Abstract
The accumulation patterns of phenolic compounds during fruit growth and ripening of Berberis buxifolia and their correlations were studied to determine the optimal time and conditions needed to obtain maximum phenolic content. Anthocyanin content increased from 1.7 to a maximum of 752.7 mg·100 g−1 FW at the end of ripening, while the flavonoid content was maximal in unripe fruits [604.0 mg (+)-catechin equivalents·100 g−1 FW]. Total phenolic compounds decreased from 968.1 to a minimum of 746.3 mg gallic acid equivalents·100 g−1 FW just as soluble solids started to accumulate; a maximum of 1522.9 mg gallic acid equivalents·100 g−1 FW was attained at the end of ripening. Both variables (1,1-diphenyl-2-picrylhydrazyl scavenging effect and reducing power) were maximum in unripe fruits, and decreased during the ripening period, although the 1,1-diphenyl-2-picrylhydrazyl scavenging effect increased again towards the end of this period. The accumulation patterns of phenols varied depending on the specific group of compounds considered, and could be correlated with fruit quality.
Introduction
During recent decades, research on healthy practices has been particularly focused on the beneficial effects that can result from the adoption of a diet rich in fruits and vegetables. In fact, these foods can help reduce the risk of chronic, degenerative and oxidative-stress-mediated diseases, such as cancer and cardiovascular and neurodegenerative diseases (Roussos et al. Citation2009). As a result, attention is now also directed towards many small fruits that are considered to be a source of organic and inorganic nutrients and metabolic-regulating factors. In addition, these fruits are particularly regarded because of their nutraceutical properties as functional foods (Béliveau & Gingras Citation2005; Kuskoski et al. Citation2005). Indeed, small fruits of cultivated Ribes, Rubus and Vaccinium species are an excellent source of natural products with antioxidant properties (Deighton et al. Citation2002), which are mainly attributed to their high polyphenolic content, especially phenolic acids, flavonoids and anthocyanins, in addition to other important natural antioxidants like ascorbic acid (vitamin C) (Béliveau & Gingras Citation2005). The antioxidant activity of phenolics is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors and singlet oxygen quenchers; they also have metal chelation potential (Kähkönen et al. Citation1999). The selection of any standardised method to evaluate antioxidant activity should meet some ‘ideal’ requirements (Prior et al. Citation2005). The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity (Shimada et al. Citation1992), the reductive ability for Fe3 +–Fe2 + transformation (Oyaizu Citation1986) in the presence of plant extracts and quantification of phenols through the Folin–Ciocalteu reagent have been shown to be simple, rapid, sensitive and precise techniques, while exhibiting a good reproducibility. The phenolic content and antioxidant activity show good correlation (Ferreyra et al. Citation2007; Turkoglu et al. Citation2007), highlighting the importance of the total phenolic content for the antioxidant activity found in several plant materials (Turkoglu et al. Citation2007).
Phenolics are much more than simple antioxidants and focus is currently turning towards other modes of action. It is important to understand how phenolics and their subgroups (e.g. flavonoids) change during fruit development, and how to measure their activity. Phenolics can be classified as flavonoid or non-flavonoid compounds. Flavonoids make up a significant portion of the phenolic compounds in small fruits, for example, grapes (Conde et al. Citation2007), and include several classes, such as proanthocyanidins (tannins), anthocyanins and flavan-3-ol monomers (i.e. catechins). Anthocyanins are the major phenolic components of soft berry fruits, and their antioxidant activity has been found to be closely related to total phenolic content (Deighton et al. Citation2002). These substances play a role in plant defence and they appear to possess beneficial effects against various diseases (Hou Citation2003). The biosynthesis of flavonoids is a tissue-specific process which is developmentally regulated. It is thought that these compounds play a variety of functions in plants, providing protection against UV radiation, participating in the defence against pathogens, providing a chemical guide to pollinating insects in flowers, being responsible for flower and fruit pigmentation, as well as playing an essential role in reproduction. In addition, flavonoids also contribute to the quality characteristics of fresh and processed food products including astringency, texture, taste and colour (Vvedenskaya & Vorsa Citation2004). Preharvest factors such as the plant genetic background, the environmental conditions during plant growth, the cultural practices employed and the stages of fruit ripening, all influence the synthesis of phenolic compounds (Kähkönen et al. Citation2001; Ferreyra et al. Citation2007) and their antioxidant capacity (Roussos et al. Citation2009), with ripening stages being even more relevant when immature berries are used, for example, cranberries in the juice industry (Çelik et al. Citation2008).
Large areas with indigenous flora which include the Berberis L. genus exist in Patagonia (Monge et al. Citation2000). At present, commercial Berberis orchards are being planted: this crop has economical potential, not only for flavour and taste of the fruit, but also because of its putative high antioxidant properties. In fact, black–blue fruits are now consumed fresh, in marmalades and jams, in non-alcoholic beverages and in ice creams.
Berberis buxifolia is a spiny evergreen shrub with a large distribution in Patagonia and particularly in Tierra del Fuego (Moore Citation1983; Orsi Citation1984). The kinetic growth behaviour of the fruit and the evolution of chemical properties during the fruiting period and different growing seasons have been studied in this species (Arena & Curvetto Citation2008), and recent research on the Berberis genus has studied the phenol content in leaves (Koncic et al. Citation2010), roots (Surveswaran et al. Citation2007; Tomosaka et al. Citation2008) and fruits (Ruiz et al. Citation2010). The beneficial effect of B. buxifolia fruits on oxidative stress induced by chloramphenicol on human blood has also been reported (Albrecht et al. Citation2010).
