Abstract
Pollination efficacy in the dioecious Coprosma spathulata was investigated in eight 900-m2 study plots. Mean fruit set was generally high (>75% in six of eight populations), aided by male-biased populations, male floral abundance and extended female receptivity in unpollinated flowers. However, pollen receipt and fruit set were both susceptible to reduction by dense understory vegetation, low abundance of males at the plot scale and in isolated females. There was a steep reduction in fruit set when females were >7 m from the nearest male, suggesting Allee effects in isolated females and in low-density populations. Although C. spathulata lives in an unpromising habitat for wind pollination, floral characteristics and flowering behaviour ensure it is surprisingly successful.
Introduction
Wind pollination (anemophily) has been reported in 18% of the world's angiosperm families, is the dominant pollination syndrome in some of them (Ackerman Citation2000) and appears to be well-suited to certain types of habitat. It is more common at higher latitudes and elevations, and in open-structured and floristically less diverse vegetation, e.g. savanna and deciduous forests (Whitehead Citation1969; Culley et al. Citation2002). Wind pollination is also relatively more common in remote island floras, e.g., Hawaii and the Juan Fernández Islands (Carlquist Citation1966; Whitehead Citation1969, Citation1983; Regal Citation1982; Anderson et al. Citation2001; Bernardello et al. Citation2001).
Wind pollination is a relatively passive process controlled primarily by microclimate factors, and pollen transport is enhanced by attributes such as open vegetation structure, close spacing of conspecifics, the production of large numbers of pollen grains, the timing of pollen release, wind velocities and relatively low humidity and rainfall during flowering (Whitehead Citation1983). Pollen transport is a function of wind velocity and the terminal velocity of a pollen grain, which is largely determined by pollen size (Whitehead Citation1969; Niklas Citation1985a). Pollen export is maximised by having large, well-exposed anthers hanging on long filaments and the production of large quantities of small pollen grains, whereas pollen capture is enhanced by structures such as bracts and sepals which deflect air currents towards the stigma (Niklas Citation1985a,b; Niklas & Buchmann Citation1985; Honig et al. Citation1992) and exposed large, finely divided stigmas (Faegri & Van der Pijl Citation1979; Proctor et al. Citation1996; Friedman & Barrett Citation2009).
Rainforests are often considered unsuitable for effective wind pollination because of low wind velocities caused partly by dense evergreen foliage, frequent rainfall and high humidity levels which reduce pollen transport distances, and vegetation structure and floristic diversity intercepts pollen travel (Whitehead Citation1969, Citation1983; Friedman & Barrett Citation2009).
The conifer/broadleaved (podocarp/angiosperm) rainforests of New Zealand are floristically diverse and primarily evergreen (Wardle Citation1991) with high humidity levels and rainfall which might render wind pollination ineffective in understory plants. For example, the annual rainfall in the Waikato region ranges from 1200 to 2400 mm (Waikato Regional Council Citation2011). Despite this, there are a number of native, anemophilous shrub species, especially members of the genus Coprosma that are a conspicuous element in many of these forests.
Coprosma species are dioecious, which makes wind pollination even more challenging by increasing the required mean pollen transport distance. Although pollen limitation is thought to be mostly confined to animal-pollinated plant species, wind-pollinated taxa can also be limited by a lack of pollen (Knapp et al. Citation2001; Kelly & Sork Citation2002; Koenig & Ashley Citation2003; Davis et al. Citation2004a,b; Friedman & Barrett Citation2009). Indeed, it has been suggested that pollen limitation in wind-pollinated species is one of the driving forces behind mast seeding, a characteristic of anemophilous trees that ensures better pollination by elevating pollen density in heavy flowering years (Nilsson & Wastljung Citation1987; Kelly Citation1994; Kelly et al. Citation2001; Koenig & Ashley Citation2003). In small populations, pollination interactions may be weakened and reproductive opportunities reduced if individuals are mate-limited (Hackney & McGraw Citation2001; Dennis Citation2002). For example, Nilsson and Wastjung (Citation1987) reported pollen limitation in low-density populations of wind-pollinated Fagus sylvatica, and Allison (Citation1990) found that reduced flower density in Taxus canadensis was also a contributing factor. When mate-finding efficiency is reduced because of low population density, an Allee effect can occur and the breeding system can influence the strength of the effect (Bessa-Gomes et al. Citation2004; Calabrese & Fagan Citation2004). An Allee effect has also been used to explain the benefit to individuals of the density of conspecifics (Stephens et al. Citation1999).
