development of recent advanced genetic technologies (Fritsche et al. Citation2018; Everett-Hincks and Henaghan Citation2019). The aim here is to:
examine the importance of consumer attitudes towards food produced from genetically modified plants or from animals fed genetically modified feed and
consider whether consumer attitudes would reduce the demand and acceptance of food produced by New Zealand pastoral farmers if genetically modified forages were included in animal feed.
To that end, the paper will undertake a systematic review and after defining terms associated with the topic, overview the relevant regulatory environment in New Zealand, review country of origin effects, seek to review societal attitudes to GM food and understand what influences those attitudes, examine the link and importance of intellectual property protection rights on attitudes towards GM technologies, determine the demand for and importance of product labelling and provide a commentary on the impact of regulation on R&D investment in New Zealand.
Definitions of genetic modification
Genetic variation amongst organisms can occur through evolutionary selection processes, man-directed selection (breeding), mutagenesis either spontaneously or accelerated through the use of mutagens, untargeted and targeted editing of genes, and the introduction of ‘foreign’ genes. Some of these are regulated in New Zealand – namely untargeted and targeted editing of genes (often referred to as gene editing or targeted gene editing), and the introduction of ‘foreign’ genes (often referred to as genetically modified (GM) or transgenic organisms)
New Breeding Technologies (NBT) (synonymous with the term New Genomic Techniques (NGT) (Parisi and Rodríguez-Cerezo Citation2021)) is another term gaining traction and includes –
genome or gene editing to modify DNA at one or more specific sites using CRISPR, Zinc Finger Nucleases or TALENs (RSNZ Citation2016);
introducing targeted changes to a small number of bases of DNA using oligonucleotide-directed mutagenesis to;
cisgenesis (transferring a gene from the same or a closely related species);
intragenesis (inserting a reorganised regulatory coding region of a gene from the same species);
using epigenetic processes to change the activity of genes without changing a DNA sequence.
Here genetic modification (GM) is defined, as it is in current New Zealand legislation, would include New Breeding Technologies (NBT) and genetic modification resulting in a transgenic organism (GMT). While definitions of what genetic modification, genetic engineering and gene editing vary the definition used here is taken from the New Zealand HSNO Act (New Zealand Legislation Citation1996) – a genetically modified organism means, unless expressly provided otherwise by regulations, any organism in which any of the genes or other genetic material –
have been modified by in vitro techniques or
are inherited or otherwise derived, through any number of replications, from any genes or other genetic material which has been modified by in vitro techniques.
Unfortunately, a relevant definition of in vitro was not provided in the subsequent regulations that resulted from the passing of the HSNO Act. In vitro is derived from Latin for ‘within glass’ and so refers to an action that happens outside of a living organism, as distinct from in vivo where the action is performed in a whole living organism.
Gene editing and genetic modification
Gene editing using the CRISPR-Cas9 (clustered, regularly interspaced, short palindromic repeats – associated protein Cas9) system allows DNA of an organism to be altered in a very precise manner and has been heralded as the most significant recent addition to the modern biotechnology toolbox (Hudson et al. Citation2019). Gene editing has been shown to provide value for medical purposes (Carroll Citation2016; Delhove et al. Citation2020), for improving plant and animal production and quality (Ellens et al. Citation2019), resistance to insect pests (Tyagi et al. Citation2020) and pathogens (Zaidi et al. Citation2018; Pixley et al. Citation2019), and to introduce sterility genes to limit the impact and population growth of introduced pest species, both invertebrate (Alphey and Bonsall Citation2018; Scott et al. Citation2018) and vertebrate (Prowse et al. Citation2017). Early in its development gene editing use for medical purposes was cautioned due to concern that the methods were neither efficient nor safe enough to ensure beneficial outcomes (Carroll Citation2016). But now the use of CRISPR to treat a host of diseases in people is moving closer to reality (Ledford Citation2020). Some of the COVID19 vaccines have used genetic engineering in their development and are deemed safe and appropriate for use (Lynas Citation2020). In 2020, the EU adopted new regulations to allow the development and testing of vaccines using genetically modified organisms (News European Parliament Citation2020). Concern has been expressed that gene editing will suffer the same fate as genetic modification through generating public mistrust and suspicion if there is not greater transparency and better oversight of how these technologies are deployed to deliver tangible benefits (Shew et al. Citation2018; Gordon et al. Citation2021; Lassoued et al. Citation2021). In New Zealand, gene editing is controlled and managed as a GM organism even where no foreign DNA remains in the edited organism (Fritsche et al. Citation2018).
Regulatory options
The advancement of NBT and in particularly gene editing provides an opportunity to review current regulatory frameworks in New Zealand and establish a future-proof framework to keep abreast of rapidly advancing technologies (Everett-Hincks and Henaghan Citation2019). They proposed a potential solution that would be product-directed legislation in contrast to process-directed legislation (e.g. evaluating agri-food products based on an examination of the actual characteristics of the new food on our health and not on the processes or techniques used to obtain the food). Interestingly, this is the approach that has been taken by FSANZ in determining the list of safe GM-produced foods listed on their website (FSANZ Citation2021). Some of these have been approved since the year 2000 and include amongst others, products derived from soybean developed with herbicide tolerance, high oleic acid, insect protection, stearidonic acid content; from canola developed with herbicide tolerance; maize developed with insect protection, herbicide tolerance, high lysine, drought tolerance, increased yield; wheat with herbicide and drought resistance; potato developed with insect and virus resistance and reduced acrylamide production; safflower with high oleic lipid content; and from lucerne developed with herbicide tolerance and reduced lignin (which would have been feed to farmed animals).
New Zealand has a process-based approach to GM regulation as has the European Union, while Canada has adopted a ‘novel product’ based approach (Smyth Citation2017a) and the United States has implemented a product-based regulation system (Ishii and Araki Citation2017). They concluded that ‘with regard to New Zealand, the amended regulations under the HSNO Act of 1996 are ambiguous regarding the details of the regulatory review of a genome-edited crop (EPA Citation2016), which may hamper communications among regulators, developers and the public’. The rationale for this cautious approach is that New Zealand exports billions of dollars of food products and market perceptions need to be balanced against the science involved. Despite the government at the time committing to continue to monitor global rules associated with the regulation of GMOs and adapt the regulatory system to be in line with international developments, nothing has changed or even been examined to determine if change might be required.
It has been proposed that regulating by product provides the advantage of being able to be much more specific about both the degree of risk that might be anticipated and the level of caution that is acceptable or required (Charo Citation2015 – quoted in Everett-Hincks and Henaghan Citation2019). Indeed, the process-based regulatory system is likely to become obsolete and irrelevant with the continued advance in gene editing technologies (Marchant and Stevens Citation2015; Everett-Hincks and Henaghan Citation2019). New Zealand urgently needs to examine the option of moving to a regulatory system based on evaluating the value and potential risk of the end product/technology rather than simply regulating the processes by which such technology is produced (Smyth Citation2017a; Genome British Columbia Citation2020).
The ability of consumers to either be able to or even want to distinguish between gene-edited plants, where there may be no detectable evidence of gene manipulation within the genome, and genetic modification where genes are introduced into the genome can be a real issue. In Japan, gene-edited plants which cannot be distinguished easily from natural non-edited plants are still viewed as genetically modified (Otsuka Citation2021), as they are in New Zealand and Europe. In these jurisdictions, naturalness is defined as the lack of artificial manipulation, while others may define it according to its similarity to nature, even though the manipulation cannot be measured. However, some consumers are willing to accept gene transfers as a protection measure, provided that the gene has come from wild germplasm of the same species (Saleh et al. Citation2021). Some countries have determined that if the genome edits are simply base pair deletions (using site-directed nucleases (SDN-1) without introduced templates to guide genome repair) or a single or multiple base pair change that is already present in nature or could occur naturally, and the end product is indistinguishable from products produced through traditional plant breeding then they do not need to be regulated. This has occurred in the USA (United States Department of Agriculture Citation2018), Australia (Mallapaty Citation2019), Brazil (CitationGenetic Literacy Project A) and Argentina (CitationGenetic Literacy Project B), but to date not in New Zealand.
Country of origin effects
New Zealand’s top trading partners are China, Australia, the USA and Japan who collectively take 58% of New Zealand’s exports (World Bank Citation2019). China, New Zealand’s largest trading partner imports about $17 billion of goods, namely dairy, meat, wood and preparations cereals, flour and starch (Ministry of Foreign Affairs and Trade [MFAT] Citation2022). In 2021, China imported 44% of dairy, 90% of logs and 41% of meat exported by New Zealand (Xinhua Citation2021). China is also Australia’s major export market (Department of Foreign Affairs and Trade Citation2021) despite Australia permitting under regulation the production of some GM crops (Ishii and Araki Citation2017). While primarily these exports are ores and minerals (Trading Economics Citation2022), China is still a top market for Australian exports of meat, wine, wool, fruit and nuts, seafood, grains and dairy (Department of Foreign Affairs and Trade Citation2021). China itself has the sixth-highest area under commercial cultivation of GM crops among 28 countries known to grow GM crops (Ishii and Araki Citation2017). Additionally, China is the largest importer of soybean (97 million MT – over 6 times greater than the next highest importer the European Union) (Shahbandeh Citation2022) most of which will come from the USA and South America where soybean crops are largely GM varieties.
In 2007, the Australian Department of Agriculture, Fisheries and Forestry (DAFF) declared that ‘marketers of GM canola and of products from livestock fed on GM materials, including GM canola, are unlikely to be disadvantaged in the Australian and world markets’ (DAFF Citation2007). This article also noted that ‘when Canada introduced GM canola, it lost access to the EU market for its canola seed. However, Canada has found ready markets for its increased canola supplies elsewhere, particularly in Mexico, the United States, Pakistan and China’. A recent analysis of opportunity costs due to some States in Australia delaying the adoption of GM canola resulted in foregone output of 1.1 million T of canola and a net economic loss to canola farmers of AU$485.6 million (Biden et al. Citation2018). However, even here the uptake of new breeding technologies in Australia will be the decision of its major exporters, since many of its major crops, such as wheat, are exported (Eriksson et al. Citation2019).
The perception of a country can have a marked effect on consumer preferences, but this can vary depending on the product category (Roth and Romeo Citation1992). In China, the country of origin of a product has an important influence on the purchase of dairy products, because of the quality and safety implications (Yang et al. Citation2018). As a result, Chinese residents show a preference towards dairy products from foreign countries, particularly Australia, New Zealand, Germany, Netherlands and the USA. Reasons for this included a lack of confidence in the Chinese dairy processing industry and their risk averse nature which seeks information on product quality and health benefits. In addition, the food scandals that occurred in the early 2000s have resulted in the Chinese approach to food safety being defined as precautionary authoritarianism and has resulted in more stringent provisions to repress violations (Bozzini and Sicurelli Citation2021). Interestingly, purchasing agents or gatekeepers place more importance on country of origin for product purchases than do consumers (Liefeld Citation1993).