However, there is a lack of information on the important role of preharvest factors on the evolution of secondary metabolism during fruit growth and ripening in B. buxifolia. Thus, the aim of this work was to study the patterns of accumulation of anthocyanin, flavonoid and phenol contents and antioxidant activity during growth and ripening of B. buxifolia fruits, and their correlations with fruit quality, to understand the optimal time and conditions needed to obtain maximum phenolic content for use in the preparation of extracts for nutraceutical purposes.
Materials and methods
Chemicals and reagents
Sodium nitrite (Merck), aluminium chloride hexahydride (AppliChem), (+)-catechin (Sigma-Aldrich), sodium carbonate (Merck), Folin–Ciocalteau reagent (Sigma-Aldrich), gallic acid (Sigma-Aldrich), DPPH (Sigma-Aldrich), butylated hydroxyanisole (Sigma-Aldrich), α-tocopherol (Sigma-Aldrich), sodium phosphate buffer (pH 6.6, Sigma-Aldrich), potassium ferricyanide (Sigma-Aldrich), trichloroacetic acid (w/v, Anedra) and ferric chloride hexahydrate (Anedra) were purchased from Global Lab S.A. (Buenos Aires, Argentina). Ascorbic acid (Cicarelli) and all other chemicals and solvents were of analytical grade and obtained from Chemit Argentina S.R.L (Buenos Aires, Argentina).
Plant material and sampling
Berberis buxifolia plants (n = 50, with a mean height of 0.91±0.15 m, with the full flower phase occurring at mid-spring for 1–2 weeks), were growing naturally in a representative area located near Ushuaia city, 54°48′S, 68°19′W (Tierra del Fuego, Argentina). Healthy and sun-exposed fruits (300 g) were collected manually every 14 days from 13 November 2007 (14 days after full flower phase; AFFP) to 4 March 2008 (126 days AFFP), and bulked as one sample for each date. The mean, maximum and minimum daily air temperatures from October to March during the 2007–2008 growing season were 8.6, 13.0 and 4.6 °C, respectively, these values being comparable with those described earlier by Arena & Curvetto (Citation2008).
Fruit growth phase definition and characterisation
Weight
Fresh and dry fruit weights were recorded the entire fruiting period (from days 14 to 126 AFFP) to define the fruit growth phases. In order to characterise the last fruit growth phases and ripening, the following variables were analysed from day 70 to 126 AFFP.
Firmness
Firmness was recorded using a digital penetrometer, Wagner Instruments Model FDI 2 (1 × 0.001 kgf), with tips of 1 mm diameter.
Soluble solids and total titratable acidity
Soluble solids were determined in fruit juice using an ATAGO N1-α refractometer with a 0–32°Brix measurement range with 0.2°Brix increments, and no temperature compensation. Total titratable acidity was measured by titration with 0.1 N NaOH solution. Total titratable acidity was expressed as malic acid, the most abundant organic acid in B. buxifolia fruits. The soluble solids/total titratable acidity ratio was also determined.
Fruit colour evolution and anthocyanin content
The percentage of fruit surface having a purple colour was recorded, while anthocyanin content was quantified using the pH differential method (Giusti & Wrolstad Citation2001). Samples (5 g) of initially frozen fruits were extracted for 24 h in 50 mL 0.1% HCl–MeOH solution at 4°C. Aliquots were then diluted from 1:5 to 1:80 to meet the Lambert–Beer's law, with either 0.025 M KCl (pH 1) or 0.4 M sodium acetate buffer (pH 4.5). Absorbance values at 510 and 700 nm were recorded with a Shimadzu 1203 UV–Vis spectrophotometer. Anthocyanin content was determined on the basis of a molar extinction coefficient of 26,900 M−1·cm−1 and a molecular mass of 449.2 g for cyanidin 3-glucoside. Anthocyanin content was determined as
where A (absorbance)=(A 510−A 700)pH1.0-(A510 –A700)pH4.5. Values were expressed in mg anthocyanin·100 g−1 fresh weight (FW), mg anthocyanin·g−1 dry weight (DW) and mg anthocyanin per fruit.
Preparation of extracts from fruits and extraction yield
Ten grams of freeze-dried fruits were extracted with 100 mL of methanol at 25°C for 24 h with continuous stirring. The residue was later extracted twice with additional volumes of 100 mL methanol for 48 h, and the three extracts were combined. The extraction yield was determined gravimetrically from the methanolic extracts from two freeze-dried fruit portions (10 g) carried out in triplicate. The extracts were then used to determine the flavonoid and total phenolic contents, as well as the DPPH scavenging activity and reducing power, as described below.
Flavonoid content
Flavonoid content was determined according to Zhishen et al. (Citation1999). Briefly, each methanolic extract (1.5 mg·250 µL−1 methanol solution) was mixed with 1.5 mL of distilled water and 75 µL of 50 g·L−1 sodium nitrite. After 5 min, 150 µL of 100 g·L−1 aluminium chloride hexahydride was added. After 6 min, 500 µL of 1 M sodium hydroxide and 275 µL of distilled water were added to the mixture. After vigorous mixing, the absorbances of the resulting solutions were measured against a blank at 510 nm using a Shimadzu 1203 UV–Vis spectrophotometer. The flavonoid content was obtained from the calibration curve prepared using (+)-catechin as standard and was expressed as mg (+)-catechin equivalents·100 g-1 FW, mg (+)-catechin equivalents·g−1 DW and mg (+)-catechin equivalents per fruit.