Gender dimorphism is common in wind-pollinated plants (Darwin Citation1877; Charlesworth Citation1993; Proctor et al. Citation1996; Friedman & Barrett Citation2009) and the sex ratio of populations of dioecious species has important consequences for reproduction (Delph Citation1999; Bessa-Gomes et al. Citation2004). A critical determinant of seed set, therefore, is the density of conspecific flowers, specifically of male flowers in gender-dimorphic species (Friedman & Barrett Citation2009), however, the relationship between male flower abundance and fruit set does not appear to have been characterised for wind-pollinated species.
Because of its low stature, Coprosma spathulata presented an opportunity to study how reproductive success is influenced by conspecific density, floral abundance in males and habitat characteristics. This study follows on from an earlier investigation of fruit set in natural and manually crossed flowers in two C. spathulata study sites during 2002 in which a high level of pollen limitation was recorded in one population (Merrett et al. Citation2007).
In this study, we investigate whether population density and habitat characteristics influence reproductive output in eight C. spathulata populations by asking: (1) how successful is wind pollination in this species; (2) is reproductive output affected by sex ratio, proximity of male plants, and male floral abundance; and (3) does the density of vegetation in the shrub layer influence fruit set?
Materials and methods
Study species
Coprosma (Rubiaceae) includes c. 100 species worldwide (Gardner Citation2002) and >50 species in New Zealand which are widely represented throughout the country (Allan Citation1961; Poole & Adams Citation1994; Parsons et al. Citation1998). All species are dioecious and anemophilous (Webb et al. Citation1999). Coprosma species occur in virtually every ecosystem from coastal to montane habitats and 22 species occur in forests, shrubland or scrub (Allan Citation1961; Poole & Adams Citation1994).
Coprosma spathulata Cunn. is an understorey shrub mostly <2 m tall which grows in lowland native forests of the northern North Island of New Zealand from the Waikato region (37°S) northwards (Allan Citation1961). It is typically found in secondary, evergreen conifer/broadleaved native forest (c. 50–100 years old), usually under a shaded, but discontinuous canopy and mainly on ridges. It flowers during late winter and early spring (August–September). Each female flower has two ovules and two exserted papillate stigmas up to 1.5 cm in length. The male flowers have four anthers that hang from the corolla on long filaments. The four anthers dehisce simultaneously within a few hours of emergence from the bud, and each anther produces c. 30,000 pollen grains, thus the pollen–ovule ratio is high (Merrett et al. Citation2007). The fruit is a two-seeded, bird-dispersed drupe (Webb & Simpson Citation2001).
Study sites
Field studies were carried out on C. spathulata populations of varying density in the Waikato region of the North Island of New Zealand during 2003. Eight 30×30 m (900 m2) plots were established within three native forests (). Four plots (HK1–HK4) were in Hakarimata Scenic Reserve, an 1800-ha area of native forest that is part of the Hakarimata Range and is characterised by steep ridge and gully systems, extending 19 km in length and 374 m a.s.l. at its highest point (Department of Lands and Survey Citation1984). Plot KR was on the Karakariki Range, a southern extension of the Hakarimata Range and with similar geography, altitude and vegetation. Three plots (PM1–PM3) were located in Pukemokemoke Bush Reserve, a 40-ha hill country, forested remnant that rises to 166 m a.s.l.
Table 1 Study sites and characteristics of eight populations of Coprosma spathulata in the Waikato region, New Zealand.
Population and habitat characteristics
In August 2003, the location of all C. spathulata plants was mapped in each plot to establish population density, the gender of each flowering adult was recorded, open flowers on all male plants were counted, and distances between male and female plants were calculated. In the small, low-density plots, the plots were either positioned to include all plants in the vicinity and thus to include all the likely potential male pollen donors, or where this was not possible, we only monitored female plants that were not near male plants located outside the plots. In the two highest density plots (HK2 and PM1), all the males were mapped in the whole 30×30 m plot, but the monitored females were all within the 10×10 m inner zone. Thus, in all cases, the female–male separation distances were not affected by unmapped male plants. The percent cover of vegetation in the shrub layer in each plot was estimated to assess whether there was any relationship between fruit set and vegetation density.
Fruit set and flowering
To measure natural fruit set, female plants in each plot were identified with numbered tags, and flowers and buds were counted on tagged branches. Fruit set was counted in December 2003 when the fruit were well developed, but not yet ripe. In total, 2910 female flowers on 83 plants were monitored for fruit set among the eight study populations ().
As a proxy measurement of the density of pollen in the air, two or three (depending on availability) mature flowers were collected from each tagged female plant in each plot, mounted and stained on microscope slides with fuchsin gel (Beattie Citation1971), and pollen grains were counted on each stigma using a compound microscope.
