Abstract
Effects of up to 46 yr retirement from grazing Chionochloa spp. rangeland were assessed in exclosure plots on the Old Man Range, Central Otago. Exclosures at 910 and 1220 m have been ungrazed since 1960, one at 1230 m since 1964, and another at 1590 m remained stock-proof from 1960 until the mid 1980s. Burning history varied between sites. Vegetation structure and composition, including invasive Hieracium spp. and topsoil chemistry, were measured in the exclosures and adjacent grazed sites. Retirement had no significant effect on topsoil chemistry but was associated with increased indigenous plant diversity. There was negligible increase in the woody component under long-term protection. Density and cover of Hieracium lepidulum were significantly higher in the ungrazed 910 and 1230 m exclosures, but not in the 1220 m exclosure. Its invasiveness decreased with altitude, probably through frosting of maturing inflorescences. H. pilosella and H. praealtum were common only on a recently burnt, heavily grazed site at 1230 m. Strategic light grazing of these grasslands may facilitate removal of flower heads of H. lepidulum and reduce its invasiveness, but maximizing recovery of snow tussock following burning is recommended to retard establishment and/or spread of H. pilosella and H. praealtum.
Introduction
As Lee et al. (Citation2006) stated, ‘non-native or alien species have invaded New Zealand in a manner that is rarely equalled in the world’ while its ‘native biodiversity is renowned for high levels of endemism, ancient lineages, local radiations, and notable taxonomic and functional absences’.
Indigenous snow tussock (Chionochloa sppFootnote1.) grasslands, are a major component of New Zealand's subalpine and alpine vegetation (Wardle Citation1991; Mark Citation1993; Mark & Dickinson Citation1997). These grasslands are also widespread at lower altitudes in the rain-shadow region of central and eastern South Island, at least since burning by Polynesians following their settlement c. 750 yr ago (Molloy et al. Citation1963; McGlone Citation2001; Mark & McLennan Citation2005; Wilmshurst et al. Citation2008). Further modifications occurred with their use as rangeland for European pastoralism from the 1850s and the associated introduction of plant species and grazing mammals (O'Connor Citation1982; Mark Citation1993, Citation1994; Mark & Dickinson Citation2003; McIntyre Citation2008).
The condition of South Island tussock-grass rangelands has long caused concern in terms of vegetation depletion (Buchanan Citation1868; Petrie Citation1912; Zotov Citation1938). Since the Crown Pastoral Land Act of 1998, extensive areas of Crown-owned land have passed from pastoral to conservation use, but for both uses, there are issues of resource sustainability, nature conservation and associated values (Martin et al. Citation1994; Lough Citation2005; Mark & Dickinson Citation2008). A major threat has been the spread of several invasive plants, particularly hawkweeds, notably Hieracium pilosella, H. praealtum and H. lepidulum (Hunter Citation1991; Treskonova Citation1991; Kerr Citation1992; Martin et al. Citation1994; Ewans Citation2004).
The optimal management to minimize hawkweed invasion, especially grazing levels and burning versus fire prevention, is not clear. Reliable evidence on the effects of management should come from long-term directed research (Mark & Dickinson Citation2003). Surprisingly, few such studies have been reported, but a set of exclosures in snow tussock rangeland on the Old Man Range, Central Otago, provides some useful evidence. Although unreplicated, being established for a different purpose, they offer one of the longest time scales of paired differential grazing, with a variety of burning histories and at a range of altitudes, the three factors recognized as affecting the invasion of several adventive hawkweed species in South Island high country (Espie Citation2001). Conclusions from informal assessments of these plots have been controversial, with differing views on the abundance of H. lepidulum. Local farmers provided much lower estimates of H. lepidulum infestation for the grazed than ungrazed plots (Mead & Elstob Citation2005, P. Espie pers. comm. 2006) which were inconsistent with visual estimates by AF Mark and others. Mead & Elstob (Citation2005) concluded from their information that absence of stock grazing promoted the establishment and consolidation of H. lepidulum, and also decreased plant diversity at most sites, so that optimal management would be achieved with continued grazing. The issues are further clouded by uncertainty on long-term vegetation trends, whether the area would naturally remain in tussock grassland or revert to a potential natural woody vegetation. These long-term plots also offer valuable evidence on this issue.
Formal sampling of the plots was therefore conducted in 2006–2008 to answer five questions:
| 1. | Does removal of grazers impede or promote invasion by local Hieracium species? | ||||
| 2. | Does burning impede or promote their invasion? | ||||
| 3. | What is the effect of grazing and/or burning on the diversity of native species, and on other exotic species? | ||||
| 4. | Are these effects consistent across altitudes? | ||||
| 5. | What was the historical vegetation, and is the vegetation showing a tendency to move to woody cover? | ||||
Site descriptions
Fenced plots were established in 1960 on Obelisk Station, Old Man Range, as part of an autecological study of rangeland snow tussocks (Chionochloa rigida and Chionochloa macra) across the Otago high country (Mark Citation1965a, Citationb, Citationc, Citationd) (). Plots of 22×22 m at 910, 1220 () and 1590 m a.s.l. were fenced with wire mesh netting, topped with barbed wire, to exclude sheep (Ovis aries L.), hares (Lepus europaeus Pallas) and rabbits (Oryctolagus cuniculus L.). The lower two exclosures have remained ungrazed until the present, whereas snow damage partially collapsed the fence at 1590 m in the mid 1980s, which was repaired in 2008. The surrounding grassland has been grazed annually by merino sheep during the snow-free months.
Figure 1 Obelisk Station showing locations of the four plot sites (•) on the Old Man Range and the Station boundary. Site map prepared by P. Espie; sourced from NZMS 260, G42 – Alexandra. Crown Copyright Reserved.
