ABSTRACT
This review explores Prionoplus reticularis, an indigenous New Zealand edible insect, commonly known as the ‘Huhu grub’. With a rich history of consumption by Māori and a presence in local food festivals, Huhu grubs are gaining attention as a potential alternative protein source. Growing global populations increase the demand for nutrient-rich foods, especially protein, driving exploration of novel food sources. Huhu grubs, abundant in essential nutrients and bioactive compounds, offer promise for contributing to future protein needs. This review outlines the nutritional qualities and health implications of consuming Huhu grubs, as well as the ecological importance of Huhu grubs in New Zealand's forests for nutrient cycling and decomposition. The potential for controlled Huhu grub farming, mitigating environmental and societal impacts of wild harvesting, would promote the use of Huhu grubs as food source, aligning with global objectives for improved food security and sustainability. This review also aims to signal the need for increased research and investment in the food value of Huhu grubs. Managed appropriately, Huhu grubs have the potential to contribute to the nutritional needs of a growing population, preserving ecosystems and fostering long-term sustainability in the food industry.
Introduction
The definition of good health has evolved beyond the absence of disease to encompass the functional physical fitness and mental well-being which is related to the nutritional status of individuals (Meiselman Citation2016). With the global population projected to reach 9 billion by 2050, there will be an increased demand for essential nutrients, and especially protein, that are well recognised to promote health and psychological resilience (FAO Citation2008, Citation2009). It is becoming increasingly evident that conventional sources of protein, derived from animal and from plant products, may not be sufficient to meet future needs, and issues of sustainability are becoming increasingly recognised. Therefore, there has been growing interest in exploring alternative sources of protein that are more sustainably produced, such as edible insect materials (Surendra et al. Citation2016; Hamm Citation2018).
Edible insect materials are rich in essential nutrients, including minerals, vitamins, lipids, and proteins (Nowak et al. Citation2016). Insect materials have functional properties desired by consumers, including high protein content in the range of 7–70% protein on a dry weight basis (Kinyuru et al. Citation2015; Churchward-Venne et al. Citation2017; Nowakowski et al. Citation2020). For instance, the house cricket (Acheta domesticus) contains 65% protein based on dry matter, which is substantially higher than that of conventional protein sources, such as milk (30%), eggs (52%), beef (50%), and soybean (45%), based on dry matter (Rumpold and Schlüter Citation2015; Churchward-Venne et al. Citation2017). Additionally, consumers are becoming increasingly interested in food with functional and bioactive compounds (Banwo et al. Citation2021). As highlighted by Chung et al. (Citation2005), physicochemical properties such as solubility, foaming capacity and emulsion stability of proteins play a crucial role in determining their specific utilisation in various food applications, as well as their digestibility and bioavailability.
This review focuses on Prionoplus reticularis, one of the indigenous edible insect species of New Zealand, that is locally known as the ‘Huhu grub’. Huhu grubs have been a traditional indigenous food of Māori (the indigenous Polynesian people of mainland New Zealand (Aotearoa)), whom it is thought originated from settlers of East Polynesia. They colonised New Zealand around the early to mid-1300s AD (Walter et al. Citation2017). Huhu grubs are traditionally either eaten raw, or cooked (e.g. fried). Traditionally, ‘Huhu’ is the Māori name for the larval stages, but more recently the larval and pupal developmental stages have become collectively referred to as Huhu grubs (Meyer-Rochow Citation2005). Folk science and the relevance of Huhu grubs in ‘te aitanga pepeke’ (the insect world) is well recognised in ‘pūrākau’ (myths and legends) in Te Ao Māori (Miller Citation1952). Huhu grubs are featured at wild food festivals in New Zealand where they are served as a delicacy. These larvae are also a food source for New Zealand indigenous birds such as the Kiwi and Kaka, as well as other wildlife, including rodents, pigs, and hedgehogs (Museum of New Zealand- Te Papa Tongarewa Citation1998). There is an increasing recognition globally of the importance of indigenous foods in combating hunger and enhancing food security, which aligns with the UN Sustainable Development Goals (Kuhnlein Citation2009). Enhancing the awareness of Huhu grubs as a food source for future development, and addressing the rearing, processing, and economics of farmed production, has the potential to enable large scale production of Huhu grubs as a food source. Wild harvesting of edible insects is considered unsustainable (Van Huis Citation2013), due in part to limited and sometimes seasonal availability, and the risk of diseases being present in wild-sourced insect material. The feasibility of cultivating specific insect species for food holds promise, provided that their nutritional value, biology, cultivation methods, and production economics are well-understood.
