Abstract
Gomortega keule is endemic to Chile and belongs to the monotypic family Gomortegaceae. Although it produces edible fruits and good-quality wood, it is not cultivated. Information on reproductive phenology may help address conservation and cultivation practices. However, there is limited knowledge about this species. This research aimed to investigate the phenology of G. keule. Inflorescences and fruits were monitored over one year in a natural population. Flower bud development extended from early January to April (summer to mid-autumn). Flowering peaked from late April to mid May, while fruitset occurred from autumn to late spring. The fruits seemed to have originated in different years, as supported by a number of observations. From these observations, a complete fruit phenology was inferred, with a notably long growth period, suggesting that 17–20 months are required from fruitset to senescent fruit fall.
Introduction
Gomortega keule (Mol.) Baillon belongs to the monotypic family Gomortegaceae and its affinity to certain families in the Laurales shows its origin in Gondwana (Renner et al. Citation2000). This evergreen tree is endemic to a small area near the coast in central Chile (Benoit Citation1989), within a biodiversity hotspot with urgent priority for conservation on a global level (Myers et al. Citation2000). Vegetation in the region has been largely replaced by plantations of exotic timber trees (Espinosa Citation1948; Le Quesne & Stark Citation2006). As a result, and also because of fires and selective logging (Serra et al. Citation1986), G. keule is endangered (Benoit Citation1989; Walter & Gillett Citation1998). This tree produces edible fruits, well known by local people from ancient times (Le Quesne & Stark Citation2006), and its wood is of excellent quality (Albert Citation1924). Despite these attributes, the species is not cultivated and its domestication has been prevented by its scarcity and difficult propagation.
There is limited information on the flowering and fruiting times of G. keule. The literature generally agrees that the tree blooms around April and May and that the fruits also ripen and fall in autumn (Ruiz & Pavon Citation1798; Espinosa Citation1948; Rodríguez et al. Citation1983; San Martín Citation1997). Reproductive phenology is important to understand the ecology and natural history of a plant species, and may help to develop conservation strategies. Details of phenology are also of paramount importance when designing agronomic practices for cultivation.
This study aims to register the phenology of G. keule throughout the year, in order to augment the knowledge on the biology of this species.
Materials and methods
Observations were undertaken from January to December 2006, in a natural population of G. keule at Ralbún (36°03′S, 72°38′W, 540 m.a.s.l.). This place is difficult to access and site visits were sometimes hindered by blocked rural roads because of fallen bridges or erosion caused by rains, especially during the winter.
Commonly, the trees of G. keule were >10 m tall with fruits occurring at the end of the branches. However, five smaller trees growing in an open site were selected, with flowers and fruits below 2 m, which allowed data collection.
The observation of flower development led to the identification of three distinct periods (flower bud development, flowering and fruitset) with a number of stages within each period. Inflorescences (8–14 per tree, total 54) were selected, labelled and monitored. The number of structures in each of the three periods of inflorescence was recorded at every field visit. Inflorescences and buds were very fragile and broke easily when manipulated for measurements, especially in the early stages. The analysis was performed using the average number of flowers or fruits per inflorescence, for all trees.
Fruits (7–19 per tree, total 63) were selected, labelled and the equatorial diameter measured. Within a single tree, one group of fruits remained green and attached to the tree throughout the year. A second group, with larger diameters, showed a change in colour and later fell. Both categories were analysed separately, because they evidenced to correspond to distinct periods in the fruit growth. Additionally, and because of the large variation in fruit diameter among trees, the analysis was performed separately for each tree.
Results and discusion
Vegetative buds activated and started to produce shoots in October. Shoots elongated and leaves expanded during summer, reaching their final size in April, when vegetative growth stopped. Actively growing shoots and young leaves usually showed a bright light green colour, turning to dark green when mature and fully expanded. Vegetative or reproductive buds (flower buds) were observed in G. keule, while mixed buds were not present.
Flowering time occurred in autumn, in agreement with the literature (Ruiz & Pavon Citation1798; Espinosa Citation1948; Rodríguez et al. Citation1983; San Martín Citation1997). Rodríguez et al. (Citation1983) mentioned five species of evergreen woody species that share their habitat with G. keule and also flower in autumn.
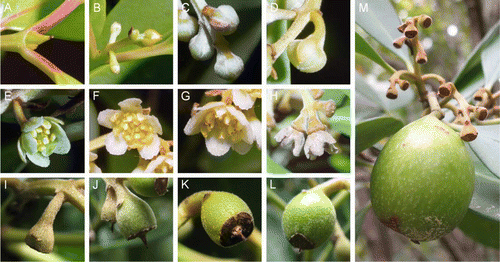
The three periods observed in this study were flower bud development, flowering and fruitset, with the stages shown in . After the dormant bud stage (A), the period of flower bud development commenced with swelling of the flower bud, which soon opened and the inflorescence elongated (B). The pedicel of the individual flower bud elongated (C) and the individual flower bud swelled (D). The period of flowering started when the tepals of the flower bud opened. The open flower (E) intiated anthesis (F) and progressed until anthers darkened (G). The period of fruitset began with drying of the tepals (H), which later fell. The thalamus showed a conic shape (I) and then swelled (J). The middle portion of the growing fruit became wider than the scar left by the tepals (K). Young structures were puberulent and hairs remained on the surface of the small fruits for several months (L), although bigger fruits lost them (M).
Figure 1 Phenological stages in Gomortega keule. A, Dormant bud. B, Elongation of inflorescence. C, Elongation of pedicel. D, Individual swollen flower bud. E, Flower opening. F, Initiation of anthesis. G, Anthers darkening. H, Dry tepals. I, Conic thalamus. J, Swollen thalamus. K, Thalamus wider than tepals scar. L, Young fruit growing. M, Unripe fruit and fruitset at the stage of conic thalamus on the same branch.
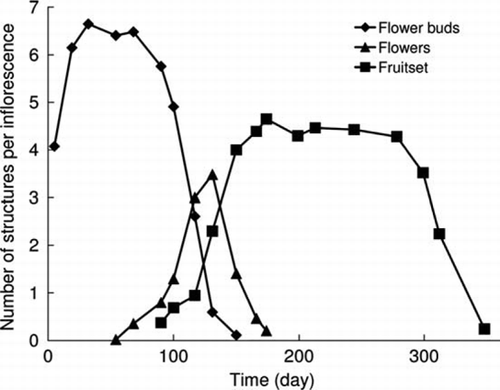
The period of flower bud development extended from the beginning of January to April (summer to mid autumn). Flowering was observed from late February to late June, with a peak from late April to mid May (day 117–131; ). The highest number of structures per inflorescence (6.6) was registered on day 32, which was rather low for the range of 6–14 flowers per inflorescence reported by Espinosa (Citation1948). Fruitset occurred from April onwards and reached values of around four structures per inflorescence from early June to early October. From October onwards, an important fall of young fruits occurred, with an average of 0.24 fruits per inflorescence at the end of the year (). Flowering out of season has rarely been observed by the authors, which agrees with Ruiz & Pavon (Citation1798) who reported flowering all year round.
Figure 2 Presence of flower buds, flowers and fruitset over time. Data correspond to the average number of structures per inflorescence (54 inflorescences, 5 trees).
Fruit ripening was observed in autumn, according to the literature (Rodríguez et al. Citation1983; San Martín Citation1997). Seven other evergreen trees that show fruit maturity in autumn have been reported to occur in the same area as G. keule (Rodríguez et al. Citation1983).