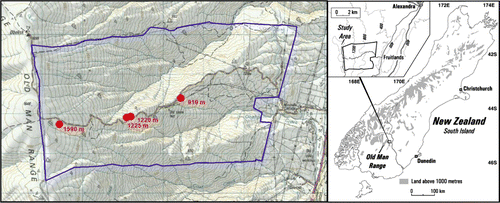
Figure 2 View east in May 2007 across two of the 1220–1230 m plots on the Old Man Range: A–A marks the fire break created in 1961; B is the area protected from burning in 1961 and unburnt since at least 1945; C is the 22×22m exclosure established in 1960 and also unburnt since at least 1945; D is the adjacent 1220 m grazed plot, beyond the exclosure; E is the area burnt in 1961 and again in 1992 (note the sparse snow tussock cover and numerous smaller tussocks of Festuca matthewsii); F is the 1230 m 20×10 m plot in the same area, which is located to the lower left foreground from the corner of the stake next to the person.

The two lower sites had not been burnt since at least 1945 (J. McCambridge, runholder, pers. comm. 1960), whereas earlier loss of snow tussock above c. 1300 m would have precluded burning here for a much longer period. The 910 m exclosure and surrounding subalpine C. rigida snow tussock grassland was burnt in a management fire in 1992. In 1961 and again in 1992, the low-alpine C. rigida tussock grassland surrounding the 1220 m exclosure was burnt, apart from a small area in and adjacent to this exclosure, where both fires were excluded by a 1961 firebreak. In 1964, an 18×10 m area of the 1961-burnt grassland, some 10 m elevation above the 1220 m plot, was experimentally re-burnt and similarly fenced to provide an additional treatment (Mark Citation1965d; Payton & Mark Citation1979; Payton et al. Citation1986). Similar plots of grazed grassland immediately adjacent to each of these three exclosures, which were permanently marked and sampled by Mead and Elstob, were also used for the present study. These six plots are referred to as: 910 m B'92, G and B'92, UG; 1220 m UB, G and UB, UG; and 1230 m B'61/'64, UG and B'61 G. A condition of the formal consent for the 1992 fire was that the exclosure areas at 1220–1230 m not be burnt. This was achieved, but following the 1992 fire, stock movement around this 1220–1230 m area was concentrated by a new fence line which limited the burnt area available to stock on the southern side of this fence in the vicinity of these study sites. This resulted in much more intensive grazing following this recent fire than in any of the previous fires. A seventh (unfenced) plot (10×20 m) was therefore established in this heavily grazed area of C. rigida tussock rangeland burnt in 1992, near the two 1230 m plots (which were unburnt at this time): this is termed the 1230 m B'61/'92, G plot. Thus the grazing contrasts available span different environments and fire disturbance histories ( and ).
Table 1 Mean values for density, per cent cover and frequency of Hieracium lepidulum in 45–121 subplots of 2×2 m in six plots on the Old Man Range, with relevant details of stock grazing and burning as indicated.
Although not an ideal experimental design, with no strict replication, these plots provide a unique opportunity to assess current differences in vegetation structure and species diversity, as well as hawkweed establishment, between the various treatments established for up to almost five decades. The study also offers an opportunity to test the prediction of Walker et al. (Citation2003, Citation2004a, Citationb) for increasing woodiness over time in tussock rangelands of the region in the absence of pastoral farming practices, particularly burning.
Methods
Snow tussock structure and biomass
The height–frequency method (Dickinson et al. Citation1992) was used to characterize the tussock canopies at the seven study sites. Four transects, each 12.5 m long and run down-slope, equidistant within each plot, were used to provide the total 50 m length required for each sample. Tussock grasses, speargrass (Aciphylla aurea) and two of the hawkweeds, H. pilosella and H. praealtum, were also recorded where present, but not distinguished because of some difficulty in their vegetative state. In the analyses for each site, the total height–frequency values for each species were summed for each 5 cm height interval, to indicate its form, and a single summed value combining all the height intervals was also derived for each species to provide an above-ground biomass index (BI; Dickinson et al. Citation1992).
Density and cover of Hieracium lepidulum
The exclosures and adjacent grazed plots were subdivided by 2×2 m temporary grids and the number of individual H. lepidulum plants in each grid cell was counted and their percentage ground cover estimated. A plant was considered an individual where it appeared to originate from one source at ground level, distinct from other plants, although it was often difficult to identify individual plants when growing close together. We classed closely adjoining stems as originating from one plant unless we could observe clear evidence of separation, an interpretation confirmed by excavating and washing three turves to isolate individual plants (Fig. A1.1). However, such grouping of closely adjacent individuals may underestimate actual plant numbers at high densities. Counts of H. lepidulum plants were converted to density per m2 to adjust for differences in plot size.
Objective assessment of the levels and associated causal factors of H. lepidulum infestation in each of the seven plots was complicated by the non-orthogonal design of treatments; the exclosures were not specifically designed for this exercise, as previously explained. A main issue was differential abundance of H. lepidulum and the main problem was the lack of true replication and obvious spatial autocorrelation within each plot. Specialist advice was therefore sought.
Use of the grid cells as pseudo-replicates of density and cover in the seven plots was unavoidable. To avoid problems of non-normality existing in the data, however, analysis of variance between the plots was performed by randomization test, with 100,000 randomizations, as described by Manly (Citation1997). Problems of possibly different distributions of residuals between treatments and of the obvious spatial autocorrelation remain, so we accepted significance only when p is very small. Subsequent planned t-tests and analysis of variance, and standard errors, using the error pooled over seven plots, were again necessarily based on the pseudo-replicates. These statistical problems were less for frequency values because a chi-squared test could be used.
Where significant differences were established between plots, the size of effects of altitude, burning and grazing were separated by fitting a general linear model to the log10 of the plot mean values. A log transformation was used because it seemed likely that the effects would be multiplicative (i.e. proportional), not additive.
Plant diversity: species composition and richness
The composition of vascular plant species in each of the seven plots was recorded and their cover estimated during a specific search in March 2006. Dissimilarity in species composition between these plots was tested, based on double square-root transformed, Bray–Curtis similarities in the computer software package PRIMER®v6 (Clarke Citation1993; Clarke et al. Citation2006). Owing to lack of replication between treatments, differences in species composition could not be directly tested, for example, using an ANOSIM test. Instead, a hierarchical clustering using group average sorting strategy was first performed to determine groups of similar species composition, using the SIMPROF test (Clarke et al. Citation2006) for significant discrimination amongst groups at p<0.05.
Species richness was expressed in terms of the number of vascular plant species recorded in each plot, whereas the Shannon diversity index (H′) was also calculated in PRIMER®v6 as: H′=− Σ i p i log (p i ), where p i is the total proportion count arising from the ith species (Clarke & Gorley Citation2006).
