Abstract
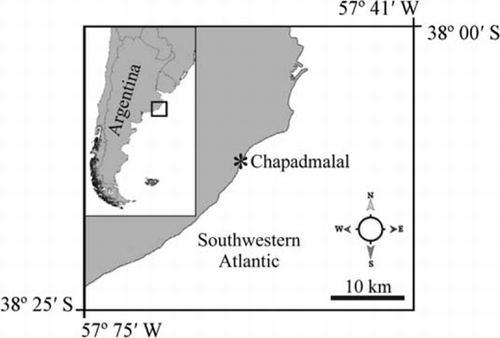
Schizymenia dubyi (Chauvin ex Duby) J. Agardh is a red alga of the order Nemastomatales that is native to Europe, Asia and Oceania. The first specimen of this seaweed from the Argentinian coast was collected in the Chapadmalal intertidal wave-cut platform (38°12′S, 57°40′W) in September 2008. Taxonomic identification of the species was made on the basis of vegetative and reproductive characters and confirmed by DNA sequence analysis of the rbcL gene. The S. dubyi samples collected in the field were represented by foliose gametophytes as well as crustose tetrasporophytes. The gametophytes were distributed in patches of c. 50% coverage of the analysed quadrants (n = 20). Gametophyte abundance was 3.1±2.5 (mean±SD) plants per quadrant (0.04 m2). All these were fertile females. Molecular results placed the specimens from Argentina within the S. dubyi clade, closely related to S. dubyi from Japan and France.
Introduction
Exotic species that spread beyond their point of introduction and become regionally abundant are termed invasive species (Richardson et al. Citation2000; Kolar & Lodge Citation2001). Seaweed invasions may result from intentional aquaculture or accidental introductions, and have been reported worldwide. Well-known examples of invasive seaweeds are Undaria pinnatifida (Harvey) Suringar and Sargassum muticum (Yendo) Fensholt, among the brown algae, and Codium fragile subspecies tomentosoides (van Goor) P.C. Silva and Caulerpa taxifolia (M. Vahl) C. Agardh, among the green algae. Invasive red algae include Grateloupia turuturu Yamada and Porphyra katadae A. Miura, among others.
Regional reviews and checklists help to document the arrival of new invasive species in specific locations (Maggs & Stegenga Citation1999; Boudouresque & Verlaque Citation2002; Orensanz et al. Citation2002; Ribera Siguan Citation2003; Castilla et al. Citation2005). The Global Invasive Species Database (http://www.issg.org/database) of the International Union for the Conservation of Nature (IUCN) provides extensive data (native/introduced ranges, references) on introduced seaweeds.
Schizymenia dubyi is distributed widely in the Indo-Pacific as well as in the north eastern Atlantic, with the Mediterranean coasts as its southernmost location (Gabriel et al. Citation2011). To date, it has not been described as an invasive species. The goal of this study is to report the presence of S. dubyi on the Mar del Plata coast, Argentina, a new location outside its known geographical range. In addition, estimates of the relative abundance of the seaweed are provided.
Materials and methods
The seaweed was identified from 22 samples, collected during spring 2008 in a Chapadmalal rocky intertidal zone (38°12′S, 57°40′W; ) in Mar del Plata, Province of Buenos Aires, Argentina. The site is formed by a compact sedimentary rock, Loess Pampeano Soil, sometimes cemented by crystalline calcium carbonate. Its colour is variable and its consistency is soft. The sampling points were located in the low intertidal region, which were exposed during extreme neap tides and covered during high spring tides (Scelzo et al. Citation1996).
Healthy whole specimens of S. dubyi in foliose gametophytic and tetrasporophytic (Haematocelis-phase) phases were collected by hand in the intertidal zone during a very low neap tide. Samples were fixed in 5% formalin in seawater and processed using standard procedures for taxonomic analysis (Ramírez Citation1982). Samples were deposited at Museo Argentino de Ciencias Naturales Bernardino Rivadavia (MACN) and Museo Nacional de Historia Natural (MNHN) of Chile, under the catalogue numbers BA 47179–47182 and SGO 158293–158298, respectively.
The taxonomic identity of S. dubyi was revised based on the vegetative and reproductive characters. Species were identified using a key for Bangiophyceae and Florideophyceae red algae species (Womersley & Kraft Citation1994), as well as by consulting marine algae herbarium international collections from the MNHN and MACN, which possess S. dubyi species with identical morphology from Australia. No examples of the genus Schizymenia were found in the MACN herbarium collection for Argentina.
For phylogenetic analyses, the gametophytes (foliose phase) used in molecular studies were desiccated in silica gel and partial rbcL genes were sequenced. Total DNA was extracted using the DNeasy Plant Mini Kit (QIAGEN, Tokyo, Japan). RbcL gene sequencing procedures were followed as described in Suzuki et al. (Citation2010). RbcL sequences from seven taxa were aligned using ClustalX (Thompson et al. Citation1997). The length of the alignment used for phylogenetic analyses was 1083 sites. Because Platoma was noted as a sister group to Schizymenia in previous rbcL trees (Gavio et al. Citation2005; Gabriel et al. Citation2010), two sequences of the genus Platoma were used as outgroup for the analyses.