Phenol content
Phenol content was determined according to the Folin–Ciocalteau method (Taga et al. Citation1984), with some modifications. Briefly, 100 µL of each methanolic extract [20 mg·5 mL−1 of 1.3% HCl in methanol/water (60:40 v/v) solution] was extracted and 2 mL of 2% (w/v) aqueous sodium carbonate solution and 100 µL of 50% Folin–Ciocalteau reagent were added to the mixture. After standing for 30 min at 24°C, the absorbances of the resulting coloured solutions were measured against a blank at 750 nm using a Shimadzu 1203 UV–Vis spectrophotometer. The phenol content was obtained from the calibration curve prepared using gallic acid as standard, and expressed as mg gallic acid equivalents·100 g−1 FW, mg gallic acid equivalents·g−1 DW and mg gallic acid equivalents per fruit.
Scavenging activity on DPPH radicals
Each methanolic extract (0.0–1.0 mg·mL−1) in methanol (2 mL) was mixed with 0.25 mL of a methanolic solution containing DPPH radicals, resulting in a final concentration of 0.1 mM DPPH. The mixture was shaken vigorously and allowed to stand in darkness for 30 min. The absorbance was then measured at 517 nm against a blank in a Shimadzu 1203 UV–Vis spectrophotometer (modified method of Shimada et al. Citation1992). A low absorbance value for the reaction mixture indicates high free radical scavenging activity. Ascorbic acid, butylated hydroxyanisole and α-tocopherol were used as antioxidant standards. The capability of methanolic extracts to scavenge DPPH radicals was calculated using the following equation:
where A blank is the absorbance of the control reaction (containing all reagents except the test compound) and A sample is the absorbance of the reaction mixture for the compound scavenging effect. To avoid anthocyanin interference, A sample was calculated by subtracting the absorbance of the fruit extracts in the absence of DPPH. The values for the DPPH scavenging effect were calculated for all methanolic extracts and antioxidant standard concentrations under study. The EC50 value (mg·mL−1) is the effective concentration of methanolic extract at which the antioxidant activity was 50%; it was obtained by interpolation from linear regression analysis between the sample concentration (0–0.5 mg·mL−1) and the DPPH scavenging effect.
Reducing power
The reducing power was determined following the method of Oyaizu (Citation1986). Each methanolic extract (0.0–5.0 mg·mL−1) in methanol (2.5 mL) was mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide, and the mixture was incubated at 50 °C for 20 min in darkness. Then, 2.5 mL of 10% trichloroacetic acid (w/v) was added and the mixture was centrifuged at 3000 rpm for 10 min. The upper layer (5 mL) was mixed with 5 mL of deionised water and 0.5 mL of 0.1% ferric chloride hexahydrate, and the absorbance was measured at 700 nm against a blank using a Shimadzu 1203 UV–Vis spectrophotometer. A high absorbance value indicates high reducing power. Ascorbic acid, butylated hydroxyanisole and α-tocopherol were used as antioxidant standards. The reducing power (RP) was calculated using the following equation:
where A maximum is the maximum absorbance value obtained with the colour solution developed by the reaction of ferricyanide salt with the compound under testing and A sample is the absorbance value obtained with the colour solution developed by the reaction of ferricyanide salt with the compound under testing for each concentration. The values of reducing power (%) were calculated for all methanolic extracts and antioxidant standard concentrations under study. The EC50 value (mg·mL−1) is the effective concentration of methanolic extract at which the reducing power was 50%; it was obtained by interpolation from linear regression analysis between sample concentration (0–1 mg·mL−1) and reducing power.
Statistical analysis
Data were analysed statistically by one-way analysis of variance (ANOVA), and means were then separated using the Tukey multiple range test at P<0.05. Linear coefficient correlations and regression analysis were performed between some pairs of variables.
Results and discussion
Fruit growth phase definition
The time course of fruit growth (fresh weight basis) from day 14 to day 126 AFFP shows a typical double sigmoid growth curve, as reported previously for this species (Arena & Curvetto Citation2008), with the highest fruit growth rate around days 42 and 56 AFFP (first phase of rapid increase), reaching a plateau (second lag phase) by day 70 AFFP (300.2 mg), which extended to day 84 AFFP (A). During the third phase of rapid increase to day 112 AFFP (when the fruits also ripen) the highest biomass was obtained (379.8 mg), a decrease in fresh biomass then occurred towards the end of the growing season. The time course evolution on the basis of dry fruit weight also showed a double sigmoid curve with a less pronounced plateau, and increased slightly to reach an average biomass of 111.1 mg·fruit−1 at day 112 AFFP (B). Fruit firmness changed significantly during the fruiting period (), with a maximum at days 70–84 AFFP, then decreasing towards the end of this period, with mean values of 0.15 and 0.19 kgf at days 112 and 126 AFFP, respectively.