This review aims to provide a summary of the currently available scientific literature of Huhu grubs, and the identification of critical knowledge gaps in relation to the potential of Huhu grubs to contribute as a sustainable alternative food source in the future.
Morphology and life cycle of the Huhu grub
The Huhu grub, the larval form of the Prionoplus reticularis beetle, is an indigenous insect of New Zealand (Miller Citation1952). Prionoplus reticularis is the largest endemic beetle in New Zealand (Hosking Citation1978), and a member of the longhorn beetles of the family Cerambycidae. The genus Prionoplus consists of only one species, Prionoplus reticularis (Williams Citation2011).
Huhu grubs were an endemic monotypic genus before European contact, but with widespread plantation of exotic conifers such as Pinus radiata (pine) following European settlement, this insect has adapted to the this exotic host (Edwards Citation1959). Huhu grubs can be found inhabiting decaying pine logs. shows an image of a Huhu grub within a cavity in decaying pine wood.
Initial research on New Zealand Huhu larvae, some decades ago, focused mainly on lifecycle and morphology aspects (Edwards Citation1959; Morgan Citation1960; Edwards Citation1961a, Citation1961b; Hosking Citation1978). The larvae are whitish in colour and can grow up to 70 mm in length. Female Huhu beetles oviposit between 250 and 300 eggs onto decaying logs, either under the bark or in crevices in the wood. The eggs hatch into larvae which bore into the wood, consuming the wood material and excreting faecal material referred to as ‘frass’. An egg develops into a small larva, which develops through what can be referred to as ‘small’, ‘medium’ and ‘large’ larval stages, and then subsequently develops into a pupa, before metamorphosis into the adult beetle () (Kavle et al. Citation2022a).
Figure 2. Representative developmental stages of Prionoplus reticularis (egg, small-, medium-, and large- larva, pupa, and adult beetle) derived from Kavle (Citation2023).
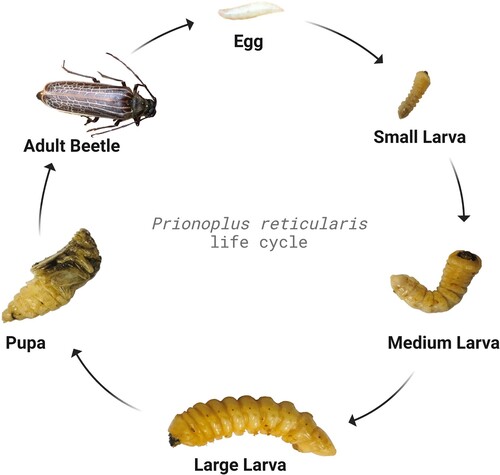
Huhu grubs live within decaying wood for about 2–3 years, before developing into the pupal stage which lasts for 25 days, before transforming into a flying adult beetle for about 2 weeks (Rogers et al. Citation2002). The timings of Huhu grub instars have not been reported. The ‘medium’ and ‘large’ larvae are the developmental stages most commonly consumed as food. The typical dimensions of these development stages are shown in .
Table 1. Anthropometric parameters of Huhu grub larvae and pupae; The data were derived from Kavle et al. (Citation2022a).