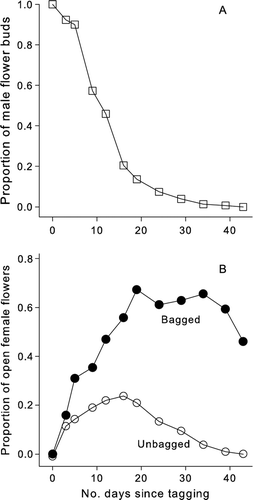
Flowering periodicity of plants of both sexes (five female and six male) was monitored on tagged branches in a representative plot (PM1). Initially, the number of buds on each branch was counted, with subsequent monitoring at 3–4-day intervals. For females, opened flowers were counted and for males, unopened buds were counted. To monitor for agamospermy and compare rates of female flower longevity in bagged and unbagged buds, an additional set of mature flower buds was enclosed in cellophane bags to exclude pollen and at each visit, open flowers were counted. Cellophane bags were used to ensure no pollen contamination of treatment flowers, and pre-treatment trials showed no evidence of internal condensation.
Statistical analysis
The sex ratios in each population were analysed using exact binomial tests on the counts of male and female plants. Fruit set and pollen receipt were analysed at two levels. At the plot level, two-factor generalised linear models (GLMs) were used to estimate the effects of male plant density and shrub layer cover on plot mean fruit set (assessed with a binomial model) and on mean pollen receipt (log-transformed to improve normality). At the plant level, blocked GLMs were used to test the effects of distance to the nearest male plant on the mean number of pollen grains per flower (using a quasi-Poisson model), and the effects of distance to the nearest male and pollen load on fruit set (again with a binomial model), using plots as blocks in both analyses. In order to characterise the relationships between pollen load and fruit set, and between these parameters and the distance to the nearest male irrespective of plot identity, logistic and quasi-Poisson regression coefficients were also estimated in single-factor analyses. These analyses use type I sum of squares, so that the first entered term takes priority in explaining variation in the dependent variable, and subsequent terms attempt to explain any residual variation. The statistical programme R version 2.10.1 was used for all analyses.
Results
There was considerable variation in C. spathulata density among the eight study plots, ranging from 13 (KR) to 259 (PM1) flowering individuals (). Populations were male-biased in seven of the eight plots, significantly so in four of them; males ranged from 28.6% (HK1) to 91.1% (HK2) (). Fruit set ranged from 15.8% in KR to 96.5% in HK4 (). Male flower abundance also varied widely, ranging from 66 flowers in KR to 3379 flowers in PM1 (). The two populations with the lowest fruit set also had the lowest number of male flowers (A). The shrub layer was variable among plots and ranged from 25% (HK3) to 95% (KR) ().
Figure 1 Relationships between fruit set, population and habitat characteristics across eight study plots containing Coprosma spathulata. A, Means of fruit set plotted against the number of male flowers per plot. B, Means of fruit set plotted against shrub cover. C, Mean number of pollen grains per flower plotted against the number of male flowers per plot. D, Mean number of pollen grains per flower plotted against shrub cover. E, Means of fruit set plotted against male ratios. F, Means of fruit set plotted against mean number of pollen grains per flower. Plots were 30×30 m (900 m2).
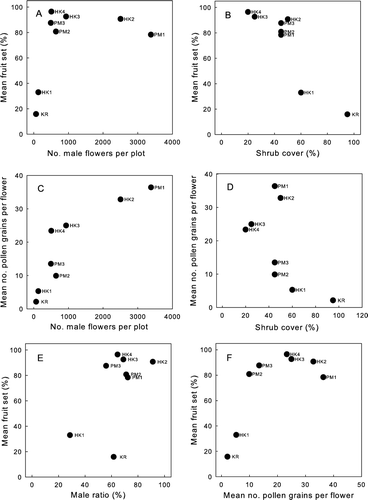
Among all plots, pollen receipt and fruit set both increased significantly with male flower abundance and, having allowed for this effect, decreased significantly with the percent of shrub cover (A–1D, ). The two plots (HK1 and KR) with the lowest population density and lowest male flower abundance had the lowest fruit set, 15.8% and 33%, respectively ().
Table 2 Multiple regression on mean number of pollen grains per flower (linear model on log-transformed data) and mean fruit set (logistic model) tested against shrub cover and the number of male flowers in each plot.