In a study undertaken in the first decade of 2000s, while European consumers were not yet ready to accept GM food, there was no relationship between countries that produce and market GM food (e.g. the USA, Canada and Argentina) and their image as a country supplying high-quality food (Knight et al. Citation2005). For European countries, trust in the ability of a country to establish and use the best quality control, traceability systems and technology available while not overcharging has been perceived as providing an excellent country of origin image (Knight et al. Citation2007a).
Attitudes towards GM foods and food derived from animals fed GM feed
In many countries, animal feed is provided in a total mixed ration containing harvested plants or plant parts (e.g. grain, cottonseed, soybean meal) and much of this is derived from GM crops (Davison Citation2010; Henseler et al. Citation2013; Turkec et al. Citation2016; Sieradzki et al. Citation2021). Most of the genetically modified traits in these crops are input traits such as herbicide resistance or insect resistance (Stein and Rodríguez-Cerezo Citation2010). But increasingly there is a move toward output traits that improve the quality and nutritive value of the plant. Currently, the GM traits being sought in New Zealand for forage plants to be consumed by animals are of the latter category (Winichayakul et al. Citation2020; Roldan et al. Citation2022). In New Zealand, animal feed is primarily pasture grazed in situ with foods derived from these being dairy or meat products. A trial, undertaken in Poland, comparing milk from animals fed either transgenic maize containing the Bt gene (MON 810) and soyabean meal produced from glyphosate-tolerant plants (Roundup Ready, MON 40-3-2) compared with feed from non-transgenic plants showed no significant differences between transgenic and non-transgenic feed treatments for productivity, milk composition and blood metabolite profiles (Furgał-Dierżuk et al. Citation2015). Additionally, the transgenic DNA sequences of MON 810 and RR soyabean meal were not detectable by genomic testing (PCR) in milk. The inability to detect transgenic events in meat or milk has been commonly reported (Furgał-Dierżuk et al. Citation2015; Flachowsky and Reuter Citation2017; Nadal et al. Citation2018; Matovu and Alçiçek Citation2021). Another extensive review has indicated that GM soybean and maize with enhanced output traits, such as nutritional characteristics affecting quality and/or quantity of proteins, amino acids, oils, and carbohydrates, can have profound effects on improving animal performance and productivity and there is no indication that GM crops are less beneficial to livestock compared with non-GM crops (Aumaitre et al. Citation2002; Deb et al. Citation2013; de Santis et al. Citation2018; Matovu Citation2021). Other studies have similarly shown no detrimental effect of using GM feeds over non-GM feeds for pigs (Sońta et al. Citation2021) and dairy cows (Castillo et al. Citation2004; Calsamglia et al. Citation2007). In a study where pigs were fed a mix of maize and GM soybean there were no differences between pigs fed the GM and non-GM diets for feed intake, weight gain, mortality and routine blood biochemistry measurements, but there was a slight increase in severe stomach inflammation amongst male and female pigs had a statistical significance for the differences of P = 0.041 and 0.034, respectively (Carman et al. Citation2013). When combining the nil and mild cases (Group A) and combining moderate and severe cases (Group B) the percentages affected for non-GM fed and GM-fed groups were for Group A 48%, 43%, and Group B 52% and 57%, respectively.
There is still a segment of society that would prefer to not purchase and consume meat from animals fed GM feed (Karasu and Öztürk Citation2020). It has been surmised that avoiding GM foods is mainly due to subjective rather than objective knowledge (Huffman et al. Citation2007; Wuepper et al. Citation2019; Ardebili and Rickertsen Citation2020). Indeed, a recent review concluded that ‘there is broad scientific consensus that all approved foods and feedstuffs that have been derived from GM plants and fish and from livestock, poultry, and fish fed diets containing GM food products are safe to eat’ (Blair and Regenstein Citation2020).
The importance of 38 key domestic and international drivers that have the potential to affect land-use change and/or practice in New Zealand have been reviewed through a survey of participants selected based on their experience and expertise in relation to New Zealand’s primary industries (n = 226 from a total of 1559 approached) (Driver et al. Citation2019). provides the predictions on 10 of these in comparison with the impact of GM technology. In summary, 17% or 26% of respondents indicated that GM technology and nanotechnology as a domestic or international issue, respectively, would have a significant impact on land-use change/practice in NZ.
Table 1. Impact of domestic and international drivers/issues on New Zealand land use change/practice. Views from a survey of 226 participants selected based on their experience and expertise in relation to New Zealand’s primary industries. Taken from Driver et al. (Citation2019) with permission from the author.
Based on responses from first-time visitors to New Zealand, it was concluded that the introduction of GM crops into New Zealand is highly unlikely to create lasting damage to perceptions in overseas markets of the image of New Zealand as a source of high-quality food products or as a highly desirable scenic and ‘clean green’ tourist destination (Knight et al. Citation2013). However, a survey published in 2003 of 120 consumers in New Zealand on the use of GM to generate food products indicated a negative response and even with the provision of additional product benefits resulting from GM foods that negative view remained unchanged (Fortin and Renton Citation2003). Having noted that it could be argued that New Zealand has not received a noticeable benefit economically for remaining GM free based on observations in two Australian states that have remained GM free – South Australia and Tasmania. In South Australia, there has been no evidence to support the view that South Australian farmers enjoy better access to European Union non-GM grain markets, and since 2012 there has been no premium for grain from South Australia despite it being the only mainland state with a GM crop moratorium (Anderson Citation2019). In Tasmania, while it was agreed that a GMO-free status does create a point of difference, there were a range of views expressed in relation to market advantage and disadvantage (Davey et al. Citation2012). One conclusion from that study was that Australian consumers were more concerned about preservatives and food colouring additives, the use of antibiotics, hormones and steroids in animals, as well as on-farm pesticide use than the use of GM foods.
In an extensive review undertaken over a decade ago, Knight (Citation2011) concluded that the introduction of GM pasture (e.g. to improve drought tolerance) into New Zealand is highly unlikely to create lasting damage to perceptions in overseas markets of the image New Zealand has for being a source of high-quality food products or as a highly desirable scenic and ‘clean green’ tourist destination. Further, based on extensive interviews with food distribution channel gatekeepers in Europe, China and India, who seem to be the key food policy decision makers (Knight et al. Citation2008), there was clear evidence that introducing specific GM technology into New Zealand will have no harmful effect on perceptions of New Zealand as a country that is the source of high-quality food and beverage imports. There was no evidence that the presence of GM in a given country downgrades that country in the eyes of distribution channel members. There was no evidence that GM applications in non-food areas (forestry or pest control) would irreparably harm New Zealand’s image in the export sector. For China in particular, as a major importer of New Zealand food products, the survey of 20 companies in five commercial centres (i.e. food distribution gatekeepers) it was concluded that Chinese consumers are likely to accept GM foods provided there are consumer benefits, a price advantage and credible governmental information concerning safety of GM foods (Knight et al. Citation2008; Knight and Gao Citation2009). A more recent survey (2016) indicated that of 2063 Chinese consumers 11.9%, 46.7% and 41.4% of respondents have a positive, neutral or negative view of GM food, respectively (Cui and Shoemaker Citation2018). Interestingly younger people (under 30) were less opposed (18.5%) than those older (>50%), but were not markedly influenced by gender, education or income.
A recent review looking at 103,084 online and print articles published in media around the world as well as 1,716,071 social media posts published between 2018 and 2020 has concluded that both social and traditional media may be moving toward a more favourable and less polarised conversation on ag-biotech overall (Evanega et al. Citation2022). Ag-biotech was defined here as technology used to generate GMOs. Despite this, they also cautioned that ‘the scientific community still faces major communication challenges in addressing gaps between traditional and social media debates and the actual scientific consensus around the safety and ability of agricultural biotechnology’. Indeed, it has been proposed, from a survey of 1000 Singaporeans, that pre-existing negative attitudes towards food from GM sources can spill over to attitudes towards other technology-enabled food, such as nano-enabled food (i.e. engineered-food containing ingredients and additives to improve the nutritional value, taste, colour, shelf-life and safety of food products, that are made using nanotechnology), suggesting that attitudes can be ingrained and hard to change (Ho et al. Citation2020).
Young consumers were surveyed in Italy and showed a strong trend toward the need to control GM experimentation, that GM-free food products are better for the environment, GM foods are unnatural products, and that GM food could be unsafe and cause allergies (Palmieri et al. Citation2020 and summarised in ). In this survey, level and type of education influenced attitudes towards GM products. In a survey of 243 undergraduate students in Italian universities, while not necessarily reflective of Italian society, it was determined that students in technical and natural science programmes (61% of respondents) had a better perception of GM products than those enrolled in social sciences programmes (23% of respondents) (Palmieri et al. Citation2020). That said, it did not necessarily result in a more positive perception towards GM-containing food. On the contrary, other studies in the USA have shown that those with higher scientific knowledge scores tend to have less negative attitudes toward GM products (Wunderlich and Gatto Citation2015). While there is reasonable evidence that knowledge/education positively benefits views on GM products, it should be acknowledged that some consumers are quite discerning in their choices and may have made informed choices not to consume GM products (Blancke et al. Citation2015). Therefore, if a person has a belief against GM products that is teleological, then the view will be firmly entrenched, and they will inevitably remain opposed irrespective of arguments put to them concerning the safety and benefits of GM products. However, even for individuals or groups opposed to GM, a country adopting GM does not necessarily ignore or obviate over their rights since they can still choose to avoid GM foods, particularly where product content labelling is required, as is the case in New Zealand (New Zealand Food Safety Citation2021).
Table 2. Perceptions of 243 Italian undergraduate students towards GM products (adapted from Palmieri et al. Citation2020, under license 5203750640373 from Elsevier).
Gatekeepers in the Indian food distribution channel indicated, a decade ago, that consumers are mainly unaware or unconcerned by the GM issue (Knight et al. Citation2008; Knight and Paradkar Citation2008). The main issue of concern was foreign companies gaining monopoly rights over intellectual property, rather than to GM foods per se. However, in a survey of young Indian consumers 58% of the students support mandatory labelling of GM foods and 39% of the students are willing to pay 10%–15% more for GM-free foods under this policy (Kajale and Becker Citation2013). A random survey of 1000 Greek citizens showed that while the overall attitude of Greek consumers towards GM food was negative, there existed a market segment of substantial size, where beliefs about GM food appeared to be positive (Arvanitoyannis and Krystallis Citation2005). In Ireland, perceptions and attitudes of university-based academic scientists to issues regarding GM food indicated that 79% of respondents believed there should be no immediate complete ban on all GM foods and their production (Morris and Adley Citation2000). An internet survey of more than 1000 respondents in Norway and the USA indicated that only between 7% and 13% were willing to pay more than a 20% premium for each of the non-GM conventional alternatives as compared to the corresponding GM alternatives (Rickertsen et al. Citation2017). However, about 90% of respondents in each country supported the practice of labelling GM food products.
