Abstract
Cololejeunea grossepapillosa (Horik.) N. Kitag. is newly recorded for New Zealand. This is the first Australasian record for this widespread species, previously known from southern and eastern Africa, and from China to Papua New Guinea. New Zealand represents the southern and eastern limits for Cololejeunea grossepapillosa, which is one of the few known species of Lejeuneaceae disjunct between tropical Africa and Asia whose distribution extends further to Australasia. Cololejeunea grossepapillosa is readily recognized by its athecal branching, two-celled first lobule tooth, prominent dome-shaped papillae on the leaf-lobe and keel, and production of 16-celled gemmae from the keel and ventral lobule surface. Cololejeunea grossepapillosa brings the number of Cololejeunea species recorded from New Zealand to 13, and highlights the contribution of widespread tropical species to the diversity of New Zealand's Lejeuneaceae flora.
Introduction
Twelve species of Cololejeunea (Spruce) Schiffn. were previously known for New Zealand, three of which [C. appressa (A. Evans) Benedix, C. cardiocarpa (Mont.) R.M.Schust. and C. floccosa (Lehm. et Lindenb.) Steph.] are widespread in Indomalaysian and Oceanic tropical regions. Within the leafy liverworts Cololejeunea is characterized by the incubously inserted, conduplicately bilobed leaves; the constricted lobule base with the lobule fused to the stem by one to three cells; the pendulous sequence of cleavage from the apical meristematic cell; and the absence of underleaves. The absence of underleaves is a highly conspicuous character, making Cololejeunea one of the most recognizable genera within the Lejeuneaceae. Cololejeunea is also unusual in having only a single row of stem medulla cells. Cololejeunea is among the most species-rich liverwort genera, and exhibits substantial morphological diversity. Aphanolejeunea A. Evans was split from Cololejeunea by Evans (Citation1911) on the basis of its small size, dimorphic leaves and thin leaf lobe cell walls, to which Schuster (Citation1955) added the formation of intramarginal gemmae, and Thiers (Citation1982) the production of athecal branches as further differences between the two genera (Pócs & Bernecker Citation2009).
Bryophytes collected as part of New Zealand's Land Use and Carbon Analysis Survey (LUCAS) included two specimens containing tiny plants growing in an unusual habitat, the inner surface of concave leaves of large mosses such as Weymouthia cochlearifolia (Schwaegr.) Dix. and Lembophyllum divulsum (Hook.f. et Wils.) Lindb. These tiny plants exhibited a number of the highly distinctive features of species previously placed in Aphanolejeunea (now synonymized with Cololejeunea) and Cololejeunea subg. Cololejeunea, in that: 1. the branches lacked a basal collar, 2. the leaves were dimorphic, 3. the leaf lobes were subisolobous, 4. the leaf lobes were prominently mamillose, 5. the plants were very small, 6. the first lobule tooth was two-celled, 7. the lobule papilla was situated on the lobule margin, and 8. the plants produced 16-celled discoidal gemmae. Although this unique combination of characters matches no species known for New Zealand or Australia (Thiers Citation1988; Pócs & Streimann Citation1999, Citation2006; McCarthy Citation2003), Cololejeunea grossepapillosa (Horik.) N.Kitag., originally described on the basis of material collected in Taiwan, agrees in all respects. However, because the quality of Horikawa's description and illustration was poor, the species remained poorly known until reported (as Aphanolejeunea grossepapillosa Horik.) new to Vietnam (Pócs Citation1969), to Papua New Guinea by Pócs et al. (Citation1994) and Pócs & Piippo (Citation1999), to Sulawesi by Eggers et al. (Citation1998), to southern China (Fujian, Yunnan and Xizang) by Zhu & So (Citation2001), and to Thailand under the name of Cololejeunea ranongensis Tixier, by Pócs & Bernecker (Citation2009). The last authors also synonymized Cololejeunea capensis S.Arnell with Cololejeunea grossepapillosa, and extending the range of this species from Southeast Asia and Papua New Guinea west to southern and eastern Africa (Pócs & Bernecker Citation2009).