The mean number of pollen grains per flower (two stigmas) ranged from 2.1 in KR to 36.4 in HK2 (). Much of the variation in pollen load and fruit set was at the plot level (, , ) reflecting the variation in mate availability among plots and likely dampening effects of the shrub layer, but even after allowing for plot effects, there was still a local effect of distance to the nearest male plant on fruit set () and a marginally non-significant effect on pollen receipt (A, ). The two plots with the lowest mean fruit set, male flowers and pollen load on flowers (HK1 and KR) also had the greatest density of vegetation in the shrub layer (D–1F, ).
Figure 2 Means and range of numbers of pollen grains recorded on female Coprosma spathulata flowers from eight study plots. The standard error of the mean for each plot is shown in parentheses.
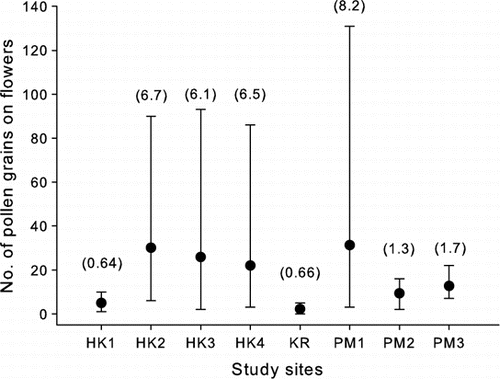
Figure 3 Relationships between the distance to the nearest male plant, pollen receipt on individual flowers (two stigmas) and fruit set in the 83 female Coprosma spathulata plants. A quasi-Poisson regression is fitted to the pollen receipt data and logistic regressions are fitted to the fruit set data. A, Pollen grains versus distance to male (P < 0.001). B, Fruit set versus distance to male (P < 0.001). C, Fruit set versus pollen grains (P < 0.001).
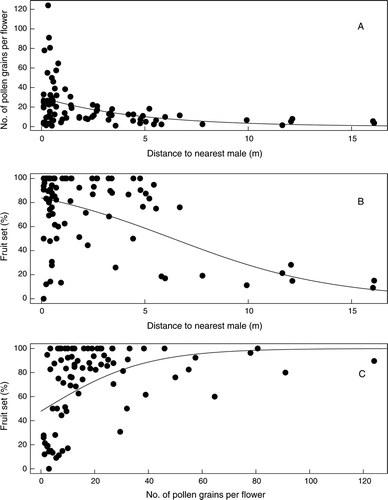
Table 3 Quasi-Poisson regression of mean number of pollen grains per flower per plant fitted to the distance to the nearest male plant.
Table 4 Multiple logistic regression of fruit set per plant fitted to the distance to the nearest male plant and the mean number of pollen grains per flower on each plant.
Because we wanted to examine the effects of local pollen supply on reproductive performance we also modelled the effects of isolation on pollen receipt and fruit set irrespective of plot using single-factor regressions (). Although most plants received multiple pollen grains per flower (median across all plots, 19.9) and had high fruit set (median 72.4%), some plants were much more pollen limited. Plants with fewer than c. 10 pollen grains per flower or more than c. 7 m from the nearest male plant were much more likely to have low fruit set. Plants at 10–16 m distance had between 10% and 30% fruit set ().
Flowering duration was spread over a six-week period in both male and female plants in plot PM1, with mean peak flowering in females occurring c. 15 days from the start of flower opening (). In male plants, there was an initial rapid burst of flowering (half the buds had opened by c. 12 days from first opening), followed by a steady reduction in the number of unopened buds. Bagged female flowers remained open and presumably receptive for considerably longer than unbagged flowers so that apart from the first count, all subsequent counts showed a significantly larger proportion of flowers in bags compared with the unbagged (). Many (52%) of the original bagged buds remained in an open-flower state when the experiment was terminated after six weeks at the end of natural flowering; unbagged flowers had all senesced by this time.
Discussion
The results of this study show that in general, anemophily is an effective pollination strategy in C. spathulata, albeit prone to reduced fruit set at low pollen density, and subjected to probable dampening effects from dense vegetation in the shrub layer. The generally high fertilisation rate in female plants, despite its relatively low stature is attributable to several features of the pollination system; male-biased sex ratios in most plots, high pollen production, low ovule number per flower, a relatively large stigmatic surface and delayed senescence of female flowers that are not immediately pollinated. Further investigation into delayed senescence of bagged female flowers compared with those manually pollinated before bagging would help confirm this as a reproductive assurance strategy. However, the same phenomenon was also noted in the dioecious, wind-pollinated Macropiper excelsum, but not in any animal-pollinated, dioecious taxa (Merrett Citation2006).