Xylophagous diet of Huhu grubs
Huhu grubs have a holometabolous lifecycle (Edwards Citation1961a), and are xylophagous (Reid et al. Citation2011), hence they thrive best in damp decaying wood. However, the mechanics of digestion of wood materials in the gut of Huhu grubs has not been studied extensively. Further, the bacterial communities associated with the gut microbiota of Huhu grubs remain largely unexplored. Viswam et al. (Citation2019) studied Huhu grub larvae under laboratory conditions and found that the composition of the Huhu grub diet influences the microbial community in the insect gut secretion (frass). Viswam et al. (Citation2019) generated a DNA fingerprint of the frass using automated ribosomal intergenic spacer analysis (ARISA), as opposed to a more powerful high-throughput sequencing technique, and hence a clear relationship between diet and bacterial community in the Huhu grub gut was not obtained.
As Huhu grubs consume an incomplete diet of decaying wood, they are expected to rely on a symbiotic association with gut microorganisms (Douglas, Citation2009), to meet their nutritional needs. A similar relationship has been demonstrated in other insects, such as dung beetles, Copris incertus (Suárez-Moo et al., Citation2020), bark beetles, Dendroctonus rhizophagus (Briones-Roblero et al., Citation2017), and a species of scarab beetle, Melolontha hippocastani (Arias-Cordero et al., Citation2012), all of whom, like the Huhu grub, share the same order (Coleoptera), and include plant material in their diets.
Wood-eating beetle juvenile stages harbour taxonomically diverse and ecologically rich gut microbes that facilitate their survival on a diet of wood tissue devoid of many essential nutrients (Scully et al. Citation2014). In general, it can be hypothesised that wood-consuming grubs obtain nutrients in one or more of the following ways: (i) by producing a suite of enzymes that can enhance lignin degradation, ferment xylose and wood sugars, (ii) by intake of wood biomass that has been partially digested by fungi growing on the decaying wood (Engel and Moran Citation2013), (iii) by the action of gut bacteria in wood-consuming grubs which ‘digest’ the wood cellulose, followed by absorption of sugars in the larval gut (Scully et al. Citation2014). Martin (Citation1983) reported that cellulose digestion by endogenous insect enzymes is rare and that microorganisms in the insect gut mediate cellulose digestion. Other wood consuming insects, also known as xylophagous insects, have evolved various mechanisms to digest and utilise wood as a food source. Wood typically consists principally of three main components: lignin, cellulose, and hemicellulose, as well as lower levels of other compounds, such as fatty acids, phenolic compounds, and resin acid (Vevere et al. Citation2020). Engel and Moran (Citation2013) have also reported that as the level of nutrients in wood biomass is low, insect larvae consuming wood require their gut microbiota to degrade cellulose into sugar intermediates that can be absorbed and converted into fatty acids.
Previous studies
summarises the previously reported studies that collectively provide a comprehensive and insightful exploration of Huhu grubs, that offer a substantial understanding of various facets of their biology and potential applications. The study by Edwards (Citation1959), to the best of our knowledge, was the first to record the presence of Huhu grubs in an exotic conifer forest, setting the stage for subsequent investigation. Subsequent studies have delved into different aspects of the Huhu grub life history, behaviour, and diet. Notably, they unveiled the enigmatic nature of the Huhu grub life cycle, including the egg incubation period of approximately 23 days, and that the larval stage lasts from 2 to 3 years.
Table 2. Table of previous studies on huhu grubs reported in the literature.
A subsequent study by Edwards (Citation1961a) reported on the phenology and flight pattern of adult Huhu beetles, using innovative methods, such as emergence cages, light trapping, and also by direct observation. This was followed by a report Edwards (Citation1961b) which improved the understanding of the Huhu grub life cycle beyond the larval stage, encompassing behaviours such as combat, copulation, and oviposition. Hutchins and Christmas (Citation1973) reported the analysis of the fatty acid content analysis of Huhu grubs, using gas–liquid chromatography, that revealed interesting information in relation to the lipids present in the Huhu grub, in relation to the lipid composition of the Huhu grub dietary source. Notably, the study found that the resin acids present in pine wood were not a component of Huhu grub lipid composition.