Bayesian analysis was performed using MrBayes 3.1.2 software (Ronquist & Huelsenbeck Citation2003). The substitution model of rbcL gene sequences was a codon position-specific rate model: first codon position, GTR + I +G; second codon position, F81 model; third codon position, HKY + I + G calculated by the hierarchical likelihood ratio tests (hLRT) using MrModeltest 2.2 software (Nylander Citation2004). Four Markov chain Monte Carlo (MCMC) iterations were performed for 1 000 000 generations, keeping one tree every 100 generations. The first 25% of the generations were discarded as burn-in, and the remaining trees were used to calculate a 50% majority-rule tree and to determine the posterior probabilities (PP) of the individual branches. The average standard deviation of split frequencies of the two MCMC iteration runs was < 0.01 for each analysis, indicating convergence. Maximum likelihood (ML) analysis was performed using RAxML v. 7.2.6 (Stamatakis 2006) implementing a GTRGAMMAI model. Bootstrap percentages (BP) in the ML analyses were calculated based on 1000 replications of heuristic searches. Maximum parsimony (MP) and neighbour joining (NJ) analyses were performed using PAUP* 4.0b10 (Swofford Citation2002). MP and NJ analyses were subjected to bootstrap resampling (5000 replicates with 100 random additions, 5000 replicates) to estimate robustness (Felsenstein Citation1985).
Twenty pictures of the sample site were randomly taken during September 2008 to estimate gametophyte abundance (presence or absence and, when present, number of individuals in quadrants of 0.04 m2). The images were analysed with Image-j software.
Results
The algal community studied is characterised by a predominance of the native red algae Ceramiaceae (Ceramium sp.), Corallinaceae (Jania sp., Bossiella sp.) and Rhodomelaceae (Polysiphonia sp.), and some green macroalgae (Ulva spp., Bryopsis sp.). The S. dubyi foliose plants collected from this community represented gametophytes with cystocarps (). Tetrasporophytes were also found close to these. All the gametophyte thalli were fertile females. They are maroon to orange in colour, gelatinous and slippery, up to 40 cm long, 20 cm wide and 1.5 mm thick. The habit of this plant was variable in morphology, with most of the fronds ovalate–lanceolate in shape and with wavy, serrated edges. The thallus was attached to the substrate by a compact and conspicuous holdfast.
Figure 2 Schizymenia dubyi individuals on the intertidal rocky shore in Chapadmalal region (Mar del Plata, Argentina).

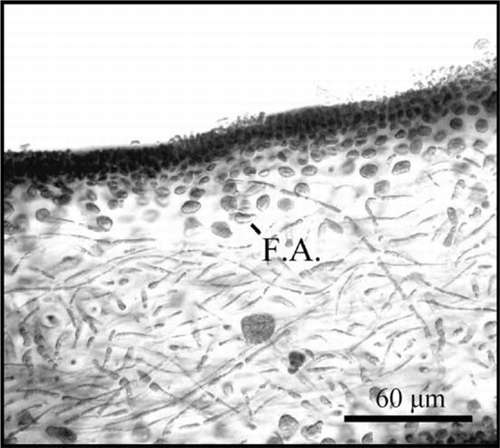
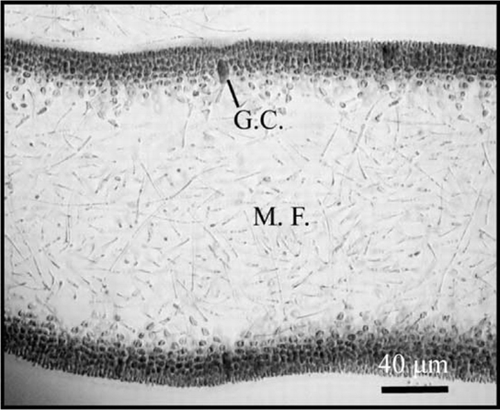
Schizymenia dubyi is generally characterised by a multiaxial thallus formed by a cortex of six to seven cell layers. This thallus is differentiated into an external cortex and an internal subcortex. The cortex is formed by two to three layers of round to subsquare cells, 20 µm in diameter and abundant glandular cells distributed along its length (). These cells are of variable morphology. Most of the cells are round to oval with an elongate stem, 120–200 µm in length and 40 µm in diameter. The subcortex is composed of three to four layers of rounded cells, 40 µm in diameter, which form bundles supported by filaments originating from a lax stringy bone.
Figure 3 Schizymenia dubyi cross-sections of filamentous medulla showing glandular cells on the cortex (G.C.), periclinals and anticlinal medular filaments (M.F.).
