Abstract
Hibiscus trionum has generally been regarded as naturalised in Australia and New Zealand. Two varieties are sometimes accepted as occurring in Australia: H. trionum var. trionum and H. trionum var. vesicarius, with the latter occasionally treated as indigenous. Following studies of the variation within H. trionum in Australia and New Zealand we propose that there are three indigenous species in this complex. Of the three species, one, Hibiscus richardsonii, occurs in coastal regions of New South Wales, Australia and the northeastern half of the North Island, New Zealand, Hibiscus tridactylites Lindley occurs in inland southern and eastern Australia, and Hibiscus verdcourtii, described herein, occurs widely in inland Australia, especially north of latitude 28°S. In addition, we provide a description and informally recognise a diploid race of H. trionum s.l. that is widespread in New Zealand. In past treatments of the species in New Zealand this diploid race has either been treated as indigenous or included with H. richardsonii under the name “H. trionum” as naturalised. A key is provided to identify these taxa.
Introduction
Hibiscus trionum L., often known by the name bladder ketmia is an extremely variable species (, ). In the broad sense, H. trionum is regarded as indigenous to Africa and Europe–west Asia and it was described from material collected from Central Africa (Allan Citation1961; Webb et al. Citation1988). H. trionum also extends to Australasia. In Australia, two varieties [var. trionum and var. vesicarius (Cav.) Hochr.] are recognised (Mitchell Citation1981; Jessop Citation1986; Walsh Citation1996). In New Zealand only the single species H. trionum is recognised (Hooker Citation1864; Kirk Citation1899; Cheeseman Citation1906, Citation1925; Allan Citation1961; Webb et al. Citation1988). There has been some debate as to whether the species in indigenous or naturalised, with some authors regarding H. trionum or portions of the variation within its Australasian range as indigenous (Hooker Citation1864; Bailey Citation1899; Kirk Citation1899; Cheeseman Citation1906, Citation1925; Blackall & Grieve Citation1956; Allan Citation1961; Cunningham et al. Citation1981; de Lange et al. Citation1999; de Lange et al. Citation2010).
Figure 1 Representative mid-stem leaves of Hibiscus species. A, H. richardsonii (Craven 10483, CANB). B, H. tridactylites (Craven 10639, CANB). C, H. verdcourtii (Craven 10638, CANB). Bar scale=25 mm. Note scale varies in parts A–C of .
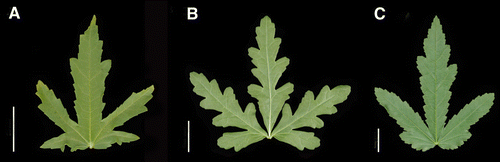
Figure 2 Flowers of Hibiscus species. A, H. richardsonii [Mayor I. (Tuhua), New Zealand]. B, H. tridactylites (Dalby, Queensland, Australia). C, H. tridactylites (Narrabri, New South Wales, Australia). D, H. verdcourtii (Emerald, Queensland, Australia). E, H. verdcourtii (St George, Queensland, Australia). F, ‘Diploid New Zealand naturalized race’ (Bream Head, North Island, New Zealand.
![Figure 2 Flowers of Hibiscus species. A, H. richardsonii [Mayor I. (Tuhua), New Zealand]. B, H. tridactylites (Dalby, Queensland, Australia). C, H. tridactylites (Narrabri, New South Wales, Australia). D, H. verdcourtii (Emerald, Queensland, Australia). E, H. verdcourtii (St George, Queensland, Australia). F, ‘Diploid New Zealand naturalized race’ (Bream Head, North Island, New Zealand.](/cms/asset/169e9a67-8159-417f-8ab3-4178de688ac0/tnzb_a_542762_o_f0002g.jpg)
Those Australian plants that have been attributed to var. trionum are distinguished from the form known there as var. vesicarius by the mid-stem and upper-stem leaves lobed to the petiole apex (i.e. there is no lamina tissue at the head of the sinus between the lobes, see B, as Hibiscus tridactylities in this figure). Those referred to as var. vesicarius always have mid-stem and upper-stem leaves with lamina tissue at the head of the sinus (see C, as Hibiscus verdcourtii in this figure). In New Zealand, although only the one species, H. trionum has been recognised, significant variation in flower shape, size and colour has been noted by some authors (de Lange & Murray Citation2002; Murray et al. Citation2008) and recently the name H. richardsonii Sweet ex Lindl. has been used for some of this variation (de Lange Citation2008; de Lange et al. Citation2010). The analysis of chromosome numbers of Australian and New Zealand plants of H. trionum s.l. found both diploid (2n=28) and tetraploid (2n=56) plants (Murray et al. Citation2008). The diploids were the first recorded for the species worldwide and in that article an indigenous status for one of the New Zealand diploids was argued. Subsequently, this has been adopted by de Lange (Citation2008), de Lange et al. (Citation2009) and de Lange et al. (Citation2010) using the name H. richardsonii for these plants (see also Nga Tipu o Aotearoa New Zealand Plants http://nzflora.landcareresearch.co.nz; accessed 4 November 2010). Another diploid, also confined to New Zealand, Murray et al. (Citation2008) was regarded as naturalised.
Australian plants of H. trionum s.l. are serious weeds in cotton (Gossypium L. spp., Malvaceae) and summer-grown cereal crops. Some of these H. trionum variants are known to support indigenous genotypes of Fusarium Link (Hypocreaceae) (Johnson & MacKinnon Citation2002; A. Becerra pers. comm. 2008) that have caused disease in eastern Australian cotton crops, necessitating changes in management systems (Kochman Citation1995; Swan & Salmond Citation2004). There is now clearly a need to resolve the biostatus of these plants (i.e. are they indigenous, naturalised or mixtures of both).