A meta-analysis, undertaken in 2005, of 25 studies that collectively report 57 valuations for GM food showed that consumers on average placed anywhere from a 42% (unweighted average using all data) to a 23% (weighted average excluding one outlier) higher value for non-GM food relative to GM food (Lusk et al. Citation2005). Yet willingness to pay a premium for non-GM processed food without any direct benefit over GM processed food was only 26% if the method used to obtain a response was non-hypothetical and carried out in person. However, the meta-analysis showed that Europeans are supposedly willing to pay, on average, 29% more for non-GM food than US consumers. A survey to test the willingness to pay for gene-edited apples showed that French consumers did not value the innovation (i.e. the apples do not become brown upon being sliced or cut) because they viewed it as a GM food, while USA consumers did value the innovations because they did not associate it with GM (Marette et al. Citation2021). Reviews have shown that US consumers tend to accept GM products more readily than their European counterparts (Wunderlich and Gatto Citation2015).
A study in 2015 of 976 randomly selected individuals almost equally split from either London (UK) or Warsaw (Poland) indicated that 19.8%, 52.5% and 27.7% were either positive, neutral or negative toward GM food products (Popek and Halagarda Citation2017). Other surveys in Europe have shown that concern about the presence of GM production in the environment has decreased from 30% (in 2002) to 19% (in 2011), while the level of concern about the use of GM ingredients in food or drinks has decreased from 63% (in 2005) to 27% (in 2019) (Ichim Citation2021). Notably, farmers in Italy were in favour of cultivating CRISPR/Cas9-modified rice that provides resistance to blast disease if it was available (Ferrari Citation2021). While the change in attitudes towards GM is positive and significant. the main issue is political and anti-GM sentiments have been and are still used as a trade barrier (Davison Citation2010; Smyth Citation2017b). While much of Europe bans the planting of GM crops, the majority of their pigs, chickens and to a lesser extent cattle rely on the importation of 15M metric tons of soybean meal annually from North and South America (Shahbandeh Citation2022) (90% of which is GM), and so rely heavily on GM technology but from outsourced production (Davison Citation2010; Henseler et al. Citation2013; Turkec et al. Citation2016; Sieradzki et al. Citation2021). Notably in 2022, the European Food Safety Authority (EFSA) authorised herbicide-tolerant GM oilseed rape, cotton and soybeans crops and also renewed the authorisation for GM cotton used for food and animal feed (ISAAA Citation2022). EFSA concluded that they are as safe as their conventional counterpart and the tested non-GM reference varieties with respect to the potential effects on human and animal health and the environment.
Other meta-analyses have also shown that consumers in general are willing to pay more for non-GM products over GM products, with a willingness to pay an extra 29%–45% more to avoid GM goods (Wunderlich and Gatto Citation2015). This willingness to pay is driven more by subjective (what the person thinks they know) rather than objective (what the person actually knows) knowledge (Rihn et al. Citation2021). In Poland, the strong distrust towards GM food has been considered irrational and with no scientific basis or fact, and primarily based on views from the internet and social media (Kubisz et al. Citation2021). In Italy, 54% of the general public believed GM foods are generally safe to eat, compared with 81% of scientist members of the Italian Association of the Agricultural Science Societies (Pappalardo et al. Citation2021). In Turkey, a survey of 1300 people indicated that the main concerns for consumers about GM foods were their carcinogenic effects to human (although there is no proof of this effect), although the use of GMOs in the health sector and in preventing environmental pollution was considered beneficial and acceptable by the consumers (Tas et al. Citation2015).
It has been propositioned that if the benefits of GM foods are made explicit to consumers, and based on scientific evidence, then the resistance to purchasing them may reduce (Knight et al. Citation2008). This was demonstrated in an Italian study where consumers were asked to rate their willingness to purchase wines from conventionally grown grapes where fungicides were used compared with fungus-resistant grapes produced by either hybridisation or genome editing (Borrello et al. Citation2021). Respondents were prepared to pay a premium price for wines from hybridised grapes compared to conventional wines (+9.14%) and with a strong discount for genome-edited wines (–21.13%). The provision of information on the hybridised or gene-edited grapes (i.e. reduced fungicidal use) showing they provided environmental benefits improved their acceptability.
The study by Lusk et al. (Citation2005) clearly shows that the method used to obtain a response, on willingness to pay more for a non-GMO food, can significantly influence the outcome. They concluded that non-hypothetical (i.e. real) valuations should be preferred over hypothetical situations when surveying for a response to acceptance or willingness to pay for non-GM foods. Estimates of consumer demand have used experimental economics where real products and real money are exchanged to reveal their true value for a product rather than using hypothetical surveys (Fox et al. Citation1998). Using this methodology, a trial was set up to determine the willingness-to-pay for non-GM corn chips over GM corn chips. Although 70% of participants were unwilling to pay more for non-GM corn chips, 20% of participants were willing to pay at least $0.25/oz. for the exchange, and 2% offered bids as high as $0.50/oz (Lusk et al. Citation2001).
A trial where stalls were set up on outskirts of urban areas in New Zealand (Queenstown), Sweden (Ystad, Skåne), Belgium (near Brussels), France (Paris), Germany (Koblenz, Rheinland-Pfalz) and the UK (Berwick-upon-Tweed) to sell conventional fruit labelled as ‘organic’, ‘spray-free genetically modified’, or ‘conventional’ (Knight et al. Citation2007b) demonstrated that a significant (and in some markets, surprisingly high) percentage of consumers in European countries appear willing to choose GM food provided there is a price advantage coupled with a consumer benefit (in this case, ‘spray-free’ status). Market-share estimates based upon prevailing market prices found a similar pattern across the six countries for the three fruit types (). This pattern consisted of the organic produce having the largest market share, followed by conventionally grown fruit, and with the spray free-GM product gaining the smallest market share. However, when the organic produce was sold at a premium, with a discount offered for the spray free-GM option, given its lower cost of inputs then there was a significant shift with organic produce losing market share in all countries, except Belgium, where it still dominated, and the spray free-GM fruit gaining the highest market share for the New Zealand, Swedish and German stalls, and reaching 30% or more for the UK and French stalls (). These changes in market share of the spray-free GM fruit between the first scenario () and the second scenario () were significant at the 99% confidence level or more for all stalls, except the Belgian stall. An online questionnaire completed by 1500 Swedish consumers in 2019 identified that there was a more positive attitude towards conventional plant breeding than to GM derived food, men expressed more positive attitudes to both conventional plant breeding and GM foods than women did, and younger consumers expressed more positive attitudes to GM foods than older consumers (Spendrup et al. Citation2021).
Table 3. Comparison of market shares in different locations for the three fruit types sold at the prevailing market price, derived from choice modelling estimations (taken from Knight et al. Citation2007b, under license 5203350048709 from Springer Nature).
Table 4. Comparison of market shares for the three fruit types in a scenario where organic is priced at a 15% premium and the spray-free GM product is discounted 15%, based on the choice modelling estimations (taken from Knight et al. Citation2007b, under license 5203350048709 from Springer Nature).
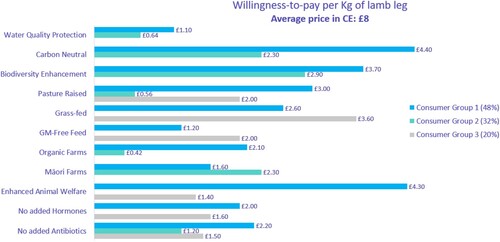
In a review article (Knight Citation2016), it was concluded that ‘it is highly unlikely that the introduction of GM plants into New Zealand would have any long-term deleterious effect on perceptions in overseas markets of food products sourced from New Zealand’ and is unlikely to affect New Zealand’s image as tourist destination, and further there is unlikely to be any premium gained for our food products by remaining GM free. A review undertaken by the previous Labour Government concluded that ‘price impacts of GM releases in New Zealand are likely to be lower than those used in the previous modelling and therefore the results for GM organism release scenarios are more likely to be a positive rather than a negative movement in GDP’ (Cullen and Hobbs Citation2003). In a wide-ranging review of over 100 estimates of consumer willingness to pay for GM food, it was concluded that consumers are willing to pay less for GM foods, the magnitude of this discount varies across countries, and the magnitude differs based on the type of genetic modification and how it affects the food product (Colson and Rousu Citation2013). In a survey undertaken in the UK willingness to pay more for GM-free and/or organic food rated relatively low compared with production being carbon neutral or having enhanced animal welfare () (Saunders et al. Citation2021).
Figure 1. UK attitudes toward willingness to pay more per kg of lamb, based on an average price of £8. Consumer Group 1 has a focus on healthy environment and healthy animals; 2, on the cultural consumer; and 3, feed focused (taken from Saunders et al. Citation2021 with permission from authors).
Using economic modeling, it was concluded that if consumers prefer non-GM products, New Zealand producers gain by focusing on those crops, but alternatively, if consumers prefer second-generation GM products, producers increase their returns by growing those crops (Saunders et al. Citation2003). If these improvements were solely aimed at improving productivity then theory, experience and expectations show that an increase in productivity does not necessarily lead to increased returns. Using genetic modification to mitigate the causes of environmental issues, such as greenhouse gas emissions, nitrogen leaching into waterways (Winichayakul et al. Citation2020; Roldan et al. Citation2022), reduced pesticide use (Saleh et al. Citation2021), increasing soil carbon (Sutherland et al. Citation2021) and/or clear health benefits to the consumer (Lusk et al. Citation2014; Boccia and Punzo Citation2021) may provide a more acceptable balance between perceived risk and benefit (Zilberman et al. Citation2018; Smith et al. Citation2021).
Education and knowledge transfer is required along with appropriate regulatory strategies to improve acceptance of GM foods (Chávez-Dulanto et al. Citation2021). One study in the USA showed that organic farmers are proactive in communicating with the public about their production practices, unlike conventional farmers who focus more on improving productivity (Masambuka-Kanchewa et al. Citation2021). A review of 543 journal articles has shown that public support for the use of GM technologies increases when the potential benefits of the technology are well articulated, there is trust in government regulatory schemes, and media delivers a positive influence that increases belief in science (Sendhil et al. Citation2021). Concerns about GM food simply benefiting food manufacturers and possibly causing allergies and illness are the two main predictors of GM food rejection (Rose et al. Citation2020). The use of concrete terms compared to abstract terms has been shown to increase support for GM foods (Tallapragada et al. Citation2021). Additionally, consumption of GM foods increases with a price discount (Shew et al. Citation2018) as demonstrated by Canadian consumers for whom, while they may mistrust GM food, price is the key attribute determining a purchasing decision (Macall et al. Citation2021).