Figure 1 Phenolic compound evolution in the days after the full flower phase in B. buxifolia fruits. A, Expressed on the basis of fresh weight (mg·100 g−1 FW). B, Expressed on the basis of dry weight (mg·g−1 DW). C, Expressed on the basis of per unit fruit (mg·fruit−1). ANT, anthocyanin content; PHE, phenol content; FLA, flavonoid content; FFW, fresh fruit weight; DFW, dry fruit weight (n = 6). Error bars represent ± 1 standard error of the mean.
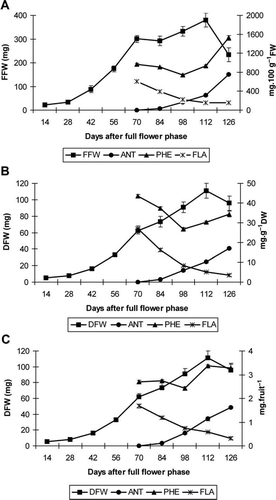
Table 1 Firmness (kgf), colour (%), soluble solids (°Brix), total titratable acidity (%) and soluble solids/total titratable acidity ratio in Berberis buxifolia fruits in the days after the full flower phase.
Soluble solids and total titratable acidity
Soluble solids increased significantly during the ripening phase, to reach a maximum (30.6°Brix) at day 126 AFFP (). Whereas total titratable acidity decreased significantly during the ripening period () from a maximum (6.3%) at day 70 AFFP. As expected, the ratio soluble solids/total titratable acidity increased significantly during the ripening phase, with the maximum (13.9) at day 126 AFFP ().
Fruit colour evolution and anthocyanin content
The percentage of the fruit surface having a purple colour changed significantly during the study period (), increasing from day 70 AFFP (20%) and attaining the highest values at days 112 (98.7%) and 126 (100%) AFFP, respectively. Anthocyanin content increased significantly during the study period in terms of fresh weight (F = 792.7, P = 0.000), dry weight (F = 284.36, P = 0.000) and on a per fruit basis (F = 296.87, P = 0.000) (A–C), reaching a maximum at day 126 AFFP in terms of both fresh and dry weight (752.7 mg·100 g−1 FW and 16.9 mg·g DW−1, respectively), and similarly on a per fruit basis (1.6 mg·fruit−1). Thus, in B. buxifolia, biosynthesis of anthocyanin compounds starts after a pronounced lag phase from fruit setting (day 84 AFFP), increases markedly during the ripening period when fruit biomass is at maximum values, and continues to increase until the end of the fruiting period, even though fruit biomass decreases. The precise conditions that initiate anthocyanin synthesis are not well established, but it has been argued that sugar accumulation provides the substrate needed for anthocyanin synthesis (Jackson Citation2008). In B. buxifolia fruits, the remarkable positive and significant correlation between anthocyanin accumulation and colour, and soluble solids and soluble solids/total titratable acidity ratio is consistent with this view (). The anthocyanin content found in B. buxifolia fruits at maturity (753 mg·100 g−1 FW) was comparable with that obtained in earlier growing seasons (Arena & Curvetto Citation2008), and similar to that reported by Ruiz et al. (Citation2010) for Berberis microphylla from southern Chile. However, the anthocyanin content found in this work was higher than that of other reddish-purple berries, such as blackcurrants (350 mg·100 g−1 FW), raspberries (55-60 mg·100 g−1 FW), strawberries (40 mg·100 g−1 FW) (Lister et al. Citation2002), and blueberries (1.2 mg·g−1 FW) (Zheng & Wang Citation2003).
Fruit methanolic extract yield
The yield of methanolic extracts increased significantly during the studied fruiting period (F = 34.61, P=0.000). Minimum values were obtained at days 70 and 84 AFFP (379.1 and 365.7 mg dry extract·g−1 DW, respectively), increasing by day 98 to 421.9 mg dry extract·g−1 DW and reaching the highest level from days 112 to 126 AFFP (453.3 and 443.2 mg dry extract·g−1 DW, respectively). The yield of methanolic extracts was positive and significantly correlated with the anthocyanin content (r = 0.779; P = 0.007).
Flavonoid content
Flavonoid content decreased significantly during the fruiting period in terms of fresh weight (F=85.6, P=0.000), dry weight (F=147.5, P=0.000) and on a per fruit basis (F=83.23, P=0.000) (A–C). The highest content was obtained at day 70 AFFP [604.0 mg (+)-catechin equivalents·100 g−1 FW, 27.1 mg (+)-catechin equivalents·g−1 DW and 1.7 mg·fruit−1], whereas the lowest contents were measured at day 126 AFFP [152.3 mg (+)-catechin equivalents·100 g−1 FW and 3.4 mg (+)-catechin equivalents·g−1 DW], following the same behaviour on a per fruit basis (0.3 mg·fruit−1). In the study by Cheel et al. (Citation2007), flavonoid content ranged between 30.0 and 123.2 mg quercetin equivalents·100 g−1 FW for Fragaria sp. fruits.
The synthesis of flavonoids (detected at 510 nm, like quercetin when they form complexes with aluminium) tends to decrease and may cease during ripening, as found for grapes, and with non-flavonoid phenolics (Jackson Citation2008). This pattern of flavonoid accumulation and subsequent decline during ripening suggests the degradation of flavonoids and their utilisation in the biosynthesis of other compounds and/or association with other cellular compounds by stable covalent links. In B. buxifolia fruits, the flavonoid content was positively and significantly correlated with the total titratable acidity, whereas it was negatively and significantly correlated with colour, soluble solids, soluble solids/total titratable acidity ratio and anthocyanin content.