Given the variation in chromosome numbers (Murray et al. Citation2008), the autogamous breeding systems (Ramsey et al. Citation2003; Seed et al. Citation2006), and the presence of consistent morphological differences (,; LAC unpublished data), it is our conclusion that the complex consists of several, perhaps many, biologically separate species. Although the morphological distinctions may be subtle, this is no reason in itself not to attempt a taxonomic resolution of the complex. We acknowledge that this revision excludes the variation exhibited by the H. trionum complex in key areas such as Africa, however, Murray et al. (Citation2008) examined chromosome variation and illustrated flowers and foliage from a range of African plants none of which match the forms found in Australasia. Although a worldwide revision of the H. trionum complex is much desired this is beyond the scope of our limited resources. What we offer is a start toward a worldwide revision of the complex, recognising in Australasia three indigenous species of the H. trionum complex: H. richardsonii (shared between Australia and New Zealand), H. tridactylites Lindl. (var. trionum of Australian authors) and H. verdcourtii Craven (var. vesicarius of Australian authors). A fourth entity, found in New Zealand but not Australia, we describe here using an informal tag name “diploid New Zealand naturalised race”. We believe that it is not indigenous to Australasia and that it may have been an early European introduction. However we have been unable to match it to any known species or lower named rank associated with H. trionum.
Taxonomy
Hibiscus L. Sp. Pl.: 693, 1 Mar 1753, nom. cons
Type species: Hibiscus syriacus L.
Key to the indigenous Australasian bladder ketmia species
1 Mid-stem and distal leaves lobed to the apex of the petiole; pedicel 0.25–0.6 mm long……H. tridactylites
1 Mid-stem and distal leaves not lobed to the apex of the petiole; pedicel 4–20 mm long……2
2 Seed glabrous, 2.5–2.8 mm long; style branches 0.6–1 mm long……H. verdcourtii
2 Seed hairy, 2.0–2.6 mm long; style branches 2.3–5.2 mm long……3
3 Seed 2.0–2.2 mm long; petal yellow, basally faintly flushed pink or with fine red striations; style branches 2.3–3 mm long……H. richardsonii
3 Seed 2.3–2.6 mm long; petal yellow with prominent basal maroon-black petal spot; style branches 2.5–5.2 mm long……H. trionum “diploid New Zealand naturalised race”
1. Hibiscus richardsonii Sweet ex Lindl., Edwards’ Bot. Reg. 11: t. 875 (1825), as ‘richardsoni’
Lectotype (chosen here): Australia: New South Wales: observed flower[ing] February, abundant on the banks of the Nepean [River], 1817, C. Fraser s.n. (BM photo seen).
= Hibiscus trionoides G. Don, Gen. Hist. 1, p. 483 (1831)
Lectotype (chosen here): Australia: New South Wales: Richmond, November 1804, G. Caley s.n. (BM photo seen).
Typification: Lectotypification of the name H. richardsonii is required, as a holotype was not cited by Lindley (Citation1825). In the protologue, Lindley referred to material cultivated in London from seed obtained at Port Macquarie, New South Wales, by John Richardson, and also to specimens collected by Charles Fraser (as Frazer) at Nepean River, New South Wales. The Fraser material, deposited in BM, is designated lectotype above, as the material is not incongruent with the protologue and it is preferable to have the name typified by herbarium specimens, in preference to the plate, i.e., t. 875, that also forms part of the protologue and would be available for typification purposes in the event that herbarium specimens could not be located.
Lectotypification of the name H. trionoides is required also, as Don (Citation1831) did not designate a holotype. Two Caley collections of the species are deposited in BM: Richmond, November 1804, and Newcastle, April 1804. The collection from Richmond is more ample, in good condition and is not incongruent with the protologue.
Description (A, A): Herb 0.2–1.0 m tall. Branchlet with fine stellate hairs 0.2–0.8 mm long and coarser stellate hairs 0.4–0.7 mm long, with sparse coarse bristles 0.3–0.6 mm long, and with sparse fine bristles 0.2–0.5 mm long. Stipules more or less persistent, 4–5 mm long. Mid-stem and distal leaves 3-lobed (rarely unlobed, or 5- or 7-lobed), lobing not extended to the apex of the petiole, the primary lobes themselves scarcely lobed, palmately veined; lamina of mid-stem leaves 20–65 mm long, 15–45 mm wide, in overall shape ovate, or broadly ovate, with fine and coarse stellate hairs, margin strongly serrate, lobe apex acute or obtuse; petiole 10–30 mm long with indumentum similar to that of the branchlet; foliar nectary absent. Flowers solitary in leaf axils, chasmogamous, pedunculate; peduncle 10–20 mm long, with fine and coarse stellate hairs, rarely also with fine bristles; pedicel 4–10 mm long, indumentum dissimilar to that of peduncle (stellate hairs longer and denser, coarse bristles present). Epicalyx 12–14-segmented, 10–13.5 mm long, segments linear, free at the base, shorter than the calyx to equally as long as the calyx. Calyx at anthesis 13–15 mm long, distinctly accrescent in fruit, with stellate hairs, fine and coarse bristles and sparse glandular hairs, without prominent marginal ribs; lobes triangular, acute at the apex; calyx nectary absent. Petal 19–26 mm long, yellow distally faintly flushed pink, base sometimes with fine pale red striations. Staminal column 7–10 mm long with the stamens distributed along the distal 4–7 mm of the column; staminal filaments 1–2.3 mm long; anthers yellow; pollen yellow. Style (including style branches) exserted 2.3–3.8 mm beyond the apex of the staminal column; style branches 5, 2.3–3 mm long; stigmas capitate, 0.8–1.2 mm across, stigmatic hairs 0.1–0.2 mm long. Ovary hairy. Fruit, capsulate. Capsule hairy, 10–12 mm long. Seed 2–2.2 mm long, subreniform, papillate-pubescent and smooth between the hair pustules. 2n=28 (Murray et al. Citation2008, p. 317, source Panui Pa; Moruya).