In recent surveys undertaken for FSANZ two asynchronous online focus groups with a mix of participants from Australia and New Zealand were asked for their views and understanding of NBTs, GM, and related topics such as trust in authorities of various types including food regulators (Ankeny and Harms Citation2021). Consumer surveyed had relatively low levels of knowledge about NBTs, however, when given basic information about NBTs the focus was less on the details of the underlying science and more on the factors associated with specific applications. A key theme to emerge was that NBTs do not represent an adequate solution to the ‘real problems’ facing society, particularly in relation to environmental issues such as climate change or broader concerns about agricultural practices. Although many participants found that specific applications of NBTs presented in the scenarios to be largely acceptable, there was a concern that the applications delivered sufficient benefit to justify their development.
A recent literature review of 146 studies on consumers’ response to the use of new breeding techniques (NBTs) in the production of food (Grant et al. Citation2021) suggests attitudes and behavioural responses to NBTs are slightly more positive than toward older forms of genetic modification (GM), but slightly more negative than toward food produced using traditional breeding techniques. They concluded that given the potential for a highly fractious debate about their value and safety in food production processes deep community engagement and deliberation will be essential.
Intellectual property rights and genetic modification
The uptake of new innovations by farmers is primarily driven through dedicated paths to the market where the intellectual property (IP) is controlled rather than through multiple paths to market where there is no IP control. Most of the NBTs currently used in developing new genetic variation have been managed through intellectual property protection processes to ensure a return back to the inventors.
The role of IP protection in stimulating the development, manufacture and sale of new technologies is hotly debated (Caradus et al. Citation2021). For plant breeders and patent owners, strong IP protection offers an effective form of security; and more importantly brings returns on investment through licences and commercialisation arrangements. When launching new products, IP protection not only provides legal security but is confirmation that the product is unique, distinctive and of value. Some view IP rights as a way to foster innovation and invention by encouraging individuals to develop/invent new ideas from which they can potentially gain a return (Scherer Citation1999; Saha and Bhattacharya Citation2011). Without the ability to capitalise on their work innovators have little incentive, other than an altruistic motive, to produce any invention.
There is an opposing view that patents and plant variety rights are ‘killing freedom to operate and crushing science with rules’, and in so doing are stifling innovation rather than encouraging it (Thiruthy Citation2017). Some believe that IP rights holders abuse the system to unfairly extend their monopoly on a technology and prevent others from using it to the benefit of the industry and the economy as a whole. There is no simple answer to whether IP protection stimulates or constrains innovation and technology transfer. However, IP protection is here to stay, and it is really up to both individuals and organisations who use IP protection to do so while delivering maximum benefit to the end-user farmer. IP protection in itself does not constrain innovation however abuse of the temporary monopoly it grants can do (Caradus et al. Citation2021).
Labelling
Certification of labels on both GM-containing and GM-free foods, despite the additional cost associated with this, will play an important role in how consumers make decisions (Berning and Campbell Citation2021; Jiang and Zhang Citation2021). In the European Union, Japan and New Zealand, it is mandatory to label food derived from GMOs if present at levels above 0.9% (Blagoevska et al. Citation2021). In Greece, a GM label on food (corn chips) evoked a deeply rooted negative attitude as more than half of participants (63%) refused to taste even a single piece of the product (Batrinou et al. Citation2008). Even when labelled ‘GM but approved by EU’ while viewed as more credible there were still 28% who refused to sample. This example indicates the importance of carefully worded labelling and use of appropriate information (Batrinou et al. Citation2005). The impact of mandatory food labelling has shown that where food labelling was not required then GM food uptake may well succeed even without any specified consumer benefit, but where labelling is required then a specified consumer benefit is essential for product uptake (Mather et al. Citation2016). In China, mandatory ‘contains GMO’ labelling was considered more important relative to the voluntary ‘non-GMO’ labelling system, although there were some differences of opinion based on perceptions of the safety of GM foods (Zheng and Wang Citation2021). The preference for labelling to enable traceability is motivated by a perception of the attributes of nutritional benefit and potential health risk, and perceived inadequacy of simple mandatory labels (Jiang and Zhang Citation2021; Zhang et al. Citation2021). In the USA, about 90% of consumers in 2015 wanted mandatory labelling for genetically modified food (Consumer Reports Citation2015), which contributed to their National Bioengineered Food Disclosure Law in 2016 (Pruitt et al. Citation2021), but this appears to have not necessarily aligned with consumer expectations (Mosier et al. Citation2020). In a study involving college students in the USA who predominantly believed that GMO products were dangerous to health and indicated that GM food labelling was important, there was no evidence of their food choices reflecting this belief (Oselinsky et al. Citation2021). Mandatory labelling in the USA of GM foods began on 1 January 2022 (Tsakok and Mengoub Citation2021), but this will not include some gene-edited technologies (Selfa et al. Citation2021). Fit-for-purpose methodology to enforce control of GM plants is available but may require additional cost and expertise (Ribarits et al. Citation2021).
Impact on R&D investment
In New Zealand, investment from the government, and to a lesser extent industry, in the application of genetic modification to solve intractable problems has been ongoing, even with no clear pathway through the regulatory environment imposed by the HSNO Act. However, in South Australia, the GM crop moratorium has been seen as a major reason for reduced public and private agricultural R&D investments (Anderson Citation2019). Similarly, in Tasmania their GMO-free status has resulted in the loss of gene technology research opportunities in some plant-based industries including canola, poppies and pastures (Davey et al. Citation2012). The challenge for New Zealand is the reluctance of researchers, due to the draconian nature of the current regulations, to apply to EPA for field trials which could be used to address societal concerns about the safety of GM technologies. This is compounded by large processor groups taking a conservative view of risk and in so doing failing to see the opportunity, and as a result, when any organisation has sought to put in an application to EPA it has effectively been vetoed by these organisations.
Concluding comments
Genetic modification (GM) has been widely adopted globally and has shown improved yield, quality and environmental impacts and has the potential to provide consumer benefits through improved product quality, nutritive value and shelf life. Public concern over GM foods is focused on their impacts on human and animal health, environmental safety, labelling and consumer choice, intellectual property rights, ethics, food safety, poverty reduction and environmental conservation. While there are factions of society who will always be against the use of GM in food production the evidence from studies attempting to understand market forces and public attitudes is that the use of GM plants in New Zealand for food production is unlikely to have long-term deleterious effects in overseas markets. However, the reputation of the country of origin of food products can have a significant influence on consumer choice. There is a need for legitimate debate that warrants research effort into providing a clearer impact analysis. In addition to having the political will to use all technologies available to provide solutions to current and future challenges, an informed discussion with major trading partners is required.
An extensive review suggests that a large segment of consumers, but not all, are willing to consume and pay for foods derived from gene-edited (including insertion, deletion and gene replacement) plants, especially if they express useful traits that the consumers perceive as beneficial for human and animal health and the environment (Beghin and Gustafson Citation2021). GM food products that focus on mitigating the causes of environmental issues, such as greenhouse gas emissions, nitrogen leaching into waterways, reduced pesticide use, increasing soil carbon and/or clear health benefits to the consumer may provide an acceptable balance between perceived risk and benefit. There has been no reliably documented loss of life or serious environmental damage that can be attributed to the use of GM technologies (Smith et al. Citation2021). All scientific published accounts would indicate that GM foods pose not greater risk than non-GM foods (Touyz Citation2013; Yang and Chen Citation2016; Giraldo et al. Citation2019), and the vast majority of studies demonstrate that the insecticidal proteins deployed in GM crops cause no unintended adverse effect on either non-target invertebrates or to natural enemies (Naranjo Citation2017; Romeis et al. Citation2019). Public support for the use of GM technologies increases when the potential benefits of the technology are well articulated, there is trust in government regulatory schemes, and media delivers a positive influence that increases belief in science. Certification of labels on both GM-containing and GM-free foods will play an important role in how consumers make decisions, where labels are used then stating the consumer benefit is essential for product uptake.
It has been argued that opposition to gene technology has taken on a life of its own and provided little benefit to anyone, and that the ethics of agricultural and food biotechnology has been drowned out by simplistic arguments about feeding the world, on one side, and a blend of fearmongering and ignorance of food systems, on the other (Thompson Citation2021). The development of new GM crops that provide either human health and nutritional benefits or assist with solving current and future environmental challenges will deliver consumer benefits (Vega Rodríguez et al. Citation2022). Quality scientific information is essential to ensure consumers understand these benefits.
In New Zealand, the use of genetic modification is regulated on a case-by case basis that has been labelled as technocratic and inconsistent with the precautionary principle of regulation. Genetic modification has continued to be used as a research tool and for medical purposes but has gained no traction, momentum or impact in improving New Zealand’s economic wellbeing. Gene editing which can precisely target changes in genes has been heralded as game changer, but there are fears it will suffer the same fate as genetic modification leading to mistrust and suspicion among vocal sectors of society. Some countries, but not New Zealand, have however decided that where gene edits are simply deletions, and the end-product is indistinguishable from products produced through traditional plant breeding then they do not need to be regulated (Fritsche et al. Citation2018; Turnbull et al. Citation2021). Additionally, because of New Zealand’s current regulatory environment opportunities are being missed in delivering new products using GM fermentation technologies (Pretorius and Boeke Citation2018; Deckers et al. Citation2020) and synthetic biology (MacDonald and Deans Citation2016; Danchin Citation2021; Macdonald Citation2022). From a regulatory viewpoint, consideration is required to focus on regulating the benefit-risk issues associated with the end-products of genetic modification rather than the processes used in their development, as occurs in some other jurisdictions (Smyth Citation2017a; Turnbull et al. Citation2021).
Disclosure statement
The author is employed by Grasslanz Technology Ltd which has an R&D investment portfolio that includes both genetic modification and gene editing of forages and microbes to provide mitigating solutions to current environmental and animal welfare issues facing both New Zealand and other pastoral economies.
References
- Alphey H, Bonsall MB. 2018. Genetics-based methods for agricultural insect pest management. Agricultural and Forest Entomology. 20:131–140. doi:10.1111/afe.12241.
- Anderson K. 2019. Independent review of the South Australian GM food crop moratorium. Report to the SA Minister for Primary Industries and Regional Development, p. 76.
- Ankeny RA, Harms R. 2021. Focus groups on consumers’ responses to the use of New Breeding Techniques (NBTs) in food production. The University of Adelaide. p. 50. [accessed 2021 Nov 26]. https://www.foodstandards.gov.au/code/proposals/Pages/p1055-definitions-for-gene-technology-and-new-breeding-techniques.aspx.