Phenolic content
The phenolic content varied significantly during the fruiting period in terms of fresh (F= 99.3, P=0.000) and dry fruit weight (F = 37.9, P=0.000), and on a per fruit basis (F = 19.07, P=0.000) (A–C). Peaks for phenolic content were different when expressed on a fresh weight, dry weight or per fruit basis. When considering phenolic content on the basis of fresh fruit weight, this decreased from day 70 AFFP to a minimum at day 98 AFFP and then increased to a maximum at day 126 AFFP (1522.9 mg gallic acid equivalents·100 g−1 FW). However, when considering phenolic content on the basis of dry fruit weight, the maximum (43.5 mg gallic acid equivalents·g−1 DW) was reached at day 70 AFFP, then decreased to a minimum at day 98 AFFP and then increased at day 126 AFFP. Peaks for phenolic content on a per fruit basis were attained at days 112 and 126 AFFP (3.4 and 3.3 mg gallic acid equivalents/fruit, respectively). Phenolic contents measured in ripe B buxifolia fruits in this study (27–34 mg·g−1 DW) were comparable with those cited for Vaccinium myrtillus (33–38 mg·g-1 DW) (Kähkönen et al. Citation2001) and Ribes nigrum (1000 mg·100 g−1 FW) (Deighton et al. Citation2002), and higher than those of other reddish-purple berries, such as Ribes rubrum (14 mg·g−1 DW), Fragaria ananassa (16–24 mg·g−1 DW) (Kähkönen et al. Citation2001), Rubus idaeus (300 mg·100 g−1 FW) (Lister et al. Citation2002) and native South American Fragaria sp. (106–268 mg·100 g−1 FW) (Cheel et al. Citation2007).
In some berries, the synthesis of phenolics begins shortly after fruit development starts, such as in grape (Jackson Citation2008), where some anthocyanins can be synthesised earlier, but most production involves other flavonoids or non-flavonoid phenolics. The phenolic content in B. buxifolia decreased significantly from the fruit growth lag phase to day 98 AFFP, reaching a minimum just when soluble solids begin to accumulate, to then begin another steeper increase to reach a maximum at the end of the ripening period. The initial decrease could be, in part, due to the decrease in tannin content, as found in grape (Conde et al. Citation2007); these particular phenolics play an important role in the defence against predators (Raffo et al. Citation2004). At the time fruits began to ripen at day 98 AFFP and phenolic content reached its minimum value, anthocyanin content was still not at a high level and the flavonoid content began to show a marked decrease. The phenolic content on a fresh weight basis showed behaviour similar to soluble solids, soluble solids/total titratable acidity ratio and anthocyanin content (), whereas the phenolic content on a dry weight basis showed similar behaviour to the flavonoid content, as shown by the significant and positive correlations found between them (). The differences in timing observed in the presentation of the phenolic peaks at the beginning and end of the ripening period could be due to the differential dry fruit weight as a percentage of fresh weight accumulated; dry fruit weight as a percentage of fresh weight doubled at day 126 AFFP (39.8%), with respect to day 70 AFFP (20.6%). Also, phenolic compound metabolism varies greatly according to the physiological stage of the fruit, and their biochemical production rates rely on the participation of many key enzymes, which are also limited by the availability of precursors. Thus, it is expected that each class of phenolic compound shows a quite different content evolution during ripening, which is seen in peaks at different harvesting dates (Raffo et al. Citation2004), and explains the differences found in the yield of methanolic extracts during ripening. Phenolic compounds are usually higher in young than in mature fruits with the exception of anthocyanins (Kähkönen et al. 2001). Typically, evolution of the phenol content in other fruit crops may show a decrease during growth. In some cases, the level of phenols continues to decrease steadily, as occurs in some species and white-coloured fruits (varieties of white grapes, mango and banana), or it may increase with maturation, as occurs in red fruits (apples) in which anthocyanins or other flavonoids accumulate (Häkkinen et al. Citation2000). Phenols decrease in both blackberries and strawberries as the fruit matures (Wang & Lin Citation2000).
Table 2 Linear correlation coefficients (r) between pairs of variables.
Scavenging activity on DPPH radicals
The DPPH scavenging effect varied during the fruiting period and with the methanolic extract concentration (). The DPPH scavenging effect at days 70 and 126 AFFP was effective at a lower concentration (0.25 mg·mL−1) than at days 84, 98 and 112 AFFP (0.50 mg·mL−1), which could be explained through the similar pattern of accumulation of phenol content and their significant and positive correlations (). The DPPH scavenging effect for standard antioxidants was effective at lower concentrations (0.10 mg·mL−1) than for fruit methanolic extracts (0.25 and 0.50 mg·mL−1) (data not shown). The effective concentration at which the DPPH radicals were scavenged by 50% (EC50) was significantly different among methanolic extracts from different days after full flower phase (). The lowest values were found at days 70 and 126 AFFP (0.13 and 0.15 mg·mL−1, respectively), whereas the highest value (0.23 mg·mL−1) was obtained at day 98 AFFP. The DPPH scavenging effect was positive and significantly correlated with total titratable acidity, with flavonoid (R 2= 40.8; y=30.6893 + 0.8693x) and phenol content (R 2 =71.1; y=−15.6222 + 1.6468x) in terms of dry fruit weight (), as found in Diospyros kaki L. cv. Mopan (Chen et al. Citation2008), although it was not correlated with anthocyanin content as found for strawberries (Cheel et al. Citation2007). The DPPH scavenging effect observed in B. buxifolia was comparable with that reported for B. vulgaris fruits (Motalleb et al. Citation2005) and for B. koreana bark (Qadir et al. Citation2009).
