Abstract
Using a combination of light (stereo and compound) and scaning electron microscopy, this study investigates the distribution and morphology of foliar trichomes of Orthosiphon labiatus (Lamiaceae) at different stages of leaf development. Three types of trichomes were observed: two glandular types (large peltate and small capitate) and one non-glandular type. Results revealed that the development of peltate trichomes was initiated in the early stages of leaf development. However, a higher number of developed peltate trichomes was observed in mature leaves, indicating an increase with age. Capitate trichomes are, by contrast, rare and sparsely distributed on both leaf surfaces. The highest density of non-glandular trichomes was observed on the abaxial leaf surface, especially along the veins, with the density decreasing with leaf maturity. This paper contributes towards the body of knowledge being assembled on the family Lamiaceae as it manifests in southern Africa.
Introduction
A vast number of angiosperm leaves are characterized by having hair-covered surfaces (Metcalfe & Chalk Citation1950). Such leaf trichomes have been proposed to have a number of functions. Non-glandular hairs reflect solar radiation off the leaf surface (Liakoura et al. Citation1999) to prevent heat damage by lowering leaf temperature (Klich Citation2000). They also decrease rates of water loss and facilitate photosynthetic gas exchange (Schreuder et al. Citation2001). Glandular trichomes, by contrast, contain or secrete a mixture of primary and secondary metabolites (including terpenes) that have a wide array of applications in pesticides, pharmaceuticals, food flavouring and fragrances (Cousins Citation1994; Maffei & Codignola Citation1990). In addition to these industrial uses, glandular trichomes found on some crop species confer resistance against insect pests. They do this by forming physical obstacles and by exuding defensive chemicals (Peter et al. Citation1995).
The Lamiaceae are well known for their often densely haired aromatic leaves (Metcalfe & Chalk Citation1950). The presence of both glandular (peltate and capitate) and non-glandular trichomes is a characteristic feature of this family (Maffei & Codignola Citation1990). The glandular trichomes are the source of aromatic, volatile oils and terpenes. As a consequence, some members of the Lamiaceae, the mint family, have since ancient times played an important role as a source of medicinal and aromatic plants of commercial importance. Mentha species were mentioned in the Code of Hammurabi some 3780 years ago (Bowman & Jordan Citation2006) and their uses were referred to by ancient scholars such as Pliny (Simpson Citation2006) and Dioscorides (Barkley Citation1974). Mint was also used as a form of money in biblical times, and, along with lavender, was widely used in countries surrounding the Mediterranean (Fernie Citation1897). In modern times, the essential oils of Lamiaceae and the structures that produce them have become a focal point for research worldwide (Ascensão et al. Citation1999; Ascensão & Pais Citation1998; Bosabalidis Citation1990; Bosabalidis & Skoula Citation1998; Bourett et al. Citation1994; Combrinck et al. Citation2007; Corsi & Bottega Citation1999; Cousins Citation1994; Gairola et al. Citation2009; Gang et al. Citation2001; Kaya et al. Citation2007; Lakusic et al. Citation2005; Maffei et al. Citation1989; Sharma et al. Citation2003; Werker Citation2000; Werker et al. Citation1985 ,Citation1993).
Because it is a genus of medicinal and commercial importance, Orthosiphon Benth. has been chosen for this study (Adam et al. Citation2009; Awale et al. Citation2003; Grosvenor et al. Citation1995; Hossain et al. Citation2008; Hsu et al. Citation2010; Hussein et al. Citation2007; Khamsah et al. Citation2006; Nguyen et al. Citation2004; Sriplang et al. Citation2007; Sumaryono et al. Citation1991; Yam et al. Citation2009). Orthosiphon stamineus Benth. [syn. O. aristatus (Blume) Miq.] from Asia is the most important ethnobotanical species in the genus and is commonly known as ‘Java Tea’, misai kuching or kumis kucing. This species is used in the treatment of various diseases, including nephritis, gout, diabetes, hypertension, rheumatism, dysuria and jaundice (Chin et al. Citation2008; Faizul et al. Citation2009; Han et al. Citation2009; Maheswari et al. Citation2008; Mariam et al. Citation1996; Ngamrojanavanich et al. Citation2006; Olah et al. Citation2003; Ong & Norzalina Citation1999).