Representative specimens: AUSTRALIA: NEW SOUTH WALES: A. Floyd 2663, 10 Dec 2009, Green Bluff, Moonee, CANB (Duplicates: CFSHB, L); J. F. Wilcox s.n., 1875, Clarence River, MEL; W. V. Fitzgerald s.n., Macleay River, MEL; R. Brown s.n., 1802–1805, Hawkesbury and Hunter Rivers, BM (Duplicates: CANB, MEL); H. Salasoo 1639, 21 Dec 1958, Lake Macquarie, Myuna Bay, NSW; A. Rodd 2428, 19 Nov 1973, NW shore, Smiths Lake, NSW; A. A. Hamilton s.n., 17 Oct 1916, headland S of Collaroy Beach, NSW; J. Liney 2348, 4 Feb 2006, E side of Italian Mine Road, 400 m S of junction with Old Mill Road, Moruya State Forest, CANB; N. Schultz 92, 15 Nov 1993, Fairhaven Point Way, 0.08 km from corner of Wallaga Lake Road, Wallaga Lake, CANB (Duplicate: NSW); E. Mullins 587, Oct 1985, Blackfellow Point, 3 km E of Bodalla, AD (Duplicates: CANB, MO); R.G. Coveny, P. Hind & R. Hancock 6202, 29 Mar 1975, Tathra, CANB (Duplicate: NSW). CULTIVATED: L.A. Craven 10483, Feb 2006, CSIRO, Canberra, [Provenance: ex E. Mullins 587, Blackfellow Point, 3 km E of Bodalla, New South Wales], CANB (Duplicate: L); L.A. Craven 10484, Feb 2006, CSIRO, Canberra, [Provenance: ex A. Rodd 2428, NW shore, Smiths Lake, New South Wales], CANB (Duplicates: A, L, P); L.A. Craven 10485, Feb 2006, CSIRO, Canberra, [Provenance: seed from P. J. de Lange, ex Mayor Island (Tuhua), New Zealand], CANB (Duplicate: A, E, L, P). NEW ZEALAND, NORTH ISLAND: NORTH AUCKLAND: T. F. Cheeseman s.n., Jan 1896, Tom Bowling Bay, AK 5232; N. Smith s.n., Feb 2002, Tapotupotu Bay, AK 256598; L. Winch s.n., 22 Feb 1995, Whangape Harbour, Whangape, AK 222103; T. Kirk 717, n.d., Whangarei, AD; L.J. Forester s.n., 26 Apr 2008, Whangarei, Kamo, AK 307165. GISBORNE: P.J. de Lange 2377, 2 Apr 1994, Hicks Bay, Haupara Point, AK 218967 (Duplicate: CHR). CAVALLI ISLANDS: G.P. Adams s.n., Oct 1969, Haraweka Island, AK 121197; A.E. Wright 3000, 3 Jan 1979, Nukutaunga Island, AK 149582. Mayor Island (TUHUA): P. Hynes s.n., 19 Nov 1955, Panui Flat, AK 43858; A.E. Wright 4364, Panui, 21 Nov 1981, AK 159471 (Duplicates: AD, CHR,HO, K); J.P. Adam s.n., 29 Mar 1986, North West slope of Crater, AK 278268; C. Jones s.n., 30 Mar 1986, Opo Bay, WAIK 9648.
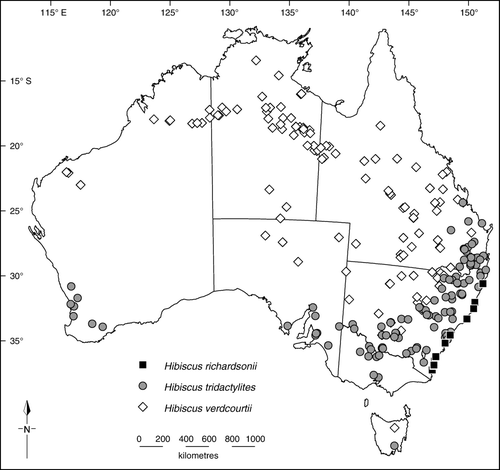
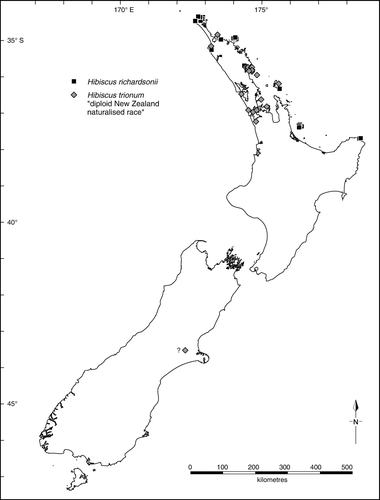
Distribution (, ): In Australia () confined to New South Wales in coastal and subcoastal country from the Clarence River district southwards to the Bermagui district. In New Zealand () H. richardsonii is confined to the North Island where it has an easterly distribution from Te Paki to Hicks Bay, including the Cavalli, Great Barrier (Aotea) and Mayor (Tuhua) islands (see de Lange et al. Citation2010).
Habitat: In Australia, recorded mainly in open sclerophyll forest of Eucalyptus (Corymbia) maculata, E. tereticornis, Banksia integrifolia or Melaleuca quinquenervia, or open woodland of Eucalyptus and Acacia; recorded substrates include sandy loam on sandstone or alluvials; recorded growing on slopes, in cleared or disturbed areas, or fringing saline mud-flats. In New Zealand, H. richardsonii is known from indigenous shrubland and sparsely vegetated slip scars associated with coastal rocky headlands, sand tombolo and boulder beaches. The only departure from this pattern is Kamo, Whangarei, where plants appeared following the removal of a house and the associated disturbance of an urban garden, and on Mayor Island (Tuhua) where plants have been found growing within a former pa site. In New Zealand Hibiscus richardsonii has on occasion been cultivated as the “white-flowered” or “unblotched” form of Hibiscus trionum, so these occurrences, in modified situations are not that surprising, and as the species is now being widely sold in specialist native plant nurseries we anticipate further such occurrences in the near future. In its indigenous habitat, H. richardsonii is commonly associated with Kunzea spp., Leptospermum scoparium s.l., Leucopogon fraseri, Asplenium flabellifolium, Cheilanthes distans, C. sieberi, Pellaea falcata, Pteridium esculentum, Arthropodium cirratum, Scandia rosifolia and Rytidosperma spp.
Conservation: In Australia (which uses the IUCN threat classification system; IUCN Citation2001) the category ‘Vulnerable’ seems appropriate for H. richardsonii because, although its overall range is large, nowhere is it common and in New South Wales it appears that none of the populations occurs within areas protected by dedicated conservation legislation. In New Zealand, which uses its own independent threat classification system (see Townsend et al. Citation2008) H. richardsonii is listed as ‘Threatened/Nationally Critical’ qualified EF (Extreme Fluctuations), Sp (Sparse), TO (Threatened Overseas) (see de Lange et al. Citation2009). A detailed conservation assessment of this species is provided by de Lange et al. (Citation2010).