Figure 2 Scavenging effect on 1,1-diphenyl-2-picrylhydrazyl radicals (%) on B. buxifolia fruit extracts from day 70 to day 126 after full flower phase and at different methanolic extract concentrations (0.0–1.0 mg·mL−1) (n = 6). Error bars represent ± 1 standard error of the mean.
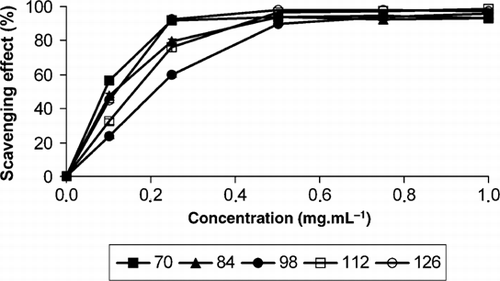
Table 3 Concentration of methanolic extracts (mg·mL−1) at which the 1,1-diphenyl-2-picrylhydrazyl radicals were scavenged by 50% (mg·mL−1) and the reducing power (mg·mL−1) was 50% in Berberis buxifolia fruits in the days after the full flower phase.
Reducing power
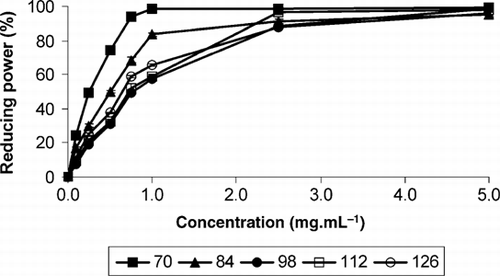
The reducing power varied during the fruiting period and with the methanolic extract concentration (). Reducing power at day 70 AFFP was effective at a lower concentration (0.75 mg·mL−1) than at days 84, 98, 112 and 126 AFFP (2.50–5.00 mg·mL−1), which might be explained by the similar pattern of flavonoid content evolution. In addition, the reducing power of standard antioxidants was effective at lower concentrations (0.10–0.75 mg·mL−1) than for fruit methanolic extracts (0.75–5.00 g·mL−1) (data not shown). The effective concentration at which the reducing power was 50% (EC50) was significantly different among the methanolic extracts obtained at different days after full flower phase (). The lowest values were found at 70 AFFP (0.36 mg·mL−1), and the highest values at day 98 AFFP (0.80 mg·mL−1), decreasing to 0.65 mg·mL−1 at day 126 AFFP. Reducing power was positively and significantly correlated with total titratable acidity, flavonoid (R 2= 80.4; y= 6.2790 + 0.6229x) and phenol content (R 2 = 82.8; y= −17.5062 + 0.9074x) in terms of dry fruit weight, as found for B. vulgaris and Berberis croatica (Koncic et al. Citation2010), whereas it was negatively and significantly correlated with anthocyanin content (). A positive and significant correlation was found between phenol content and antioxidant activity measured through the TEAC assay in B. microphylla fruits (Ruiz et al. Citation2010). The described relationships might explain the maximum DPPH scavenging effect and reducing power values obtained at day 70 AFFP, as well as the minimum values for DPPH scavenging effect at day 98 AFFP, and the contribution of the flavonoid and phenolic content, which are responsible at least in part for the demonstrated antioxidant activity of B. buxifolia methanolic extracts. Although the DPPH scavenging effect of B. buxifolia extracts was more effective at lower concentrations (0.50 mg·mL−1) than it was for reducing power (0.75–5.00 mg·mL−1), there was a strong relationship between both methods with regard to the antioxidant activity, as shown by the significant linear correlation found between both parameters (). It is noteworthy that the DPPH scavenging effect and reducing power of B. buxifolia fruit extracts showed maximum values at concentrations scarcely higher than the standard antioxidant.
Figure 3 Reducing power (%) on B. buxifolia fruit extracts from day 70 to day 126 after full flower phase and at different methanolic extract concentrations (0.0–5.0 mg·mL−1) (n = 6). Error bars represent ± 1 standard error of the mean.
The antioxidant activity of the fruits and their phenolic composition involve complex relationships, and thus are very difficult to describe using statistical tools (Kähkönen et al. Citation2001). The antioxidant activities attributed to these substances show divergences within subgroups of compounds, hence antioxidant properties of a single compound within a group can vary remarkably. Therefore, equal levels of one specific compound do not necessarily mean an equal antioxidant response as a whole. Synergism, i.e. the ability of an antioxidant compound to improve when summed with the activity of another, is another reason for the divergences. Differences may also arise from the methodology used to assay the antioxidant activity, for example, whereas the oxygen radical absorbance capacity (ORAC) method has positive correlations with anthocyanins in blueberry extracts (Prior et al. Citation1998), the DPPH method does not show any correlation with anthocyanins in strawberries (Cheel et al. Citation2007; Ferreyra et al. Citation2007), thus explaining the complex relationship between the antioxidant activity of the fruits and their phenolic composition (Kähkönen et al. Citation2001).