Orthosiphon, a genus related to Ocimum L., encompasses > 40 species found throughout Africa and Asia (Retief Citation2000). Nine species of Orthosiphon have been reported from southern Africa (Codd Citation1964 ,Citation1985) and include the African endemic Orthosiphon labiatus N.E.Br.; commonly known as ‘pink sage’. This variable species is a strongly aromatic, twiggy herb or subshrub restricted to, but widespread within, southern Africa (Codd Citation1985; Joffe Citation1993; Retief & Herman Citation2003). Except for a cursory mention in the taxonomic literature (Bremekamp Citation1933; Codd 1964, 1985; Verdoorn Citation1943), there has been no detailed investigation into the foliar trichomes of O. labiatus. Given that the glandular trichomes of this species may be the source of chemicals that have been shown to have some effect against Mycobacterium species in the Mycobacterium tuberculosis complex (the cause of tuberculosis) (McGaw et al. Citation2008), O. labiatus is a good candidate for further study at all levels. Studies such as the one here, which are aimed at elucidating the nature of microscopic structures (potentially the source of beneficial secretions) are particularly important.
With each new species of Lamiaceae studied, researchers are building a more complete picture of the complex micromorphology of this family. Within the published scientific literature, a comprehensive body of knowledge is being built on the vestiture of the Lamiaceae: its structure, function, ecological and evolutionary significance.
Materials and methods
The leaves of O. labiatus were collected during the flowering stage in April 2008 from wild-growing plants at the University of KwaZulu-Natal, Westville Campus, Durban, South Africa. The identification of this material was verified and a voucher specimen (Nicholas and Bhatt 2993) was deposited in the Ward Herbarium (herbarium acronym UDW). Leaves of different developmental stages were selected for the present study, i.e. Stage I – very young leaves from first node, Stage II – young leaves harvested from the second and third nodes, and Stage III – fully expanded/mature leaves harvested from the fourth and fifth nodes. Five plants of O. labiatus were sampled and five leaves from each plant which were at different developmental stages were used for study. Fresh leaves were examined and photographed using a stereomicroscope (Nikon AZ 100).
For the light-microscopy studies, semi-thin sections of leaf material embedded in Spurr's (Citation1969) low-viscosity resin were used. Monitor sections ranging in thickness from 0.5 to 2 µm, were cut with glass knives and fixed by heat onto pre-cleaned glass slides with drops of distilled water. The sections were stained with 0.5% Toluidine Blue-0 made up in 0.1% sodium carbonate at pH 11.1 (Feder & O'Brien, Citation1968). Sections were stained for ~ 1 min over heat, washed briefly in distilled water and mounted in immersion oil. Slides were viewed and photographed with a Leitz light microscope.
For scanning electron microscopy, leaf segments at different developmental stages were prepared by rapid quenching in liquid nitrogen and freeze drying in an Edwards Modulyo Freeze dryer at -60 °C in a vacuum of 10-2 Torr for ~ 48 h. Leaf segments were secured with carbon conductive tape on brass stubs which were sputter coated with gold for 4 min. Observations were carried out with a Jeol 6100 scanning electron microscope at 12 kV and a working distance of 15 mm.
Results
Stereomicroscopy and scanning electron microscopy studies revealed that leaves possess three types of trichome: non-glandular or acicular hairs, peltate trichomes and capitate trichomes.
Non-glandular or acicular hairs
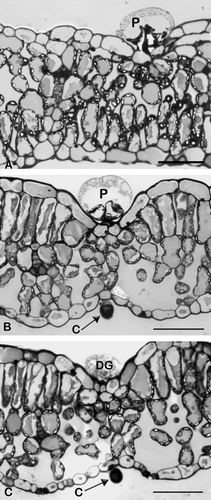
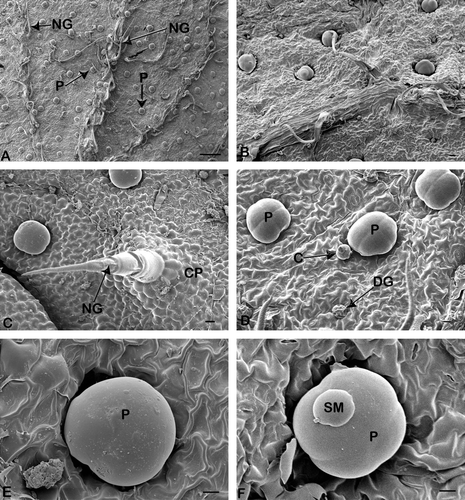
Non-glandular or acicular hairs are sharply pointed and swept in the direction of the leaf apex. They appear to be especially abundant along the veins, particularly on the abaxial surface of very young leaves (A and A), and were also evident on the serrated leaf margins (A,B). Mature non-glandular trichomes were observed in the early stages of leaf development and consist of approximately three to five cells (C). These trichomes are supported by a cellular pedestal formed by a group of epidermal cells arranged in a circle around the bases (C). Wart-like structures were observed on the surface of these trichomes. In the present study, new trichome formation was not observed in the later stages of leaf development.
Fig. 1 Stereo-micrograph showing the distribution of trichomes on an Orthosiphon labiatus leaf. (A) Distribution of non-glandular trichomes on leaf vein (bar = 50 µm), (B) leaf margin (bar = 100 µm), (C) glandular trichomes (bar = 100 µm).

Fig. 2 Scanning electron micrographs of portions of Orthosiphon labiatus leaves showing the two types of glandular trichomes. (A) Leaf vein with numerous glandular peltate (P) and non-glandular (NG) trichomes (bar = 200 µm). (B) Sparse distribution of non-glandular trichomes of mature leaf (bar = 20 µm). (C) Fully developed non-glandular (NG) trichomes on leaf surface supported by cellular pedestal (CP) (bar = 20 µm). (D) Peltate (P), capitate (C) and degenerative peltate (DG) trichome. (E) Peltate glandular trichomes (P) showing four head cells (bar = 10 µm). (E) Mature peltate trichome (P) (bar = 10 µm). (F) Secretory material (SM) on peltate trichome (P) (bar = 10 µm).
Peltate trichomes
Peltate trichomes were observed on both the adaxial and abaxial leaf surfaces (C and A). Studies of transverse sections showed the peltate trichomes to consist of a basal cell embedded in the epidermis, a short stalk cell and a large spherical secretory head (A). This head consists of four secretory cells arranged in a single layer (D), with an enlarged subcuticular chamber within which secretory material accumulates (B). Fully developed peltate trichomes were observed in very young leaves; however, their density was lowest at this developmental stage. Some degenerative peltate trichomes were also observed in all stages of leaf development. It appears that the peltate trichomes secrete their materials, and subsequently become shrivelled and degenerate (D). The peltate trichomes appear to become completely distended as they fill with the secretory material (oil); especially in the mature leaves (A). These mature peltate trichomes were particularly abundant on mature leaves (E). Exuded secretion on the peltate trichomes was observed with scanning electron microscopy (F). Observations with scanning electron microscopy indicated that a uniform distribution of peltate trichomes exists on both leaf surfaces and that they are mostly concentrated on the intervein areas.
Capitate trichomes
Capitate trichomes consist of a single basal cell which is implanted in the epidermal layer, a stalk cell and a more or less spherical secretory head (B and B). The capitate trichomes were rare and sparsely distributed (D).
Discussion
Trichomes are unicellular or multicellular appendages that originate from cells of the aerial epidermis (Werker Citation2000). They vary considerably in morphology, location, ability to secrete and mode of secretion, and different types of trichomes can be produced by the same plant. Orthosiphon labiatus leaves possess one non-glandular and two glandular trichome types on both the adaxial and abaxial leaf surfaces – similar to other species of the family Lamiaceae (Gairola et al. 2009).