Notes: Both Cunningham (Citation1840) and Raoul (Citation1846) recorded Hibiscus trionum (as Hibiscus vesicarius Cav.) from northern New Zealand. Cunningham (Citation1840) suggested that the species might not be indigenous and that it may have come from England (‘An planta vere ind igena? Forsan ex Anglia introduca’). We have not seen specimens supporting either record so cannot place what species Cunningham and Raoul had observed. Nevertheless, subsequent collections made by other botanists from near one of the locations where Cunningham had recorded H. vesicarius, i.e. from near a Maori village opposite the Cavalli Islands, comprise only H. richardsonii, suggesting that it was probably this species which Cunningham had observed.
This species has been treated within the concept of H. trionum var. trionum by Australian botanists, as demonstrated by herbarium annotations. It is unfortunate but understandable that Bentham (Citation1863), who presumably had limited materials with which to work (as he cited only six herbarium collections of Australian origin), adopted a broad circumscription of the H. trionum complex in Flora Australiensis, as his flora has been very influential until recent times. Nonetheless, H. richardsonii is so distinctive that it is surprising that Bentham's placement of the coastal New South Wales plant has not been questioned until now.
2. Hibiscus tridactylites Lindl. in Mitchell: Three expeditions into the interior of Eastern Australia 1: 85 (1838)
Lectotype (chosen here): Australia: New South Wales: between the Gwydir and Namoi Rivers, longitude c. 150°E, 1832, T.L. Mitchell s.n. (CGE photo seen).
Typification: No specimens are cited in the protologue but there is a specimen collected by Mitchell in the Lindley Herbarium at CGE. This is designated lectotype above.
Description (B, B,C): Herb 0.15–0.4(–1.3) m tall. Branchlet with fine stellate hairs 0.4–0.8 mm long and coarse stellate hairs 0.2–0.8 mm long, with sparse fine bristles 0.25–0.4 mm long. Stipules more or less persistent, 4–6 mm long. Mid-stem and distal leaves 3-lobed, lobing extended to the apex of the petiole, the primary lobes themselves strongly lobed, palmately veined; lamina of mid-stem leaves 20–90 mm long, 25–80 mm wide, in overall shape ovate, broadly ovate, or suborbicular, with stellate hairs and fine bristles, margin sparsely serrate, lobe apex rounded; petiole 20–50 mm long with indumentum similar to that of the branchlet; foliar nectary absent. Flowers solitary in leaf axils, chasmogamous, pedunculate; peduncle 15–60 mm long, with stellate hairs and with sparse fine bristles; pedicel 0.25–0.6 mm long, indumentum dissimilar to that of peduncle (the hairs slightly denser and longer). Epicalyx 10–13-segmented, 6.5–11 mm long, segments linear, free at the base, shorter than the calyx. Calyx at anthesis 11–14 mm long, distinctly accrescent in fruit, with stellate hairs and coarse bristles, without prominent marginal ribs; lobes triangular, acute at the apex; calyx nectary absent. Petal 22–30 mm long, yellow or cream with a large purplish basal petal spot. Staminal column straight. Staminal column at the apex 5-toothed. Staminal column 7–12 mm long with the stamens distributed along the distal 1.6–4 mm of the column; staminal filaments 2–3 mm long; anthers yellow; pollen yellow. Style (including style branches) exserted 2–3 mm beyond the apex of the staminal column; style branches 5, 0.7–0.9 mm long; stigmas capitate, 0.4–0.6 mm across, stigmatic hairs 0.2–0.3 mm long. Ovary hairy. Fruit capsulate. Capsule hairy, 12–16 mm long. Seed 2.3–2.5 mm long, subreniform, papillate–pubescent and smooth between the hair pustules. 2n = 56 (Murray et al. Citation2008, p. 317, source Narrabri; Dalby). .
Representative specimens: AUSTRALIA: WESTERN AUSTRALIA: G.J. Keighery 15653, 7 Feb 1999, Lake Claremont, Nedlands, CANB (Duplicate: PERTH); A.J. Egan s.n., 11 Jan 1950, Bedfordale, PERTH. SOUTH AUSTRALIA: R.J. Bates 3635, 20 Feb 1984, Wynne Vale, Adelaide, AD (Duplicate: PRE). QUEENSLAND: A.R. Bean 9737, 11 Feb 1996, 0.5 km SW of Bancroft, via Monto, CANB (Duplicate: BRI). NEW SOUTH WALES: C.W.E. Moore 3633, 18 Feb 1966, 40 km E of Hay, CANB; J.M. Dalby, W. Bishop & R.G. Coveny 88/24, 16 Apr 1988, 1.4 km S of the Deniliquin–Finley road, on the Aratula road heading towards Tocumwal, MEL, (Duplicate: NSW); R. Medd 160193, 27 Jan 1982, “Cavan”, 16 km W from Merriwa on the Dubbo road, NSW; M. Gray & P. Michael 6279, 24 Jan 1969, near Brungle, between Tumut and Gundagai, CANB (Duplicate: BRI,); J.R. Hosking 1424, 19 Feb 1997, grounds of Tangaratta Stockfeeds Pty Ltd, NW of Tamworth, CANB (Duplicates: MEL, NE, NSW, TARCH); R. Martin s.n., 21 Jan 1974, Kilgra, N of Kyogle, NSW; C.J. Dunn, J. Plat & R.G. Coveny 50, 27 Mar 1987, 9 km from Inverell on the Yetman road, AD (Duplicates: BRI, MEL, NSW); I.R. Telford 10760, 23 Dec 1988, “Invergowrie”, 15 km W of Armidale, CANB (Duplicate: BISH). VICTORIA: R.V. Smith 67/64, 10 Mar 1967, Birchip, MEL; J. Turner s.n., 27 Feb 1979, Lockington, c. 25 km SW of Echuca, MEL. CULTIVATED: L.A. Craven 10473, 14 Jan 2005, CSIRO, Canberra, [Provenance: seed from S. Johnson, Dalby, Queensland], CANB; CSIRO, Canberra, 14 Jan 2005, [Provenance: seed from S. Johnson, Narrabri, New South Wales], L.A. Craven 10478 (CANB); L.A. Craven 10639, Mar 2005, CSIRO, Canberra, [Provenance: seed from S. Johnson, Narrabri, New South Wales], CANB (Duplicates: A, L); L.A. Craven 10640, Mar 2005, CSIRO, Canberra, [Provenance: seed from S. Johnson, Dalby, Queensland], CANB (Duplicates: A, L); D.I. Morris 7869, 11 Dec 1978, Tasmania, New Town Research Laboratories, [Provenance: Sheffield Area School], HO.
Distribution (): Western Australia, South Australia, Queensland, New South Wales, Victoria, Tasmania: the southwest region of Western Australia, and from the Tropic of Capricorn upland region in Queensland southwards to Victoria and southeast South Australia, possibly naturalised in Tasmania.
Habitat: In Australia, commonly found in disturbed areas associated with agricultural activities (such as cotton, soybean, grain sorghum or other crops), on roadsides or in wastelands; usually associated with grasses (such as Cynodon dactylon, danthonia [Rytidosperma spp.] and needle-grasses [Austrostipa spp.]), or in disturbed open woodland with herbs (such as Portulaca oleracea); recorded as growing in black, brown or grey clay, red clay loam, or brown or grey sand; recorded landscapes are undulating country, often associated with road, river or irrigation channel embankments.
Conservation: Hibiscus tridactylites can be regarded as being of Least Concern according to the IUCN Red List categories and criteria (IUCN, Citation2001) as it is a widespread species.
Notes: The original range of H. tridactylites is presumed to be the Northwest Slopes region of New South Wales, but an exact understanding of the original distribution may never be achievable. The species now occurs widely in southern Australia, probably as a result of the movement of agricultural machinery, livestock and escape from cultivation, as this species is often cultivated (at least in the Canberra region of the Australian Capital Territory). The common name of this species in Australia is narrow leaf bladder ketmia.
3. Hibiscus verdcourtii Craven, sp. nov.
Diagnosis: A H. tridactylite Lindley lamina foliorum non lobata ad apice petiolo, et a H. richardsonii Sweet ex Lindley semine glabro et 2.5–2.7 8mm longo, epicalyce 12–14-segmentato, et ramis styli 0.6–1 mm longis differt.
Type: Australia. Northern Territory: c. 168 km SW of ‘Calvert Hills’ on road to ‘Creswell Downs’, Lat. 17°50′S, Long. 136°15′E, 16 May 1974, R. Pullen 9279 (CANB holo!, DNA iso n.v.).
Description (C, D,E): Herb 0.25–0.8(–1.8) m tall. Branchlet with fine stellate hairs 0.4–1.1 mm long, with very sparse coarse bristles 0.5–0.7 mm long, and with fine bristles 0.4–0.5 mm long. Stipules more or less persistent, 3–4 mm long. Mid-stem and distal leaves 3-lobed (shallowly or deeply), lobing not extended to the apex of the petiole, the primary lobes themselves scarcely lobed, palmately veined; lamina of mid-stem leaves 25–138 mm long, 15–94 mm wide, in overall shape ovate to broadly ovate or narrowly ovate, with fine stellate hairs and fine bristles, margin strongly serrate, lobe apex rounded or acute; petiole 10–55 mm long with the indumentum dissimilar to that of branchlet (not markedly, petioles with more bristles); foliar nectary absent. Flowers solitary in leaf axils, chasmogamous, pedunculate; peduncle 5–9 mm long, with fine stellate hairs and fine bristles; pedicel 4–20 mm long, indumentum generally similar to that of the peduncle but the hairs denser. Epicalyx 9–10-segmented, 7.5–12 mm long, segments linear to subulate, free at the base, shorter than the calyx. Calyx at anthesis 10–20 mm long, distinctly accrescent in fruit, with stellate hairs, without prominent marginal ribs; lobes triangular, acute or acuminate at the apex; calyx nectary absent. Petal 20–35 mm long, yellow, fading to white in older flowers, generally with basal petal spot present [pallid in southern populations (D) and strongly reddish in northern populations (E)]. Staminal column 7.5–10 mm long with the stamens distributed along the distal 3.5–5.5 mm of the column; staminal filaments 2.3–3 mm long; anthers yellow; pollen yellow. Style exserted 2–4 mm beyond the apex of the staminal column; style branches 5, 0.6–1 mm long; stigmas capitate, 0.6–0.8 mm across, stigmatic hairs 0.05–0.2 mm long. Ovary hairy. Fruit capsulate. Capsule hairy, 11–13 mm long. Seed 2.5–2.7 mm long, subreniform, glabrous, smooth. 2n = 56 (Murray et al. Citation2008, p. 317, source Emerald, St. George). .
Representative specimens: WESTERN AUSTRALIA: M. Lazarides 6294, 12 Jul 1959, 8 km NE of Lamboo Station, Kimberleys, CANB; R. Pullen 10748, 15 Apr 1977, between old Ord River Station and the Water Recording Station, Ord River, CANB (Duplicate: PERTH, WIR); J.N. Hutchinson 23, 14 Jun 1968, Nicholson Station, E of Halls Creek and just W of the Northern Territory border, PERTH. NORTHERN TERRITORY: K. Brennan 1837, 14 Feb 1992, Gimbat, at SE end of Fisher Airstrip, DNA; L.A. Craven, J. Grace & G. Second 8523, 12 May 1987, Borroloola Racecourse swamp, CANB; A.O. Nicholls 755, 30 Mar 1968, 1.6 km N of Bore 37, Brunette Downs, DNA; R.A. Perry 1505, 23 Jun 1948, 48 km E of Alexandria Station, on bank of Bore tank 14, CANB. SOUTH AUSTRALIA: F.J. Badman 679, 3 Mar 1984, 6 km SW of Beresford, Yard waterhole, AD. QUEENSLAND: P.L. Harris 153, 15 Mar 1987, 18 km NW of Mount Isa, BRI; L.A. Craven & J.McD. Stewart 9781, 7 Jun 1997, 32 km S of the Lake Nash turnoff on the Camooweal-Urandangi road, CANB (Duplicates: A, AK, BRI, L, P); L.G. Adams 1184, 24 Jul 1964, 24 km NW of Moray Downs Station, South Kennedy District, CANB (Duplicates: BRI, K); I.G. Champion, A.B. Pollock & G. Morgan 1035, 29 Mar 1994, Homevale Station adjacent to fenceline track at watercourse crossing 3.7 km due E of Mt Robert Station, AD (Duplicate: BRI). NEW SOUTH WALES:, C.W.E. Moore 6252, 9 Mar 1973, “Westmere”, c. 12 km NW of Louth CANB; L.A. Craven & G. Whitbread 7445, 5 May 1982, 32 km from Brewarrina on the Goodooga road, CANB. CULTIVATED: L.A. Craven 10474, 14 Jan 2005, CSIRO, Canberra, [Provenance: seed from S. Johnson, Emerald, Queensland], CANB; L.A. Craven 10477, 14 Jan 2005, CSIRO, Canberra, [Provenance: seed from S. Johnson, St. George, Queensland], CANB; L.A. Craven 10637, Mar 2005, CSIRO, Canberra, [Provenance: seed from S. Johnson, St. George, Queensland], CANB (Duplicate: L); CSIRO, Canberra, [Provenance: seed from S. Johnson, Emerald, Queensland], L.A. Craven 10638, Mar 2005, A (Duplicate: CANB).