Conclusions
The accumulation patterns of phenolic compounds during fruit growth and ripening in B. buxifolia varied depending on the specific group considered, i.e. anthocyanins, flavonoids or phenols, and can be correlated with fruit quality characteristics including fruit weight, firmness and colour, soluble solids, total titratable acidity and their ratios, which are responsible for astringency, texture, taste and colour. This, together with the variation in antioxidant activity during fruiting and the definition of adequate terms of reference, i.e. biomass of secondary metabolites on the basis of fresh or dry fruit weight, or on a per fruit unit basis, are of considerable importance when compared with other species, or from a practical point of view when the nutraceutical value of a fruit has to be examined. Spectrophotometric methods to quantify phenolic contents are simple, fast and quite accurate, although they do not characterise a specific component. Although this study was carried out during a single, but representative, growing season, knowledge of the accumulation patterns of these secondary metabolites during B. buxifolia fruit growth and ripening will allow us to understand the effect of preharvest factors such as light and fertilisation on this period, and contribute to selecting the most appropriate practices and assigning the best alternative uses (beginning of ripening for fresh market, ending of ripening for industrial processing, etc.). Irrespective of differences due to the methodology used to study the antioxidant responses of the methanolic fruit extracts, and taking into consideration the antioxidant responses by comparison with widely used standard antioxidant substances and with other small fruit species, B. buxifolia fruit appeared to possess excellent antioxidant activity during the second and third fruit growth phases (lag phase and the time when fruit ripening occurs, respectively), which may contribute to its functional value, acquiring relevance and applicability for nutraceutical purposes.
Acknowledgements
This work received funds from the Consejo Federal de Ciencia y Tecnología (PFIP) and INTA.
References
- Albrecht , C , Pellarin , G , Rojas , MJ , Albesa , I and Eraso , AJ . 2010 . Beneficial effect of Berberis buxifolia Lam, Zizyphus mistol Griseb and Prosopis alba extracts on oxidative stress induced by chloramphenicol . Medicina-Buenos Aires , 70 ( 1 ) : 65 – 70 .
- Arena , ME and Curvetto , N . 2008 . Berberis buxifolia fruiting: kinetic growth behavior and evolution of chemical properties during the fruiting period and different growing seasons . Scientia Horticulturae , 118 : 120 – 127 .
- Béliveau R , Gingras D 2005 . Les aliments contre le cancer . Montréal (Québec), Éditions Trécarré .
- Çelik , H , Özgen , M , Serçe , S and Kaya , C . 2008 . Phytochemical accumulation and antioxidant capacity at four maturity stages of cranberry fruit . Scientia Horticulturae , 117 : 345 – 348 .
- Cheel , J , Theoduloz , C , Rodríguez , JA , Caligari , PDS and Schmeda-Hirschmann , G . 2007 . Free radical scavenging activity and phenolic content in achenes and thalamus from Fragaria chiloensis ssp. chiloensis, F. vesca and F.×ananassa cv. Chandler . Food Chemistry , 102 : 36 – 44 .
- Chen , XN , Fan , JF , Yue , X , Wu , XR and Li , LT . 2008 . Radical scavenging activity and phenolic compounds in persimmon (Diospyros kaki L. cv. Mopan) . Journal of Food Science , 73 ( 1 ) : 24 – 28 .
- Conde , C , Silva , P , Fontes , N , Dias , ACP , Tavares , RM , Sousa , MJ , Agasse , A , Delrot , S and Gerós , H . 2007 . Biochemical changes throughout grape berry development and fruit and wine quality . Food , 1 ( 1 ) : 1 – 22 .
- Deighton , N , Stewart , D , Davies , HV , Gardner , PT , Duthie , GG , Mullen , W and Crozier , A . 2002 . Soft fruit as sources of dietary antioxidants . Acta Hortculturae , 585 : 459 – 465 .
- Ferreyra , RM , Viña , SZ , Mugridge , A and Chaves , AR . 2007 . Growth and ripening effects on antioxidant capacity on strawberry cultivar Selva . Scientia Horticulturae , 112 : 27 – 32 .
- Giusti MM , Wrolstad RE 2001 . Unit F1.2.1–13: anthocyanins. Characterization and measurement with UV–visible spectroscopy . In: Wrolstad RE Current protocols in food analytical chemistry . New York , Wiley . doi: 10.1002/0471142913.faf0102s00
- Häkkinen , SH , Kärenlampi , SO , Mykkänen , HM and Törrönen , AR . 2000 . Influence of domestic processing and storage on flavonol contents in berries . Journal of Agriculture and Food Chemistry , 48 ( 7 ) : 2960 – 2965 .
- Hou , DX . 2003 . Potential mechanisms of cancer chemoprevention by anthocyanins . Current Molecular Medicine , 3 : 149 – 15 .
- Jackson , RS . 2008 . Wine science: principles and applications , 751 Burlington , MA : Academic Press .
- Kähkönen , MP , Hopia , AI and Heinonen , M . 2001 . Berry phenolics and their antioxidant activity . Journal of Agriculture and Food Chemistry , 49 : 4076 – 4082 .
- Kähkönen , MP , Hopia , AI , Vuorela , HJ , Rauha , J-P , Pihlaja , K , Kujala , TS and Heinonen , M . 1999 . Antioxidant activity of plant extracts containing phenolic compounds . Journal of Agriculture and Food Chemistry , 47 : 3954 – 3962 .