Further insights were gained from experiments treating Huhu grub eggs and larvae at elevated temperatures under controlled atmosphere conditions (Dentener et al. Citation1999). The results of this study contributed to a wider consideration in relation to the need to treat wood for export to achieve eradication of infecting animal, fungal and plant species. Rogers et al. (Citation2002) reported that an artificial diet containing pine sawdust could substantially reduce the duration of the larval stage in a laboratory setting, which facilitated subsequent laboratory based taxonomic investigations. Reid et al. (Citation2011) and Williams (Citation2011) reported on exploring the microbiome of Huhu grubs, providing novel insights into metabolically active and inactive bacteria in the Huhu grub gut. This research substantially contributed to knowledge of Huhu grub gut-associated bacteria, that are crucial for digesting components of the nutritionally poor wood-based diet consumed by the Huhu grub, and reported the predominant presence of Candida shehatae, a fungal community hemicellulose degrader, indicating prospects for biological conversion of lignocelluloses. A pivotal dimension of this research focused on rearing Huhu grubs on either non-degraded pine blocks (lignocellulose), or cotton (cellulose), to contribute to a better understanding of the gut microbiome composition in relation to these diets, and led to the isolation of novel lignocellulolytic isolates, offering potential applications in sustainable industries.
Up until 2011, Huhu grub research was generally limited to various studies focussing on rearing, anatomic biology, and gut ecology. However, apart from the work of Hutchins and Christmas (Citation1973), who studied the fatty acid composition of P. reticularis Huhu larvae, no other published studies had reported a comprehensive nutritional profile of Huhu. Kavle et al. (Citation2022a) reported the proximate composition of Huhu grubs (26.2–30.5% protein, 32.1–58.4% fat, and 1.5–3.2% ash on a dry weight (DW) basis) and mineral content, revealing that Huhu grubs contain substantial nutrients and an array of essential and non-essential minerals (manganese (37.5 mg/kg DW), magnesium (1306.7 mg/kg DW), phosphorus (3970.0 mg/kg DW), iron (28.0 mg/kg DW), copper (8.9 mg/kg DW), and zinc (53.6 mg/kg DW)), which indicated that Huhu grubs can be considered to be a safe and nutritious food option.
An investigation of Huhu grub development stages revealed variations in the fatty acid profile and lipid nutritional indices, with pupae exhibiting the highest lipid content. This highlighted that the lipid composition and nutritional characteristics change during the Huhu grub life cycle (Kavle et al. Citation2022b). A following study analysed protein extracts from Huhu grub larvae and pupae (386.7 and 411.7 mg/g protein) and revealed substantial essential amino acid content and favourable techno-functional properties, such as foaming and emulsifying capacities, indicating potential for use of Huhu grub material in food technology applications (Kavle et al. Citation2023).
Collectively, the studies listed in provide a holistic and multifaceted portrait of the Huhu grub, summarising knowledge ranging from natural habitat (Edwards Citation1959, Citation1961a, Citation1961b; Hutchins and Christmas Citation1973; Dentener et al. Citation1999), to chemical composition (Rogers et al. Citation2002; Williams Citation2011; Kavle et al. Citation2022a, Citation2023), and indicating potential to contribute as an alternative food source, and the potential application in diverse industries.
Collectively, this research is prompting increasing public interest, as a result of various outreach activities, including engaging with audiences from schools, colleges, universities, and the public, and reports in the media, as well as scientific conference presentation.