Distribution (): Western Australia, South Australia, Queensland, New South Wales: the Pilbara and Kimberley regions of Western Australia and from there eastwards to Queensland and southeastwards to northern South Australia, extending southwards to the Riverina region of New South Wales. A weed of cultivation, especially cotton crops.
Habitat: In Australia, H. verdcourtii is often found in open woodland or forest (of Eucalyptus and/or Acacia), in grassland (of Triodia, Dichanthium, Astrebla, Eriachne, etc.) or wasteland and disturbed areas (with weeds such as Xanthium); recorded substrates are grey, red, brown or black cracking clay, black basalt or brown loams; landscapes include floodplains, lake beds, drainage or irrigated areas, flat or low relief gilgai plains and margins of cultivated fields.
Conservation: Hibiscus verdcourtii can be regarded as of Least Concern according to the IUCN Red List categories and criteria (IUCN, Citation2001) as it is a widespread species.
Notes: This species is common and widespread across northern Australia but apparently it is extending its range southwards in eastern Australia; presumably this expansion has been facilitated by agricultural activities. The common name of this species in Australia is wide leaf bladder ketmia.
Etymology: The epithet honours Bernard Verdcourt (1925-) whose prolific published research (Verdcourt, Citation1997, Citation2002) includes contributions to the account of Malvaceae for Flora of Tropical East Africa (Verdcourt & Mwachala Citation2009).
4. Hibiscus trionum “diploid New Zealand naturalised race”
Description (F): Herb 0.2–1.8 m tall. Branchlet covered in fine stellate (rarely bifid) hairs 0.2–0.9 mm long and coarser stellate (rarely bifid) hairs 0.8–1.5 mm long, with sparse coarse bristles 0.3–1.0 mm long, and with sparse fine bristles 0.2–0.8 mm long. Stipules more or less persistent, 4–9 mm long. Mid-stem and distal leaves 3–(5–7)-lobed, lobing not extended to the apex of the petiole, the primary lobes either deeply lobed or scarcely so, palmately veined; lamina of mid-stem leaves 16–70 mm long, 18–58 mm wide, in overall shape ovate, or broadly ovate, adaxial and abaxial surfaces sparsely covered with fine and coarse stellate and bifid hairs, margin mostly strongly serrate, sometimes crenate usually sparsely hairy (hairs simple), lobe apex acute or obtuse; petiole 3–24 mm long with indumentum similar to that of the branchlet; foliar nectary absent. Flowers solitary in leaf axils, chasmogamous, pedunculate; peduncle 8–30 mm long, with fine and coarse stellate hairs, rarely also with fine bristles; pedicel 4–6 mm long, indumentum similar to that of peduncle. Epicalyx 12–16-segmented, 10–14 mm long, segments linear–lanceolate, margins serrated, free at the base, shorter than the calyx to equally as long as the calyx. Calyx at anthesis 13–18 mm long, distinctly accrescent in fruit, with stellate hairs, fine and coarse bristles, and sparse glandular hairs, without prominent marginal ribs; lobes broadly triangular, acute at the apex; calyx nectary absent. Petal 36–48 mm long, 18–26 mm wide, pale lemon–yellow to yellow with a prominent maroon–black basal petal spot, 9–30 mm long. Staminal column 6–15 mm long with the stamens distributed along the distal 4–8 mm of the column; staminal filaments 1.0–2.2 mm long maroon–black; anthers dark yellow to pale orange; pollen yellow to orange–yellow (when dry). Style (including style branches) exserted 3.8–9.4 mm beyond the apex of the staminal column, maroon–black; style branches 5, 2.5–5.2 mm long; stigmas maroon–black, capitate, 0.9–1.3 mm across, stigmatic hairs 0.1–0.2 mm long. Ovary hairy. Fruit capsulate. Capsule hairy, 7.8–10.9 mm long. Seed 2.3–2.6 mm long, subreniform, finely papillate–pubescent and smooth between the hair pustules. 2n = 28 (de Lange & Murray Citation2002, p. 4; Murray et al. Citation2008, p. 317, source for both counts Bream Head).
Representative specimens: NEW ZEALAND: NORTH ISLAND: NORTH AUCKLAND: H. Carse s.n., Nov 1897, Rangaunu Heads, AK 102922; D. Petrie, May 1897, Ahipara Bay, WELT SP028214; H. Carse s.n., 18 Dec 1918, Ahipara, CHR 296410; L. J. Forester s.n., 12 Dec 1995, Tutukaka, AK 228985; T. Kirk s.n., Whangarei, WELT SP028217 (Duplicate: AK 11395); D.V.G. Woods s.n., Whangarei, Parua Bay, AK 121687; W.R. Sykes 237/72, 12 Feb 1972, Whangarei Heads, Aubrey Hill, CHR 228767; W. Parr s.n., 4 Dec 2000, Whangarei Heads, Bream Head Scenic Reserve, AK 253689; B. Waller s.n., 7 Feb 2002, Kaipara, South Head, AK 255759; H.H. Allan s.n., 27 Mar 1935, Piha, “G. Stream”, CHR 17740–17741; P.J. de Lange 6678 & T. J. de Lange, 24 Jun 2006, Auckland City, Western Springs, Motions Road, AK 297111; I. Chapman s.n., 23 Apr 1970, Waiuku, Waiuku Primary School Grounds, AK 122931. HAURAKI GULF ISLANDS: E.K. Cameron 1035, 2 Jan 1982, Hen and Chicken Islands, Lady Alice Island, AK 270087; P.J. de Lange 6798, 11 Mar 2006, Waiheke Island, Matiatia Bay, AK 297935; T. Kirk s.n., Dec 1867, Great Barrier Island (Aotea), Whangapoua, WELT SP028221. SOUTH ISLAND: CANTERBURY: D. Petrie s.n. & J.B. Armstrong, Canterbury Plains WELT SP028221.