Indicators of grazing and recent burning
The contributions of all plant species plus snow tussock (C. rigida) seedlings and three of the non-living components (dead material, litter, bare soil) to average plot dissimilarities between the main treatments (grazed/ungrazed and burnt/unburnt since 1964) were also explored in PRIMER®v6, using the SIMPER (‘SIMilarity PERcentages’) analysis, based on a square root transformation of the cover values. All of the < 1% cover values were standardised as 0.1% cover.
Results
Grassland structure
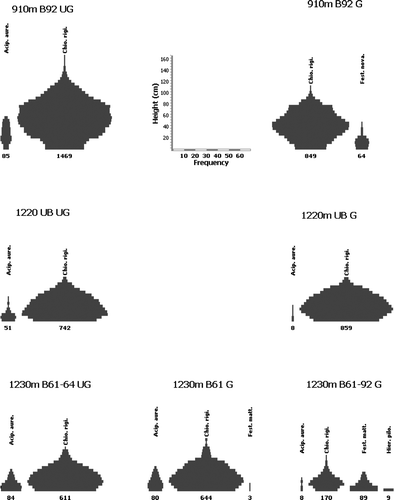
The largest biomass index (BI=1469) was recorded for C. rigida at the 910 m ungrazed site (), substantially greater than that in the adjacent grazed plot (BI=849) where Festuca novae-zelandiae was also present (BI=64). Such an ungrazed/grazed difference was not present at 1220 m, with similar values for the two plots (BI=742 and 859), or at 1230 m. However, stature of the C. rigida tussocks was greatest at 910 m (max height=110 cm), greater than at 1220 m (75 cm). At 1230 m, the C. rigida tussocks in the two plots unburnt since the mid 1960s were again similar between the ungrazed and grazed (BI=611 and 644) plots and were of heights similar to those at the nearby 1220 m site, whereas the overall stature of those at the adjacent grazed and recently burnt site was considerably smaller (BI=170; max height=65 cm). Tussocks of Festuca matthewsii at this latter site were also much more abundant (BI=89) than at any of the nearby sites (). The speargrass Aciphylla aurea, was present at all but one of the sites and, except at 1230 m, was much more conspicuous on the ungrazed plots (BI > 50). Hieracium pilosella and H. praealtum were recorded only on the 1230 m recently burnt and heavily grazed site, where they had a combined BI value of 9 ().
Figure 3 Diagrammatic representation of height–frequency values (see scales at top centre) for the tussock species (Chionochloa rigida, Festuca novae-zelandiae, Festuca matthewsii) plus speargrass (Aciphylla aurea) and hawkweed (Hieracium pilosella and H. praealtum combined, as Hier. pilo.) at seven sites on the Old Man Range. The altitude and differential burning (UB=unburnt; B=burnt during the year(s) specified) and grazing (UG=ungrazed; G=grazed) treatments are shown for each site. The total above-ground biomass index for each species is shown at the base of its kite diagram.
Infestation of Hieracium lepidulum
There were highly significant differences in density, frequency and per cent cover of H. lepidulum in the seven plots (), for density and cover, using the randomization test with the pseudo-replicates, and for frequency using a chi-squared test. Analysis of variance and t-tests indicated that the density was significantly higher (p <0.001) in the ungrazed plots at both 910 m and 1230 m, but not significant between the pair of 1220 m plots. The significance values for cover were similar to those for density, except for a lower order of difference (p <0.01) between the three plots at 1230 m.
The effects of altitude, burning since 1945, and grazing were separated by fitting a general linear model to the log10 of the plot mean values. This model indicated that at the higher altitudes (1220–1230 m) the density was 17.5% of that at the lowest altitude (910 m), whereas the cover was 9.5% and the frequency 82.4% of that at these lower altitude plots. Note also that the species was not recorded at the highest altitude (1590 m) plot, or in its vicinity, when scanned in 2008.
Grazing, according to the model, reduced the density of H. lepidulum plants to 24.4% of that in the ungrazed areas, cover to 22.2%, and frequency to 82.4%. Burning in the early 1960s (i.e. 1961 and 1964) increased the density to 11.1 times that associated with a longer period (>60 yr) since burning, whereas cover increased 12.2 times and frequency 3.0 times compared with the longer period since burning. With the most recent (1992) burning, the density of H. lepidulum plants was 5.2 times that in the plots last burnt before 1945, its cover 4.7 times and its frequency 2.0 times greater than that on the earlier burnt plots.
The model explained 87.3% of the variation in density, 92.9% of that for cover and 87.1% of the variation in frequency. It was not possible to examine both the interaction and the three levels of burning in the general linear model, because there were insufficient plots (and thus degrees of freedom) to separate them. The residuals from the model, however, gave an indication of interactions. For example, the model predicted that the combination of low altitude (giving higher abundance of H. lepidulum), recent burning (also giving higher abundance) and lack of grazing (giving higher abundance) would result in a density of 15.7 plants 4 m−2, whereas the density with this combination was in fact 34.1 plants 4 m−2: a positive interaction between burning and lack of grazing. Similar effects were seen for density and cover.
Observations of the H. lepidulum plants in all plots during late autumn (early May) over three years (2006–2008) revealed apparent frosting of the flower heads in most plants in the 1220 and 1230 m plots, with no sign of successful seeding there. By contrast, inflorescences in the two 910 m plots showed little sign of frost damage, and some full fruits were present. A similar pattern was observed in early March, 2009. Stock grazing of the H. lepidulum inflorescences at all four grazed plots appeared to be negligible over this period.
The mouse-ear hawkweed, H. pilosella, and king devil, H. praealtum were not assessed in the same detail as H. lepidulum, with only estimates of combined cover in each of the seven plots. They were not recorded in either plot at the 910 m site, and their cover values were minor (<1%) in all except the grazed 1230 m plot which had been burned in both 1961 and 1992, and heavily grazed following the latter fire. Here H. pilosella and H. praealtum were both conspicuous and relatively abundant, with an estimated combined cover of c. 3%.
