Abstract
Gender dimorphism was studied in the Nationally Critical threatened species Pachycladon stellatum using a cultivated population that was raised from seed collected from Yeo Stream, Marlborough. On the basis of flower morphology two sexual morphs were identified. Flowers on male plants have larger petals, sepals, filaments and anthers, and the anthers are dehiscent and produce abundant pollen. Female plants have a larger stigma and the anthers are indehiscent and produce no fertile pollen. Male plants produce seeds and therefore P. stellatum is considered to be gynodioecious.
Introduction
A feature of the New Zealand flora is the prevalence of gender dimorphism with 83 (23%) genera of seed plants having gender dimorphism, and it has arisen autochthonously in 17 of the 83 genera (Webb et al. Citation1999). The most common forms of dimorphism are dioecism (65 genera; 71% of dimorphic genera) and gynodioecism (26 genera; 28%) (Webb et al. Citation1999). Within the Brassicaceae gender dimorphism is rare worldwide with gynodioecism known in the Mediterranean Hirschfeldia incana (Horovitz & Galil Citation1972). Dioecism is known in the New Zealand endemic Lepidium sisymbrioides and L. solandri (Kirk Citation1899; Heenan et al. Citation2007) and gynodioecy in Pachycladon wallii (Garnock-Jones Citation1991, as Cheesemania wallii). Furthermore, a single female (male sterile) plant and two male (hermaphrodite) plants of Pachycladon stellatum were noted by Heenan & Garnock-Jones (Citation1999, as Cheesemania stellata). These authors also observed male sterility in single plants of P. fastigiatum and P. latisiliquum (both species then in the genus Cheesemania).
In reference to gynodioecism in P. wallii, Webb et al. (Citation1999) commented ‘that in a few cases the record of gender dimorphism is based on a small sample, as for example in Cheesemania (Garnock-Jones Citation1991), and in such cases more research is needed to determine whether dimorphism is fully established in populations’. Another problem is that the observations made by Heenan & Garnock-Jones (Citation1999) on P. stellatum, P. fastigiatum, and P. latisiliquum were based on dried herbarium material and accurate measurements of all floral parts were not able to be made; for P. stellatum only filament length measurements were made and pollen presence or absence assessed. Gender dimorphism in these species of Pachycladon is not easily studied in the field as based on many years of experience they occur on steep and difficult to access alpine bluffs, usually grow in small and scattered populations (typically 3–20 plants), and few (often only 1–2) if any plants will flower in wild populations at any one time.
P. stellatum, for example, is especially difficult to study as it naturally occurs in very low numbers in a restricted geographic area in the upper Awatere River, Marlborough, and is classified as a Nationally Critical threatened species (de Lange et al. Citation2009). There are, for example, 23 plants on herbarium sheets in the Allan Herbarium (CHR) with only four of these in flower. One solution to study the breeding system of rare species is to examine a cohort of cultivated plants, and this approach was used for P. stellatum as reported here.
Materials and methods
A population of 12 plants were cultivated in a glass house at the experimental nursery, Landcare Research, Lincoln. These plants were raised from seed collected in 1999 from the Yeo Stream, upper Awatere River, Marlborough (42°09′30′′S, 173°15′89′′E). Floral characters measured (in mm) were: petal length, petal width, sepal length, sepal width, short filament length, long filament length, anther length, stigma width, and pollen presence or absence noted. These characters are comparable to those used for P. wallii by Garnock-Jones (Citation1991).
Results and discussion
This study has confirmed gender dimorphism (gynodioecism) in P. stellatum and builds on the earlier report of a male sterile plant in this species (Heenan & Garnock-Jones Citation1999). The cultivated population of the study plants of P. stellatum has gender bias with 10 male (hermaphrodite) plants (e.g. CHR 533014) and two female (male sterile) plants (e.g. CHR 533015). The flowers of male plants have several floral parts with dimensions that exceed those of female plants. Male plants have flowers with larger sepals, petals, filaments and anthers, and the anthers dehisce normally and produce abundant pollen (; ). In female plants the filaments are only half as long as those in male plants and they lack male function as the anthers are indehiscent and do not produce any pollen. Female flowers also have a wider diameter stigma than male flowers (; ).