Distribution (): New Zealand, North and South Islands. This race is more common and better established in the northern North Island (from Auckland City north).
Habitat: Mostly known from urban areas where it grows in wasteland, roadside verges and as a garden escape. Plants have sometimes been collected from less modified ‘indigenous’ habitats where they give the impression of being ‘native’. However, even at these sites an appraisal of the local history highlights the past usage of these ‘wild’ areas as picnic spots and former holiday beaches and camping grounds and at all sites there are other garden escapes and cultigens present as persistent relics. Over the last 30 years this pattern has gradually been disrupted through increased cultivation of this race as “native hibiscus” sometimes in an understandable but misguided conservation measure resulting from publications that have treated this race as indigenous (Eagle Citation1975, Citation2006; Metcalfe Citation1993; Brunsden & Brunsden Citation2004) and/or threatened (Given Citation1981). Even now, plants of this race can be found for sale in New Zealand garden centres and specialist nurseries as ‘native hibiscus’. As a result, further naturalisations of this race are likely, including those that stem from misguided translocations to wild and/or secure reserves, e.g. Tiritiri Matangi Island Scientific Reserve (see AK 299054) as part of well-intended but unnecessary conservation measures.
Notes: As with de Lange & Murray (Citation2002) and Murray et al. (Citation2008) we have assumed that despite the cytological evidence, this diploid race of H. trionum is naturalised to New Zealand. As noted in the Introduction, it is beyond the scope of this article to determine from where this race has reached New Zealand and where it is truly indigenous. Only a critical, worldwide revision will be able to resolve the exact status of this race and its relationship to H. trionum s.s. In New Zealand, such a situation is not that unusual, for example, Geranium gardneri (de Lange et al. Citation2005) was first informally recognised and described from New Zealand as Geranium solanderi “coarse hairs” by Gardner (Citation1984). Subsequently it was found to be an indigenous Australian plant which had naturalised itself to New Zealand and Norfolk Island (de Lange et al. Citation2005).
As noted under H. richardsonii, we have not seen specimens supporting the earlier Cunningham (Citation1840) and Raoul (Citation1846) records of bladder ketmia from New Zealand. Nor have we seen those Colenso records that Hooker (Citation1864) mentioned. Interestingly, Hooker described a plant whose flowers were ‘yellow with a purple eye’ which does not match H. richardsonii fitting better the “diploid New Zealand naturalised race” described here. However, it is not clear if Hooker based his description on New Zealand specimens or used a general description for the species as it was known to him (his description implies the later). Therefore, until the collections on which Hooker based his comments come to light we can only work with herbarium specimens available to us.
It would seem that the “diploid New Zealand naturalised race” was first collected in New Zealand by Thomas Kirk in 1867 from Great Barrier Island (Aotea Island) where it has not since been seen in the wild. Based on herbarium evidence, it was then collected by Kirk again, probably in 1868, from Whangarei Harbour. In the mid 1800s Whangarei Harbour was already an important port in the northern North Island and was an early point of naturalisation for a number of plant species (see Webb et al. Citation1988; Heenan & de Lange Citation2004). We therefore suspect that this diploid race of H. trionum may have been one of these. Whangarei is also notable as the only place in New Zealand where the diploid race has become thoroughly naturalised, even establishing into primarily indigenous habitats including on some adjacent offshore islands. In these areas, including Lady Alice Island (see especially, Cameron Citation1984, Citation1991), it gives the impression of being ‘indigenous’. Nevertheless, all of these ‘wild’ occurrences occur in sites that were once modified by pioneering Europeans who were known to have planted a range of garden ornamentals in such sites presumably to ‘civilise’ and enhance the wild forest of their favoured picnic sites (Cameron Citation1991; R. Brassey pers. comm. 2010).
Acknowledgements
We thank Alex Floyd, John Knight and Jennifer Liney for their efforts in locating populations of H. richardsonii and for obtaining materials for our study. David Mallinson assisted us in our search for this species in southern New South Wales. Alex George and Juliet Wege were extremely helpful in obtaining images of types and possible types deposited in British herbaria. Colleen Keena provided information on Bladder Ketmias cultivated in Australia. Nunz Knerr and Jeremy Rolfe kindly prepared the distribution maps. We thank Peter Heenan and Robert Brassey for their comments on an earlier draft of this article. The Directors and Curators of the following herbaria are thanked for the opportunity of studying collections in their care: AD, AK, CHR, BRI, CANB, DNA, MEL, NSW, PERTH, WAIK, WELT.
References
- Allan HH 1961 . Flora of New Zealand 1 . Wellington , Government Printer .
- Bailey , FM . 1899 . The Queensland flora Part 1 , Brisbane : H. J. Diddams .
- Bentham G 1863 . Hibiscus trionum. Flora Australiensis 1 . London : Reeve . P. 210 .
- Blackall , WE and Grieve , BJ . 1956 . How to know Western Australian wildflowers. Part 2 , Perth : University of Western Australia Press .
- Brunsden , G and Brunsden , L . 2004 . A photographic guide to wildflowers of New Zealand , Auckland : New Holland .
- Cameron , EK . 1984 . Vascular plants of the three largest Chickens (Marotere) Islands: Lady Alice, Whatupuke, Coppermine: north-east New Zealand . Tane , 30 : 53 – 75 .
- Cameron , EK . 1991 . Additional vascular plant records for the Chickens Islands, north-east New Zealand . Tane , 33 : 87 – 88 .
- Cheeseman , TF . 1906 . Manual of the New Zealand flora , 1st edition , Wellington : Government Printer .
- Cheeseman , TF . 1925 . Manual of the New Zealand flora , 2nd edition , Wellington : Government Printer .