- Koncic , MZ , Kremer , D , Karlovic , K and Kosalec , I. 2010 . Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat . Food and Chemical Toxicology , 48 ( 8–9 ) : 2176 – 2180 .
- Kuskoski , EM , Asuero , AG , Troncoso , AM , Mancini-Filho , J and Fett , R . 2005 . Aplicación de diversos Métodos químicos para determinar actividad antioxidante en pulpa de frutos . Ciência e Tecnología de Alimentos, Campinas , 25 ( 4 ) : 726 – 732 .
- Lister , CE , Wilson , PE , Sutton , KH and Morrison , SC . 2002 . Understanding the health benefits of blackcurrants . Acta Horticulturae , 585 : 443 – 449 .
- Monge , A , Chorghade , M , Erhardt , PW , Genellin , CR , Koga , N , Lindberg , P , Perun , TJ , Topliss , JG , Trivedi , BK and Wermuth , CG . 2000 . Medicinal chemistry in the development of societies: biodiversity and natural products . European Journal of Medicinal Chemistry , 35 : 1121 – 1125 .
- Moore DM 1983 . Flora of Tierra del Fuego . Oswestry, UK, Anthony Nelson & Missouri Botanical Garden .
- Motalleb , G , Hanachi , P , Kua , SH , Fauziah , O and Asmah , R . 2005 . Evaluation of phenolic content and total antioxidant activity in Berberis vulgaris fruit extract . Journal of Biological Sciences , 5 ( 5 ) : 648 – 653 .
- Orsi MC 1984 . Berberidaceae . In: Correa MN Flora Patagónica. Secc. 4a. Tomo VIII . . Buenos Aires , , Argentina , INTA . Pp. 325 – 348 .
- Oyaizu , M . 1986 . Studies on product of browning reaction prepared from glucose amine . Japanese Journal of Nutrition , 44 : 307 – 315 .
- Prior , RL , Cao , G , Martin , A , Sofic , E , McEwen , J , O'Brien , C , Lischner , N , Ehlenfeldt , M , Kalt , W , Krewer , G and Mainland , CM . 1998 . Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species . Journal of Agriculture and Food Chemistry , 46 : 2686 – 2693 .
- Prior , RL , Wu , X and Schaich , K . 2005 . Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements . Journal of Agricultural and Food Chemistry , 53 : 4290 – 4302 .
- Qadir , SA , Kwon , MC , Han , JG , Ha , JH , Chung , HS , Ahn , J and Lee , HY . 2009 . Effect of different extraction protocols on anticancer and antioxidant activities of Berberis koreana bark extracts . Journal of Bioscience and Bioengineering , 107 ( 3 ) : 331 – 338 .
- Raffo , A , Paoletti , F and Antonelli , M . 2004 . Changes in sugar, organic acid, flavonol and carotenoid composition during ripening of berries of three seabuckthorn (Hippophae rhamnoides L.) cultivars . European Food Research and Technology , 219 ( 4 ) : 360 – 368 .
- Roussos , PA , Denaxa , N-K and Damvakaris , T . 2009 . Strawberry fruit quality attributes after application of plant growth stimulating compounds . Scientia Horticulturae , 119 : 138 – 146 .
- Ruiz , A , Hermosin-Gutierrez , I , Mardones , C , Vergara , C , Hertlitz , E , Vega , M , Dorau , C , Winterhalter , P and von Baer , D . 2010 . Polyphenols and antioxidantactivity of calafate (Berberis microphylla) fruits and other native berries from southern Chile . Journal of Agricultural and Food Chemistry , 58 ( 10 ) : 6081 – 6089 .
- Shimada , K , Fujikawa , K , Yahara , K and Nakamura , T . 1992 . Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion . Journal of Agriculture and Food Chemistry , 6 : 945 – 948 .
- Surveswaran , D , Cai , Y-Z , Corke , H and Sun , M . 2007 . Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants . Food Chemistry , 102 : 938 – 953 .
- Taga , MS , Miller , EE and Pratt , DE . 1984 . Chia seeds as a source of natural lipid antioxidants . Journal of the American Oil Chemists Society , 61 : 928 – 993 .
- Tomosaka , H , Chin , YW , Salim , AA , Keller , WJ , Chai , H and Douglas Kinghom , A . 2008 . Antioxidant and cytoprotective compounds from Berberis vulgaris (barberry) . Phytotherapy Research , 22 ( 7 ) : 979 – 981 .
- Turkoglu , A , Duru , ME , Mercan , N , Kivrak , I and Gezer , K . 2007 . Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murril . Food Chemistry , 101 : 267 – 273 .
- Vvedenskaya , IO and Vorsa , N . 2004 . Flavonoid composition over fruit development and maturation in American cranberry, Vaccinium macrocarpon Ait . Plant Science , 167 : 1043 – 1054 .
- Wang , SY and Lin , H-S . 2000 . Antioxidant activity in fruits and leaves of blackberry, raspberry and strawberry varies with cultivar and developmental stage . Journal of Agriculture and Food Chemistry , 48 ( 2 ) : 140 – 146 .
- Zheng , W and Wang , SY . 2003 . Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries . Journal of Agriculture and Food Chemistry , 51 : 502 – 509 .
- Zhishen , J , Mengcheng , T and Jianming , W . 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .