Abstract
Geological and climatic changes, coupled with long-distance dispersals, have resulted in relatively recent origins and radiations of many New Zealand plant lineages. Several have extensive morphological but low genetic variation, rendering taxonomic resolution difficult. This study presents population-level phylogenies and networks for the New Zealand species of Plantago (Plantaginaceae) using DNA sequences from internal transcribed spacer (ITS) regions of the nuclear ribosomal genes. The data suggest that the two P. spathulata subspecies, and a 16-ploid entity (P. sp. ‘Sylvester’), should be recognized at species rank. However, there was no evidence for divergence of: two P. raoulii forms; P. lanigera and P. novae-zelandiae; and two P. triandra subspecies. Several species and subspecies boundaries require revision with additional data (e.g. chromosome counts, morphological data, and additional DNA loci) needed. The high morphological variation but low sequence divergence found here could be caused by various factors, including incomplete speciation and/or hybridization.
Introduction
It has previously been shown using molecular phylogenetic approaches that many plant groups in New Zealand are of recent origin, and that the timing of lineage splitting is often correlated with recent geological and climatic events. Contributing factors include the Pleistocene glaciation cycles (Trewick & Morgan-Richards Citation2005; McDowall Citation2008; McCulloch et al. Citation2010), final uplift of the Southern Alps ~5 Mya (Cox & Findlay Citation1995; Batt et al. Citation2000; Haase et al. Citation2007), and prevalent long-distance dispersal events (Winkworth et al. Citation1999, Citation2002a). As a result of the recent, and in some cases incomplete, speciation within some New Zealand plant groups, detecting species boundaries may be difficult using only data from morphological characters. Many New Zealand genera are large and have high morphological variation both within and among species, e.g. Myosotis (Winkworth et al. Citation1999) and Veronica (Garnock-Jones & Lloyd Citation2004, as Parahebe; Bayly & Kellow Citation2006, as Hebe). Such variation can lead to taxonomic uncertainty. Molecular phylogenetic data are increasingly being used to more effectively detect patterns of evolution and speciation (e.g. Wagstaff & Garnock-Jones Citation1998; Lockhart et al. Citation2001; Smissen et al. Citation2003; Meudt & Simpson Citation2007) and to help bring more precision to the systematics of many New Zealand plant groups (e.g. Heenan et al. Citation2002; Glenny Citation2004; Smissen et al. Citation2004; Perrie & Brownsey Citation2005; Ford et al. Citation2007; Meudt & Bayly Citation2008; Meudt et al. Citation2009).
Plantago (Plantaginaceae) is a large genus of wind-pollinated plants, with over 200 species distributed worldwide. Eight species native to New Zealand were recognized in the most recent taxonomic treatment (Sykes Citation1988a) (). The molecular phylogenetic study of Tay et al. (2010) investigated biogeographic patterns of the Australasian species of Plantago, sampling 20 of a total of 32 species and using three independent DNA sequencing markers. In the study, it was proposed that New Zealand Plantago have arrived at least three times through long-distance dispersal, probably from Australia, between 2.291 and 0.5 Mya. In addition, it was also proposed in the study that a habitat shift from alpine regions into lowland and coastal areas occurred before the most recent inferred dispersal from Australia to New Zealand. Recent speciation subsequent to these events has generated several closely related species complexes in both Australia and New Zealand.
Table 1 Conspectus of previous taxonomic treatments of New Zealand Plantago. In this article we have followed the most recent (Sykes 1988a)
Species delimitation and taxonomy based on morphology within Plantago has been difficult (Rock Citation1920; Rahn Citation1996; Sykes Citation1988a). On a worldwide scale, as well as within Australasia, there is variation both among and within Plantago species with respect to ploidy and chromosome numbers (Groves & Hair Citation1971; Rahn Citation1996; Dhar et al. Citation2006), as well as morphological characters. Within some species, ecotypes are known to occur in different habitats and there is also a high degree of plasticity in many morphological characters, whereas among other species, few morphological differences exist. Rahn (Citation1996) reported that hair and seed morphology appeared to be the most useful characters for the genus, whereas characters such as leaf size, shape, hairiness and teeth, scape length, and the number of flowers can vary within a population according to environmental factors (Sykes Citation1988a). Thus previous New Zealand Flora treatments have differed in the number and circumscriptions of species of Plantago recognized (). In this study, we focus on the taxonomy and boundaries of New Zealand species that have not been satisfactorily addressed in previous studies. Specifically, there are four species or species complexes that are studied here; these are discussed in detail below.
Plantago spathulata subsp. spathulata and subsp. picta
Sykes (Citation1988a) included two subspecies under P. spathulata: subsp. spathulata (found on southern North Island coasts, and both coasts and inland in the South Island) and subsp. picta (endemic to the coastal North Island regions of the Poverty Bay region and East Cape). These were previously treated as two species (P. spathulata and P. picta; ) and both have the same chromosome number of 2n=48 (Groves & Hair Citation1971; Murray et al. Citation2010). Separation of the two entities was based on morphological characters, i.e. a persistent taproot with no adventitious roots and scapes up to 7 cm long in P. picta vs. only adventitious roots and scapes of 3–12–(22) cm long in P. spathulata. Sykes (Citation1988a) recognized these entities at the lower rank of subspecies because populations of the two entities were found close to one another geographically and the characters used by Moore (Citation1961) to distinguish the two species were inconsistent due to morphological plasticity within populations. One representative of each of these two entities was included in the study of Tay et al. (2010). Although they were found in the same moderately supported clade, they were not sister to one another. Clearly more sampling within both subspecies is warranted for additional molecular and morphological studies to determine their taxonomic status.
Two forms of P. raoulii, plus P. sp. ‘Sylvester’
Plantago raoulii has been noted to have two different forms; one widespread on both the North and South Islands, and the other common only in Taranaki and around the Cook Strait (AP Druce collections and annotations in CHR; C Ogle personal communication; Sykes Citation1988a). The latter is described as having thicker leaves that are also wider and shorter. Both forms can be found in coastal regions and at altitudes of up to 1500 m. In addition, another undescribed but tag-named entity, P.sp. ‘Sylvester’ (Druce Citation1993), may be a species of polyploid origin with 2n=96 (Groves & Hair Citation1971; Murray et al. Citation2010). The single individual of P.sp. ‘Sylvester’ sampled in Tay et al. (2010) was sister to an individual of P. raoulii with high support, suggesting that it may be closely related to or perhaps derived from P. raoulii. Determining whether P.sp. ‘Sylvester’ is indeed a new species is a priority and requires further sampling and study.