- Cunningham A 1840 . Florae insularum Novae Zelandiae precursor. IV . Annals of Natural History 22 – 26 .
- Cunningham , GM , Mulham , WE , Milthorpe , PL and Leigh , JH . 1981 . Bladder ketmia. Plants of western New South Wales , Sydney, NSW : Government Printing Office .
- de Lange , PJ . 2008 . Hibiscus richardsonii – the rediscovery of a native hibiscus in New Zealand . New Zealand Botanical Society Newsletter , 93 : 13 – 15 .
- de Lange , PJ , Gardner , RO , Sykes , WR , Crowcroft , GM , Cameron , EK , Stalker , F , Christian , ML and Braggins , JE . 2005 . Vascular flora of Norfolk Island: some additions and taxonomic notes . New Zealand Journal of Botany , 43 : 563 – 596 .
- de Lange , PJ , Heenan , PB , Given , DR , Norton , DA , Ogle , CC , Johnson , PN and Cameron , EK . 1999 . Threatened and uncommon plants of New Zealand . New Zealand Journal of Botany , 37 : 603 – 628 .
- de Lange , PJ , Heenan , PB , Norton , DA , Rolfe , JR and Sawyer , JWD . 2010 . Threatened plants of New Zealand , Christchurch : Canterbury University Press .
- de Lange , PJ and Murray , BG . 2002 . Contributions to a chromosome atlas of the New Zealand floras – 37. Miscellaneous families . New Zealand Journal of Botany , 40 : 1 – 23 .
- de Lange , PJ , Norton , DA , Courtney , SP , Heenan , PB , Barkla , JW , Cameron , EK , Hitchmough , R and Townsend , AJ . 2009 . Threatened and uncommon plants of New Zealand (1998 revision) . New Zealand Journal of Botany , 47 : 61 – 96 .
- Don , G . 1831 . Hibiscus trionoides . A General History of Dichlamydeous Plants , 1 : 483
- Eagle , AL . 1975 . Eagle's trees and shrubs of New Zealand in colour , Auckland : Collins .
- Eagle , AL . 2006 . Eagle's complete trees and shrubs of New Zealand , Wellington : Te Papa Press .
- Gardner , RO . 1984 . Geranium solanderi and allies in New Zealand . New Zealand Journal of Botany , 22 : 127 – 134 .
- Given , DR . 1981 . Rare and endangered plants of New Zealand , Auckland : Reed .
- Heenan , PB and de Lange , PJ . 2004 . Alternanthera denticulata (Amaranthaceae) in New Zealand: a new addition to the indigenous or naturalised flora? . New Zealand Journal of Botany , 42 : 739 – 745 .
- Hooker , JD . 1864 . Handbook of the New Zealand flora. Part I , London : Reeve .
- IUCN 2001 . IUCN Red List categories and criteria, version 3.1 . Prepared by the IUCN Species Survival Commission, Gland, Switzerland, and Cambridge, United Kingdom .
- Jessop , JP . 1986 . “ Hibiscus L ” . In Flora of South Australia 2 , 4th edition , Edited by: Jessop , JP and Toelken , HR . 829 – 831 . Adelaide : South Australian Government Printing Division .
- Johnson S , MacKinnon L 2002 . Plant protection interactions with weeds. Section F5. 1–5.2 . In: Johnson SB , Charles GW , Roberts GN , Taylor IN WEEDpak, a guide for integrated management of weeds in cotton . Narrabri : Australian Cotton Cooperative Research Centre . http://www.cottoncrc.org.au/content/Industry/Publications/Weeds/WEEDpak/F1FarmHygiene.aspx [accessed 11 August 2010] .
- Kirk , T . 1899 . The students’ flora of New Zealand and the outlying islands , Wellington : Government Printer .
- Kochman , JK . 1995 . Fusarium wilt in cotton – a new record in Australia . Australasian Plant Pathology , 24 : 74
- Lindley , J . 1825 . Hibiscus richardsoni . Edward's Botanical Register , 11 : t. 875
- Metcalfe , L . 1993 . The cultivation of New Zealand plants , Auckland : Godwit Press .
- Mitchell , AS . 1981 . “ Hibiscus L ” . In Flora of central Australia , Edited by: Jessop , JP . 208 – 210 . Sydney : Reed .
- Murray , BG , Craven , LA and de Lange , PJ . 2008 . New observations on chromosome number variation in Hibiscus trionum s.l. (Malvaceae) and their implications for systematics and conservation . New Zealand Journal of Botany , 46 : 315 – 319 .
- Ramsey , M , Seed , L and Vaughton , G . 2003 . Delayed selfing and low levels of inbreeding depression in Hibiscus trionum (Malvaceae) . Australian Journal of Botany , 51 : 275 – 281 .
- Raoul , E . 1846 . Choix de Plantes de la Nouvelle-Zélande , Paris : Fortin Masson et Cie .
- Seed , L , Vaughton , G and Ramsey , M . 2006 . Delayed autonomous selfing and inbreeding depression in the Australian annual Hibiscus trionum var. vesicarius (Malvaceae) . Australian Journal of Botany , 54 : 27 – 34 .
- Swan , L and Salmond , G . 2004 . Options for managing Fusarium wilt with crop rotations . Australian Cottongrower , 26 : 8 – 10 .
- Townsend , AJ , de Lange , PJ , Norton , DA , Molloy , J , Miskelly , C and Duffy , C. 2008 . The New Zealand Threat Classification System manual , Wellington : Department of Conservation .
- Verdcourt , B . 1997 . A list of publications and new taxa described (1936–1996) , Maidenhead, , UK : Author .
- Verdcourt , B . 2002 . A list of publications and new taxa described, Supplement 1 (1997–2001) , Maidenhead, , UK : Author .
- Verdcourt , B and Mwachala , GM . 2009 . “ Malvaceae ” . In Flora of tropical East Africa , Edited by: Beentje , HJ and Ghazanfar , SA . 1 – 170 . London : Royal Botanic Gardens .
- Walsh , NG . 1996 . “ Hibiscus ” . In Flora of Victoria. Volume 3 , Edited by: Walsh , NG and Entwistle , TJ . 332 – 333 . Melbourne : Inkata Press .
- Webb CJ , Sykes WR , Garnock-Jones PJ 1988 . Flora of New Zealand IV . Christchurch : Botany Division, DSIR .