Studies of trichome type, size and density in the Lamiaceae have shown that although they can be variable within genera (Dinç et al. Citation2009), within species (especially in size and density) (Grubešic et al. Citation2007) and between different organs of the same plant (Kahraman et al. Citation2010), most differences are species specific in nature and can often be of taxonomic significance (Bini Maleci & Servettaz Citation1991; Navarro & El Oualidi Citation2000) even at the subspecific level (Giuliani et al. Citation2008).
Bosabalidis and Skoula (Citation1998) reported that in Origanum intercedens Rech.f., also an aromatic lamiaceous species, the non-glandular trichomes are diverse in morphology, anatomy and microstructure. They may be unicellular or multicellular, branched or unbranched. In O. labiatus, non-glandular trichomes were single, uniseriate and multicellular, and more abundant on the leaf veins and margins; particularly on the abaxial surface of very young leaves. Similar observations have been made in Plectranthus laxiflorus Benth. (Bhatt et al. unpublished data). As in other species of Lamiaceae, non-glandular trichomes of O. labiatus were supported by a cellular pedestal formed by a group of epidermal cells. It has been suggested that this basal cellular pedestal provides mechanical support (Ascensão et al. 1999) and serves as a point of connection between the trichome and the epidermal layer (Payne Citation1978). The presence of warts on the trichomes is indicative of maturity (Werker Citation2000). In the present study, we observed warts on the trichomes of very young leaves and this therefore indicates that non-glandular trichomes mature at the early stages of leaf development. Warty trichomes are a characteristic of the Lamiaceae and might also be common in other families of the Asterid clade, as defined by the Angiosperm Phylogeny Group (Citation2009); Naidoo et al. unpublished data). The higher density of non-glandular trichomes on the abaxial leaf surface suggests that they prevent water loss by shielding and trapping air over the stomata (Wagner Citation1991), and also reflect UV light and maintain leaf temperature (Lakusic et al. 2005). However, the density of non-glandular trichomes decreased with leaf maturity. A similar pattern of trichome distribution was observed in other species of the family Lamiaceae (Ascensão & Pais Citation1998, Ascensão et al. 1999; Corsi & Bottega Citation1999).
We also observed the presence of glandular trichomes (peltate and capitate) on both the adaxial and abaxial leaf surface of O. labiatus. Similar observations were made for Salvia officinalis L. (Corsi & Bottega Citation1999), Plectranthus ornatus Codd (Ascensão et al. 1999), Mentha arvensis L. (Sharma et al. 2003) and Tetradenia riparia (Gairola et al. 2009). Peltate trichomes were mostly found on the intervein laminar of the leaves of O. labiatus. A similar type of distribution of peltate trichomes was reported in Plectranthus madagascariensis Benth. (Ascensão & Pais Citation1998).
The voluminous appearance of the peltate trichomes (E) may be due to the accumulation of secretory materials, which mostly occurred in the latter stages of leaf development. Although, mature peltate trichomes were observed in very young leaves, they were fewer in number. This was also confirmed by light microscopy studies (A,B) which clearly showed the large subcuticular space present in the peltate trichome. Our results are consistent with those of Gang et al. (2001) who reported a similar pattern of distribution of peltate trichomes in Ocimum basilicum L. (Sweet Basil). The peltate trichome head consists of four central secretory cells. This type of peltate trichome with four-headed cells has been reported in other species of the Lamiaceae (Bosabalidis Citation1990; Kaya et al. 2007; Werker et al. 1993). It is reported that peltate trichomes have a characteristic spherical head due to the development of a large subcuticular space where secretory products accumulate prior to secretion (Ascensão et al. 1999) As the cells fill with secretory material, the secretory cells are no longer visible. Our findings are in agreement with Bourett et al. (1994) who reported that mature oil-producing peltate trichomes were more common on the mature leaf surface of Nepeta racemosa Lam. These glands were sutureless due to the accumulation of secretory material in the subcuticular space. Similar observations are applicable to peltate trichomes of O. labiatus which have a spherical or slightly flattened head, possibly indicating that they accumulate secretory products. Degenerative peltate trichomes were observed on both leaf surfaces. It is believed that these degenerative peltate trichomes may indicate a loss of turgidity because of the release of accumulated secretory products. Similar degenerative peltate trichomes have also been reported in Lippia scaberrima Sond. (Combrinck et al. 2007).