Plantago lanigera and P. novae-zelandiae
Plantago lanigera as circumscribed by Sykes (Citation1988a), was previously treated as two distinct species (e.g. Moore Citation1961, P. lanigera and P. novae-zelandiae; ). Various morphological characters were used by Moore (Citation1961) to differentiate between the two entities, such as crowded long hairs, rhomboid-shaped lamina and bracts <2 mm long in P. lanigera vs. sparse long hairs, elliptic lamina, and bracts 2.5–3 mm long in P. novae-zelandiae. Sykes (Citation1988a) recognized only one species (P. lanigera) because these morphological distinctions were considered inconsistent and populations of the two forms display overlapping character states. In a subsequent study (Spence & Sykes Citation1989), it was shown that several quantitative morphological characters were significantly different between the two entities (inflorescence height, seed size, shape and weight, and number of leaves per plant, capsules per inflorescence and ovules and seeds per capsule). Although the ranges of most of these characters overlap (and therefore cannot conclusively be used to separate one species from the other), differences in seed size, shape and weight roughly correspond to the two entities (Spence & Sykes Citation1989). In addition, vouchers for chromosome counts of 2n=12 were identified as either P. lanigera or P. novae-zelandiae, whereas vouchers for counts of 2n=24 were always identified as P. novae-zelandiae (Spence & Sykes Citation1989). Tay et al. (2010) included five individuals of this species complex in their study and called all of them P. lanigera due to difficulties distinguishing the two species. Three of these have subsequently been confirmed to match P. lanigera seed characters (the two Sugarloaf Pass specimens do not have seeds; HM Meudt personal observation). Four of the sampled individuals formed a monophyletic group with high support, whereas one individual from Sugarloaf Pass instead grouped in the same clade as individuals of P. raoulii, P. spathulata and P.sp. ‘Sylvester’. This might suggest that two species are indeed present, but the issue needs further testing because none of the five individuals sampled in Tay et al. (2010) can be definitively identified as P. novae-zelandiae.
Plantago triandra subsp. triandra and subsp. masoniae
Two subspecies of P. triandra were recognized in the most recent taxonomic treatment (Sykes Citation1988a). Subsp. masoniae (found in coastal areas) is described as having leaves that are fleshier and flowers that are sessile and smaller than those of subsp. triandra (mostly found inland at higher altitudes). Moore (Citation1961) did not recognize two distinct subspecies because morphological characters used to distinguish between the coastal and inland forms were regarded as inconsistent and also very plastic. A third entity in this complex, P. hamiltonii (Kirk Citation1879) was treated as a synonym of P. triandra by Cheeseman (Citation1906, Citation1925) and Moore (1961). Finally, P. unibracteata (previously known by the illegitimate name P. uniflora Hook.f.; Rahn Citation1996, ) was shown to be very closely related to the two subspecies of P. triandra with high support (Tay et al. Citation2010), which may indicate that P. unibracteata might also be a part of this species complex.
The aim of this study was therefore to investigate species delimitations within these four Plantago species groups. To do this, we extensively sampled individuals within each species and employed phylogenetic and network analyses utilizing sequences of the nuclear ribosomal DNA internal transcribed spacer (ITS) region. Specifically, we aimed to use the ITS data to answer the following questions: (1) Are P. spathulata subsp. spathulata and subsp. picta genetically distinct, and if so, which rank is more appropriate? (2) Can two forms of P. raoulii be distinguished, and what is the status of P.sp. ‘Sylvester’? (3) Can P. novae-zelandiae be distinguished from P. lanigera? (4) Is there genetic evidence to separate two species or subspecies within P. triandra? The present study contributes to a revision of the New Zealand species of Plantago by complementing morphological and chromosomal analyses already undertaken (e.g. Groves & Hair Citation1971; Spence & Sykes Citation1989) and those currently in progress (HM Meudt unpublished data; Murray et al. Citation2010).
Materials and methods
Sample collection
Collection locations of samples, along with voucher information and Genbank accession numbers for DNA sequences are presented in . Samples were collected from natural populations across the known distribution and habitat ranges of all recognized native New Zealand species and subspecies, including the tag-named P.sp. ‘Sylvester’ (Groves & Hair Citation1971; Druce Citation1993). In total, material was collected from 43 localities. For each species, a maximum of 28 individuals (1–10 per locality) were obtained from up to 14 separate localities. These were supplemented with individuals representing an additional seven species from Australia and one additional species from the Amsterdam and St. Paul Islands (P. stauntoni). Inclusion of these was based on a previously reconstructed phylogeny, in which these non-New Zealand species were placed in clades with the New Zealand species (Tay et al. Citation2010). Thus, a total of 122 Australasian ITS sequences (including 114 from New Zealand) are presented in our analyses here. Of these, 98 were sequenced specifically for this study and 24 were sourced from other molecular studies (Rønsted et al. Citation2002; Hoggard et al. Citation2003; Tay et al. Citation2010).
Table 2 Details of the New Zealand Plantago species sequenced for ITS in this study.
Molecular techniques
Tissue samples were mostly preserved in silica gel from field collections, but one was obtained from an existing herbarium specimen. DNA extractions and PCR amplifications were performed as per Tay et al. (2010). The ITS marker (ITS1, 5.8S nuclear rDNA and ITS2) was chosen because it typically provides an appropriate level of variation for plant phylogenetic studies (White et al. Citation1990; Álvarez & Wendel Citation2003), even at shallow phylogenetic levels (Álvarez & Wendel Citation2003); it was proven to be a useful marker in a previous study (Tay et al. Citation2010); and ITS sequences for Plantago species outside Australasia are available on Genbank. The primers used to amplify the ITS DNA region are ITS28CC: CGCCGTTACTAGGGGAATCCTTGTAAG (Wagstaff & Garnock-Jones Citation1998), and ITS5: GGAAGTAAAAGTCGTAACAAGG (White et al. Citation1990).
Dataset alignment and phylogenetic analyses
The program MEGA v3.1 (Kumar et al. Citation2004) was used to assemble and align the forward and reverse sequences for each individual. The New Zealand species were shown to belong to three separate lineages within Plantago in both the concatenated and expanded ITS data sets of Tay et al. (2010). Thus, in the present study, data sets were created and analysed separately for these three groups called Groups I, II and III following Tay et al. (2010; see ) (). Because ITS copies within an individual may show intraindividual sequence polymorphism, with some copies remaining in the genome as pseudogenes, the sequences included were checked for conserved regions in the 5.8S rDNA (see Harpke & Peterson Citation2008). All of our sequences contained conserved regions and are unlikely to be pseudogenes.