Ecological implications of harvesting Huhu grubs
Huhu grubs are considered to be contributors to decomposition and nutrient cycling in New Zealand's forests. By boring into decaying wood, Huhu grubs, along with fungi, contribute to decomposition of the wood (Martin Citation1983), which releases nutrients, including nitrogen, phosphorus, and potassium, back into the soil environment. Released nutrients then become available for uptake by plants and other organisms, contributing to maintaining a healthy ecosystem (Koshila et al. Citation2019), including essential elements that are recycled and made available to other organisms in the ecosystem (Jankielsohn Citation2018).
Harvesting Huhu grubs from the wild can impact the environment. While small-scale and sustainable harvesting practices may have minimal effects, large-scale harvesting can lead to the depletion of Huhu grub populations, which can affect local ecosystems, including wild bird and lizard communities consuming Huhu grubs as a food source. In addition to wild harvesting of insect material such as Huhu grubs, that may be subject to variation in availability, there is also the risk of disease contamination of material obtained from the wild. Huhu grub farming offers potential advantages by using controlled environment conditions and not impinging on wild populations, and could contribute to a stable alternative food supply, which can contribute to local economies, and mitigate negative societal and ecological impacts associated with unsustainable wild harvesting.
However, it is important to note that Huhu grub farming on an industrial scale has several challenges, including the development of optimal farming conditions with an appropriate timescale, as well as genetic diversity considerations. Most of the studies to date have focused on Huhu grubs developing in pine wood, and further research is needed to explore potential variations in behaviour and biology in different habitats, such as non-pine indigenous tree wood. As is evident from the above considerations, several challenges need to be addressed through further investigation, before Huhu grubs can be produced at farmed industrial scale for contribution to alternative food supply.
Future work
Conservation and ecological impact: As Huhu grubs have an important role in forest ecosystems, future research should be conducted to assess population dynamics, ecological impact, and interaction with other organisms. Understanding how changes in their population might affect other species and ecosystem processes can aid in developing conservation strategies to protect these insects and maintain ecosystem balance.
Farming of Huhu grubs: Limited research has been conducted on the commercial farming of Huhu grubs, with only a few studies addressing this aspect (Rogers et al. Citation2002; Williams Citation2011). To date, there have been no reports of establishment of commercial production. Further investigation and experimentation are necessary to establish viable farming practices for this species. It has been reported that in a laboratory setting insects often exhibit accelerated life cycles (Rogers et al. Citation2002). Valuable insights could be gleaned from established practices used in the cultivation of analogous wood-boring insects, such as the sago weevil and African palm weevil, which have been successfully commercialised in various regions worldwide.
Impact of processing on sensory quality and consumer acceptance: It is important to consider the impact of processing, formulation, and the form in which insect materials are used for food, such as the fortification of processed foods with insect material in powder form, or as intact grubs/insects, on the sensory characteristics and acceptability of an insect material such as the Huhu grub. Factors such as taste, texture, appearance, and aroma play a crucial role in consumer acceptance of conventional and novel foods (Balzan et al. Citation2016). Moreover, research needs to be focused on assessing the sensory characteristics of foods fortified with Huhu grub (protein) extracts, to evaluate consumer acceptance and explore potential applications in large-scale food processing. This would involve conducting sensory evaluations, consumer surveys, and preference studies. Moreover, it will be important to conduct a cost–benefit analysis to determine the feasibility of incorporating Huhu grub material, such as protein extracts, in the formulation of processed foods.
Conclusion
In conclusion, this review underscores the value of Huhu grubs (P. reticularis) as a highly nutritious edible insect material that could potentially contribute to food security and diversity in New Zealand. With a rich history of consumption by the indigenous Māori people in New Zealand, and their increasing recognition as a viable food source, Huhu grubs offer promising prospects for contributing to addressing nutritional needs in a sustainable manner.