In O. labiatus capitate trichomes were distributed on both leaf surfaces, however, they appear to be very rare and sparse. The capitate trichomes consist of a single basal cell which is implanted in the epidermis, a stalk cell and a rounded secretory head. These capitate trichomes are similar in structure and morphology to those described by Werker et al. (1985) in their study of selected species of Lamiaceae. The round glandular head of these capitate trichomes develops only a small subcuticular space and it is assumed that the secretion is probably discharged through micropores (Kaya et al. 2007). It has been suggested that the compounds secreted by capitate trichomes are mostly excreted to the immediate environment (Kaya et al. 2007).
Our study contributes to the knowledge of the morphology, distribution and type of trichomes in angiosperms (in particular Lamiaceae), and will lend support to further studies on the chemical constituents of the glandular trichomes of O. labiatus. However, detailed anatomical and ultrastructural studies may be helpful in interpreting the function of glandular and non-glandular trichomes.
Acknowledgements
This research was carried out with financial support from the National Research Foundation-Thuthuka, South Africa. We wish to thank Dr J-W. Smith, P. Maartens and S. Eggers of the Electron Microscope Unit, UKZN-Westville campus for technical assistance. Our special thanks to Mr A. Rajh for assistance with the photographic plates. Mr M. Ngwenya of the KwaZulu-Natal Herbarium (South African National Biodiversity Institute) is thanked for help with identifying the species used for this study.
References
- Adam , Y , Somchit , MN , Sulaiman , MR , Nasaruddin , AA , Zuraini , A , Bustamam , AA and Zakaria , ZA . 2009 . Dieuretic properties of Orthosiphon stamineus Benth . Journal of Ethnopharmacology , 124 : 154 – 156 .
- Angiosperm Phylogeny Group (APG) 2009 . An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III . Botanical Journal of the Linnean Society 161 : 105 121 .
- Ascensão , L , Mota , L and Castro , MDM . 1999 . Glandular trichomes on the leaves and flowers of Plectranthus ornatus: morphology, distribution and histochemistry . Annals of Botany , 84 : 437 – 447 .
- Ascensão , L and Pais , MS . 1998 . The leaf capitate trichomes of Leonotis leonurus: histochemistry, ultrastructure and secretion . Annals of Botany , 81 : 263 – 271 .
- Awale , S , Tezuka , Y , Banskota , AH and Kadota , S . 2003 . Nitric oxide inhibitory isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia . Journal of Natural Products , 66 : 255 – 258 .
- Barkley , TM . 1974 . “ History of taxonomy ” . In Vascular plant systematics , Edited by: Radford , AE , Dickison , WC , Massey , JR and Bell , CR . 13 – 34 . New York, Harper & Row. Pp .
- Bini Maleci , L and Servettaz , O . 1991 . Morphology and distribution of trichomes in Italian species of Teucrinum setc. Chamaedrys (Labiatae) – a taxonomic evaluation . Plant Systematics and Evolution , 174 : 83 – 91 .
- Bosabalidis , AM . 1990 . Glandular trichomes in Satureja thymbra leaves . Annals of Botany , 65 : 71 – 78 .
- Bosabalidis , AM and Skoula , M . 1998 . A comparative study of the glandular trichomes on the upper and lower leaf surfaces of Origanum intercedens Rech . Journal of Essential Oil Research , 10 : 277 – 286 .
- Bourett , TM , Howard , RJ , O'Keefe , DP and Hallahan , DL . 1994 . Gland development on leaf surface of Nepeta racemosa . International Journal of Plant Science , 155 : 623 – 632 .