- Ardebili AT, Rickertsen K. 2020. Personality traits, knowledge, and consumer acceptance of genetically modified plant and animal products. Food Quality and Preference. 80:Article 103825. doi:10.1016/j.foodqual.2019.103825.
- Arvanitoyannis IS, Krystallis A. 2005. Consumers’ beliefs, attitudes and intentions towards genetically modified foods, based on the ‘perceived safety vs. benefits’ perspective. International Journal of Food Science & Technology. 40:343–360.
- Aumaitre A, Aulrich K, Chesson A, Flachowsky G, Piva G. 2002. New feeds from genetically modified plants: substantial equivalence, nutritional equivalence, digestibility, and safety for animals and the food chain. Livestock Production Science. 74:223–238. doi:10.1016/S0301-6226(02)00016-7.
- Batrinou AM, Dimitriou E, Liatsos D, Pletsa V. 2005. Genetically modified foods: the effect of information. Nutrition & Food Science. 35:148–155. doi:10.1108/00346650510594895.
- Batrinou AM, Spiliotis V, Sakellaris G. 2008. Acceptability of genetically modified maize by young people. British Food Journal. 110:250–259.
- Bawa AS, Anilakumar KR. 2013. Genetically modified foods: safety, risks and public concerns—a review. Journal of Food Science and Technology. 50:1035–1046. doi:10.1007/s13197-012-0899-1.
- Beghin JC, Gustafson CR. 2021. Consumer valuation of and attitudes towards novel foods produced with new plant engineering techniques: a review. Sustainability. 13:11348. doi:10.3390/su132011348.
- Berning J, Campbell BL. 2021. Market simulations of consumer preferences for the introduction of GM tomatoes. International Food and Agribusiness Management Review. 24:71–88. doi:10.22434/IFAMR2019.0218.
- Biden S, Smyth SJ, Hudson D. 2018. The economic and environmental cost of delayed GM crop adoption: the case of Australia's GM canola moratorium. GM Crops & Food. 9:13–20. doi:10.1080/21645698.2018.1429876.
- Blagoevska K, Ilievska G, Jankuloski D, Dimzoska BS, Crceva R, Angeleska A. 2021. The controversies of genetically modified food. In: IOP Conference Series: Earth and Environtal Sci. 854: 012009. IOP Publishing. https://iopscience.iop.org/article/10.1088/1755-1315/854/1/012009/meta.
- Blair R, Regenstein JM. 2020. GM food and human health. In: Anderson V, editor. Genetically modified and irradiated food. Academic Press; p. 69–98. doi:10.1016/B978-0-12-817240-7.00005-X.
- Blancke S, Van Breusegem F, De Jaeger G, Braeckman J, Van Montagu M. 2015. Fatal attraction: the intuitive appeal of GMO opposition. Trends in Plant Science. 20:414–418. doi:10.1016/j.tplants.2015.03.011.
- Boccia F, Punzo G. 2021. A choice experiment on consumer perceptions of three generations of genetically modified foods. Appetite. 161:105158.
- Borrello M, Cembalo L, Vecchio R. 2021. Role of information in consumers’ preferences for eco-sustainable genetic improvements in plant breeding. PLoS ONE. 16:e0255130. doi:10.1371/journal.pone.0255130.
- Bozzini E, Sicurelli D. 2021. Precautionary authoritarianism and the contested governance of Chinese food safety. Contemporary Politics. 27:336–355. doi:10.1080/13569775.2021.1891681.
- Calsamglia S, Hernandez B, Hartnell GF, Phipps RR. 2007. Effects of corn silage derived from a genetically modified variety containing two transgenes on feed intake, milk production, and composition, and the absence of detectable transgenic deoxyribonucleic acid in milk in Holstein dairy cows. Journal of Dairy Science. 90:4718–4723. doi:10.3168/jds.2007-0286.
- Caradus JR. 2008. An opportunity lost and sorting fact from fiction. Proceedings NZ Grassland Association. 70:1–6.
- Caradus JR, Tumilson CA, Lin JY. 2021. Intellectual property protection – stimulating or constraining innovation and technology transfer? Procs Joint X1 Intl Rangeland Congr and XXIV Int Grassl Congr (IRC/IGC), Mombasa, Kenya, 25 to 29 October 2021. p. 4. https://uknowledge.uky.edu/igc/24/7-2/10/.
- Carman JA, Vlieger HR, Ver Steeg LJ, Sneller VE, Robinson GW, Clinch-Jones CA, Haynes JI, Edwards JW. 2013. A long-term toxicology study on pigs fed a combined genetically modified (GM) soy and GM maize diet. Journal of Organic Systems. 8:38–54.
- Carroll D. 2016. Genome editing: progress and challenges for medical applications. Genome Medicine. 8:120. doi:10.1186/s13073-016-0378-9.
- Castillo AR, Gallardo MR, Maciel M, Giordano JM, Conti GA, Gaggiotti MC, Quaino O, Gianni C, Hartnell GF. 2004. Effects of feeding rations with genetically modified whole cottonseed to lactating Holstein cows. Journal of Dairy Science. 87:1778–1785. doi:10.3168/jds.S0022-0302(04)73333-0.
- Charo A. 2015. “The Governance of Human Gene Editing” (speech to the International Summit on Human Gene Editing: a global discussion, Washington DC, 1–3 December 2015).
- Chávez-Dulanto PN, Thiry AAA, Glorio-Paulet P, Vögler O, Carvalho FP. 2021. Increasing the impact of science and technology to provide more people with healthier and safer food. Food and Energy Security. 10:e259. doi:10.1002/fes3.259.
- Colson G, Rousu MC. 2013. What do consumer surveys and experiments reveal and conceal about consumer preferences for genetically modified foods? GM Crops & Food. 4:158–165. doi:10.4161/gmcr.26322.
- Consumer Reports. 2015. Consumers Want Mandatory Labeling for GMO Foods. [accessed 2021 Nov 11]. https://www.consumerreports.org/food-safety/consumers-want-mandatory-labeling-for-gmo-foods/.
- Coyle FJ, Fairweather JR. 2005. Challenging a place myth: New Zealand's clean green image meets the biotechnology revolution. Area. 37:148–158. doi:10.1111/j.1475-4762.2005.00617.x.
- Cui K, Shoemaker SP. 2018. Public perception of genetically-modified (GM) food: a nationwide Chinese consumer study. npj Science of Food. 2:Article 10. doi:10.1038/s41538-018-0018-4.
- Cullen M, Hobbs ML. 2003. Government response to the Royal Commission on Genetic Modification: economic analysis results and HSNO Act implications. p. 18 [accessed 2021 Dec 5]. https://www.treasury.govt.nz/sites/default/files/2007-09/pol03-77.pdf.
- DAFF. 2007. Biotechnology – market acceptance of GM Canola. [accessed 2022 April 17]. https://www.awe.gov.au/sites/default/files/sitecollectiondocuments/ag-food/biotech/gm-market-acceptance-final.pdf.
- Danchin A. 2021. In vivo, in vitro and in silico: an open space for the development of microbe-based applications of synthetic biology. Microbial Biotechnology. 15:1751–7915. doi:10.1111/1751-7915.13937.
- Davey L, Goodwin T, Bennett J. 2012. Market advantage of Tasmania’s GMO-free status. Devonport: Macquarie Franklin. p. 67.
- Davison J. 2010. GM plants: science, politics and EC regulations. Plant Science. 178:94–98. doi:10.1016/j.plantsci.2009.12.005.
- de Santis B, Stockhofe N, Wal J-M, Weesendorp E, Lallès J-P, van Dijk J, Kok E, De Giacomo M, Einspanier R, Onori R, et al. 2018. Case studies on genetically modified organisms (GMOs): potential risk scenarios and associated health indicators. Food and Chemical Toxicology. 117:36–65. doi:10.1016/j.fct.2017.08.033.
- Deb R, Sajjanar B, Devo K, Reddt KM, Prasad R, Kumar S, Sharma A. 2013. Feeding animals with GM crops: boon or bane? Indian Journal of Biotechnology. 12:311–322. http://nopr.niscair.res.in/handle/123456789/21854.
- Deckers M, Deforce D, Fraiture M-A, Roosens NHC. 2020. Genetically modified micro-organisms for industrial food enzyme production: an overview. Foods (Basel, Switzerland). 9:Article 326. doi:10.3390/foods9030326.
- Delhove J, Osenk I, Prichard I, Donnelley M. 2020. Public acceptability of gene therapy and gene editing for human use: a systematic review. Human Gene Therapy. 31:20–46. https://www.liebertpub.com/doi/abs/10.1089/hum.2019.197.
- Department of Foreign Affairs and Trade. 2021. China Market Insights 2021. [accessed 2022 April 17]. https://www.dfat.gov.au/sites/default/files/china-market-insights-2021.pdf.
- Dinica V. 2021. Transitioning to what? The role of genetic-engineering in New Zealand’s (circular) bioeconomy debates. Journal of Environmental Policy & Planning. 23:194–212. doi:10.1080/1523908X.2021.1893161.
- Driver T, Saunders C, Duff S, Saunders J. 2019. The Matrix of Drivers: 2019 Update. Report for Our Land and Water National Science Challenge Agribusiness and Economics Research Unit (AERU), Lincoln University. p. 139. [accessed 2021 Nov 29]. https://ourlandandwater.nz/wp-content/uploads/2020/01/Matrix_OurLandandWaterScienceChallenge-TheMatrixofDrivers3-2019.pdf.
- Edwards S. 2017. Research into genetically modified organisms in New Zealand: an examination of a sociotechnical controversy. Case Studies in the Environment. 1:1–8. Electronic ISSN 2473-9510.
- Eichelbaum T, Allan J, Fleming J, Randerson R. 2001. Report of the Royal Commission on Genetic Modification. Report of the Royal Commission on Genetic Modification. Wellington, New Zealand.
- Ellens KW, Levac D, Pearson C, Savoie A, Strand N, Louter L, Tibelius C. 2019. Canadian regulatory aspects of gene editing technologies. Transgenic Research. 28:165–168. doi:10.1007/s11248-019-00153-2.
- EPA. 2016. The Environmental Protection Authority (EPA): GMO regulations clarified. [accessed 2021 Dec 5]. https://www.beehive.govt.nz/release/gmo-regulations-clarified.
- Eriksson D, Kershen D, Nepomuceno A, Pogson BJ, Prieto H, Purnhagen K, Smyth S, Wesseler J, Whelan A. 2019. A comparison of the EU regulatory approach to directed mutagenesis with that of other jurisdictions, consequences for international trade and potential steps forward. New Phytologist. 222:1673–1684. doi:10.1111/nph.15627.