Vascular plant diversity: species composition and richness
Cluster analysis of the vascular plant species composition in the seven plots () discriminated three groups significantly different from each other (p <0.05): Group 1, 910 m altitude, burnt in 1992, grazed and ungrazed; Group 2, 1230 m, burnt in 1961 and 1992, and heavily grazed; and Group 3, the two unburnt plots, grazed and ungrazed, at 1220 m plus the two 1230 m plots, unburnt since 1964, both grazed and ungrazed ().
Figure 4 Dendrogram from cluster analysis of species composition in the six study plots on the Old Man Range. Groups of plots with significantly dissimilar species composition (p<0.05), are shown in bold; the remainder are non-significantly dissimilar Plots are identified by: altitude (m); unburned (UB) or burned (B) with year(s); and grazed (G) or ungrazed (UG).
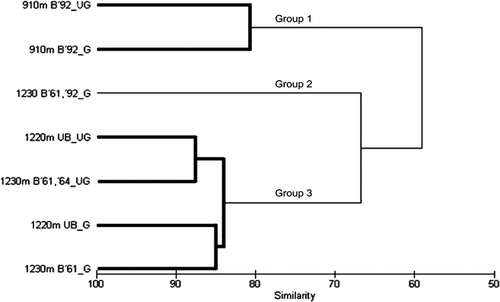
Table 2 List of vascular species (39 native; 12 exotic) and their estimated cover in seven plots at three altitudes (910 m, 1220 m and 1230 m) on the Old Man Range, representing different burning (B) and grazing histories since 1945, as indicated.
The two 910 m plots separated at 63% similarity, whereas the 1230 m plot, burnt in 1961 and 1992 and grazed, separated from the other four plots at similar altitude, unburnt since the early 1960s at 67% similarity. Separation between the two 910 m plots was at 81% similarity. The two ungrazed plots at 1220–1230 m separated from the two grazed plots these altitudes at 84% similarity. All these separations were significant at p <0.05, while the remainder were not ().
Species richness, as expressed by the number of vascular species present, was greater (≥ 30) in the three ungrazed (exclosure) plots irrespective of altitude, plus the 1230 m grazed-burnt ‘61 plot (). Of the remaining three plots, the recently burnt and heavily grazed plot at 1230 m had the fewest (23) species.
Differences in species diversity (Shannon Index) were relatively small () with the largest value recorded for the 1230 m recently burnt (1961–1992) and heavily grazed plot. This was driven by the much greater cover here of F. matthewsii, Poa colensoi and Raoulia subsericea, and much lower cover of C. rigida than in any of the other plots. In the remaining six plots, species diversity generally followed species richness. The exotic component, which ranged from 5 to 10 species, was higher in the two low-altitude plots and the only other recently (1992) burnt (and grazed) plot ().
Species indicators for grazing and recent burning treatments
Plot dissimilarities between the two main treatments (grazed/ungrazed and burnt/unburnt since 1964) averaged 38.2% between the pre- and post-1964 burning treatments and 24.0% between the grazed and ungrazed treatments (). Chionochloa rigida was the primary indicator species, for both the burning and grazing treatments. Among the 11 species (plus litter) which contributed most (>50%) to the dissimilarity between the burning treatments, five (and litter) were more abundant in the unburnt plots, most notably C. rigida (including seedlings), Gentianella bellidifolia, Aciphylla aurea and Gaultheria depressa, and the exotic H. pilosella (including H. praealtum). By contrast, Poa colensoi, Raoulia subsericea, Festuca matthewsii and the exotic grasses Agrostis capillaris and Holcus lanatus were more abundant in the burnt plots (A).
Table 3 Results of a SIMPER analysis of dissimilarities: (a) between burnt and unburnt plots and (b) between ungrazed (fenced) and grazed (unfenced) plots (b: below) among seven plots at 910 m, 1220 m and 1230 m on the Old Man Range.
Chionochloa rigida, together with Aciphylla aurea, Celmisia lyallii, Pentachondra pumila, Dracophyllum uniflorum and two exotics, Agrostis capillaris and Lotus pedunculatus were all more abundant on the ungrazed plots. Bare soil was one of the primary indicators, being more important in the grazed plots, while Poa colensoi, Raoulia subsericea and tussocks of Festuca matthewsii were the best plant indicators of grazing (B). There were substantially more C. rigida tussock seedlings in the two ungrazed (exclosure) plots at 1220–1230 m than in any of the grazed plots, even though their cover in all three was < 1% (see ).
In addition to the restriction of Celmisia lyallii, Dracophyllum uniflorum and Pentachondra pumila to the ungrazed (exclosure) plots at 1220 m and 1230 m, other notable features in the 1220 m exclosure were the occurrence (and restriction to it) of the generally common high-alpine cushion species Dracophyllum muscoides, Celmisia sessiliflora and Phyllachne colensoi, albeit as single cushions.
Discussion and conclusions
Experimental design
Despite the limitations imposed by the experimental design (lack of true replication), the exclosures associated with this study span a prolonged (five-decade) period, and special caution was used in interpreting significance from results of the stastical analyses. There is the danger with an essentially unreplicated study that the paired treatment plots: grazed, and ungrazed mammal-proof exclosures, might have been intrinsically different from the beginning. This seems unlikely because the pairs are essentially adjoining, so that any differences would probably have been in the soil. However, single bulk samples of topsoil (0–10 cm), each of 20 random, 2.5 cm diameter cores collected in March 2005, both from within and alongside the exclosures at 910, 1220 and 1590 m, showed no significant differences associated with the differential grazing at a particular altitude in any of 12 chemical factors analysed: pH, Ca, K, Mg, Na, Al, available P and sulfate S, organic S, total N, total P, anion storage (analyses by Cribbles Analytical Laboratories, Hamilton; P. Espie, pers. comm. 2008). It is unlikely, therefore, that the plot pairs were different initially.
Tussock cover
When protected from grazing, C. rigida tussocks were vigorous, especially at 910 m where it attained a biomass index (BI) of 1469, a value considerably higher than the maximum of 1143 recorded for essentially pristine low-alpine snow tussock grasslands in Fiordland National Park by Mark et al. (Citation2008).
A reduction in the size of C. rigida tussocks due to grazing could be seen only where there had been a recent (1992) fire, at 910 m and 1230 m, consistent with the impact on the species of grazing just after burning described by Mark (Citation1965d, Citation1994). At both sites, the reduction of C. rigida cover was accompanied by increased abundance of unpalatable fescue tussocks: F. novae-zelandiae at 910 m and F. matthewsii at 1230 m.
Hawkweed (Hieracium spp.) invasion and establishment
Several species of hawkweed (Hieracium spp.) have been actively invading New Zealand hill and high-country indigenous grasslands for several decades and are now major environmental pests in many central and eastern South Island rangeland and conservation areas (Hunter Citation1991; Rose & Frampton Citation1999; Espie Citation2001). H. lepidulum, tussock hawkweed, ‘occur[s] throughout the high country, with localised areas of high abundance’ according to Espie (Citation2001). It is also locally invasive in indigenous Nothofagus solandri var. cliffortioides forest (Wiser et al. Citation1998), and is considered to have ‘a growth advantage over tussock grasses, shrubs, and trees, at least in some ecological circumstances’ (Radford et al. Citation2009). H. pilosella, mouse-ear hawkweed, together with H. praealtum, king devil, are considered by Espie (Citation2001) to be ‘among the most abundant tussock grassland species in the moderate to low rainfall areas of the South Island high country’ while ‘further increases in distribution and abundance seem likely for all species’.
No hawkweeds were recorded when the exclosure plots were established and each site sampled in 1959–1964 using 1000 point intercepts in each of two strata per plot (Mark Citation1965a, Citationd). AF Mark first observed H. lepidulum in the area c. 1995 while H. pilosella and H. praealtum established much later, and even today all three are far less abundant here than in several other parts of Central Otago.
Abundance of H. lepidulum decreased with altitude. It has not been recorded in the 1590 m exclosure near the crest of the Old Man Range, or in the adjacent cushionfield, a degraded Chionochloa macra grassland, although occasional plants can be found in this habitat elsewhere on the range. This altitudinal effect may be caused partly by the observed frost-killing of the maturing inflorescences, so reducing the availability of viable seed.
High infestation of H. lepidulum occurred only in ungrazed plots, but lack of grazing alone does not allow significant invasion, as seen in the ungrazed 1220 m exclosure. Probably the open ground created by burning provides an opportunity for H. lepidulum to invade. The C. rigida tussock cover would have re-established much more quickly without post-fire grazing (Mark Citation1965d; Payton & Mark 1979), but it is clear that, once established, H. lepidulum is not inhibited by a tall and dense tussock cover, as Rose and Frampton (Citation1999) also found in Marlborough. Its shade tolerance is confirmed by its invasion of mountain beech (Nothofagus solandri var. cliffortioides) forests, particularly species-rich sites within c. 300 km of our study area (Wiser et al. 1998) and generally similar forest < 100 km distant (Rob Roy Valley, Mt Aspiring National Park since the mind 1970s; AF Mark, pers. obs.). The much reduced invasion when grazing is allowed after burning is unlikely to be because of herbivory of the vegetative parts, for sheep do not seem to graze them, but may remove inflorescences and thus retard spread through seed, its only means of reproduction (P Espie, unpublished contract report for Obelisk Station, 2006).
The greater extent of invasion in the 910 m ungrazed/burnt 1992 plot than in any of the 1220–1230 m plots could be because of greater vigour of H. lepidulum at lower altitude and/or greater propagule rain there, particularly after the 1992 burn than after the 1961 or 1964 burn when the species was absent ot at least sparse in the area.
Information on H. pilosella and H. praealtum (recorded jointly) from this study was inadequate to draw conclusions, although our results are consistent with the general observation of Espie (Citation2001) that H. pilosella ‘establishes best in sites with low vegetation stature, from where, even in the presence of grazing, it can spread to dominate the surrounding plants and bare ground’. Rose & Frampton (Citation1999) also reported that H. pilosella was much more adversely affected than H. lepidulum by a dense and overhanging cover of tall tussock. At the five higher altitude study sites (1220–1230 m) on the Old Man Range, the cover of H. pilosella exceeded 1% only on the open, recently (1992) burnt and heavily grazed site where it provided 3% cover, and H. praealtum had its only observed presence.
As with the Rose et al. (Citation1995) study in the Harper–Avoca catchment, mid-Canterbury, the invasive patterns of the three Hieracium spp. does not allow differentiation between the several hypotheses for their invasion, invoked to date. Ecosystem degradation, as proposed by Treskonova (Citation1991), involving reduction in cover of Chionochloa sp., an increase of Festuca sp. cover, and ‘reduced diversity of alpine and grassland species’, is certainly implied with the apparently recent establishment of H. pilosella and H. praealtum, and reduced diversity of indigenous plants on the recently burnt and subsequently heavily grazed 1230 m burnt 1961–1992 site where the biomass index for C. rigida snow tussock is relatively small (170). However, there was no evidence for the soil degradation associated with infestations of these two species elsewhere (McIntosh Citation1997), and ecosystem degradation could not account for the high abundance of H. lepidulum in the 910 m ungrazed, burnt-1992 plot.
Our findings are consistent with the conclusion of Duncan et al. (Citation1997) that distribution and abundance of Hieracium spp. over much of the South Island rangeland is dependent on site features (a function of the environment and past management), plus the size of the propagule rain.
Vascular plant diversity and indicator species
Vascular plant richness was generally greater in the ungrazed exclosures than the adjacent grazed areas, regardless of their burning history. Indeed, the maximum richness of 36 species was recorded in the plot at 1220 m that had been unburnt since 1945 (61 yr) and ungrazed since 1960 (46 yr). Several species were restricted to this plot, including some high-altitude cushion species (Celmisia sessiliflora, Dracophyllum muscoides, Phyllachne colensoi), which may now reach their lower limits on the range at this site. The larger herb Celmisia lyallii, which comprised c. 6% of the cover in the 1220 m exclosure, the subshrub Pentachondra pumila and the occasional erect shrubs Ozothamnus leptophyllus and Dracophyllum uniflorum, which are all restricted to the two exclosures at 1220–1230 m, are otherwise rare in the vicinity.