- Bowman , BJ and Jordan , R . 2006 . “ Natural products ” . In Remington: the science and practice of pharmacy , 21st edition , Edited by: Troy , DB . Baltimore, MD, Lippincott Williams & Wilkins .
- Bremekamp , CEB . 1933 . New or otherwise noteworthy plants from the northern Transvaal . Annals of the Transvaal Museum , 15 : 233 – 264 .
- Chin , JH , Abas , HH and Sabariah , I . 2008 . Toxicity study of Orthosiphon stamineus Benth. (Misai Kucing) on Sprague–Dawley rats . Tropical Biomedicine , 25 : 9 – 16 .
- Codd , LE . 1964 . The South African species of Orthiosiphon . Bothalia , 8 : 149 – 162 .
- Codd , LE . 1985 . The genus Orthosiphon . Flora of Southern Africa , 28 ( 4 ) : 229 – 236 .
- Combrinck , S , Du Plooy , GM , McCrindle , RI and Botha , BM . 2007 . Morphology and histochemistry of the glandular trichomes of Lippia scaberrima (Verbenaceae) . Annals of Botany , 99 : 1111 – 1119 .
- Corsi , G and Bottega , S . 1999 . Glandular hairs of Salvia officinalis: new data on morphology, localization and histochemistry in relation to function . Annals of Botany , 84 : 657 – 664 .
- Cousins , DJ . 1994 . Medicinal, essential oil, culinary herb and pesticidal plants of the Labiatae , Wallingford, CAB International .
- Dinç , M , Pinar , NM , Dogu , S and Yildirimli , S . 2009 . Micromorphological studies of Lallemantia L. (Lamiaceae) species growing in Turkey . Acta Biologica Cracoviensia series Botanica , 51 : 45 – 54 .
- Faizul , FM , Aminudin , N , Kadir , HA and Tayyab , S . 2009 . Bilirubin lowering potential of Orthosiphon stamineus in temporarily jaundice adult rats . African Journal of Pharmacy and Pharmacology , 3 : 359 – 361 .
- Feder , N and O'Brien , TP . 1968 . Plant microtechnique: some principles and new methods . American Journal of Botany , 55 : 123 – 142 .
- Fernie , WT . 1897 . “ Lavender ” . In Herbal simples approved for modern uses of cure , 2nd edition , 321 – 324 . Facsimile 2009 by BiblioBazaar .
- Gairola , S , Naidoo , Y , Bhatt , A and Nicholas , A . 2009 . An investigation of the foliar trichomes of Tetradenia riparia (Hochst.) Codd (Lamiaceae): An important medicinal plant of southern Africa. Flora – Morphology, Distribution . Functional Ecology of Plants , 204 : 325 – 330 .
- Gang , DR , Wang , J , Dudareva , N , Nam , KH , Simon , JE , Lewinsohn , E and Pichersky , E . 2001 . An investigation of the storage and biosynthesis of phenylpropenes in Sweet Basil . Plant Physiology , 125 : 539 – 555 .
- Giuliani , C , Pellegrino , R , Tirillini , B and Maleci Bini , L . 2008 . Micromorphological and chemical characterizations of Stachys recta L. serpentine (Fiori) Arrigoni in comparison to Stachys recta L. subsp. recta (Lamiaceae) . Flora , 203 : 376 – 385 .
- Grosvenor , PW , Supriono , A and Gray , DO. 1995 . Medicinal plants from Riau Province, Sumatra, Indonesia. Part 2: antibacterial and antifungal activity . Journal of Ethnopharmacology , 45 : 97 – 111 .
- Grubešic , RJ , Vladimir-Knezvic , S , Kremer , D , Kalodera , Z and Vukovic , J. 2007 . Trichome micromorphology in Teucrium (Lamiaceae) species growing in Croatia . Biologia , 62 : 148 – 156 .
- Han , CJ , Hj Hussin , A and Ismail , S . 2009 . Effects of Orthosiphon stamineus leaf extracts on hepatic cytochrome P450, UGT and GST activity in STZ-induced diabetic rats . Journal for the Advancement of Science and Arts , 1 : 1 – 8 .
- Hossain , MA , Ismail , Z , Rahman , A and Kang , SC. 2008 . Chemical composition and antifungal properties of the essential oils and crude extracts of Orthosiphon stamineus Benth . Industrial Crops and Products , 27 : 328 – 334 .
- Hsu , C-L , Hong , B-H , Yu , Y-S and Yen , G-C . 2010 . Antioxidant and anti-inflammotory effects of Orthosiphon aristatus and its bioactive compounds . Journal of Agricultural and Food Chemistry , 58 : 2150 – 2156 .
- Hussein , AA , Jacobus , JM , Meyer , LJ and RodrIguez , B . 2007 . Bioactive diterpenes from Orthosiphon labiatus and Salvia africana-lutea . Journal of Natural Products , 70 : 293 – 295 .
- Joffe , P . 1993 . The gardeners guide to South African plants , Cape Town, Tafelberg Publishers .
- Kahraman , K , Celep , F and Dogan , M . 2010 . Anatomy, trichome morphology and palynology of Salvia chrysophylla Stapf (Lamiaceae) . South African Journal of Botany , 76 : 187 – 195 .
- Kaya , A , Demirci , B and Baser , KHC . 2007 . Micromorphology of glandular trichomes of Nepeta congesta Fisch. and Mey. var. congesta (Lamiaceae) and chemical analysis of the essential oils . South African Journal of Botany , 73 : 29 – 34 .
- Khamsah , SM , Akowah , G and Zhari , I . 2006 . Antioxidant activity and phenolic content of Orthosiphon stamineus Benth. from different geographical origin . Journal of Sustainability Science and Management , 1 : 14 – 20 .
- Klich , MG . 2000 . Leaf variations in Elaeagnus angustifolia related to environmental heterogeneity . Environment and Experimental Botany , 44 : 171 – 183 .
- Lakusic , B , Lakusic , D , Jancic , R and Stevanovi( , B . 2005 . Morpho-anatomical differentiation of the Balkan populations of the species Teucrium flavum L. (Lamiaceae) . Flora , 201 : 108 – 119 .
- Liakoura , V , Stavrianakou , S , Liakopoulos , G , Karabourniotis , G and Manetas , Y . 1999 . Effects of UV-B radiation on Olea europaea: comparisons between a greenhouse and a field experiment . Tree Physiology , 19 : 905 – 908 .
- Maffei , M , Chialva , F and Sacco , T . 1989 . Glandular trichomes and essential oils in developing peppermint leaves . New Phytologist , 111 : 707 – 716 .
- Maffei , M and Codignola , A . 1990 . Photosynthesis, photorespiration and herbicide effect of terpene production in peppermint (Mentha piperita L.) . Journal of Essential Oil Research , 2 : 275 – 286 .
- Maheswari , C , Maryammal , R and Venkatanarayana , R . 2008 . Hepatoprotective activity of ‘Orthosiphon stamineus’ on liver damage caused by paracetamol rats . Jordan Journal of Biological Science , 1 : 105 – 108 .
- Mariam , A , Asmawi , MZ and Sadikun , A. 1996 . Hypoglycaemic activity of the aqueous extract of Orthosiphon stamineus . Fitoterapia , 67 : 465 – 468 .
- McGaw , LJ , Lall , N , Meyer , JJM and Eloff , JN . 2008 . The potential of South African plants against Mycobacterium infections . Journal of Ethnopharmacology , 119 : 482 – 500 .
- Metcalfe , CR and Chalk , L . 1950 . Anatomy of dicotyledons – leaves, stem, and wood in relation to taxonomy , Oxford : Clarendon Press .
- Navarro , T and El Oualidi , J . 2000 . Trichome morphology in Teucrium L. (Labiatae). A taxonomic review . Anales Jardin Botánico de Mardrid , 57 : 277 – 297 .