All but one of the plots had >50% males, and in one case the proportion of male flowering plants was over 90%. Male-biased sex ratios are common in dioecious species (Delph Citation1999), and usually develop post-fertilisation as a result of factors such as higher costs of reproduction and consequently higher rates of mortality in females (Lloyd & Webb Citation1977) and greater rates of herbivory affecting female plants (Ågren et al. Citation1986). Whatever the cause in this case, the high density of male plants combined with male floral abundance and high pollen counts per flower raises pollen density.
In C. spathulata, the pollen production of each flower is c. 120,000 grains (Merrett Citation2006) and because there are two ovules, the pollen–ovule ratio (P/O) is consequently very high at 60,185 (Merrett et al. Citation2007). P/O values are considered an indicator of the efficiency of pollen transfer, therefore very high P/O values in C. spathulata suggest inefficiency (Cruden Citation1977). The P/O of C. spathulata is substantially above the median value for other wind-pollinated species (22,150) (Cruden Citation2000), and the effective ratio may be even higher since in most of the study populations male plants outnumber female plants.
Each C. spathulata ovule is served by a densely papillate stigma with a relatively large stigmatic surface. Assuming a cross-section of c. 2 mm diameter, the surface area of each stigma is > 1 cm2, even without allowing for the papillae. Long feathery stigmas are common in wind-pollinated plants (Whitehead Citation1969; Ackerman Citation2000), but this is a large surface area for an anemophilous plant and unusual outside cereals like maize which have very long stigmatic tassels. The female flowers are also unusually long-lived and, as shown by the bagging experiments, can remain open for several weeks if pollination does not occur, thus increasing the chance of eventually trapping sufficient pollen to set seed. Delayed senescence of unpollinated flowers appears to be common in animal-pollinated plants (Primack Citation1985; Ashman & Schoen Citation1994) and presumably gains reproductive assurance at the expense of maintaining flowers in receptive condition (Ashman & Schoen Citation1994), but to our knowledge it has not been previously reported as a tactic to increase pollination efficacy in wind-pollinated plants.
Despite these features that assist pollination efficiency, fruit set is not uniformly high in C. spathulata. The plot with a female-biased population and the plot with 95% shrub cover had the lowest mean fruit set, and isolated females were strongly pollen limited. Significant pollen limitation has been shown previously with hand-pollinations in two populations of this species (Merrett et al. Citation2007). Allee effects are limitations on reproduction that occur at low population densities, and they arise in C. spathulata because low fruit set indicates pollen limitation in isolated females. Pollen limitation is experimentally tested by a comparison of supplemental hand-pollination with natural pollination to distinguish resource limits to seed production versus limits due to pollen supply (Bierzychudek Citation1981; Ashman et al. Citation2004), but few such tests have been performed in wind-pollinated shrubs (Knight et al. Citation2005; Friedman & Barrett Citation2009).
In wind-pollinated trees, pollen limitation has been inferred by the identification of significant correlations between seed set and either tree density or nearest-neighbour distance in Taxus canadensis and Pinus contorta (Smith et al. Citation1988; Allison Citation1990). Similarly, reduced population densities and insufficient pollen supply increased the risk of reproductive failure in two oak species (Quercus douglasii, Q. lobata) (Knapp et al. Citation2001; Kelly & Sork Citation2002). Among herbs, Steven and Waller (Citation2007) found that seed set was reduced with increased pollen donor distance in low-density populations of two Thalictrum species, but not in high-density populations. However, some studies have found no effect of male density on fruit set (Berry & Gorchov Citation2004).
This study allows identification of the density threshold below which Allee effects become important. Coprosma spathulata females at more than c. 10 m from the nearest male had very low fruit set. Similarly, work on the dioecious Rumex nivalis showed that seed set was sensitive to increasing distance to males at local scales of < 2 m (Stehlik & Barrett Citation2006; Stehlik et al. Citation2008; Friedman & Barrett Citation2009). These patterns suggest that the colonisation and establishment of new populations may be difficult while plant densities remain low (Smith et al. Citation1988; Stevenson Citation2007). For dioecious species such as C. spathulata to overcome Allee effects, males need to be present in close proximity to females and produce abundant pollen. Allee effects like this were identified as retarding the rate of invasion of Spartina alterniflora in estuaries which was slow until vegetative spread of colonists was sufficient to raise pollen densities (Davis et al. Citation2004a; Taylor et al. Citation2004).
Seedlings were recorded in all of the C. spathulata study plots (Merrett Citation2006) indicating that even when fruit set was low, there is potential population recruitment from previous reproductive success, and there was suitable habitat for seed germination and establishment. However, ongoing monitoring would be required to determine their long-term survival.