The other two ungrazed exclosures contained 32 and 30 vascular plant species, whereas the four grazed plots contained somewhat fewer species (23, 25, 30, 32). The indigenous forbs Gentianella bellidioides and Craspedia uniflora, together with the low shrub Gaultheria depressa, were the best indicators of grassland that had been long (61 yr) unburnt. Chionochloa rigida was more abundant on the ungrazed plots and its seedlings were also more abundant there. However, the widespread and usually common rangeland native species Rytidosperma pumilum, Poa colensoi, Raoulia subsericea and Festuca matthewsii were all more abundant in the grazed plots. Exotic species varied from five to ten between the seven plots, being generally richer at the lower altitude (910 m) site and in the recently burnt, heavily grazed plot at 1230 m. Indeed, three exotics, Hieracium pilosella, Rumex acetosella and Holcus lanatus, were among the best indicators of more recent burning.
Persistence of snow tussock dominance
The continued dominance of narrow-leaved snow tussock and the rarity of erect shrubs in any of the exclosures, given the absence of burning in one for at least 61 yr and of mammalian grazing since 1959 (47 yr) might be considered surprising given the now widely held view that areas of low- to mid-altitude tussock grassland in the South Island rain-shadow rangeland region, have been derived from a woody, mostly forest cover which was removed by not-infrequent burning during the period following Polynesian settlement c. 750 yr BP (McGlone Citation2001; Wilmshurst et al. 2008; McWethy Citation2010). McGlone (Citation2001) considered that in presettlement times grassland was ‘mostly patchy within the woody ecosystems, occurring on limited areas of droughty or low-nutrient soils and wetlands, or temporarily after infrequent fire or other disturbance’. Walker et al. (2003; 2004a, b) put the case more strongly, predicting that forest would have covered the montane and subalpine zones while ‘shrublands may have dominated above the regional treeline’. Tussock grasses were considered relevant by Walker et al. (2003, p. 58) only in ‘the alpine tussock-shrubland zone [which] is restricted to the highest elevations in Central Otago … [as on] the summits of Old Man, Old Woman, and Pisa Ranges, and the Garvie Mountains’. They further indicated that ‘at low elevations, grassland was probably confined to floodplains and local areas of shallow or permanently moist soils’. Extensive grasslands below treeline would probably have been maintained by fire, but Rogers et al. (Citation2007) found no evidence for this prior to settlement (after 1000 BP).
The only evidence from the Old Man Range exclosure plots for reversion to the historical cover supposed by Walker et al. (2003, 2004a, b) is the rare occurrence of the erect shrubs of Dracophyllum uniflorum and Ozothamnus leptophyllus in the 1220 m exclosure and surrounding long (61-yr) unburnt (since 1945) areas of C. rigida tussock grassland. This sparse woody invasion parallels the increased dominance of snow tussock over 30 yr of non-intervention reserve management described by Mark & Dickinson (Citation2003) for the Black Rock Reserve at 690–770 m on the Lammerlaw Range, along the eastern margin of the region considered by Walker et al. (2003, 2004a, b). The ecotypic differentiation of C. rigida across c. 800 m of altitude (Mark Citation1965c) in the study area, with its growth and mast flowering attuned to altitudinal variation in temperature on the range (Mark Citation1965a, Citationb, Citationc), is surely evidence for its long-term presence on the mid-slopes on the Old Man Range and presumably in similar areas: such genetic differentiation would be most unlikely within the c. 700-yr period of human occupation, given the assumed great longevity and very slow turnover of these tussocks (Mark Citation1993, Citation2005). Additional verification of the long-term presence of at least localized grassland vegetation on the mountain slopes in the region comes from the number of specialist Lepidoptera species associated with lowland to montane tussock grassland in inland South Island (Patrick & Dugdale Citation2000), and the species richness of indigenous moths in the rain-shadow tussock grasslands, from low altitude to the high-alpine zone (Patrick Citation2004).
The alternative view of Wardle (Citation1991), pp. 244–245) was that the intermontane basins in Central Otago, South Canterbury and Marlborough were probably dominated by short tussock grassland in pre-settlement times, while ‘large Chionochloa tussocks were widely dominant on the older, more acid soils, to lower altitudes than today’.
Overall, the evidence is consistent with a natural (i.e. pre-human) grassland component through the altitudinal sequence of vegetation on the mountains of the South Island rain-shadow region, most likely as part of a mosaic with woody components, moulded by physiography and infrequent natural fires, described by McGlone et al. (Citation2003) as ‘maintaining an open mosaic of forest, scrub and grasses’ occurring ‘between the dense coastal forests of Southland/Otago and the scrub of the interior’, and also assumed by Mark & Dickinson (Citation2003) for the Lammerlaw Range on the basis of 29 yr of monitoring a mid-altitude snow tussock grassland reserve.
Management implications
Three aggressive species of Hieracium are now present in the study area. Implications for management derived from this study are not clear cut, and would vary in relation to both altitude and the long-term plans for land use.
Burning, followed soon with heavy and selective grazing by sheep, will promote unpalatable and/or grazing-tolerant indigenous species such as Festuca novae-zelandiae, F. matthewsii, Poa colensoi and Raoulia subsericea, as well as the establishment and spread of several exotic species, particularly all three Hieracium species considered here (H. lepidulum, H. pilosella, H. praealtum), at the expense of the typical indigenous flora and the otherwise dominant snow tussock (Chionochloa spp.) cover. Ideally, fire should be prevented or at least carefully managed at infrequent intervals and combined with at least two years of post-burn relief from grazing.
Complete protection from stock grazing and burning for a number of decades may induce the establishment of several indigenous species which are otherwise rare or absent, and also maintain a strong dominance of snow tussock. However, occasional fire is probably inevitable. Autecological studies of the rangeland snow tussocks, C. rigida and C. macra, including their short- and long-term responses to the burning and grazing components of pastoral farming, have emphasized the importance of avoiding grazing for one and preferably two years after burning the grassland. This facilitates recovery of the tussock canopy since young tussock regrowth is particularly vulnerable to grazing with its enhanced nutrient levels and palatability (Payton et al. Citation1986). Severe grazing during this period can seriously deplete the nutrient status of the tussocks and thus jeopardize their recovery and even survival (Mark Citation1994). Exclusion of grazers following fire also permits the post-burn induction of flowering in snow tussocks which replenishes their seed bank (Mark Citation1965d). Such management would maintain more natural snow tussock physiognomy and thus minimize invasion of hawkweeds.
However, if complete destocking associated with burning is followed by establishment or spread of tussock hawkweed, H. lepidulum, light grazing of sheep after the immediate post-fire period could retard its spread through grazing of seed heads. This species is clearly less aggressive above c. 1000 m, at least in part through frosting of its seed heads which thus retards its spread. Nevertheless, it can obviously establish with or without grazing by sheep and it is clearly a potentially serious threat to the snow tussock grasslands in the South Island high country whether managed for pastoral or conservation purposes.
Acknowledgements
We thank Peter Espie of AgScience Ltd. for providing results of the soil analyses and the location map, as well as for many discussions and field assistance; also Bert Elstob and Nicky Mead, lessees of Obelisk Station at the time of our field work, for their field assistance and, together with Guy Mead, for useful discussions. We also thank Allistair Campbell, the current lessee, for discussions and also approval to publish our findings. Pascale Michel and Stefan Porter, Botany Department, University of Otago, assisted with some of the statistical analyses and compilation of the diagrams. The Hellaby Indigenous Grasslands Research Trust made a considerable contribution to the cost of this and associated projects.
Notes
1Plant names follow the Allan Herbarium (2000).
References
- Allan Herbarium 2000 . Nga Tipu o Aotearoa–New Zealand plant names database. Lincoln, New Zealand, Landcare Research . http://nzflora.landcareresearch.co.nz (accessed June 2008 ).
- Buchanan , J . 1868 . Sketch of the botany of Otago . Transactions of the New Zealand Institute , 1 : 22 – 53 .
- Clarke , KR . 1993 . Non-parametric multivariate analyses of change in community structure . Australian Journal of Ecology , 18 : 117 – 143 .
- Clarke KR , Gorley RN 2006 . Primer V6: user manual/tutorial. Plymouth, UK, PRIMER-E Ltd .
- Clarke , KR , Somerfield , PJ and Chapman , MG . 2006 . On resemblance measures for ecological studies, including taxonomic dissimilarities and zero-adjusted Bray–Curtis coefficient for denuded assemblages . Journal of Experimental Marine Biology and Ecology , 330 : 55 – 80 .
- Dickinson , KJM , Mark , AF and Lee , WG . 1992 . Long-term monitoring of non-forest communities for biological conservation . New Zealand Journal of Botany , 30 : 163 – 179 .
- Duncan , RP , Colhoun , KM and Foran , BD . 1997 . The distribution and abundance of Hieracium species (hawkweeds) in the dry grasslands of Canterbury and Otago . New Zealand Journal of Ecology , 21 : 51 – 62 .
- Espie P 2001 . Hieracium in New Zealand: ecology and management . Mosgiel, New Zealand, AgResearch .
- Ewans R 2004 . Effects of removing grazing from native grasslands in the eastern South Island of New Zealand . Science Internal Series 168. Wellington, Department of Conservation .
- Hunter , GG . 1991 . The distribution of hawkweeds (Hieracium spp.) in the South Island, indicating problem status . Journal of the New Zealand Mountain Lands Institute Review , 48 : 21 – 31 .
- Kerr , CJ . 1992 . The high country in transition: some implications for occupiers and administrators . Tussock Grasslands and Mountain Lands Institute Review , 49 : 32 – 50 .
- Lee W , Allen RB , Tompkins DM 2006 . Paradise lost – the last major colonization . In : Allen RB , Lee WG , Biological invasions in New Zealand . Berlin, Springer-Verlag . PP. 1 13 .
- Lough R , 2005 . High country landscape management forum. Proceedings . Dunedin, Otago Regional Council 122 Pp.
- McGlone , MS . 2001 . The origin of the indigenous grasslands of southeastern South Island in relation to pre-human woody ecosystems . New Zealand Journal of Ecology , 25 : 1 – 15 .
- McGlone M , Wardle P , Worthy T 2003 . Environmental change since the last glaciation . In : Darby J , Fordyce RE , Mark A , Probert K , Townsend C . The natural history of southern New Zealand . Dunedin, University of Otago Press . Pp. 105 128 .
- McIntosh , P . 1997 . Nutrient changes in tussock grasslands, South Island, New Zealand . Ambio , 2626 : 147 – 151 .
- McIntyre R 2008 . Whose high country? A history of the South Island high country of New Zealand . Auckland, Penguin Books .
- McWethy , DB , Whitlock , C , Wilmshurst , JM , McGlone , MS , Fromont , M , Xun , L , Dieffenbacher- Frall , A , Hobbs , WO , Fritz , SC and Cook , ER . 2010 . Rapid landscape transformation in South Island, New Zealand, following initial Polynesian settlement . Proceedings of the National Academy of Science of the USA , 107 : 21343 – 21348 .
- Manly BFJ 1997 . Randomisation, bootstrap and Monte Carlo methods in biology. , 2nd edition . London, Chapman and Hall .
- Mark , AF . 1965a . The environment and growth rate of narrow-leaved snow tussock, Chionochloa rigida in Otago . New Zealand Journal of Botany , 3 : 73 – 103 .
- Mark , AF . 1965b . Flowering, seeding and seedling establishment of narrow-leaved snow tussock, Chionochloa rigida . New Zealand Journal of Botany , 3 : 180 – 193 .
- Mark , AF . 1965c . Ecotypic differentiation in Otago populations of narrow-leaved snow tussock, Chionochloa rigida . New Zealand Journal of Botany , 3 : 277 – 299 .
- Mark , AF . 1965d . Effects of management practices on narrow-leaved snow tussock, Chionochloa rigida . New Zealand Journal of Botany , 3 : 300 – 319 .
- Mark AF 1993 . Indigenous grasslands of New Zealand . In : Coupland RT , Natural grasslands, Eastern hemisphere. Ecosystems of the World 8B . Amsterdam, Elsevier . P. 361 410 .
- Mark , AF . 1994 . Effects of burning and grazing on sustainable utilisation of upland snow tussock (Chionochloa spp.) rangelands for pastoralism in South Island, New Zealand . Australian Journal of Botany , 42 : 149 – 161 .