- Ngamrojanavanich , N , Manakit , S , Pornpakakul , S and Petsom , A . 2006 . Inhibitory effects of selected Thai medicinal plants on Na+, K + ATPase . Fitoterapia , 77 : 481 – 483 .
- Nguyen , MTT , Awale , S , Tezuka , Y , Chang , C-H and Kadota , S . 2004 . Staminane- and isopimarane-type diterpenes from Orthosiphon stamineus of Taiwan and their nitric oxide inhibitory activity . Journal of Natural Products , 67 : 654 – 658 .
- Olah , N-K , Radu , L , Mogo(an , C , Hanganu , D and Gocan , S . 2003 . Phytochemical and pharmacological studies on Orthosiphon stamineus Benth. (Lamiaceae) hydroalcoholic extracts . Journal of Pharmaceutical and Biomedical Analysis , 33 : 117 – 123 .
- Ong , HC and Norzalina , J . 1999 . Malay herbal medicine in Gemenchen, Negri, Sembilan, Malaysia . Fitoterapia , 70 : 10 – 14 .
- Payne , WW . 1978 . A glossary of plant hair terminology . Brittonia , 30 : 239 – 255 .
- Peter , AJ , Shanower , TJ and Romeis , J . 1995 . The role of plant trichomes in insect resistance: a selective review . Phytophaga , 7 : 41 – 63 .
- Retief , E . 2000 . Lamiaceae. In: Leistner OA ed. Seed plants of southern Africa: families and genera . Strelitzia , 10 : 323 – 334 .
- Retief , E and Herman , PPJ . 2003 . Lamiaceae. In: Germishuizen G, Meyer NL eds. Plants of southern Africa: an annotated checklist . Strelitzia , 14 : 584 – 600 .
- Schreuder , MD , Brewer , CA and Heine , C . 2001 . Modelled influences of non-exchanging trichomes on leaf boundary layers and gas exchange . Journal of Theoretical Biology , 210 : 23 – 32 .
- Sharma , S , Sangwan , NS and Sangwan , RS . 2003 . Development process of essential oil glandular trichome collapsing in menthol mint . Current Science , 84 : 544 – 550 .
- Simpson , MG. 2006 . Plant systematics . Elsevier Academic Press , Burlington, MA . 308 310 .
- Spurr , AR . 1969 . A low viscosity epoxy embedding medium for electron microscopy . Journal of Ultrastructure Research , 26 : 31 – 43 .
- Sriplang , K , Adisakwattana , S , Rungsipipat , A and Yibchok-anun , S . 2007 . Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin-induced diabetic rats . Journal of Ethnopharmacology , 109 : 510 – 514 .
- Sumaryono , W , Proksch , P , Wray , V , Witte , L and Hartmann , T . 1991 . Qualitative and quantitative analysis of the phenolic constituents from Orthosiphon aristatus . Planta Medica , 57 : 176 – 180 .
- Verdoorn , IC . 1943 . “ Nautochilus labiatus ” . In Flowering plants of Africa , Edited by: Phillipps , EP . Pretoria, Van Schaik .
- Wagner , GJ . 1991 . Secreting glandular trichomes: more than just hairs . Plant Physiology , 96 : 675 – 679 .
- Werker , E , Ravid , U and Putievsky , E . 1985 . Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae . Israel Journal of Botany , 34 : 31 – 45 .
- Werker , E . 2000 . Trichome diversity and development . Advances in Botanical Research , 31 : 1 – 35 .
- Werker , E , Putievsky , E , Ravid , U , Dudai , N and Katzir , I . 1993 . Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae) . Annals of Botany , 71 : 43 – 50 .
- Yam , MF , Ang , LF , Basir , R , Salman , IM , Ameer , OZ and Asmawi , MZ. 2009 . Evaluation of the anti-pyretic potential of Orthosiphon stamineus Benth. standardized extract . Inflammopharmacology , 17 : 50 – 54 .