A recent molecular phylogeny resolved Aphanolejeunea nested within Cololejeunea (Wilson et al. Citation2007), such that maintenance of the genus without considerable splitting of Cololejeunea was untenable. Aphanolejeunea was formally synonymized with Cololejeunea by Pócs and Bernecker (Citation2009), who listed and proposed combinations in Cololejeunea for all species of Aphanolejeunea including Aphanolejeunea grossepapillosa. Unfortunately, Pócs and Bernecker (Citation2009) overlooked a previously existing combination for this species: Cololejeunea grossepapillosa (Horik.) N.Kitag., Hikobia, Suppl. 1: 68. 1981, which is the valid combination. Here we extend the range of C. grossepapillosa to New Zealand on the basis of two collections made in the Rotoroa Ecological District of the South Island. We include a description and illustration based on the New Zealand plants.
Cololejeunea grossepapillosa (Horik.) N.Kitag. Hikobia, Suppl. 1: 68. 1981.
≡ Aphanolejeunea grossepapillosa Horik. J. Sci. Hiroshima Univ. Ser. B, Div. 2, 1: 92. 1932.
Type: Taiwan, Tanan, Mt. Arisan, Numaro-daira, 1928, Noguchi (holotype: HIRO!).
= Aphanolejeunea capensis (S.Arnell) S.Arnell Hep. S. Afr.: 172. 1963.
= Cololejeunea capensis S.Arnell Bot. Not. Lund 106: 163. 1953.
Type: South Africa, Western Cape Province: Knysna, Deepwall Forest, Arnell 1790 (holotype: BOL).
= Cololejeunea ranongensis Tixier Nat. Hist. Bull. Siam Soc. 3: 4–5: 556. 1970.
Type: Thailand, Ranong, Namphy Ron, Tixier 1970. (HOLOTYPE: PC).
Description (): Plants tiny, shoots to 250 µm wide and 3 mm long, forming diffuse bright green patches on the leaves and stems of large epiphytic mosses. Stems 20–25 µm diameter, with five to six cortical and one medulla cell row, all walls unthickened except for weak triangular trigones composed of primary wall at cell junctions, medulla cell three times as long as cortical cells. Branching sporadic, athecal. Leaves remote, dimorphic, with normal and reduced leaves intermixed without transitional forms, full-sized leaves with lobes ovate–rotund, 110–130 µm long by 95–120 µm wide along axis perpendicular to length, apex rounded, rarely obtuse, subisolobous, lobules on full sized leaves 0.8× lobe area carinal region broadly inflated, with concave lobes, leaves appearing more or less spherical. Lobe cells prominently papillose, papillae 10–16 µm high, cells isodiametric, 9–15 µm diameter, cell walls unthickened. Ocelli and vitta absent. Lobule first tooth moniliform, of two elongated cells, the lower cell fused with adjacent proximal lobule cell for 0.66 its interior length, lobule papilla fused to lobule margin at junction between basal cell of first tooth and adjacent cell. Lobule second tooth unicellular, formed by an inflated, elongate cell orientated almost perpendicular to the first lobule tooth, cells of the lobule antical margin also elongate, to 4× long as wide. Carinal region cells smooth, keel cells papillose as for lobe cells. Lobule fused to stem via a single, inflated cell. Explanate lobules on reduced leaves rectangular, plane, without obvious teeth. Oil-bodies not known. Asexual reproduction by discoidal gemmae, comprised of 16 cells, with entire margins and no adhesive cells, produced haphazardly from lobe, keel and ventral cells of lobule. Initial phase of gemma development characterized by marked swelling of progenitor cell. Progenitor cell then either produces gemma, or alternatively a shoot primordium that gives rise to a vegetative shoot, whose origin is from the lobule or lobe surface. Sexuality, sexual reproductive structures and sporophyte unknown from New Zealand material.
Specimens seen: NEW ZEALAND, SOUTH ISLAND: Rotoroa Ecological District, Spencer Ecological Region, Buller River catchment, Upper reaches of Maggie Creek, 41°48.554′S 172°44.154′E, 765 m, M. Todd LUCAS-353, 13 Oct 2009, AK 314641; Springs Junction, Upper Grey River catchment, Mary Maruia Saddle, 42°22.927′S 172°9.031′E, 510 m, M. Todd LUCAS-613, 29 Oct 2009, AK 314638.