Figure 1 Flowers of P. stellatum. A, B, Female flower with large stigma and sterile anthers that lack pollen (one petal removed in B). C, D, Male flowers with dehiscent anthers and abundant pollen and an immature but small stigma (one petal removed in D).
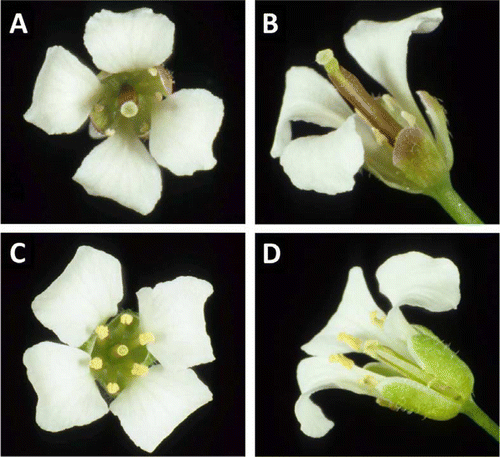
Table 1 Sizes of flower parts in female (male sterile) and male (hermaphrodite) plants of P. stellatum.
Although not specifically measured, when cultivated male plants were isolated and they set abundant seed and this indicates that P. stellatum is gynodioecious. There are also several specimens in the Allan Herbarium with abundant fruit and these too are likely to be male plants. One wild-collected plant in the Allan Herbarium (CHR 405236) is female with an estimated 750 flowers of which 35 (4.66%) have elongated siliques and set seed. It is not known whether the flowers that have set seed have been cross-pollinated by a male P. stellatum plant or if the male sterility is inconstant and some flowers produced anthers with viable pollen. On this female plant most of the siliques that are elongated and with seed are at similar positions on the inflorescence and it is possible that these flowered synchronously and were pollinated by the same insect pollen vector.
The results reported for P. stellatum are similar to those of Garnock-Jones (Citation1991) for two wild populations of P. wallii, where there was gender dimorphism (gynodioecy) and the flowers of male plants were larger than those of female plants. Furthermore, and as noted by Garnock-Jones (Citation1991) in his study of P. wallii, predominance of male plants also supports P. stellatum being gynodioecious. Previously, pollen:ovule ratios have been determined for several Pachycladon species, and the male plants of P. stellatum have a ratio of (1:525) (Mitchell & Heenan Citation2002). This ratio is low and infers selfing, but these authors concluded the flower morphology is consistent with outcrossing and the species is therefore regarded as having a mixed mating system.
This second record of gender dimorphism in Pachycladon is of significance in that P. wallii and P. stellatum belong to different species groups and are not sister species in the phylogenetic analyses (Heenan et al. Citation2002; Heenan & Mitchell Citation2003). P. stellatum is monocarpic, has stout terminal inflorescences, toothed lanceolate leaves, restricted to greywacke substrate in northern South Island (inland Marlborough), and related to other greywacke species such as P. fastigiatum and P. enysii. In comparison, P. wallii is polycarpic, the inflorescences lateral, leaves are deeply lobed, restricted to schist substrate in southern South Island (southern Otago and northern Southland), and it is sister to P. novae-zelandiae in the phylogenetic analyses. The extent of gender dimorphism in Pachycladon remains unclear, and further studies are required to see if it occurs in other species as suggested for P. fastigiatum and P. latisiliquum by Heenan & Garnock-Jones (Citation1999). Another unusual breeding system has also been described in P. exile. This species is known from a single limestone outcrop in North Otago and has hermaphrodite flowers but can reproduce by matromorphy—or meiotic parthenogenesis and endo-replication (Bicknell et al. Citation2009). The biological significance of gynodioecism and matromorphy in three of the most geographically restricted species of Pachycladon remains unclear and requires further study.