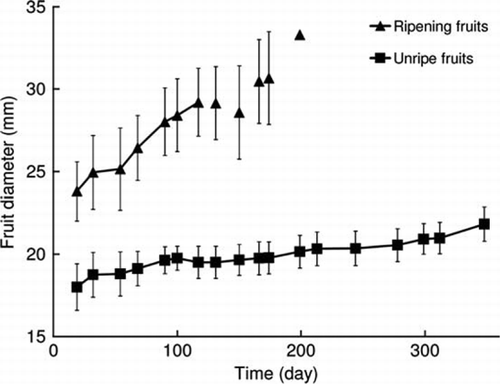
The two groups of fruits observed () seemed to belong to two different cohorts of fruits originating in successive and independent flowering events. This idea is supported by the following five observations. First, the group of unripe fruits remained green and attached to the branch all year, indicating immaturity. Second, the initial fruit diameter in the unripe group (18.0±1.4 mm; day 19) was different from the diameter in the ripening group (23.8±1.8 mm), at the same time. Also, ripening fruits changed colour from green to yellow and fell throughout the year, indicating maturity and senescence. In addition, the final diameter of fruits in the unripe group (21.8±1.0; day 348) is smaller than, albeit close to, the initial diameter of fruits in the ripening group, suggesting continuity. This continuity seems sensible if the evolution of the diameter in the unripe group was to follow a curve analogous to that of the ripening fruits (). Finally, the diameter of the newly formed fruits (2.81±0.22 mm; day 278; 27 fruits measured on the same tree of ) suggests continuity with the unripe group at the beginning of the year. Precisely because of this fifth observation, and since there is a large gap between the diameter of the newly formed fruits and that of the fruits that would ripen the next season, a direct continuity for the last two groups of fruits does not seem reasonable. The M shows an unripe fruit and fruitset on the same branch on day 299 (26 October).
Figure 3 Evolution of fruit diameter over time. Data correspond to means of fruit diameter±SD in a single tree on which 10 ripening fruits and 4 unripe fruits were recorded. Triangles without lines indicate that one or more fruits fell, the average being calculated with fewer than 10 fruits; the last triangle corresponds to one fruit.
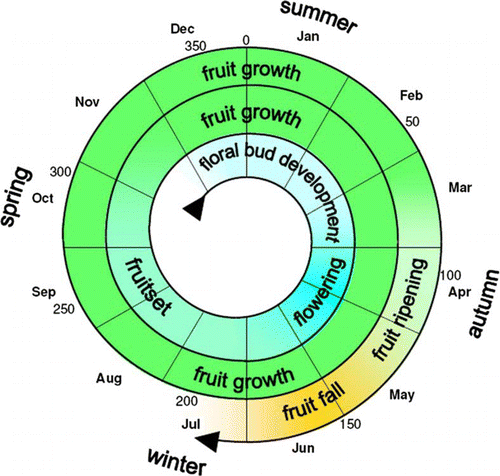
The long time span for fruit growth that is inferred from the observations () is unexpected. Especially notable would be the presence of three cohorts of developing offspring on the mother tree from April to June, simultaneously bearing flowers, unripe and ripening fruits. Interestingly, this agrees with the observation that fruits can be found throughout the year (Ruiz & Pavon Citation1798), and that flowers and fruits in different developmental stages may occur on the same branch (Le Quesne & Stark Citation2006).
Figure 4 Proposed reproductive phenology of Gomortega keule, inferred from a 1-year observation period. The numbers indicate the numerical day of the year (1 = 01 January).
Although fruit tree crops generally have a period of six or fewer months from anthesis to fruit ripening (Gil Citation2006), there are some cases of long-time ripening fruits. Certain Citrus spp. (grapefruit) may take more than 18 months to mature (Ladaniya Citation2008), whereas avocado in the coolest growing region of the USA (California) can take 18–22 months from flowering to maturity (Whiley et al. Citation2002). Cocos nucifera also shows a long period (14 months) of fruit growth (Rachel et al. Citation2010). The woody fruit of the Amazonian tree Bertholletia excelsa takes 14 months from pollination to fruit maturity (Wadt et al. Citation2005). Among Chilean species, Gevuina avellana, a tree with edible nuts, has a period from flowering to fruit maturity of around one year (Doll et al. Citation2005).
The development of flower buds of G. keule was observed to begin in the summer, with a flowering peak in autumn. Fruitset occurred during an extended period from autumn to late spring. Fruit growth would span beyond 12 months, ending with maturation and fruit fall in autumn. The suggestion that G. keule has a very long period of fruit development (17–20 months) from fruitset to ripening and senescent fall, would be outstanding and intriguing. It is therefore of interest to conduct further research monitoring the growth of fruits during their whole lifetime, in order to obtain more complete information on the subject.
Acknowledgements
The authors thank to Andrea Garrido for field assistance and Roberto Muñoz, CELCO S.A., for field permission. Fundación para la Innovación Agraria, Gobierno de Chile, partly funded the project FIA-ES-C-2005-2-F-162.
References
- Albert F 1924 . Materias primas vegetales y animales . Sociedad Imprenta y Litografía Universo , Santiago . 35 p.
- Benoit I 1989 . Libro rojo de la flora terrestre de Chile . Corporación Nacional Forestal , Santiago 157 p.
- Doll , U , San Martín , J , Ravanal , C , Cifuentes , S and Muñoz , M . 2005 . Potential fruit production evaluation of Gevuina avellana, during one season (1999–2000) in the coastal dryland of the VII Region . Bosque , 26 : 87 – 96 .
- Espinosa , M . 1948 . Estudios botánicos. 2. Nomenclatura del queule . Boletín del Museo Nacional de Historia Natural , 24 : 52 – 79 .
- Gil GF 2006 . Fruticultura: la producción de fruta . Ediciones Universidad Católica de Chile , Santiago 590 p.
- Ladaniya MS 2008 . Citrus fruit: biology, technology and evaluation . Academic Press , London 576 p.
- Le Quesne , C and Stark , D . 2006 . “ Gomortega keule (Mol.) Baillon ” . In Las especies arbóreas de los bosques templados de Chile y Argentina, Autoecología , Edited by: Donoso , C . 277 – 284 . Valdivia : Marisa Cuneo Ediciones .
- Myers , N , Mittermeier , RA , Mittermeier , CG , Da Fonseca , GA and Kent , J . 2000 . Biodiversity hotspots for conservation priorities . Nature , 403 : 853 – 858 .
- Rachel , AR , Jean-Louis , KK , Alexia , P , Jean , N and Ernest , K . 2010 . Physicochemical characteristics of kernel during fruit maturation of four coconut cultivars (Cocos nucifera L.) . African Journal of Biotechnology , 9 : 2136 – 2144 .
- Renner , SS , Foreman , DB and Murray , D . 2000 . Timing transantarctic disjunctions in the Atherospermataceae (Laurales): evidence from coding and noncoding chloroplast sequences . Systematic Biology , 49 : 579 – 591 .
- Rodríguez R , Matthei O , Quezada M 1983 . Flora arbórea de Chile . Editorial Universidad de Concepción Concepción 408 p.
- Ruiz H , Pavon J 1798 . Systema vegetabilium florae, peruvianae et chilensis . Typis Gabrielis de Sancha , Madrid 455 p.
- San Martín , J . 1997 . Bioecology of Gomortega keule (Mol.) Baillon (Gomortegaeae): a relict of temperate forest from Chile . Noticiero de Biologia , 5 : 131
- Serra M , Gajardo R , Cabello A 1986 . Ficha técnica de especies amenazadas: Gomortega keule (Mol.) Baillon ‘Keule’ (Gomortegaceae), especie en peligro Corporación Nacional Forestal , Santiago 18 p.
- Wadt , LH , Kainer , KA and Gomes-Silva , DA . 2005 . Population structure and nut yield of a Bertholletia excelsa stand in Southwestern Amazonia . Forest Ecology and Management , 211 : 371 – 384 .
- Walter KS , Gillett HJ 1998 . 1997 IUCN red list of threatened plants . IUCN – The World Conservation Union , Cambridge 862 p.
- Whiley AW , Schaffer B , Wolstenholme B 2002 . The avocado: botany, production, and uses . CABI Publishing , Oxton-Wallingford 500 p.