- Evanega S, Conrow J, Adams J, Lynas M. 2022. The state of the ‘GMO’ debate - toward an increasingly favorable and less polarized media conversation on ag-biotech? GM Crops & Food. 13:38–49. doi:10.1080/21645698.2022.2051243.
- Everett-Hincks J, Henaghan M. 2019. Gene editing in aotearoa – legal considerations for policy makers. Victoria University of Wellington Law Review. 50:515–549. doi:10.26686/vuwlr.v50i3.5990.
- Ferrari L. 2021. Farmers’ attitude toward CRISPR/Cas9: the case of blast resistant rice. Agribusiness. 1–20. doi:10.1002/agr.21717.
- Flachowsky G, Reuter T. 2017. Future challenges feeding transgenic plants. Animal Frontiers. 7:15–23. doi:10.2527/af.2017.0114.
- Food and Drug Administration. 2022. Science and history of GMOs and other food modification processes. [accessed 2022 April 27]. https://www.fda.gov/food/agricultural-biotechnology/science-and-history-gmos-and-other-food-modification-processes.
- Fortin DR, Renton MS. 2003. Consumer acceptance of genetically modified foods in New Zealand. British Food Journal. 105:42–58. doi:10.1108/00070700310467483.
- Fox JA, Shogren JF, Hayes DJ, Kliebenstein JB. 1998. CVM-x: calibrating contingent values with experimental auction markets. American Journal of Agricultural Economics. 80:455–465.
- Fritsche S, Poovaiah C, MacRae E, Thorlby G. 2018. A New Zealand perspective on the application and regulation of gene editing. Frontiers in Plant Science. 9:1323. doi:10.3389/fpls.2018.01323.
- FSANZ. 2021. Food Standard Australia New Zealand – current GM applications and approvals. [accessed 2021 Dec 5; 2022 April 24]. https://www.foodstandards.govt.nz/consumer/gmfood/applications/Pages/default.aspx#Table1.
- Furgał-Dierżuk I, Strzetelski J, Twardowska M, Kwiatek K, Mazur M. 2015. The effect of genetically modified feeds on productivity, milk composition, serum metabolite profiles and transfer of tDNA into milk of cows. Journal of Animal and Feed Sciences. 24:19–30. doi:10.22358/jafs/65649/2015.
- Gbashi S, Adebo O, Adebiyi JA, Targuma S, Tebele S, Areo OM, Olopade B, Odukoya JO, Njobeh P. 2021. Food safety, food security and genetically modified organisms in Africa: a current perspective. Biotechnology and Genetic Engineering Reviews. 37:30–63. doi:10.1080/02648725.2021.1940735.
- Genetic Literacy Project A. Brazil: crops / food. [accessed 2021 Nov 14] https://crispr-gene-editing-regs-tracker.geneticliteracyproject.org/brazil-crops-food/.
- Genetic Literacy Project B. Argentina: crops / food. [accessed 2021 Nov 14] https://crispr-gene-editing-regs-tracker.geneticliteracyproject.org/argentina-crops-food/.
- Genome British Columbia. 2020. Regulation of genetically modified and genetically engineered products in Canada. [accessed 2021 Nov 7]. https://www.genomebc.ca/infobulletins/regulation-of-genetically-modified-and-genetically-engineered-products-in-canada.
- Giraldo PA, Shinozuka H, Spangenberg GC, Cogan NO, Smith KF. 2019. Safety assessment of genetically modified feed: is there any difference from food? Frontiers in Plant Science. 10:Article 1592. doi:10.3389/fpls.2019.01592.
- Gordon DR, Jaffe G, Doane M, Glaser A, Gremillion TM, Ho MD. 2021. Responsible governance of gene editing in agriculture and the environment. Nature Biotechnology. 39:1055–1057. doi:10.1038/s41587-021-01023-1.
- Grant WJ, Bray H, Harms R, Ankeny RA, Leach J. 2021. Consumer responses to the use of NBTs in the production of food: a systematic literature review. Australian National University. p. 79. [accessed 2021 Nov 26]. https://www.foodstandards.gov.au/code/proposals/Pages/p1055-definitions-for-gene-technology-and-new-breeding-techniques.aspx.
- Henseler M, Piot-Lepetit I, Ferrari E, Mellado AG, Banse M, Grethe H, Parisi C, Hélaine S. 2013. On the asynchronous approvals of GM crops: potential market impacts of a trade disruption of EU soy imports. Food Policy. 41:166–176. doi:10.1016/j.foodpol.2013.05.005.
- Ho SS, Goh TJ, Chuah AS, Leung YW, Bekalu MA, Viswanath K. 2020. Past debates, fresh impact on nano-enabled food: a multigroup comparison of presumed media influence model based on spillover effects of attitude toward genetically modified food. Journal of Communication. 70:598–621. doi:10.1093/joc/jqaa019.
- Howard R. 2022. We need to talk about GMO. [accessed 2022 April 29]. https://businessdesk.co.nz/article/opinion/we-need-to-talk-about-gmo.
- Hudson M, Mead ATP, Chagné D, Roskruge N, Morrison S, Wilcox PL, Allan AC. 2019. Indigenous perspectives and gene editing in Aotearoa New Zealand. Frontiers in Bioengineering and Biotechnology. 11:1–9. doi:10.3389/fbioe.2019.00070.
- Huffman WE, Rousu M, Shogren JF, Tegene A. 2007. The effects of prior beliefs and learning on consumers’ acceptance of genetically modified foods. Journal of Economic Behavior & Organization. 63:193–206.
- Ichim MC. 2021. The more favorable attitude of the citizens toward GMOs supports a new regulatory framework in the European Union. GM Crops & Food. 12:18–24. doi:10.1080/21645698.2020.1795525.
- ISAAA. 2018. International Service for the Acquisition of Agri-biotech Applications (ISAAA): global status of commercialized biotech/GM crops in 2018: biotech crops continue to address the challenges of increased population and climate change. Brief 54. [accessed 2022 April 23]. https://www.isaaa.org/resources/publications/briefs/54/default.asp.
- ISAAA. 2022. European Commission Authorizes 3 GM Crops; Renews Authorization for GM Cotton. [accessed 2022 April 23]. https://www.isaaa.org/kc/cropbiotechupdate/article/default.asp?ID=19411.
- Ishii T, Araki M. 2017. A future scenario of the global regulatory landscape regarding genome-edited crops. GM Crops & Food. 8:44–56.
- Jiang D, Zhang G. 2021. Marketing clues on the label raise the purchase intention of genetically modified food. Sustainability. 13:9970. doi:10.3390/su13179970.
- Kaefer F. 2016. Origins and meaning of ‘Clean, Green’ New Zealand. [accessed 2022 April 27]. https://placebrandobserver.com/country-reputation-origins-clean-green-new-zealand/.
- Kajale DB, Becker TC. 2013. Determinants of consumer support for mandatory labeling of genetically modified food in India: a student survey. British Food Journal. 115:1597–1611. doi:10.1108/BFJ-12-2011-0302.
- Kannan S, Najjar D. 2020. Therapeutic gene editing is here, can regulations keep up? MIT Science Policy Review. 1:64–75.
- Karasu K, Öztürk E. 2020. The effects of genetically modified feeds on consumers’ preferences in buying broiler meat. Austin Journal of Nutrition & Metabolism. 7:Article 1087.
- Knight J, Paradkar A. 2008. Acceptance of genetically modified food in India: perspectives of gatekeepers. British Food Journal. 110:1019–1033.
- Knight JC. 2016. GM crops and damage to country image: much ado about nothing? Acta Hortic. 1124. In: Panis et al. editors. Proc. III International Genetically Modified Organisms in Horticulture Symposium - Past, Present and Future. p. 23–31. doi:10.17660/ActaHortic.2016.1124.4.
- Knight JG. 2011. New Zealand’s ‘clean green’ image: will GM plants damage it? ISBN 978-1-877156-45-8. p. 68.
- Knight JG, Clark A, Mather DW. 2013. Potential damage of GM crops to the country image of the producing country. GM Crops & Food. 4:151–157. doi:10.4161/gmcr.26321.
- Knight JG, Gao H. 2009. Chinese gatekeeper perceptions of genetically modified food. British Food Journal. 111:56–69.
- Knight JG, Holdsworth DK, Mather DW. 2007a. Country-of-origin and choice of food imports: an in-depth study of European distribution channel gatekeepers. Journal of International Business Studies. 38:107–125.
- Knight JG, Holdsworth DK, Mather DW. 2008. GM food and neophobia: connecting with the gatekeepers of consumer choice. Journal of the Science of Food and Agriculture. 88:739–744.
- Knight JG, Mather DW, Holdsworth DK. 2005. Impact of genetic modification on country image of imported food products in European markets: perceptions of channel members. Food Policy. 30:385–398.
- Knight JG, Mather DW, Holdsworth DK, Ermen DF. 2007b. Acceptance of GM food—an experiment in six countries. Nature Biotechnology. 25:507–508.
- Kubisz P, Dalton G, Majewski E, Pogodzińska K. 2021. Facts and myths about GM food—the case of Poland. Agriculture. 11:791. doi:10.3390/agriculture11080791.
- Lassoued R, Phillips PWB, Macall DM, Hesseln H, Smyth SJ. 2021. Expert opinions on the regulation of plant genome editing. Plant Biotechnology Journal. 19:1104–1109. doi:10.1111/pbi.13597.
- Ledford H. 2020. Quest to use CRISPR against disease gains ground. Nature. 577:156.
- Lee L, Giesbrecht A. 2021. A welfare analysis on GM crop market structures and existing regulations. Journal of Student Research. 10:1–10.
- Liefeld JP. 1993. Experiments on country-of-origin effects: review and meta-analysis of effect size. In: Papadopoulos C, Heslop L, editors. Product-country images: impact and role in international marketing. New York: International Business Press; p. 117–146.
- Lusk JL, Daniel MS, Mark DR, Lusk CL. 2001. Alternative calibration and auction institutions for predicting consumer willingness to pay for nongenetically modified corn chips. Journal of Agricultural and Resource Economics. 26:40–57.
- Lusk JL, Jamal M, Kurlander L, Roucan M, Taulman L. 2005. A meta-analysis of genetically modified food valuation studies. Journal of Agricultural and Resource. 30:28–44.
- Lusk JL, Roosen J, Bieberstein A. 2014. Consumer acceptance of new food technologies: causes and roots of controversies. Annual Review of Resource Economics. 6:381–405.
- Lynas M. 2020. Yes, some COVID vaccines use genetic engineering. Get over it. Alliance for Science. [accessed 2021 Dec 5]. https://allianceforscience.cornell.edu/blog/2020/12/yes-some-covid-vaccines-use-genetic-engineering-get-over-it/.