Of interest in regard to the general biology of P. stellatum is that it has a restricted distribution and occurs in small and scattered populations and few, if any, plants will flower at one time (Heenan & Mitchell Citation2003). During five field trips into the upper Awatere River catchment and Yeo Stream between 1997 and 2011 only eight populations of P. stellatum have been located and these comprised approximately 2, 3, 5, 5, 6, 6, 8, and 35 plants (Heenan & Garnock-Jones Citation1999; unpubl. data). Any female plant (male sterile) that flowers will require pollen from a male plant of P. stellatum, or another Pachycladon species, for it to be pollinated, fertilised, and set seed. As P. stellatum has small populations with few plants that flower synchronously it is not surprising that wild interspecific hybrids between P. stellatum and P. fastigiatum have been collected from Yeo Stream, Marlborough (Yogeeswaran et al. Citation2011). These hybrids were collected from a bluff growing among other plants referable to P. stellatum, and this suggests it was a female plant of P. stellatum that was crossed with pollen from P. fastigiatum to produce the hybrids; at this site P. fastigiatum grows about 80 m from P. stellatum.
Acknowledgements
I thank David Purcell for tending the cultivated plants and Debby Redmond for technical assistance. Funds for this research were provided by the Ministry of Science and Innovation.
References
- Bicknell , RA , Heenan , PB , Dawson , MI , Fletcher , PJ and Christey , MC . 2009 . Matromorphy in Pachycladon exile (Brassicaceae) revealed by interspecific hybridization . New Zealand Journal of Botany , 47 : 139 – 148 .
- de Lange , PJ , Norton , DA , Courtney , SP , Heenan , PB , Barkla , JW , Cameron , EK , Hitchmough , R and Townsend , AJ . 2009 . New Zealand extinct, threatened and at risk vascular plant list . New Zealand Journal of Botany , 47 : 61 – 96 .
- Garnock-Jones , PJ . 1991 . Gender dimorphism in Cheesemania wallii (Brassicaceae) . New Zealand Journal of Botany , 29 : 87 – 90 .
- Heenan , PB and Garnock-Jones , PJ . 1999 . A new species combination in Cheesemania (Brassicaceae) from New Zealand . New Zealand Journal of Botany , 37 : 235 – 241 .
- Heenan , PB and Mitchell , AD . 2003 . Phylogeny, biogeography, and adaptive radiation of Pachycladon (Brassicaceae) in the mountains of South Island, New Zealand . Journal of Biogeography , 30 : 1737 – 1749 .
- Heenan , PB , Mitchell , AD and Koch , M . 2002 . Molecular systematic of the New Zealand Pachycladon (Brassicaceae) complex: generic circumscription and relationships to Arabidopsis s. l. and Arabis s. l . New Zealand Journal of Botany , 40 : 543 – 562 .
- Heenan , PB , Mitchell , AD , McLenachan , PA , Lockhart , PJ and de Lange , PJ . 2007 . Natural variation and conservation of Lepidium sisymbrioides Hook.f. and L. solandri Petrie (Brassicaceae) in South Island, New Zealand, based on morphological and DNA sequence data . New Zealand Journal of Botany , 45 : 237 – 264 .
- Horovitz , A and Galil , J . 1972 . Gynodioecism in east Mediterranean Hirschfeldia incana (Cruciferae) . Botanical Gazette , 133 : 127 – 131 .
- Kirk T 1899 . The students’ flora of New Zealand and outlying islands . Government Printer , Wellington 37 p .
- Mitchell , AD and Heenan , PB . 2002 . Genetic variation within the Pachycladon (Brassicaceae) complex based on fluorescent AFLP data . Journal of the Royal Society of New Zealand , 32 : 427 – 443 .
- Webb , CJ , Lloyd , DG and Delph , LF . 1999 . Gender dimorphism in indigenous New Zealand seed plants . New Zealand Journal of Botany , 37 : 119 – 130 .
- Yogeeswaran K , Voelckel C , Joly S , Heenan PB 2011 . Pachycladon . In : Kole C Wild crop relatives: genomic and breeding resources, oilseeds. Vol 3: Wild Relatives of Oilseeds . Tokyo , Springer-Verlag . Pp. 227 – 249