- Mark AF 2005 . Fifty years of snow tussock grassland research applied to high country landscape . In : Lough RS . High Country Landscape Management Forum Proceedings . Dunedin, Otago Regional Council . Pp. 43 53 .
- Mark AF , Dickinson KJM 1997 . New Zealand alpine ecosystems . In : Wielgolaski FE . Polar and alpine tundra. Ecosystems of the World 3 . Amsterdam, Elsevier . P. 311 343 .
- Mark , AF and Dickinson , KJM . 2003 . Temporal responses over 30 years to removal of grazing from a mid-altitude snow tussock grassland reserve, Lammerlaw Ecological Region, New Zealand . New Zealand Journal of Botany , 41 : 655 – 668 .
- Mark , AF and Dickinson , KJM . 2008 . Maximizing water yield with indigenous non-forest vegetation: a New Zealand perspective . Frontiers in Ecology and the Environment , 6 : 25 – 34 .
- Mark , AF and McLennan , B . 2005 . The conservation status of New Zealand's indigenous grasslands . New Zealand Journal of Botany , 43 : 245 – 270 .
- Mark , AF , Porter , S , Piggott , JJ , Maegli , T and Dickinson , KJM . 2008 . Altitudinal patterns of vegetation, flora, life forms, and environments in the alpine zone of the Fiord Ecological Region, New Zealand . New Zealand Journal of Botany , 46 : 205 – 237 .
- Martin G , Garden P , Meister A , Penno W , Sheath G , Stephenson G , Urquhart R , Mulcock C , Lough R 1994 . South Island High Country Review. Final report of the working party on sustainable land management to the Ministers of Conservation, Agriculture and Environment . Dunedin, South Island High Country Review Working Party, Otago Regional Council .
- Mead N , Elstob B 2005 . Managing Obelisk, a semi-arid high country run . In : Lough RS , High Country Landscape Management Forum Proceedings. Dunedin, Otago Regional Council . Pp. 77 78 .
- Molloy , BPJ , Burrows , CJ , Cox , JE , Johnston , JA and Wardle , P . 1963 . Distribution of subfossil forest remains in eastern South Island, New Zealand . New Zealand Journal of Botany , 1 : 68 – 77 .
- O'Connor , KF . 1982 . Implications of past exploitation and current developments to the conservation of South Island tussock grasslands . New Zealand Journal of Ecology , 5 : 97 – 107 .
- Patrick , BH . 2004 . Conservation of New Zealand's tussock grassland moth fauna . Journal of Insect Conservation , 8 : 199 – 208 .
- Patrick BH , Dugdale JS 2000 . Conservation status of the New Zealand Lepidoptera. Science for Conservation . 136. Wellington, Department of Conservation .
- Payton , IJ , Lee , WG , Dolby , R and Mark , AF . 1986 . Nutrient concentrations in narrow-leaved snow tussock (Chionochloa rigida) after spring burning . New Zealand Journal of Botany , 24 : 529 – 537 .
- Payton , IJ and Mark , AF . 1979 . Long-term effects of burning on growth, flowering and carbohydrate reserves in narrow-leaved snow tussock (Chionochloa rigida) after spring burning . New Zealand Journal of Botany , 17 : 43 – 54 .
- Petrie D 1912 . Report on the grass-denuded lands of Central Otago. New Zealand Department of Agriculture, Industries and Commerce Bulletin 23 . Wellington, Government Printer .
- Radford , I , Dickinson , KJM and Lord , JM . 2009 . Does the invader Hieracium lepidulum have a comparative growth advantage over co-occurring plants? High leaf area and low metabolic costs as invasive traits . New Zealand Journal of Botany , 47 : 395 – 403 .
- Rogers , GM , Walker , S , Basher , LM and Lee , WG . 2007 . Frequency and impact of Holocene fire in eastern South Island, New Zealand . New Zealand Journal of Ecology , 31 : 129 – 142 .
- Rose , AB and Frampton , CM . 1999 . Effects of microsite characteristics on Hieracium seedling establishment in tall- and short-tussock grasslands, Marlborough . New Zealand Journal of Botany , 37 : 107 – 118 .
- Rose , AB , Platt , KH and Frampton , CM . 1995 . Vegetation change over 25 years in a New Zealand short-tussock grassland: effects of sheep grazing and exotic invasions . New Zealand Journal of Ecology , 19 : 163 – 174 .
- Treskonova , M . 1991 . Changes in the structure of tall tussock grassland and infestation by species of Hieracium in the Mackenzie country, New Zealand . New Zealand Journal of Ecology , 15 : 65 – 78 .
- Walker S , Lee WG , Rogers GM 2003 . The woody vegetation of Central Otago, New Zealand: its present and past distribution and future restoration needs . Science for Conservation. 226. Wellington, Department of Conservation .
- Walker , S , Lee , WG and Rogers , GM . 2004a . The woody vegetation of Central Otago, New Zealand . New Zealand Journal of Botany , 42 : 589 – 612 .
- Walker , S , Lee , WG and Rogers , GM . 2004b . Pre-settlement woody vegetation of Central Otago, New Zealand . New Zealand Journal of Botany , 42 : 613 – 646 .
- Wardle P 1991 . The vegetation of New Zealand . Cambridge, Cambridge University Press .
- Wilmshurst , JM , Anderson , AJ , Higham , TFG and Worthy , TH . 2008 . Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat . Proceedings of the National Academy of Science of the USA , 105 : 7676 – 7680 .
- Wiser , SK , Allen , RB , Clinton , PW and Platt , KH . 1998 . Community structure and forest invasion by an exotic herb over 23 years . Ecology , 79 : 2071 – 2081 .
- Zotov , VD . 1938 . Survey of the tussock grasslands of the South Island, New Zealand; preliminary report . New Zealand Journal of Science and Technology , A20 : 212 – 244 .
Appendix
Figure A1.1 One of three samples of Hieracium lepidulum collected from 1230 m Old Man Range, showing what was assumed to be two plants in the field (top), and which was confirmed with washing (bottom). Each of the two plants had a tightly interconnected root mass, but apparently functioned as separate individuals; no root grafts were seen.