However, this review also highlights critical gaps in knowledge that necessitate further research and attention. Firstly, there is a need for studies exploring culturally appropriate ways of harnessing the value of this unique insect material by learning from traditional Māori practices. Additionally, the ecological impact of harvesting Huhu grubs and their role in forest ecosystems requires thorough investigation to devise effective conservation strategies. Furthermore, the scalability and efficiency of Huhu grub farming needs comprehensive research into optimal rearing techniques, nutritional composition, and integration into the food supply chain. Understanding the sensory attributes and consumer acceptance of Huhu grub-based products is crucial for their successful incorporation into mainstream food processing. In essence, while Huhu grubs hold considerable promise as a sustainable protein source, addressing the above knowledge gaps is essential for their successful utilisation.
Acknowledgements
The first author (R.R Kavle) is grateful to the University of Otago for a Ph.D. Scholarship to research this topic. The help of City Forest Dunedin, who allowed access to decaying logs to enable harvesting of Huhu grubs, is also acknowledged. The research project was discussed with the Ngāi Tahu Research Consultation Committee about research on indigenous Huhu grubs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
- Arias-Cordero E, Ping L, Reichwald K, Delb H, Platzer M, Boland W. 2012. Comparative evaluation of the gut microbiota associated with the below-and above-ground life stages (larvae and beetles) of the forest cockchafer, Melolontha hippocastani. PLoS One. 7(12):e51557.
- Balzan S, Fasolato L, Maniero S, Novelli E. 2016. Edible insects and young adults in a north-east Italian city an exploratory study. British Food Journal. 118(2):318–326. doi:10.1108/BFJ-04-2015-0156.
- Banwo K, Olojede AO, Adesulu-Dahunsi AT, Verma DK, Thakur M, Tripathy S, Singh S, Patel AM, Gupta AK, Aguilar CN, Utama GL. 2021. Functional importance of bioactive compounds of foods with potential health benefits: A review on recent trends. Food Bioscience. 43:101320. doi:10.1016/j.fbio.2021.101320.
- Briones-Roblero CI, Hernández-García JA, Gonzalez-Escobedo R, Soto-Robles LV, Rivera-Orduña FN, Zúñiga, G. 2017. Structure and dynamics of the gut bacterial microbiota of the bark beetle, Dendroctonus rhizophagus (Curculionidae: Scolytinae) across their life stages. PloS One. 12(4):e0175470.
- Chung M, Lei B, Li-Chan E. 2005. Isolation and structural characterization of the major protein fraction from NorMan flaxseed (Linum usitatissimum L.). Food Chemistry. 90(1-2):271–279. doi:10.1016/j.foodchem.2003.07.038.
- Churchward-Venne TA, Pinckaers PJ, van Loon JJ, van Loon LJ. 2017. Consideration of insects as a source of dietary protein for human consumption. Nutrition Reviews. 75(12):1035–1045. doi:10.1093/nutrit/nux057.
- Dentener PR, Lewthwaite SE, Rogers DJ, Miller M, Connolly PG. 1999. Mortality of huhu (Prionoplus reticularis) subjected to heat and controlled atmosphere treatments. New Zealand Journal of Forestry Science. 29(3):473–483.
- Douglas AE. 2009. The microbial dimension in insect nutritional ecology. Functional Ecology. 23(1):38–47.
- Edwards JS. 1959. Host range in Prionoplus reticularis White. Transactions of the Royal Society of New Zealand. 87:315–318.
- Edwards JS. 1961a. Observations on the biology of the immature stages of Prionoplus reticularis White (Col. Ceramb.). Transactions of the Royal Society of New Zealand. 88:727–731.
- Edwards JS. 1961b. Observations on the ecology and behaviour of the huhu beetle, Prionoplus reticularis White.(Col.: Ceramb.). Transactions of the Royal Society of New Zealand. 88:733–741.
- Engel P, Moran NA. 2013. The gut microbiota of insects – diversity in structure and function. FEMS Microbiology Reviews. 37(5):699–735. doi:10.1111/1574-6976.12025.
- FAO. 2008. Chapter 2: Global dairy sector: status and trends.[accessed 2022 February 2]. http://www.fao.org/3/i1522e/i1522e02.pdf.