The first group (Group I) includes the two subspecies of P. spathulata (4 localities, 15 individuals), P. raoulii (7 localities, 24 individuals), and P.sp. ‘Sylvester’ (1 locality, 12 individuals) from New Zealand; five Australian species (P. varia, P. debilis, P. hispida, P. paradoxa and P. cladarophylla); one species from the Amsterdam and St. Paul Islands in the South Indian Ocean (P. stauntoni); and one species, P. triantha (one locality, one individual), native to both New Zealand and Australia. Group I also contains individuals from Eyre Mountains and Sugarloaf Pass (see for locations) that were originally identified as P. lanigera based on morphological characters but do not form a clade with the other samples of P. lanigera, which are instead placed in Group II. Group II contains P. lanigera s.l. (13 localities, 28 individuals), P. obconica (2 localities, 7 individuals) and P. aucklandica (1 locality, 1 individual). We identified specimens of P. lanigera s.l. to either P. lanigera s.s. or P. novae-zelandiae using seed size, shape and number following Spence & Sykes (Citation1989). Because this was only possible for fruiting specimens with seeds, we assigned 15 individuals to P. lanigera s.s. and 1 to P. novae-zelandiae, whereas 12 individuals could not be assigned and were identified as ‘P. lanigera?’ (). Finally, Group III consists of P. unibracteata (6 localities, 10 individuals) and the two subspecies of P. triandra (6 populations, 15 individuals).
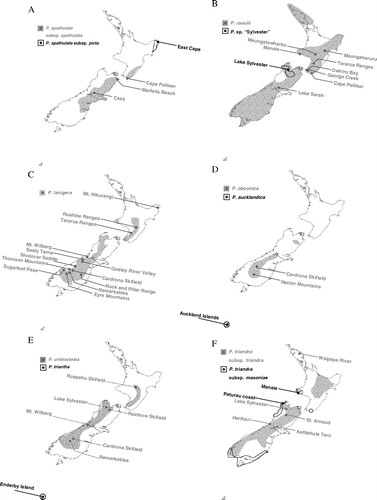
Fig. 1 Distributions of New Zealand Plantago species based on herbarium specimen locality information (Allan Herbarium, Landcare Research, Christchurch, New Zealand) and locations of samples collected for this study. Group I: (A) P. spathulata, (B) P. raoulii and P. sp. ‘Sylvester’. Group II: (C) P. lanigera, (D) P. obconica and P. aucklandica. Group III: (E) P. unibracteata and P. triantha, (F) P. triandra.
A model for each of the three data sets was estimated using MODELTEST v3.7 (Posada & Crandall Citation1998); these are presented in Supplementary (available online only), along with other data statistics. MRBAYES v3.1.1 (Huelsenbeck & Ronquist Citation2001) was used to conduct heuristic searches under a maximum likelihood (ML) criterion to check initial topology of these groups. Analyses were run with 1,000,000 generations, yielding 10,000 trees, with a final standard deviation that was <0.01 for each (the first 25% of the trees were discarded as the ‘burn-in’). A 50% majority rule consensus tree of the resulting 7,500 trees was made. To improve the efficiency of subsequent ML analyses, sequences of individuals from the same population that clustered in the same clade were removed from all three data sets. ML analyses were then run on the each of the three reduced data sets using PAUP* v4.0b10 (Swofford Citation2002) with 100 replicates of random sequence addition and TBR branch swapping. Nonparametric bootstrap support was assessed using 200 replicates, with random sequence addition and TBR branch swapping.
Complex evolutionary patterns, such as those arising from hybridization, introgression and species radiations, may not be properly displayed on traditional bifurcating trees (Kumar et al. Citation2004; Huson & Bryant Citation2006). Therefore, SPLITSTREE v4.8 (Huson & Bryant Citation2006) was used to conduct neighbour-net analyses on each of the data sets in order to better visualize relationships among the New Zealand species. Neighbour-net analyses use genetic distances based on the neighbour-joining method of Saitou and Nei (Citation1987) to compute networks of splits (bipartitions of the data set) and convert them to a splits graph. This allows conflicting signals of species similarities to be visualized if they are present in a data set. Bootstrap analyses were not conducted for the neighbour-net analyses because the purpose of the split networks was primarily to visualize conflicts of the phylogenetic analyses.
Results
In general, there was little interspecific genetic variation among the Australasian Plantago. Uncorrected p-distances between all pairwise comparisons of Australasian species ranged from 0 to 4.3% (results not shown), and several Australasian species pairs had identical ITS sequences: Plantago debilis (GenBank accession: FJ024608) and P. hispida (AJ548967), P. daltonii (AJ548968) and P. tasmanica (AJ548970), and introduced species P. major (called ‘P.sp.’ in Tay et al. Citation2010; FJ024620) and a sampled lodged in Genbank as P. asiatica (AY101862). Very little intraspecific variation was found within the New Zealand species. Most species displayed <1% sequence divergence (). The highest intraspecific sequence divergences were found within P. raoulii (4.58%) and within P. lanigera s.l. (6.40%).
Table 3 ITS DNA sequence statistics for the native New Zealand Plantago species sequenced for this study
For each of the three separate data sets, Bayesian and ML analyses had congruent topologies and equivalent support values; therefore only the phylogenies recovered using ML are presented here, but with both ML bootstrap values ( BP) and Bayesian posterior probabilities (PP) shown on branches (A, 3A and 4A). Support values <50% were considered to be low and are not shown in the figures. Support values of > 70% (ML) or > 95% (PP) were considered to indicate high support.
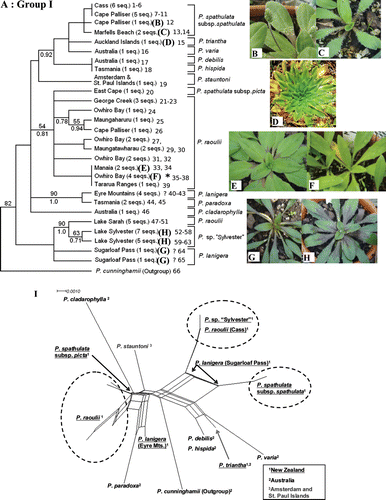
Fig. 2 (A) One of the reconstructed maximum likelihood (ML) trees of Group I, which comprises lowland (including coastal) and alpine/subalpine species (tree score=−1087.60). Each sequence represents an individual plant. Numbers appended at the end of the sequences correspond to the individual Genbank accession numbers presented in Table 2. ML bootstrap values are displayed above branches, whereas Bayesian posterior probabilities are displayed below branches (only support values >50% are shown). *Narrow-leaved samples of P. raoulii; ? samples of P. lanigera s.l. that could not be identified to either P. lanigera or P. novae-zelandiae. (B,C) Habit of P. spathulata subsp. spathulata. (D) P. triantha from the Auckland Islands. (E,F) Two different forms of P. raoulii. (G) Specimen identified as P. lanigera from Sugarloaf Pass. (H) Specimen from Lake Sylvester (P. sp. ‘Sylvester’). Pictures presented here are from plants collected from the wild that have been cultivated in the greenhouse for ~1 year. (I) Neighbour-net graph of Group I ITS sequences. Species native to New Zealand are underlined.
Group I
ML analyses yielded six trees (−ln likelihood score=−1087.60) that differed only by rearrangements in the P. raoulii–P. spathulata subsp. picta clade. Plantago raoulii had the most sequence divergence within the group (4.58%), whereas P.sp. ‘Sylvester’ only had 0.49% sequence divergence and there was 2.45% sequence divergence between the two subspecies of P. spathulata. In general, clades within Group I were not well resolved, and only two nodes receive > 90 BP and > 0.95PP (A). The individual samples largely clustered by species, but the phylogeny suggests that the two subspecies of P. spathulata as currently circumscribed are not sister taxa (A). Plantago spathulata subsp. spathulata forms a clade with P. triantha, P. varia, P. debilis and P. hispida (<50 BP, 0.92PP), whereas P. spathulata subsp. picta clusters with the majority of the P. raoulii samples (54 BP, 0.81PP), but neither of these clades received high support.
Plantago raoulii is also not monophyletic, and is separated into two groups concurrent with geographic locations. The North Island P. raoulii are grouped together in a clade with P. spathulata subsp. picta (54 BP, 0.81PP), whereas the sole sample of the South Island P. raoulii forms a clade with P.sp. ‘Sylvester’ (90 BP, 1.0PP). One P. lanigera population from the Eyre Mountains forms a clade with the Australian P. paradoxa (90 BP, 1.0PP).
Neighbour-net analysis of this clade (I) had a similar topology to the phylogenetic analysis, and also reveals several points of conflict among the data. This is particularly true regarding the individuals of P.sp. ‘Sylvester’, South Island P. raoulii, P. lanigera from Sugarloaf Pass and P. spathulata subsp. spathulata, as well as P. lanigera from the Eyre Mountains.
Group II
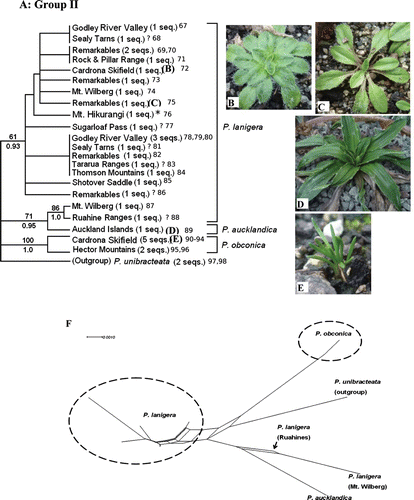
ML analyses resulted in 24 trees (−ln likelihood score=−1065.00) where the only differences were rearrangements among P. lanigera s.l. samples. Among all the Australasian species, P. lanigera s.l. had the highest intraspecific sequence divergence (6.40%; ). Three main clades were moderately to highly supported within this group (A), but relationships among them were not well resolved. The first clade contains most of the P. lanigera s.l. samples (61 BP, 0.93PP) with no support for any relationships within the clade. The second clade has two populations of P. lanigera s.l. (Mt. Wilberg, Ruahine Ranges, 86 BP, 1.0PP), which together are sister to P. aucklandica (71 BP, 0.95PP). The third clade contains the two populations of P. obconica (100 BP, 1.0PP). There does not appear to be any geographical pattern to the ITS sequence data. The neighbour-net analysis (F) is consistent with the ML tree topology in the finding of the three main lineages in this group with little conflict among them.
Fig. 3 (A) One of the reconstructed maximum likelihood (ML) trees of Group II, a clade of alpine/subalpine species (tree score=−1065.00). Each sequence represents an individual plant. Numbers appended at the end of the sequences correspond to the individual Genbank accession numbers presented in Table 2. ML bootstrap values are displayed above branches, whereas Bayesian posterior probabilities are displayed below branches (only support values >50% are shown). (B,C) Two different forms currently classified under P. lanigera s.l. (previously P. lanigera and P. novae-zelandiae respectively). (D) P. aucklandica, endemic to the Auckland Islands. (E) Habit of P. obconica. *Samples that were identified to be P. novae-zelandiae. ? Samples of P. lanigera s.l. that could not be identified to either P. lanigera or P. novae-zelandiae. All other P. lanigera sequences are P. lanigera s.s. Pictures presented here are from plants collected from the wild that have been cultivated in the greenhouse for ~1 year. (F) Neighbour-net graph of Group II ITS sequences.
Group III
ML trees (−ln likelihood score=−976.76) provided very little resolution for relationships within Group III (A). There were three clades of P. unibracteata (all with <50 BP), and two clades of P. triandra (51 BP and <50 BP). The only difference among the three resulting ML trees was the placement of one P. unibracteata individual from Mt. Wilberg (data not shown). Intra- and interspecific sequence divergence was low for both species in Group III. Sequences within each species differed by fewer than 10 nucleotide substitutions out of the 656 bp of ITS sequence. Sequence divergence between the two species was represented by extremely low uncorrected p-distances (0.2–0.8%). Although two clades were found within P. triandra, these do not correspond to the two subspecies of P. triandra (following the morphology-based taxonomy of Sykes Citation1988a). In fact, sampled individuals from the two populations of subsp. masoniae cluster with different populations of subsp. triandra, and some share identical ITS sequences (i.e. Paturau Coast, Kettlehole Tarn and Harihari individuals).
Fig. 4 (A) One of the reconstructed maximum likelihood (ML) trees of Group III, a clade of alpine/subalpine and coastal species (tree score=−976.76). Each sequence represents an individual plant. Numbers appended at the end of the sequences correspond to the individual Genbank accession numbers presented in Table 2. ML bootstrap values are displayed above branches, whereas Bayesian posterior probabilities are displayed below branches (only support values >50% are shown). (B) P. unibracteata. (C) P. triandra subsp. triandra. (D) P. triandra subsp. masoniae. Populations of P. triandra marked with * indicate populations of subsp. masoniae; all others are subsp. triandra. Pictures presented here are from plants collected from the wild that have been cultivated in the greenhouse for ~1 year. (E) Neighbour-net graph of Group III ITS sequences.
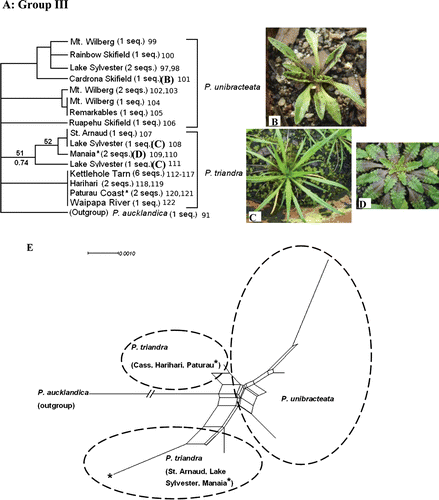
Similar groups are recovered in the neighbour-net analysis of Group III (E), and the large number of conflicting splits in the middle of the graph confirms that P. unibracteata and the two subspecies of P. triandra are unresolved using ITS data.
Discussion
The aim of this study was to clarify species boundaries of New Zealand Plantago using ITS sequence data, with an emphasis on four specific species or species complexes that have presented taxonomic problems in the past. The resulting ITS phylogenies and networks provided some insight into species limits of New Zealand Plantago and are further discussed in the context of current taxonomy, with support from cytological data and geographical distributions.
Taxonomic questions
Are P. spathulata subsp. spathulata and subsp. picta genetically distinct, and if so, which rank is most appropriate?
In the ML tree (A), P. spathulata subsp. picta appears to be more divergent from P. spathulata subsp. spathulata and closer to P. raoulii than expected based on morphology and previous classifications. Sykes (Citation1988b) noted that the main difference between the two subspecies is a persistent taproot in subsp. picta compared with a short-lived taproot with many adventitious roots in subsp. spathulata; although they may also be differentiated by hairs, either on the keels of bracts and sepals (subsp. spathulata) or only on the margins of bracts and sepals (subsp. picta) (Moore Citation1961). This is mirrored in Rahn's (1996) morphological phylogeny, where subsp. picta is separated from subp. spathulata by only five steps (three characters). These are: absence/presence of adventitious roots, whether or not the sepals are glabrous abaxially, and 1–4 ovules per ovary with a rudiment of an upper compartment on the adaxial side of the placenta (vs. an ovary with 5 ovules). This suggests that although the two taxa are genetically divergent, they may share many plesiomorphic character states. The divergence of the two subspecies and the placement of subsp. picta as closely related to the South Island population of P. raoulii are also evident in the neighbour-net analysis of Group I (I). In addition, the subspecies are allopatric: subsp. picta is endemic to the East Cape and the Poverty Bay Coast in the North Island, whereas subsp. spathulata is found in the Wairarapa coast and throughout the South Island (Sykes Citation1988a). Our phylogenetic analyses suggest that the two taxa are distinct lineages that are not each other's closest relatives. However, DNA sequences from additional subsp. picta samples should be obtained, and reliable and consistent morphological differences should be found before deciding on the rank of these two entities.
Can two forms of P. raoulii be distinguished, and what is the status of P. sp. ‘Sylvester’?
There are reportedly two sympatric forms of P. raoulii (Sykes Citation1988a; C Ogle personal communication) distinguished by broad vs. narrow leaves. These two distinct forms were collected for this study (E,F). However, both ML and neighbour-net analyses indicated that there was no genetic differentiation between populations of P. raoulii consistent with this morphological characterization. Thus, it appears that the two forms of P. raoulii may simply be ecotypes or represent plasticity or genetic polymorphisms.
The phylogenetic and network analyses show that South Island and North Island P. raoulii are genetically distinct. It is also possible that the South Island individuals sampled from Lake Sarah, Cass that we identified as P. raoulii may in fact be better placed with P.sp. ‘Sylvester’ from Lake Sylvester; they group together with high support, both were found in similar habitats (bogs among Schoenus), and they have similar morphology and flowering times in cultivation (PJ Garnock-Jones personal observation) However, an individual of P. raoulii from a location near to Cass had 2n=48 (Groves & Hair Citation1971). The chromosome number of the individuals we sampled there is unknown. We need further studies and additional samples of P. raouliifrom the South Island, as well as chromosome counts of the Lake Sarah population, in order to clarify this observation.
We hypothesize that the 16-ploid (2n=96) P.sp. ‘Sylvester’ (H) might represent an allopolyploid resulting from hybridization between P. raoulii and P. spathulata subsp. spathulata. Some morphological characteristics are consistent with this hypothesis: P.sp. ‘Sylvester’ plants resemble P. spathulata in that two of their seeds are longer than the rest, and they both possess broad glabrous corolla lobes (Moore Citation1961); whereas they resemble P. raoulii in seed characteristics (having a total of four vertical seeds) and habit (Sykes Citation1988a). P.sp. ‘Sylvester’ has a chromosome number of 2n=96, whereas both P. spathulata and P. raoulii have chromosome numbers of 2n=48 (Groves & Hair Citation1971; Murray et al. Citation2010). The individuals of P.sp. ‘Sylvester’ sampled here cluster with the individuals of P. raoulii from Lake Sarah (A,I) and in the neighbour-net analysis (I) these are at almost equal distances from the North Island P. raoulii and P. spathulata subsp. spathulata. However, both ITS and chloroplast DNA phylogenetic analyses of Tay et al. (2010) placed P.sp. ‘Sylvester’ in the Group I clade together with P. spathulata and P. raoulii, and therefore are not informative regarding its putative allopolyploid nature. Nevertheless, our data suggest that P.sp. ‘Sylvester’ is genetically distinct from other species of New Zealand Plantago. This and its unique chromosome number suggest that it may be a distinct species. Further detailed studies of the morphology, geographic range and genetic distinctiveness (using AFLP) of P.sp. ‘Sylvester’ are currently underway to fully resolve its status (HM Meudt unpublished data).
Can P. novae-zelandiae be distinguished from P. lanigera?
In our analyses, individuals identified a priori as P. lanigera s.l. appear to form three separate lineages (Groups I and II; A and 3A): (1) the majority comprise a large clade (61 BP, 0.93PP) in Group II (including one of two sampled individuals of P. novae-zelandiae), (2) two individuals (from within larger samples of populations from Mt. Wilberg and Ruahine Ranges) are highly supported as sister to P. aucklandica in Group II, and (3) all individuals (except one) from Sugarloaf Pass and Eyre Mountains (identified as ‘P. lanigera?’ due to lack of fruiting material) cluster with P. raoulii, P. paradoxa and P.sp. ‘Sylvester’ in Group I. Nevertheless, relationships within the three lineages were unresolved. Thus, there is no evidence to refute the conspecificity of P. lanigera and P. novae-zelandiae (Sykes Citation1988a). There appears to be genetic interchange among populations, and some conflict is noted for the placement of P. lanigera individuals in the neighbour-net analysis (F). For several of the populations sampled (Mt. Wilberg, Remarkables, Sealy Tarns, Godley River Valley, Sugarloaf Pass) individuals did not all have the same ITS sequences and thus did not necessarily group according to geographic location. Two scenarios might explain this finding: either there are two morphologically cryptic species that are sympatric in these localities (and perhaps in other locations; Spence & Sykes Citation1989), or more ITS sequence variation exists within than between populations. The latter appears to be more plausible. Because the ITS marker exists as multiple copies within an individual, it is possible for divergent paralogues (e.g. functional sequence variants or pseudogenes) to be amplified during PCR. This scenario is unlikely but must be considered; the use of additional markers in future phylogenetic work would be valuable in exploring this issue.
The morphology of the Eyre Mountains and Sugarloaf Pass individuals that cluster within Group I is certainly peculiar, because they resemble the specimens collected from Lake Sylvester (P.sp. ‘Sylvester’) with respect to coloration, leaf shape and plant size (G,H). The position of the Sugarloaf Pass individuals, in particular in the neighbour-net analysis (I) between P. spathulata subsp. spathulata and a clade of P.sp. ‘Sylvester’ and P. raoulii (from Cass), lends support to this interpretation. Plantago lanigera from the Eyre Mountains, in contrast, is closest to P. paradoxa and P. raoulii. It is possible that we have misidentified these individuals and they are not in fact P. lanigera or P. novae-zelandiae; four individuals from this population have been shown to be hexaploid (2n=48), which would suggest this is the case (Murray et al. Citation2010). Their unexpected placements in the ML and neighbour-net analyses may also be a result of complex history perhaps involving hybridization. Further sampling and a study of morphological characters should help clarify the taxonomy of these individuals.
Is there genetic evidence to separate two species or subspecies within P. triandra?
Sampling in this study included populations referable to both P. triandra subsp. triandra and subsp. masoniae based on morphology. Although two clades of P. triandra were resolved, each contained samples from both subspecies (A). Populations of the two were also not separable in the neighbour-net analysis (E). Sykes (Citation1988b) distinguished subsp. masoniae as having fleshier, broader leaves with an obtuse apex and a scape that does not elongate at fruiting (although these characters overlap) compared with subsp. triandra. Morphological differences between coastal and inland plants appear to be consistent in situ (see C,D), however, some components relating to the thickness of leaves, size, shape and even whether the leaves are toothed or entire appear to be plastic depending on environment and seasonal changes (authors’ personal observation from plants in the greenhouse). Thus, although two morphological groups can be distinguished in the field, we found no genetic evidence for the separation of these into separate subspecies. Several populations of subsp. triandra even share identical ITS sequences with individuals from a population of subsp. masoniae. These two morphological forms appear to be ecotypes that are also morphologically plastic and therefore recognition at the subspecies rank may not be warranted. This question might be clarified by reciprocal clone transplants in the field.
Furthermore, the interspecific p-distance range between P. triandra (both subspecies) and P. unibracteata is extremely low () and results in no resolution between the two in the ML phylogeny (A). Samples of P. triandra and P. unibracteata were inseparable based on ITS sequence data (A,E), even though they have different chromosome counts (2n=48 vs. 2n=60). Wider population sampling of both species and the use of more informative markers (such as AFLPs) will be necessary to clarify the taxonomy and relationship of P. triandra and P. unibracteata.
Conclusions and future directions
Taxonomy within the large, worldwide genus Plantago has been historically difficult because of substantial morphological plasticity and the simplification of flowers associated with wind pollination. The New Zealand Plantago species are no exception, because the vegetative morphology of plants in cultivation can change dramatically according to environmental and seasonal differences (ML Tay unpublished data). In this study, it was found that the Australasian species of Plantago have very little interspecific ITS sequence divergence, even though morphological variation may exist. As a result, within the three main Australasian Plantago groups presented here, there is little resolution and support for any resolved clades in ML analyses of ITS sequences, and neighbour-net graphs also indicate unresolved splits (Figures ).
Nevertheless, the ITS data revealed two genetically distinct lineages in P. spathulata. There was no evidence to support recognition of two subspecies of P. triandra, P. novae-zelandiae as separate from P. lanigera, or two forms within P. raoulii. Based on analyses of ITS sequences from 12 individuals of P.sp. ‘Sylvester’ and observations of morphological similarity, we propose that this tag-named entity is an allopolyploid species closely related to P. raoulii and P. spathulata subsp. spathulata. This hypothesis needs to be tested using additional appropriate techniques such as cytological examination.
Although the analyses of ITS sequence data presented here were able to partly answer some taxonomic questions regarding New Zealand Plantago, several questions remain about species boundaries. Although ITS is the most commonly used marker in plant phylogenetic studies (Álvarez & Wendel Citation2003) – including those of Plantago (Rønsted et al. Citation2002; Hoggard et al. Citation2003; Tay et al. Citation2010) – it may not be the most suitable marker for investigating genetic variation among recently diverged species. ITS sequence divergence in New Zealand Plantago is highest within populations, followed by between populations. There is the least amount of variation between species. Plausible explanations for this unexpected pattern could be incomplete speciation within many lineages, or that the plants are undergoing range expansion around the North and South Islands. Plantago plants are wind-pollinated, and their mucilaginous seeds are probably biotic-dispersed, as evidenced by widespread dispersal and the presence of many cosmopolitan species in the genus. This suggests that genetic exchange could occur over long distances.
Neighbour-net analyses of the ITS sequences indicated that New Zealand Plantago may have had a complex evolutionary history involving hybridization, which requires further investigation. Allopolyploidy – hybridization and genome doubling – has played a big part in the evolution of New Zealand Plantago (Tay et al. Citation2010), perhaps allowing rapid speciation to occur. A wide range of ploidy levels (2n=12, 24, 48, 60, 72 and 96; Groves & Hair Citation1971; Murray et al. Citation2010), is evident among New Zealand Plantago and such genome mismatches might act as post-zygotic reproductive barriers where distributions overlap. Most sympatric species have different chromosome numbers (). Different flowering times may also present pre-zygotic barriers to genetic exchange, thus promoting differentiation. Finally, deviations from assumptions of tree-like evolutionary histories could also be important, especially in wind-pollinated plants such as Plantago. Additional chromosome counts (Murray et al. Citation2010) will be crucial to further understanding the evolutionary history and taxonomy of New Zealand Plantago, particularly for the P. lanigera–P. novae-zelandiae, P. raoulii–P.sp. ‘Sylvester’ and P. triandra–P. unibracteata pairs.
Table 4 Comparison of sympatry vs. allopatry and chromosome numbers for all native species of Plantago in New Zealand.
The lack of resolution and low support for clades in ML analyses of ITS sequence data for New Zealand Plantago may be a result of low sequence divergence, which coupled with variable morphology, is consistent with the pattern found in most New Zealand plants that have undergone recent speciation (e.g. Winkworth et al. Citation1999, Citation2002b). Analyses of other faster-evolving loci, such as low-copy nuclear DNA markers (e.g. Ishikawa et al. Citation2009) and/or additional chloroplast DNA markers, may prove more effective than the ITS sequences reported here. Other molecular data such as amplified fragment length polymorphisms (AFLPs) are increasingly being applied for plant groups with low genetic variability (e.g. Wolff & Morgan-Richards Citation1999; Meudt & Bayly Citation2008). It is hoped that an AFLP study currently underway for New Zealand Plantago (HM Meudt unpublished data), when integrated with a morphological study (HM Meudt unpublished data) and chromosome data (Murray et al. Citation2010) will be able to improve resolution of species relationships, clarify species boundaries and more fully address the outstanding taxonomic issues in this group.
More generally, our findings indicate some caveats about the widespread use of ITS sequences in plant systematics. First, it is clear that ITS sequences can vary within species, populations or even within individual plants; this suggests that phylogenetic results where a single sample has been used to represent a species might be contingent upon the selection of sample sequences. Second, such variation demonstrates that although in some genera morphological evolution has preceded at a faster rate than sequence divergence in ITS (e.g. Sophora, Mitchell & Heenan Citation1999; hebes [Veronica spp.], Wagstaff et al. Citation2002; Senecio, Pelser et al. Citation2003; New Zealand Craspedia, Ford et al. Citation2007), in other genera, such as Plantago, the reverse may be the case, making ITS a data source to be used with caution in species delimitation and species radiation studies. Third, assumptions of tree-like patterns of evolution in ITS and other sequences might not be justified in groups that are rapidly evolving, show frequent hybridization or have mechanisms of long-distance pollen and seed dispersal.
Supplementary Table 1 for Tay article (10.1080/0028825X.2010.518318)
Download PDF (72 KB)Acknowledgements
We thank Lara Shepherd, Vincent Woo and Stephanie Greaves at the Victoria University Molecular Ecology Laboratory, and Lesley Milicich for technical support. We also thank Peter Beveridge, Barbara Briggs, Rewi Elliot, Kerry Ford, Peter Heenan, Rodney Lewington, Peter Lockhart, Bill Malcolm, Nancy Malcolm, Colin Ogle, Leon Perrie, Ines Schönberger, Lara Shepherd, Barry Sneddon, Rilka Taskova, Vanessa Thorn and Mike Thorsen for help with sample collection; Barbara Briggs (National Herbarium of New South Wales, NSW) and the Allan Herbarium, Landcare Research, Lincoln, Canterbury (CHR), for supplying information about the morphology and geographic distributions of Plantago during the course of this research; and the Department of Conservation for collecting permits. For financial support, we acknowledge the following: the Morton family (the Alison Morton Postgraduate Scholarship for Ecology and Marine Biology to MLT), New Zealand Foundation for Research Science and Technology through the OBI Defining New Zealand's Land Biota, the Wellington Botanical Society Student Awards to MLT, and Victoria University of Wellington (VUW Post Graduate Scholarship to MLT; VUW Small Grants Scheme).
References
- Álvarez , I and Wendel , JF . 2003 . Ribosomal ITS sequences and plant phylogenetic inference . Molecular Phylogenetics and Evolution , 29 : 417 – 434 .
- Batt , GE , Braun , J , Kohn , BP and McDougall , I . 2000 . Thermochronological analysis of the dynamics of the Southern Alps, New Zealand . Geological Society of America Bulletin , 112 : 250 – 266 .
- Bayly , MJ and Kellow , A . 2006 . An illustrated guide to New Zealand hebes , Wellington : Te Papa Press .
- Cheeseman , TF . 1906 . Manual of the New Zealand flora , Wellington : Government Printer .
- Cheeseman , TF . 1925 . Manual of the New Zealand flora , 2nd edn , Wellington : Government Printer .
- Cox , SC and Findlay , RH . 1995 . The Main Divide Fault Zone and its role in formation of the Southern Alps, New Zealand . New Zealand Journal of Geology and Geophysics , 38 : 489 – 499 .
- Dhar , MK , Friebe , B , Kaul , S and Gill , BS . 2006 . Characterization and physical mapping of ribosomal RNA gene families in Plantago . Annals of Botany , 97 : 541 – 548 .
- Druce AP 1993 . Indigenous vascular plants of New Zealand, 9th revision . Landcare Research, Lower Hutt. Unpublished list held at WELT .
- Ford , KA , Ward , JM , Smissen , RD , Wagstaff , SJ and Breitwieser , I . 2007 . Phylogeny and biogeography of Craspedia (Asteraceae : Gnaphalieae) based on ITS, ETS and psbA-trnH sequence data . Taxon , 56 : 783 – 794 .
- Garnock-Jones , PJ and Lloyd , DG . 2004 . A taxonomic revision of Parahebe (Plantaginaceae) in New Zealand . New Zealand Journal of Botany , 42 : 181 – 232 .
- Glenny , D . 2004 . A revision of the genus Gentianella in New Zealand . New Zealand Journal of Botany , 42 : 361 – 530 .
- Groves , BE and Hair , JB . 1971 . Contributions to a chromosome atlas of the New Zealand flora – 15 miscellaneous families . New Zealand Journal of Botany , 9 : 569 – 575 .
- Haase , M , Marshall , B and Hogg , I . 2007 . Disentangling causes of disjunction on the South Island of New Zealand: the Alpine fault hypothesis of vicariance revisited . Biological Journal of the Linnean Society , 91 : 361 – 374 .
- Harpke , D and Peterson , A . 2008 . 5.8S motifs for the identification of pseudogenic ITS regions . Botany , 86 : 300 – 305 .
- Heenan , PB , Mitchell , AD and Koch , M . 2002 . Molecular systematics of the New Zealand Pachycladon (Brassicaceae) complex: generic circumscription and relationship to Arabidopsis sens. lat. and Arabis sens. lat . New Zealand Journal of Botany , 40 : 543 – 562 .
- Hoggard , RK , Kores , PJ , Molvray , M , Hoggard , GD and Broughton , DA . 2003 . Molecular systematics and biogeography of the amphibious genus Littorella (Plantaginaceae) . American Journal of Botany , 90 : 429 – 435 .
- Hooker , JD . 1864 . Handbook of the New Zealand flora , Reeve : London .
- Huelsenbeck , JP and Ronquist , F . 2001 . MRBAYES: Bayesian inference of phylogenetic trees . Bioinformatics , 17 : 754 – 755 .
- Huson , DH and Bryant , D . 2006 . Application of phylogenetic networks in evolutionary studies . Molecular Biology and Evolution , 23 : 254 – 267 .
- Ishikawa , N , Yokoyama , J and Tsukaya , H . 2009 . Molecular evidence of reticulate evolution in the subgenus Plantago (Plantaginaceae) . American Journal of Botany , 96 : 1627 – 1635 .
- Kirk , T . 1879 . Descriptions of new plants . Transactions of the New Zealand Institute , 11 : 463 – 466 .
- Kumar , S , Tamura , K and Nei , M . 2004 . MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment . Briefings in Bioinformatics , 5 : 150 – 163 .
- Lockhart , PJ , McLenachan , PA , Havell , D , Glenny , D , Huson , D and Jensen , U . 2001 . Phylogeny, radiation, and transoceanic dispersal of the New Zealand alpine buttercups: molecular evidence under split decomposition . Annals of the Missouri Botanic Garden , 88 : 458 – 477 .
- McCulloch , GA , Wallis , GP and Waters , JM . 2010 . Onset of glaciation drove simultaneous vicariant isolation of alpine insects in New Zealand . Evolution , 64 : 2033 – 2043 .
- McDowall , RM . 2008 . Process and pattern in the biogeography of New Zealand – a global microcosm? . Journal of Biogeography , 35 : 197 – 212 .
- Meudt , HM and Bayly , MJ . 2008 . Phylogeographic patterns in the Australasian genus Chionohebe (Veronica s.l. Plantaginaceae) based on AFLP and chloroplast DNA sequences . Molecular Phylogenetics and Evolution , 47 : 319 – 338 .
- Meudt , HM , Lockhart , PJ and Bryant , D . 2009 . Species delimitation and phylogeny of a New Zealand plant species radiation . BMC Evolutionary Biology , 9 : 111
- Meudt , HM and Simpson , BB . 2007 . Phylogenetic analysis of morphological characters in Ourisia (Plantaginaceae): taxonomic and evolutionary implications . Annals of the Missouri Botanical Garden , 94 : 554 – 570 .
- Mitchell , AD and Heenan , PB . 1999 . Sophora sect. Edwardsia (Fabaceae): further evidence from nrDNA sequence data of a recent and rapid radiation around the Southern Oceans . Botanical Journal of the Linnean Society , 140 : 435 – 441 .
- Moore LB 1961 . Plantaginaceae Allan HH Flora of New Zealand 1 . Wellington , Government Printer 780 86 .
- Murray BG , Meudt HM , Tay ML , Garnock-Jones PJ 2010 . New chromosome counts in New Zealand species of Plantago (Plantaginaceae) . New Zealand Journal of Botany 48 : 197 204 .
- Pelser , PB , Gravendeel , B and van der Meijden , R . 2003 . Phylogeny reconstruction in the gap between too little and too much divergence: the closest relatives of Senecio jacobaea (Asteraceae) according to DNA sequences and AFLPs . Molecular Phylogenetics and Evolution , 29 : 613 – 628 .
- Perrie , LR and Brownsey , PJ . 2005 . Insights into the biogeography and polyploid evolution of New Zealand Asplenium from chloroplast DNA sequence data . American Fern Journal , 95 : 1 – 21 .
- Posada , D and Crandall , KA . 1998 . MODELTEST: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Rahn , K . 1996 . A phylogenetic study of the Plantaginaceae . Botanical Journal of the Linnean Society , 120 : 145 – 198 .
- Rock , JF . 1920 . The genus Plantago in Hawaii . American Journal of Botany , 7 : 195 – 210 .
- Rønsted , N , Chase , MW , Albach , DC and Bello , MA . 2002 . Phylogenetic relationships within Plantago (Plantaginaceae): evidence from nuclear ribosomal ITS and plastid trnL-F sequence data . Botanical Journal of the Linnean Society , 139 : 323 – 338 .
- Saitou , N and Nei , M . 1987 . The neighbour–joining method: A new method for reconstructing phylogenetic trees . Molecular Biology and Evolution , 4 : 406 – 425 .
- Smissen , RD , Breitwieser , I and Ward , JM . 2004 . Phylogenetic implications of trans-specific chloroplast DNA sequence polymorphism in New Zealand Gnaphalieae (Asteraceae) . Plant Systematics and Evolution , 249 : 37 – 53 .
- Smissen , RD , Garnock-Jones , PJ and Chambers , GK . 2003 . Phylogenetic analysis of ITS sequences suggests a Pliocene origin for the bipolar distribution of Scleranthus (Caryophyllaceae) . Australian Systematic Botany , 3 : 301 – 315 .
- Spence , JR and Sykes , WR . 1989 . Are Plantago novae-zelandiae L. Moore and P. lanigera Hook. f. (Plantaginaceae) different? . New Zealand Journal of Botany , 27 : 499 – 502 .
- Swofford DL 2002 . PAUP* . Phylogenetic analysis using parsimony (*and other methods) . Version 4.0b10 . Sunderland, MA , Sinauer Associates .
- Sykes WR 1988a . Plantaginaceae Webb CJ , Sykes WR , Garnock-Jones PJ Flora of New Zealand Vol. IV . Christchurch , Botany Division DSIR 942 955 .
- Sykes , WR . 1988b . Notes on New Zealand Plantago species . New Zealand Journal of Botany , 26 : 321 – 323 .
- Tay , ML , Meudt , HM , Garnock-Jones , PJ and Ritchie , PA . 2010 . DNA sequences from three genomes reveal multiple long-distance dispersals and non-monophyly of sections in Australasian Plantago (Plantaginaceae) . Australian Systematic Botany , 23 : 47 – 68 .
- Trewick , SA and Morgan-Richards , M . 2005 . After the deluge: mitochondrial DNA indicates Miocene radiation and Pliocene adaptation of tree and giant weta (Orthoptera : Anostostomatidae) . Journal of Biogeography , 32 : 295 – 309 .
- Wagstaff , SJ , Bayly , MJ , Garnock-Jones , PJ and Albach , DC . 2002 . Classification, origin, and diversification of the New Zealand hebes (Scrophulariaceae) . Annals of the Missouri Botanical Garden , 89 : 38 – 63 .
- Wagstaff , SJ and Garnock-Jones , PJ . 1998 . Evolution and biogeography of the Hebe complex (Scrophulariaceae) inferred from ITS sequences . New Zealand Journal of Botany , 36 : 425 – 437 .
- White TJ , Bruns T , Lee S , Taylor J 1990 . Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics Innis M , Gelfand C , Sninsky J , White T PCR protocols : a guide to methods and applications . San Diego, CA , Academic Press 315 322 .
- Winkworth , RC , Grau , J , Robertson , AW and Lockhart , PJ . 2002b . The origins and evolution of the genus Myosotis L. (Boraginaceae) . Molecular Phylogenetics and Evolution , 24 : 180 – 193 .
- Winkworth , RC , Robertson , AW , Ehrendorfer , F and Lockhart , PJ . 1999 . The importance of dispersal and recent speciation in the flora of New Zealand . Journal of Biogeography , 26 : 1323 – 1325 .
- Winkworth , RC , Wagstaff , SJ , Glenny , D and Lockhart , PJ . 2002a . Plant dispersal NEWS from New Zealand . Trends in Ecology & Evolution , 17 : 514 – 520 .
- Wolff K , Morgan-Richards M 1999 . The use of RAPD data in the analysis of population genetic structure: case studies of Alkanna (Boraginaceae) and Plantago (Plantaginaceae) Hollingsworth PM , Bateman RM , Gornall RJ Molecular systematics and plant evolution . New York , Taylor & Francis 51 74 .
- Xia , X and Xie , Z . 2001 . DAMBE: software package for data analysis in molecular biology and evolution . Journal of Heredity , 92 : 371 – 373 .