An alternative strategy isolated females can adopt is agamospermy (Baker Citation1955), but since this only clones more females, it will not restore sexual reproduction in the absence of males, and apomixis remains rare in dioecious plants (Dupont Citation2002). Heenan and colleagues (Citation2002, Citation2003) have found evidence for agamospermy in other New Zealand Coprosma species but there is no evidence that it occurs in C. spathulata.
Native forest succession after disturbance leads to increasing species diversity and abundance in the ground and shrub layer (Wardle Citation1991). For understory, wind-pollinated taxa such as C. spathulata, as well as other forest-dwelling Coprosma species, the density of the vegetation in the shrub layer is likely to have a negative impact on pollen transport; wind velocity would be reduced and stems and leaves would intercept pollen grains, resulting in lower fruit set. The habitat itself might also become increasingly less likely to have open areas suitable for seed germination unless further disturbance events such as tree fall, opened new habitat for colonisation. An investigation into environmental variables such as temperature, humidity, rainfall, and wind direction and velocity in relation to fruit set in individual female plants and pollen transport in males could be useful in identifying whether these factors also influence variation in reproductive output in this species.
We have shown that although C. spathulata lives in an unpromising habitat for wind pollination, floral characteristics and flowering behaviour ensures it is surprisingly successful.
Acknowledgements
We thank the Department of Conservation and private landowners for giving access or permits to study sites, and Donna Worthy, Louise Duncan, Mark Smale and Neil Fitzgerald for help with fieldwork. Thanks also to Dave Kelly (Canterbury University) for comments on an earlier draft, Zlatko Kovacic (Open Polytechnic) for additional statistical advice, and two anonymous referees for their comments. This work was supported by the New Zealand Foundation for Research, Science and Technology under Public Good Science Fund (contracts CO9X0004, CO9X0503).
References
- Ackerman , JD . 2000 . Abiotic pollen and pollination: ecological, functional, and evolutionary perspectives . Plant Systematics and Evolution , 222 : 167 – 185 .
- Ågren , J , Elmqvist , T and Tunlid , A . 1986 . Pollination by deceit, floral sex-ratios and seed set in dioecious Rubus chamaemorus L . Oecologia , 70 : 332 – 338 .
- Allan HH 1961 . Flora of New Zealand , Vol. 1 . Wellington : Government Printer . 1085 p.
- Allison , TD . 1990 . Pollen production and plant density affect pollination and seed production in Taxus canadensis . Ecology , 71 : 516 – 522 .
- Anderson , GJ , Bernardello , G , Stuessy , TF and Crawford , DJ . 2001 . Breeding system and pollination of selected plants endemic to Juan Fernandez Islands . American Journal of Botany , 88 : 220 – 233 .
- Ashman , T-L , Knight , TM , Steets , JA , Amarasekare , P , Burd , M , Campbell , DR , Dudash , RM , Johnston , MO , Mazer , SJ , Mitchell , RJ , Morgan , MT and Wilson , WG . 2004 . Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences . Ecology , 85 : 2408 – 2421 .
- Ashman , TL and Schoen , DJ . 1994 . How long should flowers live? . Nature , 371 : 788 – 790 .
- Baker , HG . 1955 . Self-incompatibility and establishment after ‘long-distance’ dispersal . Evolution , 9 : 347 – 349 .
- Beattie , AJ . 1971 . A technique for the study of insect-borne pollen . Pan-Pacific Entomologist , 47 : 82
- Bernardello , G , Anderson , GJ , Stuessy , TF and Crawford , DJ . 2001 . A survey of floral traits, breeding systems, floral visitors, and pollination systems of the angiosperms of the Juan Fernandez Islands (Chile) . Botanical Review , 67 : 255 – 308 .
- Berry , EJ and Gorchov , DL . 2004 . Reproductive biology of the dioecious understorey palm Chamaedorea radicalis in a Mexican cloud forest: pollination vector, flowering phenology and female fecundity . Journal of Tropical Ecology , 20 : 369 – 376 .
- Bessa-Gomes , C , Legendre , S and Clobert , J . 2004 . Allee effects, mating systems and the extinction risk in populations with two sexes . Ecology Letters , 7 : 802 – 812 .
- Bierzychudek , P . 1981 . Pollinator limitation of plant reproductive effort . American Naturalist , 117 : 838 – 840 .
- Calabrese , JM and Fagan , WF . 2004 . Lost in time, lonely, and single: reproductive asynchrony and the Allee effect . American Naturalist , 164 : 25 – 37 .