- FAO. 2009. How to feed the world in 2050. Rome, Italy: Food and Agriculture Organization.
- Hamm MW. 2018. Sustainable protein provisioning. Nature Sustainability. 1(12):733–734. doi:10.1038/s41893-018-0196-8.
- Hosking G. 1978. Prionoplus reticularis, White:(Coleoptera: Cerambycidae), Huhu beetle. Forest Service., New Zealand: Forest Research Institute.
- Hutchins R, Christmas P. 1973. Fatty acid composition of huhu larvae (Prionoplus reticularis) in relation to their food source. New Zealand Journal of Science. 16(1):155–159.
- Jankielsohn A. 2018. The importance of insects in agricultural ecosystems. Advances in Entomology. 6(2):62–73. doi:10.4236/ae.2018.62006.
- Kavle RR. 2023. Nutritional composition, protein functionalities, transcriptomics, and in silico discovery of bioactive peptides from Prionoplus reticularis (huhu) [Doctoral thesis]. Dunedin, New Zealand: University of Otago.
- Kavle RR, Carne A, Bekhit AE-DA, Kebede B, Agyei D. 2022a. Macronutrients and mineral composition of wild harvested Prionoplus reticularis edible insect at various development stages: nutritional and mineral safety implications. International Journal of Food Science & Technology. 57(10):6270–6278. doi:10.1111/ijfs.15545.
- Kavle RR, Carne A, Bekhit AE-DA, Kebede B, Agyei D. 2022b. Proximate composition and lipid nutritional indices of larvae and pupae of the edible Huhu beetle (Prionoplus reticularis) endemic to New Zealand. Journal of Food Composition and Analysis. 110:104578. doi:10.1016/j.jfca.2022.104578.
- Kavle RR, Nolan PJ, Bekhit AE-DA, Carne A, Morton JD, Agyei D. 2023. Physicochemical characteristics, techno-functionalities, and amino acid profile of Prionoplus reticularis (huhu) larvae and pupae protein extracts. Foods (basel, Switzerland). 12(2):417. doi:10.3390/foods12020417.
- Kinyuru JN, Mogendi JB, Riwa CA, Ndung'u NW. 2015. Edible insects—a novel source of essential nutrients for human diet: Learning from traditional knowledge. Animal Frontiers. 5(2):14–19.
- Koshila R, Anusuya S, Balachandar M, Muthukumar T. 2019. Microbial interactions in soil formation and nutrient cycling. In: Varma A, Choudhary D, editor. Mycorrhizosphere and pedogenesis. In: Varma A, Choudhary D, editor. Mycorrhizosphere and pedogenesis. Singapore: Springer; 363–382. https://doi.org/10.1007/978-981-13-6480-8_21.
- Kuhnlein HV. 2009. Introduction: Why are Indigenous Peoples’ food systems important and why do they need documentation. In: Kuhnlein HV, Erasmus B, Spigelski D, editor. Indigenous peoples’ food systems: the many dimensions of culture, diversity and environment for nutrition and health. Rome: United Nations Food and Agriculture Organisation; p. 1–7.
- Martin MM. 1983. Cellulose digestion in insects. Comparative Biochemistry and Physiology Part A: Physiology. 75(3):313–324. doi:10.1016/0300-9629(83)90088-9.
- Meiselman HL. 2016. Quality of life, well-being and wellness: measuring subjective health for foods and other products. Food Quality and Preference. 54:101–109. doi:10.1016/j.foodqual.2016.05.009.
- Meyer-Rochow V. 2005. Chapter 19, Traditional food insects and spiders in several ethnic groups of Northeast India, Papua New Guinea, Australia, and New Zealand. In: Paoletti MG, editor. Ecological implications of mini livestock: potential of insects, rodents, frogs and snails. Enfield, New Hampshire: Science Publishers Enfiel; p. 389–414.