- Macall DM, Williams C, Gleim S, Smyth SJ. 2021. Canadian consumer opinions regarding food purchase decisions. Journal of Agriculture and Food Research. 3:100098. doi:10.1016/j.jafr.2020.100098.
- MacDonald IC, Deans TL. 2016. Tools and applications in synthetic biology. Advanced Drug Delivery Reviews. 105(Part A):20–34. doi:10.1016/j.addr.2016.08.008.
- Macdonald N. 2022. Is this the technology to win Kiwis over to genetic engineering? [accessed 2022 April 25]. https://www.stuff.co.nz/environment/128185542/is-this-the-technology-to-win-kiwis-over-to-genetic-engineering?cid=app-android.
- Mahaffey H, Taheripour F, Tyner WE. 2016. Evaluating the economic and environmental impacts of a global GMO ban. Paper prepared for presentation at the 2016 Agricultural and Applied Economics Association Annual Meeting, Boston, Massachusetts, July 31-August 2 2016. p. 34. https://ageconsearch.umn.edu/record/235591.
- Mallapaty S. 2019. Australian gene-editing rules adopt ‘middle ground’. Nature. [accessed 2021 Nov 14]. doi:10.1038/d41586-019-01282-8.
- Marchant GE, Stevens YA. 2015. A new window of opportunity to reject process-based biotechnology regulation. GM Crops & Food. 6:233–242.
- Marette S, Disdier A-C, Beghin JC. 2021. A comparison of EU and US consumers’ willingness to pay for gene-edited food: evidence from apples. Appetite. 159:105064. doi:10.1016/j.appet.2020.105064.
- Masambuka-Kanchewa F, Rumble J, Buck E. 2021. Exploring differences in communication behaviors between organic and conventional farmers. Journal of Agriculture, Food Systems, and Community Development. 10:205–219. doi:10.5304/jafscd.2021.103.018.
- Mather D, Vikan R, Knight J. 2016. Marketplace response to GM animal products. Nature Biotechnology. 34:236–238.
- Matovu J. 2021. Does the inclusion of second generation genetically modified plants in feeds have any effect on animal performance? Turkish Journal of Agriculture – Food Science and Technology. 9:1799–1807. doi:10.24925/turjaf.v9i10.1799-1807.4104.
- Matovu J, Alçiçek A. 2021. Investigations and concerns about the fate of transgenic DNA and protein in livestock. 5th International Students Science Congress Proceedings, p. 1–10. doi:10.52460/issc.2021.011.
- McGuinness Institute. 2013. An overview of genetic modification in New Zealand 1973–2013: the first forty years. Project 2058; Report 16, September 2013, p. 140. [accessed 2021 Nov 10] http://www.mcguinnessinstitute.org/project-2058-reports/.
- Ministry of Foreign Affairs and Trade (MFAT). 2022. Key facts on New Zealand-China trade. [accessed 2022 April 17] https://www.mfat.govt.nz/en/trade/free-trade-agreements/free-trade-agreements-in-force/nz-china-free-trade-agreement/key-facts-on-new-zealand-china-trade/.
- Morris SH, Adley CC. 2000. Genetically modified food issues: attitudes of Irish university scientists. British Food Journal. 102:669–691. doi:10.1108/00070700010362040.
- Mosier SL, Rimal A, Ruxton MM. 2020. A song of policy incongruence: the missing choir of consumer preferences in GMO-labeling policy outcomes. The Review of Policy Research. 37:511–534. doi:10.1111/ropr.12391.
- Muzhinji N, Ntuli V. 2021. Genetically modified organisms and food security in Southern Africa: conundrum and discourse. GM Crops & Food. 12:25–35. doi:10.1080/21645698.2020.1794489.
- Nadal A, De Giacomo M, Einspanier R, Kleter G, Kok E, McFarland S, Onori R, Paris A, Toldrà M, van Dijk J, et al. 2018. Exposure of livestock to GM feeds: detectability and measurement. Food and Chemical Toxicology. 117:13–35. doi:10.1016/j.fct.2017.08.032.
- Naranjo SE. 2017. Displacement of native natural enemies by introduced biological control agents in agroecosystems: a series non-target effect or not? In: Mason PG, Gillespie DR, Vincent C, editors. Proceedings of the 5th international symposium on biological control of arthropods, Langkawi, Malaysia. CABI International; p. 46–49.
- Naranjo SE, Hellmich RL, Romeis J, Shelton AM, Velez AM. 2020. The role and use of genetically engineered insect-resistant crops in IPM systems. In: Kogan M, Heinrichs E, editors. Integrated management of insect pests: current and future developments. Cambridge (UK): Burleigh Dodds Science Publishing; p. 1–58. doi:10.19103/AS.2019.0047.10.
- New Zealand Food Safety. 2021. Labelling requirements for genetically modified food. [accessed 2022 April 17]. https://www.mpi.govt.nz/food-business/labelling-composition-food-drinks/specific-product-labelling/labelling-requirements-for-genetically-modified-food/#:~:text=If%20the%20food%20you%20sell,of%20the%20Food%20Standards%20Code.
- New Zealand Legislation. 1996. Hazardous Substances and New Organisms Act 1996. [accessed 2021 Dec 5]. https://legislation.govt.nz/act/public/1996/0030/latest/DLM381228.html.
- New Zealand Productivity Commission . 2021. New Zealand firms: reaching for the frontier. Final report. Available at www.productivity.govt.nz/inquiries/frontier-firms/.
- News European Parliament. 2020. Parliament to allow COVID-19 vaccines to be developed more quickly. [accessed 2021 Dec 5]. https://www.europarl.europa.eu/news/en/press-room/20200706IPR82731/parliament-to-allow-covid-19-vaccines-to-be-developed-more-quickly.
- Oselinsky K, Johnson A, Lundeberg P, Johnson Holm A, Mueller M, Graham DJ. 2021. GMO food labels do not affect college student food selection, despite negative attitudes towards GMOs. International Journal of Environmental Research and Public Health. 18:1761. doi:10.3390/ijerph18041761.
- Otsuka Y. 2021. Consumer movements confronted by naturalness in gene editing in Japan. East Asian Science, Technology and Society: An International Journal. 15:24–45. doi:10.1080/18752160.2021.1877442.
- Palmieri N, Simeone M, Russo C, Perito MA. 2020. Profiling young consumers’ perceptions of GMO products: a case study on Italian undergraduate students. International Journal of Gastronomy and Food Science. 21:100224. doi:10.1016/j.ijgfs.2020.100224.
- Pappalardo G, D’Amico M, Lusk JL. 2021. Comparing the views of the Italian general public and scientists on GMOs. International Journal of Food Science and Technology. 56:3641–3650. doi:10.1111/ijfs.14993.
- Parisi C, Rodríguez-Cerezo E. 2021. Current and future market applications of new genomic techniques. EUR 30589 EN. Publications Office of the European Union: Luxembourg, p. 52, JRC123830. doi:10.2760/02472.
- Pixley KV, Falck-Zepeda JB, Giller KE, Glenna LL, Gould F, Mallory-Smith CA, Stelly DM, Stewart Jr CN. 2019. Genome editing, gene drives, and synthetic biology: will they contribute to disease-resistant crops, and who will benefit? Annual Review of Phytopathology. 57:165–188.
- Popek S, Halagarda M. 2017. Genetically modified foods: consumer awareness, opinions and attitudes in selected EU countries. International Journal of Consumer Studies. 41:325–332. doi:10.1111/ijcs.12345.
- Pretorius IS, Boeke JD. 2018. Yeast 2.0—connecting the dots in the construction of the world's first functional synthetic eukaryotic genome. FEMS Yeast Research. 18(4):Article foy032. doi:10.1093/femsyr/foy032.
- Prowse TAA, Cassey P, Ross JV, Pfitzner C, Wittmann TA, Thomas P. 2017. Dodging silver bullets: good CRISPR gene-drive design is critical for eradicating exotic vertebrates. Proceedings of the Royal Society B: Biological Sciences. 284:20170799. https://royalsocietypublishing.org/doi/abs/10.1098/rspb.2017.0799.
- Pruitt JR, Melton KM, Palma MA. 2021. Does physical activity influence consumer acceptance of gene edited food? Sustainability. 13:7759. doi:10.3390/su13147759.
- Purnhagen KP, Clemens S, Eriksson D, Fresco LO, Tosun J, Qaim M, Visser RGF, Weber APM, Justus Wesseler HH, Zilberman D. 2021. Europe’s farm to fork strategy and Its commitment to biotechnology and organic farming: conflicting or complementary goals? Trends in Plant Science. 26:600–606. doi:10.1016/j.tplants.2021.03.012.
- Ribarits A, Narendja F, Stepanek W, Hochegger R. 2021. Detection methods Fit-for-purpose in enforcement control of genetically modified plants produced with novel genomic techniques (NGTs). Agronomy. 11:61. doi:10.3390/agronomy11010061.
- Rickertsen K, Gustavsen GW, Nayga RM. 2017. Consumer willingness to pay for genetically modified vegetable oil and salmon in the United States and Norway. AgBioForum. 20:1–11.
- Rihn A, Khachatryan H, Wei X. 2021. Perceived subjective versus objective knowledge: consumer valuation of genetically modified certification on food producing plants. PLoS ONE. 16(8):e0255406. doi:10.1371/journal.pone.0255406.
- Roberts RJ. 2018. The nobel laureates’ campaign supporting GMOs. Journal of Innovation & Knowledge. 3:61–65. doi:10.1016/j.jik.2017.12.006.
- Roldan MB, Cousins G, Muetzel S, Zeller WE, Fraser K, Salminen J-P, Blanca A, Kaur R, Richardson K, Maher D, et al. 2022. Condensed tannins in white clover (Trifolium repens) foliar tissues expressing the transcription factor TaMYB14-1 bind to forage protein and reduce ammonia and methane emissions in vitro. Frontiers in Plant Science. 12:777354. doi:10.3389/fpls.2021.777354.
- Rolleston W. 2010. An industry viewpoint – why it’s time to relax the regulations. AgScience. 36:7–9.
- Romeis J, Naranjo SE, Meissle M, Anthony M, Shelton AM. 2019. Genetically engineered crops help support conservation biological control. Biological Control. 130:136–154. doi:10.1016/j.biocontrol.2018.10.001.
- Rose KM, Brossard D, Scheufele DA. 2020. Of society, nature, and health: how perceptions of specific risks and benefits of genetically engineered foods shape public rejection. Environmental Communication . 14:Article 7. doi:10.1080/17524032.2019.1710227.
- Roth MS, Romeo JB. 1992. Matching product category and country image perceptions: a framework for managing country-of-origin effects. Journal of International Business Studies. 23(3):477–497.