- Carlquist , S . 1966 . The biota of long-distance dispersal. IV. Genetic systems in the floras of oceanic islands . Evolution , 20 : 433 – 455 .
- Charlesworth , D . 1993 . Why are unisexual flowers associated with wind pollination and unspecialized pollinators . American Naturalist , 141 : 481 – 490 .
- Cruden , RW . 1977 . Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants . Evolution , 31 : 32 – 46 .
- Cruden , RW . 2000 . Pollen grains: why so many? . Plant Systematics and Evolution , 222 : 143 – 165 .
- Culley , TM , Weller , SG and Sakai , AK . 2002 . The evolution of wind pollination in angiosperms . Trends in Ecology & Evolution , 17 : 361 – 369 .
- Darwin C 1877 . The different forms of flowers on plants of the same species . London : Murray . 352 p.
- Davis , HG , Taylor , CM , Civille , JC and Strong , DR . 2004a . An Allee effect at the front of a plant invasion: Spartina in a Pacific estuary . Journal of Ecology , 92 : 321 – 327 .
- Davis , HG , Taylor , CM , Lambrinos , JG and Strong , DR . 2004b . Pollen limitation causes an Allee effect in a wind-pollinated invasive grass (Spartina alterniflora) . Proceedings of the National Academy of Sciences USA , 101 : 13804 – 13807 .
- Delph , LF . 1999 . “ Sexual dimorphism in life history ” . In Gender and sexual dimorphism in flowering plants , Edited by: Geber , MA , Dawson , TE and Delph , LF . 149 – 173 . Berlin : Springer .
- Dennis , B . 2002 . Allee effects in stochastic populations . Oikos , 96 : 389 – 401 .
- Department of Lands and Survey 1984 . Management plan for Hakarimata Scenic Reserve . Hamilton , , New Zealand : Department of Lands and Survey .
- Dupont , Y . 2002 . Evolution of apomixis as a strategy of colonization in the dioecious species Lindera glauca (Lauraceae) . Population Ecology , 44 : 293 – 297 .
- Faegri , K and Van der Pijl , L . 1979 . The principles of pollination ecology , 244 Oxford : Pergamon Press .
- Friedman , J and Barrett , SCH . 2009 . Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants . Annals of Botany , 103 : 1515 – 1527 .
- Gardner , R . 2002 . The genus Coprosma (Rubiaceae) in New Guinea . Candollea , 57 : 97 – 130 .
- Hackney , EE and McGraw , JB . 2001 . Experimental demonstration of an Allee effect in American ginseng . Conservation Biology , 15 : 129 – 136 .
- Heenan , PB , Dawson , MI and Bicknell , RA . 2002 . Evidence for apomictic seed formation in Coprosma waima (Rubiaceae) . New Zealand Journal of Botany , 40 : 347 – 355 .
- Heenan , PB , Molloy , BPJ , Bicknell , RA and Luo , C . 2003 . Levels of apomictic and amphimictic seed formation in a natural population of Coprosma robusta (Rubiaceae) in Riccarton Bush, Christchurch, New Zealand . New Zealand Journal of Botany , 41 : 287 – 291 .
- Honig , MA , Linder , HP and Bond , WJ . 1992 . Efficacy of wind pollination: pollen load size and natural microgametophyte populations in wind-pollinated Staberoha banksii (Restionaceae) . American Journal of Botany , 79 : 443 – 448 .
- Kelly , D . 1994 . The evolutionary ecology of mast seeding . Trends in Ecology and Evolution , 9 : 465 – 470 .
- Kelly , D , Hart , DE and Allen , RB . 2001 . Evaluating the wind pollination benefits of mast seeding . Ecology , 82 : 117 – 126 .
- Kelly , D and Sork , VL . 2002 . Mast seeding in perennial plants: why, how, where? . Annual Review of Ecology Evolution and Systematics , 33 : 427 – 447 .
- Knapp , EE , Goedde , MA and Rice , KJ . 2001 . Pollen-limited reproduction in blue oak: implications for wind pollination in fragmented populations . Oecologia , 128 : 48 – 55 .
- Knight , TM , Steets , JA , Vamosi , JC , Mazer , SJ , Burd , M , Campbell , DR , Dudash , MR , Johnston , MO , Mitchell , RJ and Ashman , TL . 2005 . Pollen limitation of plant reproduction: pattern and process . Annual Review of Ecology Evolution and Systematics , 36 : 467 – 497 .
- Koenig , WD and Ashley , MV . 2003 . Is pollen limited? The answer is blowin’ in the wind . Trends in Ecology and Evolution , 18 : 157 – 159 .
- Lloyd , DG and Webb , CJ . 1977 . Secondary sex characters in plants . Botanical Review , 43 : 177 – 216 .
- Merrett MF 2006 . Breeding systems and reproduction of indigenous shrubs in fragmented ecosystems . Unpublished PhD thesis , Palmerston North , , New Zealand : Massey University . 232 p.
- Merrett , MF , Robertson , AW and Peterson , PG . 2007 . Pollination performance and vulnerability to pollination breakdown of sixteen native shrub species from New Zealand . New Zealand Journal of Botany , 45 : 579 – 591 .
- Niklas , KJ . 1985a . The aerodynamics of wind pollination . Botanical Review , 51 : 328 – 386 .
- Niklas , KJ . 1985b . Wind pollination – a study in controlled chaos . American Scientist , 73 : 462 – 470 .
- Niklas , KJ and Buchmann , SL . 1985 . Aerodynamics of wind pollination in Simmondsia chinensis (Link) Schneider . American Journal of Botany , 72 : 530 – 539 .
- Nilsson , SG and Wastljung , U . 1987 . Seed predation and cross-pollination in mast-seeding beech (Fagus sylvatica) patches . Ecology , 68 : 260 – 265 .
- Parsons MJ , Douglass P , Macmillan BH 1998 . Current names list for wild gymnosperms, dicotyledons and monocotyledons (except grasses) in New Zealand as used in Herbarium CHR . Lincoln : Manaaki Whenua Press . 206 p.
- Poole AL , Adams NM 1994 . Trees and shrubs of New Zealand. , Rev. edn . Lincoln : Manaaki Whenua Press . 256 p.
- Primack , RB . 1985 . Longevity of individual flowers . Annual Review Ecology and Systematics , 16 : 15 – 37 .
- Proctor , M , Yeo , P and Lack , A . 1996 . The natural history of pollination , 479 Portland : Timber Press .
- Regal , PJ . 1982 . Pollination by wind and animals: ecology and geographic patterns . Annual Review of Ecology and Systematics , 13 : 497 – 524 .
- Smith , CC , Hamrick , JL and Kramer , CL . 1988 . The effects of stand density on frequency of filled seeds and fecundity in lodgepole pine (Pinus contorta Dougl.) . Canadian Journal of Forest Research , 18 : 453 – 460 .
- Stehlik , I and Barrett , SCH . 2006 . Pollination intensity influences sex ratios in dioecious Rumex nivalis, a wind-pollinated plant . Evolution , 60 : 1207 – 1214 .
- Stehlik , I , Friedman , J and Barrett , SCH . 2008 . Environmental influence on primary sex ratio in a dioecious plant . Proceedings of the National Academy of Sciences USA , 105 : 10847 – 10852 .
- Stephens , PA , Sutherland , WJ and Freckleton , RP . 1999 . What is the Allee effect? . Oikos , 87 : 185 – 190 .
- Steven , JC and Waller , DM . 2007 . Isolation affects reproductive success in low-density but not high-density populations of two wind-pollinated Thalictrum species . Plant Ecology , 190 : 131 – 141 .
- Stevenson , PR . 2007 . A test of the escape and colonization hypotheses for zoochorous tree species in a western Amazonian forest . Plant Ecology , 190 : 245 – 258 .
- Taylor , CM , Davis , HG , Civille , JC , Grevstad , FS and Hastings , A . 2004 . Consequences of an Allee effect in the invasion of a Pacific estuary by Spartina alterniflora . Ecology , 85 : 3254 – 3266 .
- Waikato Regional Council 2011 . Our climate . http://www.waikatoregion.govt.nz/environmental-information (accessed 7 July 2011) .
- Wardle , P . 1991 . Vegetation of New Zealand , 672 Cambridge : Cambridge University Press .
- Webb , CJ , Lloyd , DG and Delph , LF . 1999 . Gender dimorphism in indigenous New Zealand seed plants . New Zealand Journal of Botany , 37 : 119 – 130 .
- Webb , CJ and Simpson , MJA . 2001 . Seeds of New Zealand gymnosperms and dicotyledons , 428 Christchurch : Manuka Press .
- Whitehead , D . 1969 . Wind pollination in the angiosperms: evolutionary and environmental considerations . Evolution , 23 : 28 – 35 .
- Whitehead , D . 1983 . “ Wind pollination: some ecological and evolutionary perspectives ” . In Pollination biology , Edited by: Real , L . 97 – 108 . Orlando : Academic Press .