- Miller D. 1952. The insect people of the Maori. The Journal of the Polynesian Society. 61(1/2):1–61.
- Modlik M, Johnston L. 2017. Huhu grubs, bull semen shots and koki: visceral geographies of regional food festivals in Aotearoa. New Zealand Geographer. 73(1):25–34. doi:10.1111/nzg.12148.
- Morgan FD. 1960. The comparative biologies of certain New Zealand Cerambycidae. New Zealand Entomologist. 2(5):26–34. doi:10.1080/00779962.1960.9722791.
- Museum of New Zealand- Te Papa Tongarewa. 1998. Huhu beetle (Prionoplus reticularis) or tunga rere. Tai Awatea. [accessed 2023 September 29].
- Nowak V, Persijn D, Rittenschober D, Charrondiere UR. 2016. Review of food composition data for edible insects. Food Chemistry. 193:39–46. doi:10.1016/j.foodchem.2014.10.114.
- Nowakowski AC, Miller AC, Miller ME, Xiao H, Wu X. 2020. Potential health benefits of edible insects. Critical Reviews in Food Science and Nutrition. 62(13):1–10.
- Reid NM, Addison SL, Macdonald LJ, Lloyd-Jones G. 2011. Biodiversity of active and inactive bacteria in the gut flora of wood-feeding huhu beetle larvae (Prionoplus reticularis). Applied and Environmental Microbiology. 77(19):7000–7006. doi:10.1128/AEM.05609-11.
- Rogers DJ, Lewthwaite SE, Dentener PR. 2002. Rearing huhu beetle larvae, Prionoplus reticularis (Coleoptera: Cerambycidae) on artificial diet. New Zealand Journal of Zoology. 29(4):303–310. doi:10.1080/03014223.2002.9518314.
- Rumpold BA, Schlüter O. 2015. Insect-based protein sources and their potential for human consumption: Nutritional composition and processing. Animal Frontiers. 5(2):20–24.
- Scully ED, Geib SM, Carlson JE, Tien M, McKenna D, Hoover K. 2014. Functional genomics and microbiome profiling of the Asian longhorned beetle (Anoplophora glabripennis) reveal insights into the digestive physiology and nutritional ecology of wood feeding beetles. BMC Genomics. 15(1):1–21. doi:10.1186/1471-2164-15-1096.
- Suárez-Moo P, Cruz-Rosales M, Ibarra-Laclette E, Desgarennes D, Huerta C, Lamelas A. 2020. Diversity and composition of the gut microbiota in the developmental stages of the dung beetle Copris incertus Say (Coleoptera, Scarabaeidae). Frontiers in Microbiology. 11:564362.
- Surendra KC, Olivier R, Tomberlin JK, Jha R, Khanal SK. 2016. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renewable Energy. 98:197–202. doi:10.1016/j.renene.2016.03.022.
- Van Huis A. 2013. Potential of insects as food and feed in assuring food security. Annual Review of Entomology. 58:563–583. doi:10.1146/annurev-ento-120811-153704.
- Vevere L, Fridrihsone A, Kirpluks M, Cabulis U. 2020. A review of wood biomass-based fatty acids and rosin acids use in polymeric materials. Polymers. 12(11):2706. doi:10.3390/polym12112706.
- Viswam JP, Lee CK, Morgan HW, McDonald IR. 2019. Laboratory rearing of huhu, Prionoplus reticularis (Cerambycidae): insights into the gut microbiome. New Zealand Journal of Zoology. 46(1):1–12. doi:10.1080/03014223.2018.1461117.
- Walter R, Buckley H, Jacomb C, Matisoo-Smith E. 2017. Mass migration and the Polynesian settlement of New Zealand. Journal of World Prehistory. 30:351–376. doi:10.1007/s10963-017-9110-y.
- Williams TC. 2011. The microflora of the Huhu grub. Hamilton, New Zealand: University of Waikato.