Recognition: Cololejeunea grossepapillosa is a small yet distinctive species possessing a combination of characters unique among Australasian Cololejeunea: 1. the prominently papillose leaf and keel surfaces; 2. the contrasting smooth lobule surface; 3. the subisolobous leaves; 4. the two-celled first lobule tooth; 5. the unicellular second lobule tooth orientated perpendicular to the first tooth; 6. the athecal branching; 7. the production of 16-celled discoidal gemmae from keel and ventral lobule surfaces; 8. the remote leaves; 9. the tiny size, shoots typically < 250 µm width. In the combination of small size, prominently papillose leaf lobe cells and absence of a vitta, C. grossepapillosa is most likely to be confused with C. hodgsoniae (Herzog) E.A.Hodgs. and C. mamillata (Angstr.) R.M.Schust., the New Zealand species of Cololejeunea subg. Cololejeunea. These species can be distinguished on the basis of the following key, whose characters are also summarized in . Species with papillose leaf lobes described by Schuster, including the putatively similar C. ellipsoidea R.M.Schust., and C. falcidentata R.M.Schust., have not been considered as no known material (including types) was available for study.
Figure 1 Cololejeunea grossepapillosa. A, Ventral view of leaf. B, Dorsal view of leaf. C, Athecal vegetative branch. D, Stem sections. E, Ventral view of shoot. F, Ventral view of leaf showing gemma initial cells. G, Gemmae. H, Gemmaling. I, Dorsal view of leaf showing gemma initial cells, papillae omitted. Scale bar A–D, F–I: 100 µm; E: 250 µm.
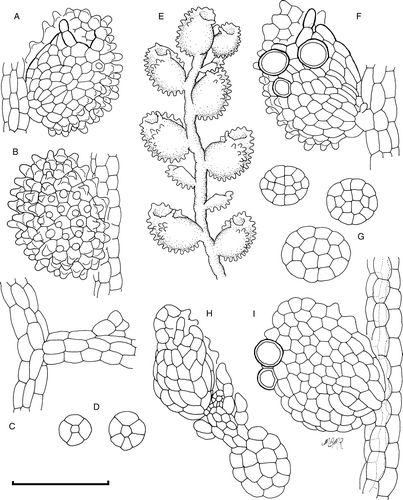
Table 1 Characters comparing Cololejeunea grossepapillosa with known New Zealand species with which it could be confused.
Key to New Zealand species of Cololejeunea with papillose leaf lobes*
1. Leaves with vitta ……… 2
1. Leaves without vitta. ……… 32. First lobule tooth of two or three cells, strongly falcate, apex often extending backwards beyond the basiscopic lobe margin, second lobule tooth indistinct … Cololejeunea floccosa.
2. First lobule unicellular or of two cells, straight, second lobule tooth distinct, the two teeth forming an obvious notch within which the lobule papilla is situated …. Cololejeunea appressa.
3. Vegetative branching athecal. Leaves subisolobous, lobules four fifths the size of the lobe, first lobule tooth two-celled, lobule papilla attached to lobule margin between first and second teeth. Gemmae production copious, 16-celled …… Cololejeunea grossepapillosa
3. Vegetative branching thecal. Leaves anisolobous, lobules half or less the size of the lobe, first lobule tooth unicellular or of two cells, lobule papilla attached inner surface of lobule behind first tooth. Gemmae present or absent .…… 4
4. Lobule without prominent lobule teeth, first lobule tooth obscure, lobule margins serrate due to papillose marginal cells, lobule papillose over entire surface, including keel and surface of carinal region …… Cololejeunea hodgsoniae.
4. Lobule with first lobule tooth prominent, two-celled, lobule margins entire, lobule papillose on keel only, surface of carinal region smooth …… Cololejeunea mamillata
*Cololejeunea ellipsoidea and C. falcidentata, species with papillose leaf lobes described by Schuster, are not included within this key.
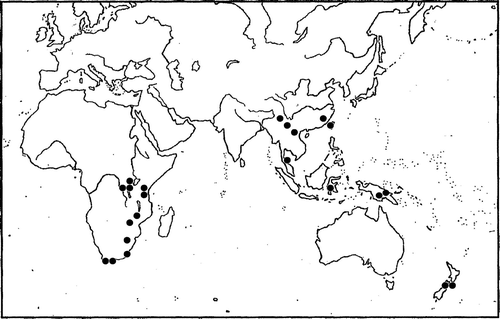
Distribution: Cololejeunea grossepapillosa is a widespread species, now known to occur in southern and eastern Africa, including Burundi, Rwanda, Zaire, Ethiopia, Tanzania, Uganda, Malawi, Zimbabwe, Sulawesi and South Africa (Eggers et al. Citation1998; Wigginton & Grolle Citation1996); and Indo-China, including Vietnam (Pócs Citation1969), southern China (Zhu & So Citation2001), Malaysia and Papua New Guinea (Pócs et al. Citation1994); and in New Zealand (). New Zealand represents the southern and eastern limits for this species. In New Zealand, Cololejeunea grossepapillosa is known from two locations between 500 and 800 masl within the Nelson Lakes region of the northern South Island. Both sites are within the Rotoroa Ecological District, and are separated by a distance of c. 140 km.
Ecology: Both sites where C. grossepapillosa occurs are in beech forest, one dominated by red beech alone, the other by red and silver beech. Cololejeunea grossepapillosa was most abundant on inner leaf surfaces of Weymouthia cochlearifolia and Lembophyllum divulsum, themselves epiphytes on beech trees. Cololejeunea grossepapillosa also grew on the leaves of other several other mosses, including Glyphothecium sciuroides (Hook.) Hampe, and as scattered shoots on bark. On moss leaves C. grossepapillosa grew alone, but on bark grew among Chiloscyphus sp., Frullania sp., Zygodon intermedius B.S.G., Plagiochila spp., Lejeunea primordialis (Hook.f. et Taylor) Taylor, Cheilolejeunea sp., Calyptopogon mnioides (Schwaegr.) Broth. and Glyphothecium sciuroides.
Conservation status: The apparent scarcity of this plant in New Zealand may have more to do with its size, occupancy of an unusual microsite, and occurrence in isolated forests on rugged terrain of the Southern Alps, than genuine scarcity. Other highly distinctive species with similar ecological preferences, for example, Austrolejeunea papillosa (Glenny) Pócs (Glenny Citation1996), had been overlooked, yet once formally recognized have proven widespread. The species associated with C. grossepapillosa are common in cool–temperate forest on the wetter western side of the South Island, and C. grossepapillosa may likewise prove to be widespread in this habitat.
Figure 2 Worldwide distribution of Cololejeunea grossepapillosa, showing disjunction between eastern and southern Africa, Asia and New Zealand. Black dots represent the approximate location of known sightings and herbarium records. The two South Island records are reported here.
Nevertheless, until such time as dedicated surveys for this species have been conducted it seems appropriate to regard this species as ‘Data Deficient’ sensu Townsend et al. (Citation2008). This assessment should be qualified ‘SO’ (Secure Overseas) as the species is known to be widely distributed throughout eastern and southern Africa, and appears widespread through southern China, Malaysia and Papua New Guinea.
Variation: Cololejeunea grossepapillosa described from China by Zhu and So (Citation2001), as Aphanolejeunea grossepapillosa, differs in several details from New Zealand plants, ncluding ovate-oblong leaf lobes. The New Zealand specimens of Cololejeunea grossepapillosa are quite typical and very similar to the South African specimens described by S. Arnell under the name of Cololejeunea capensis, in having very high round papillae on the leaf surface. Although they differ in their more rotund leaf shape, variation in leaf shape exists in both African (see Pócs Citation1984: 250, figs 32Fig. 38) and Asian populations (Horikawa Citation1932: 92, fig. 16 and Pócs & Piippo Citation1999: 90, fig. 3), and ovate to rotund leaf lobes may even occur within one specimen, including the type. The height of leaf papillae and the length of the lobule tooth (one or two cells) are also variable, independent of the geographical origin of the specimen.
Leaf margins of Asian specimens vary from crenulated to papillose, and are rarely subentire (Zhu & So Citation2001). The leaf margins of New Zealand specimens are consistently papillose. Although the gemmae of Asian specimens are also 16-celled, they have three adhesive cells, and often crenulate margins, neither of which have yet been observed in New Zealand material.
Table 2 Properties influencing dispersability for New Zealand Cololejeunea.
Discussion
Cololejeunea grossepapillosa brings the number of Cololejeunea species reported for New Zealand to 13, based on Glenny (Citation1998) with recent additions by Renner & Braggins (Citation2006) and Renner & Glenny (Citation2009). Seven of these species are either endemic, or confined to southern temperate Australasia. The remaining six species are widespread overseas, and four of these have been discovered in New Zealand within the past 20 years. New Zealand is recognized for having both high numbers of species and high species richness in its liverwort flora (von Konrat et al. Citation2008). However, New Zealand is unusual among regions with high species richness in having a low percentage of Lejeuneaceae in its overall liverwort flora. Two explanations for this feature of New Zealand's flora were suggested by von Konrat et al. (2008), either New Zealand has a genuine lack of Lejeuneaceae or the family within New Zealand is understudied.
The Cololejeunea flora of Australia has increased over the past 20 years from 17 species (Thiers Citation1988) to 36 (Renner, unpublished data). Like New Zealand, this has also been by the addition of widespread extraterritorial species, suggesting that the Lejeuneaceae floras of both Australia and New Zealand are understudied. However, Australia, and in particular northeast Queensland from where most of the new records arise, has a much higher proportion of Lejeuneaceae compared to its total flora, with > 50% of species reported from Queensland belonging to the Lejeuneaceae, This is approximately five times the percentage contribution of Lejeuneaceae to the New Zealand flora. The number of Cololejeunea recognized for Australia has increased by 19 species, which is nearly five times the number of Cololejeunea species added to the New Zealand flora over the same period. This increase in species numbers is proportion to the contribution of the Lejeuneaceae to the flora of Australia as compared with New Zealand. This supports the view of von Konrat et al. (2008) that both factors could be at play in New Zealand, in that New Zealand's liverwort flora is both genuinely species poor, but also understudied. Further support for the view that New Zealand's Lejeuneaceae flora is understudied can be derived from the recent discovery of several new species in genera other than Cololejeunea (Glenny Citation1996; Renner et al. Citation2009 Renner et al. Citation2010; Renner Citation2010).
Gradstein et al. (Citation1983) postulated that highly dispersible species should possess five properties. First, they should be autoicous, so that sexual reproduction can occur in isolated individuals, they should also produce spores in large quantities. Second, they should reproduce asexually, to enhance local recruitment. Third, they should have spores resistant to desiccation and freezing, to facilitate dispersal by cyclonic weather systems (van Zanten Citation1978, van Zanten & Pócs Citation1981). Fourth, they should have small spores that are capable of suspension within slow moving air columns, Gradstein et al. (Citation1983) suggested spores > 17 µm might be unsuitable for long distance aerial dispersal. Finally, they should be ecologically ‘weedy’ species capable of colonizing bare substrates associated with disturbance, including bare rock, and bark. In their analysis of taxa exhibiting ranges disjunct between Africa and South America, Gradstein et al. (Citation1983) observed that approximately half the taxa were dioicous, two thirds were not known to produce gemmae, and one fifth had spores > 20 µm. For the suite of African–American taxa studied, there did not appear to be good agreement between expectation and observation with regards dispersability. A comparison between New Zealand Cololejeunea species endemic to southern temperate Australasia, and those widespread overseas yields a similar result. All species are autoicous, and all bar two produce gemmae, at least occasionally. Two of the Australasian endemics are obligate epiphylls, so are largely confined to hyper-humid microsites, typically associated with forest interiors. However, there does not appear to be correlation with ecology, with two other endemics being colonizers of microsites associated with disturbance, while two of the widespread taxa are obligate or facultative epipmuscicols, so are also associated with hyper-humid environments. However, there may be a relationship between distribution in the tropics and distribution in New Zealand. Cololejeunea appressa, and C. floccosa, species widespread at low altitude through subtropical and tropical regions, are confined to the upper third of the North Island, a distribution paralleled by at least one other taxon with tropical affinities, Stenolejeunea acuminata R.M.Schust. It is possible that the thermal tolerance of these tropical species permits them to occur in the warmest parts of New Zealand. In this regard, C. grossepapillosa may be atypical. Rather than being a lowland element whose thermal tolerance allows it to occur in the far north of New Zealand, this tropical–montane species is disjunct in sites that may be thermally equivalent, in southern New Zealand. Cololejeunea grossepapillosa shares with more than 35 other liverwort species a distribution disjunct between tropical Africa and Asia (Pócs Citation1976), but is unusual in that it is one of the few species whose distribution extends further to Australasia. Two other species exhibiting this disjunction, Acrolejeunea aulacophora (Mont.) Steph. and Acrolejeunea pycnoclada (Taylor) Schiffn., are not known to occur in New Zealand. Within the New Zealand Lejeuneaceae flora C. grossepapillosa appears to have no biogeographic parallels.
The occurrence of widespread tropical species of Lejeuneaceae within New Zealand, even in unexpected settings such as South Island beech forest, emphasizes the importance of taking a global perspective when establishing the identity of novel entities in New Zealand. This is an impediment given the fragmentary and scattered nature of literary resources, herbarium collections and type specimens (literally), in addition to the complicated web of nomenclatural confusion within which the Lejeuneaceae is still enmeshed (Gradstein Citation2006).
Acknowledgements
The collections of C. grossepapillosa were made during the remeasurement of Carbon and Biodiversity monitoring plots by Wildland Consultants Ltd for New Zealand's Ministry for the Environment. We thank the curators of the herbarium HIRO for making the type of Cololejeunea grossepapillosa available to the second author for study, and two anonymous referees for their reviews that resulted in improvements to the submitted manuscript.
References
- Eggers , J , Frahm , J-P and Pursell , RA . 1998 . New bryophyte taxa for tropical countries, II . Tropical Bryology , 14 : 81 – 84 .
- Evans , AW . 1911 . Hepaticae of Puerto Rico X. Cololejeunea, Leptocolea and Aphanolejeunea . Bulletin of the Torrey Botanical Club , 38 : 251 – 286 .
- Glenny , D . 1996 . Nephelolejeunea papillosa, a new liverwort species from New Zealand, with notes on the distribution of Kymatolejeunea bartlettii Grolle . New Zealand Journal of Botany , 34 : 195 – 198 .
- Glenny , D . 1998 . A revised checklist of New Zealand liverworts and hornworts . Tuhinga , 10 : 119 – 149 .
- Gradstein , SR . 2006 . Stephani's Species Hepaticarum revisited . Willdenoia , 36 : 557 – 563 .
- Gradstein , SR , Pócs , T and Vána , J . 1983 . Disjunct Hepaticae in tropical America and Africa . Acta Botanica Hungarica , 29 : 127 – 171 .
- Horikawa , Y . 1932 . Studies on the Hepaticae of Japan VI . Journal of Science of Hiroshima University, Series B , Division 2 (Botany) 1 ) : 77 – 94 .
- McCarthy , P . 2003 . Catalogue of Australian liverworts and hornworts. Flora of Australia Supplementary Series Number 21 , Canberra, Australian Biological Resources Study 137 p .
- Pócs , T . 1969 . Adatok Észak-Vietnám Mohaflórájához. V. Fifth contribution to the bryoflora of North Vietnam . Botanikai Közlemények , 56 : 139 – 147 .
- Pócs , T . 1976 . Correlations between the tropical African and Asian bryofloras, I . Journal of the Hattori Botanical Laboratory , 41 : 95 – 106 .
- Pócs , T . 1984 . New or little known epiphyllous liverworts. III. The genus Aphanolejeunea Evans in Tropical Africa . Cryptogamie, Bryologie Lichénologie , 5 : 239 – 267 .
- Pócs , T and Bernecker , A . 2009 . Overview of Aphanolejeunea (Jungermanniopsida) after 25 years . Polish Botanical Journal , 54 : 1 – 11 .
- Pócs , T and Piippo , S . 1999 . Bryophyte flora of the Huon Peninsula, Papua New Guinea. LXIV. Aphanolejeunea (Lejeuneaceae, Hepaticae) . Acta Botanica Fennica , 165 : 85 – 102 .
- Pócs , T and Streimann , H . 1999 . Epiphyllous liverworts from Queensland, Australia . Bryobrothera , 5 : 165 – 172 .
- Pócs , T and Streimann , H . 2006 . Contributions to the bryoflora of Australia, I . Tropical Bryology , 27 : 19 – 24 .
- Pócs , T , Mizutani , M and Piippo , S . 1994 . Bryophyte flora of the Huon Peninsula, Papua New Guinea. LXV. Preliminary contributions on Lejeuneaceae (Hepaticae) 1 . Annales Botanici Fennici , 31 : 179 – 190 .
- Renner MAM 2010 . Another new Austrolejeunea (Lejeuneaceae: Jungermanniopsida) from New Zealand's subalpine environs . The Bryologist 113 : 781 787 .
- Renner , MAM and Braggins , JE . 2006 . Hepatic records for Australia and New Zealand . Australian Bryological Newsletter , 52 : 11 – 13 .
- Renner , MAM , Brown , EA and Wardle , GM . 2009 . Evidence for species recognition on the basis of a single specimen: Nephelolejeunea carcharias sp. nov. (Lejeuneaceae: Jungermanniopsida) . Systematic Botany , 34 : 615 – 624 .
- Renner MAM , Brown EA , Wardle GM 2010 . The Lejeunea tumida species group (Lejeuneaceae: Jungermanniopsida) in New Zealand . Australian Systematic Botany 23 ( 6 ): 443 462 .
- Renner , MAM and Glenny , DS . 2009 . Cololejeunea floccosa (Lehm. & Lindenb.) Steph. new to New Zealand . Australian Bryological Newsletter , 57 : 17
- Schuster , RM . 1955 . North American Lejeuneaceae II. Paradoxae: the genera Aphanolejeunea and Leptocolea . Journal of the Elisha Mitchell Society , 71 : 126 – 148 .
- Thiers , BM . 1982 . Branching in the Lejeuneaceae I: a comparison of branch development in Aphanolejeunea and Cololejeunea . The Bryologist , 85 : 104 – 109 .
- Thiers , BM . 1988 . The Australia species of Cololejeunea . Beiheft zur Nova Hedwigia , 90 : 113 – 146 .
- Townsend , AJ , de Lange , PJ , Norton , DA , Molloy , J , Miskelly , C and Duffy , C . 2008 . The New Zealand Threat Classification System manual , Wellington, Department of Conservation .
- van Zanten , BO . 1978 . Experimental studies on trans-oceanic long-range dispersal of moss spores in the Southern Hemisphere . Journal of the Hattori Botanical Laboratory , 44 : 455 – 482 .
- van Zanten , BO and Pócs , T . 1981 . “ Distribution and dispersal of Bryophytes ” . In Advances in bryology, Volume 1 , Edited by: Schultze-Motel , W . 479 – 562 . Gybtt, Strauss & Cramer .
- von Konrat MJ , Hagborg A , Söderström L , Renner MAM 2008 . Early land plants today: global patterns of liverwort diversity, distribution, and floristic knowledge . In : H Mohamed , B Bakar , Boyce AN , PKY , Lee , Bryology in Asia in the new millennium. Proceedings of the World Conference of Bryology, Petaling Jaya, Malaysia,, 23–27 July 2007. Univ. of Malaya, Int. Ass. Bryol. and Malaysian Ministry of Natural Resources . Pp. 425 438 .
- Wigginton , M and Grolle , R. 1996 . Catalogue of the Hepaticae and Anthocerotae of sub-Saharan Africa . Bryophytorum Bibliotheca , 50 : 1 – 267 .
- Wilson , R , Gradstein , SR , Schneider , H and Hienrichs , J . 2007 . Unravelling the phylogeny of Lejeuneaceae (Jungermanniopsida): evidence for four main lineages . Molecular Phylogenetics and Evolution , 43 : 270 – 282 .
- Zhu , R-L and So , ML . 2001 . Epiphyllous liverworts of China . Nova Hedwigia, Beiheft , 121 : 1 – 418 .