- RSNZ. 2010. Genetically modified forages – emerging issues. p. 6. [accessed 2021 Oct 25]. https://www.royalsociety.org.nz/assets/documents/publications-policy-2009-emerging-issues-gm-forages.pdf.
- RSNZ. 2016. Gene editing: evidence update. p. 9. [accessed 2021 Dec 5]. https://www.royalsociety.org.nz/assets/documents/Gene-editing-evidence-update2.pdf.
- Saha CN, Bhattacharya S. 2011. Intellectual property rights: an overview and implications in pharmaceutical industry. Journal of Advanced Pharmaceutical Technology & Research. 2:88–93.
- Saleh R, Bearth A, Siegrist M. 2021. How chemophobia affects public acceptance of pesticide use and biotechnology in agriculture. Food Quality and Preference. 91:104197. doi:10.1016/j.foodqual.2021.104197.
- Sanmugam S, Sivakumar S, Gobalakrishnan T, Sarawanan T, Abeweera PR, Sandrasaigaran P. 2021. Perception and acceptance of genetically modified foods in Malaysia. Malaysian Journal of Science Advanced Technology. 1:144–150. https://mjsat.com.my/index.php/mjsat/article/view/29.
- Saunders C, Dalziel P, Tait P, Driver T, Guenther M, Rutherford P, Saunders J. 2021. A global perspective on New Zealand's agriculture and horticultural brand. Invited address to the Sustainable and Profitable farming – what is our brand conference. New Zealand Institute of Agricultural and Horticultural Science (NZIAHS) forum, ‘Sustainable and profitable farming – what is our brand?” Lincoln University, Canterbury.
- Saunders C, Kaye-Blake W, Cagatay S. 2003. Economic impacts on New Zealand of GM crops: result from partial equilibrium modelling. Research Report No. 261, Agribusiness and Economics Research Unit, Lincoln University, New Zealand. http://dspace.lincoln.ac.nz/handle/10182/743.
- Scheitrum D, Schaefer KA, Nes K. 2020. Realized and potential global production effects from genetic engineering. Food Policy. 93:101882. doi:10.1016/j.foodpol.2020.101882.
- Scherer FM. 1999. New perspectives on economic growth and technological innovation. Washington (DC): Brookings Institution Press.
- Scott MJ, Gould F, Lorenzen M, Grubbs N, Edwards O, O’Brochta D. 2018. Agricultural production: assessment of the potential use of Cas9-mediated gene drive systems for agricultural pest control. Journal of Responsible Innovation. 5:S98–S120. doi:10.1080/23299460.2017.1410343.
- Selfa T, Lindberg S, Bain C. 2021. Governing gene editing in agriculture and food in the United States: tensions, contestations, and realignments. Elementa: Science of the Anthropocene. 9:1. doi:10.1525/elementa.2020.00153.
- Sendhil R, Yadav S, Prashat PR, Ragupathy R. 2021. Consumer perception and preference towards genetically modified (GM) foods: bibliometric evidence and policy imperatives. agriRxiv. Wallingford. 2021 Jul 5. p. 42. doi:10.31220/agriRxiv.2021.00061.
- Shahbandeh M. 2022. Import volume of soybeans worldwide in 2021/22, by country. [accessed 2022 April 27]. https://www.statista.com/statistics/612422/soybeans-import-volume-worldwide-by-country/.
- Shew AM, Nalley LL, Snell HA, Nayga RM, Dixon BL. 2018. CRISPR versus GMOs: public acceptance and valuation. Global Food Security. 19:71–80. doi:10.1016/j.gfs.2018.10.005.
- Sieradzki Z, Mazur M, Król B, Kwiatek K. 2021. Prevalence of genetically modified soybean in animal feedingstuffs in Poland. Journal of Veterinary Research. 65:93–99. doi:10.2478/jvetres-2021-0012.
- Smith V, Wesseler JHH, Zilberman D. 2021. New plant breeding technologies: an assessment of the political economy of the regulatory environment and implications for sustainability. Sustainability. 13:3687. doi:10.3390/su13073687.
- Smyth SJ. 2017a. Canadian regulatory perspectives on genome engineered crops. GM Crops & Food. 8:35–43.
- Smyth SJ. 2017b. Genetically modified crops, regulatory delays, and international trade. Food and Energy Security. 6:78–86. doi:10.1002/fes3.100.
- Sońta M, Rekiel A, Więcek J, Batorska M, Puppel K. 2021. Alternative protein sources vs. GM soybean meal as feedstuff for pigs—meat quality and health-promoting indicators. Animals. 11:Article 177. doi:10.3390/ani11010177.
- Spendrup S, Eriksson D, Fernqvist F. 2021. Swedish consumerś attitudes and values to genetic modification and conventional plant breeding – the case of fruit and vegetables. GM Crops & Food. 12:342–360. doi:10.1080/21645698.2021.1921544.
- Stein A, Rodríguez-Cerezo E. 2010. International trade and the global pipeline of new GM crops. Nature Biotechnology. 28:23–25. doi:10.1038/nbt0110-23b.
- Sutherland C, Gleim S, Smyth SJ. 2021. Correlating genetically modified crops, glyphosate use, and increased carbon sequestration. Sustainability. 13:11679. doi:10.3390/su132111679.
- Tallapragada M, Hardy BW, Lybrand E, Hallman WK. 2021. Impact of abstract versus concrete conceptualization of genetic modification (GM) technology on public perceptions. Risk Analysis. 41:976–991. doi:10.1111/risa.13591.
- Tas M, Balci M, Yüksel A, Sahin Yesilçubuk N. 2015. Consumer awareness, perception and attitudes towards genetically modified foods in Turkey. British Food Journal. 117:1426–1439. doi:10.1108/BFJ-01-2014-0047.
- Thiruthy N. 2017. Open source—is it an alternative to intellectual property? The Journal of World Intellectual Property. 20:68–86.
- Thompson P. 2021. Food system transformation and the role of gene technology: an ethical analysis. Ethics & International Affairs. 35:35–49. doi:10.1017/S0892679421000034.
- Tourism NZ. 2009. Pure as celebrating 10 years of 100% Pure New Zealand. [accessed 27 April 2022]. https://www.tourismnewzealand.com/media/1544/pure-as-celebrating-10-years-of-100-pure-new-zealand.pdf.
- Touyz LZG. 2013. Genetically modified foods, cancer, and diet: myths and reality. Current Oncology. 20:59–61. doi:10.3747/co.20.1283.
- Trading Economics . 2022. Australia exports to China. [accessed 2022 April 17]. https://tradingeconomics.com/australia/exports/china#:~:text=Australia%20Exports%20to%20China%20was,COMTRADE%20database%20on%20international%20trade.
- Tsakok I, Mengoub FE. 2021. Genetically modified organisms: promising or problematic for food security? A review of major developments in selected industrialized countries part I. Policy Brief, Plocy Centre for the New South. p. 12. https://www.policycenter.ma/sites/default/files/PB_21-02_Tsakok-Mengoub.pdf.
- Turkec A, Lucas SJ, Karlik E. 2016. Monitoring the prevalence of genetically modified (GM) soybean in Turkish food and feed products. Food Control. 59:766–772. doi:10.1016/j.foodcont.2015.06.052.
- Turnbull C, Lillemo M, Hvoslef-Eide TAK. 2021. Global regulation of genetically modified crops amid the gene edited crop boom – a review. Frontiers in Plant Science. 12:630396. doi:10.3389/fpls.2021.630396.
- Tyagi S, Kesiraju K, Saakre M, Rathinam M, Raman V, Pattanayak D, Sreevathsa R. 2020. Genome editing for resistance to insect pests: an emerging tool for crop improvement. ACS Omega. 5:20674–20683. doi:10.1021/acsomega.0c01435.
- United States Department of Agriculture. 2018. Sectary perdue issues USDA statement on plant breeding innovation. USDA Press Releases. [accessed 2021 Nov 14]. https://www.usda.gov/media/press-releases/2018/03/28/secretary-perdue-issues-usda-statement-plant-breeding-innovation.
- Vega Rodríguez A, Rodríguez-Oramas C, Sanjuán Velázquez E, Hardisson de la Torre A, Rubio Armendáriz C, Carrascosa Iruzubieta C. 2022. Myths and realities about genetically modified food: a risk-benefit analysis. Applied Sciences. 12: Article 2861. doi:10.3390/app12062861.
- Winichayakul S, Beechey-Gradwell Z, Muetzel S, Molano G, Crowther T, Lewis S, Xue H, Burke J, Bryan G, Roberts NJ. 2020. In vitro gas production and rumen fermentation profile of fresh and ensiled genetically modified high–metabolizable energy ryegrass. Journal of Dairy Science. 103:2405–2418. doi:10.3168/jds.2019-16781.
- World Bank. 2019. New Zealand Trade. World Integrated Trade Solution. [accessed 2022 April 17]. https://wits.worldbank.org/CountrySnapshot/en/NZL.
- Wuepper D, Wree P, Ardali G. 2019. Does information change German consumers’ attitudes about genetically modified food? European Review of Agricultural Economics. 46:53–78.
- Wunderlich S, Gatto KA. 2015. Consumer perception of genetically modified organisms and sources of information. American society for nutrition. Advances in Nutrition. 6:842–851. doi:10.3945/an.115.008870.
- Xinhua. 2021. China continues to receive largest share of New Zealand exports: statistics. [accessed 2022 April 17] http://www.xinhuanet.com/english/asiapacific/2021-07/26/c_1310086422.htm.
- Yang R, Ramsaran R, Wibowo S. 2018. An investigation into the perceptions of Chinese consumers towards the country-of-origin of dairy products. International Journal of Consumer Studies. 42:205–216. doi:10.1111/ijcs.12403.
- Yang YT, Chen B. 2016. Governing GMOs in the USA: science, law and public health. Journal of the Science of Food and Agriculture. 96:1851–1855. doi:10.1002/jsfa.7523.
- Zaidi SS, Mukhtar MS, Mansoor S. 2018. Genome editing: targeting susceptibility genes for plant disease resistance. Trends in Biotechnology. 36:898–906. doi:10.1016/j.tibtech.2018.04.005.
- Zhang M, Fan Y, Chen C, Cao J, Pu H. 2021. Consumer perception, mandatory labeling, and traceability of GM soybean oil: evidence from Chinese urban consumers. GM Crops & Food. 12:36–46. doi:10.1080/21645698.2020.180785.
- Zheng Q, Wang HH. 2021. Do consumers view the genetically modified food labeling systems differently? “contains GMO” versus “Non-GMO” labels. The Chinese Economy. doi:10.1080/10971475.2021.1890356.
- Zilberman D, Holland TG, Trilnick I. 2018. Agricultural GMOs—what we know and where scientists disagree. Sustainability. 10:1514. doi:10.3390/su10051514.