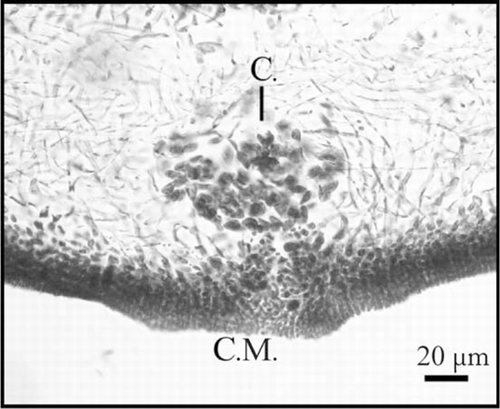
The medulla is formed by thin filaments that cross the thallus periclinaly and anticlinaly (). The gametophytes have differential reproductive cells in the external cortex. Such cells develop from a structure in the form of an ampulla in separate branches (condition not procarpic) (). After fertilisation, cystocarps develop a specialised opening or carpostome through the cortex, with a delicate pericarp surrounding the mass of carpospores ().
Figure 5 Schizymenia dubyi cross-sections of cystocarps showing specialised opening or carpostome (C.) and mass of carpospores (C.M.).
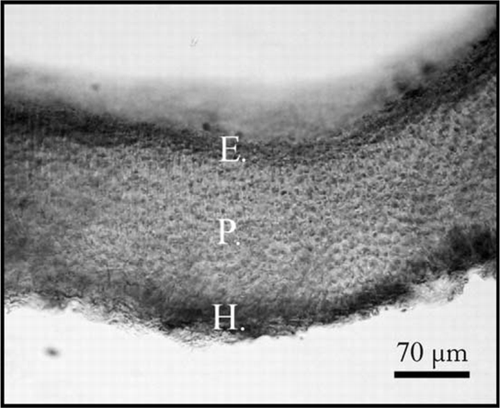
Tetrasporophyte plants develop a crust, a dark red thallus of c. 185 µm in height. The crust presents a single-layer hypothallus of cells, 8 m in diameter and 20 µm in height, a perithallus of six to eight cell layers with sub-square-shaped cells, 10 µm in length and 6 µm in diameter. The epithallus is comprised of several layers of well-pigmented spherical cells, 2.4 µm in diameter ().
Figure 6 Schizymenia dubyi cross-sections of a tetraesporophyte showing hypothallus (H), epithallus (E) and perithallus (P).
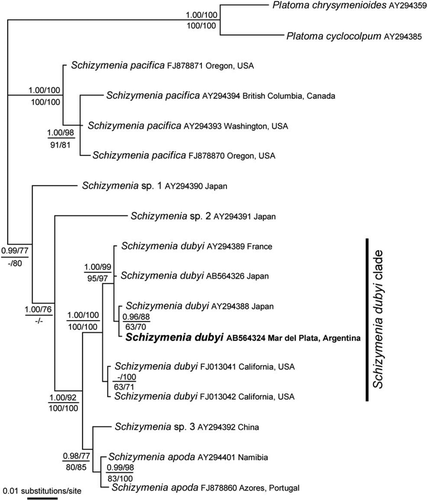
Molecular analysis of the rbcL gene was conducted on seven taxa in the Schizymeniaceae (). Schizymenia dubyi from Argentina, France, Japan and the Pacific coast of the USA formed a clade with full statistical support (1.00 PP and 100% BP). The sequence differences among the S. dubyi clade were 0.5% (5 bp).
Figure 7 Bayesian tree based on rbcL gene sequences. The corresponding posterior probabilities (>0.94) are shown (top left). Numbers shown at the top right indicate bootstrap values (>50%) from maximum likelihood. Numbers shown at the bottom indicate bootstrap values (>50%) from maximum parsimony (left) and neighbour joining (right). In bold, samples of the exotic population detected in Argentina.
In the sampled upper intertidal, gametophytes of S. dubyi were distributed in patches of c. 50% presence in the analysed quadrants. Abundance was estimated to be 3.1±2.5 (mean±SD) plants per quadrant (0.04 m2).
Discussion
This study reports for the first time the presence of the red algae S. dubyi on the Argentinian coast. Until now, the distribution of S. dubyi was only known to be along the North Atlantic coast from Iceland (Caram & Jónsson Citation1972), the north of England to Morocco, Madeira and the Atlantic Islands (Levring Citation1974; Neto et al. Citation2001; John et al. Citation2004), throughout the Mediterranean (France, Italy & Spain), and in the North Pacific (Japan, Korea, China, North America) up to the Australian coast (Womersley & Kraft Citation1994). RbcL analyses show that the specimens from Argentina are included in the S. dubyi clade and are close to S. dubyi from Japan and France. This result indicates the possible origin of the specimens that landed on the Argentinian coast, but the introduction vector of S. dubyi is still unknown. However, the most probable colonisation path of S. dubyi would be its attachment to the hull of transoceanic vessels or as sporelings transported within ballast water or attached to ballast rocks in the international port of Mar del Plata. Its introduction for aquaculture purposes is improbable because this activity is rare or non-existent in Argentina, and S. dubyi is not considered to be an economically important species, unlike Porphyra, Undaria or Laminaria which are extensively cultured in Japan. Accidental introduction associated with cultured shellfish is also improbable because in San Antonio Oeste the closest mussel farms are c. 1000 km to the south.
The accidental or deliberate introduction of marine exotic species may result in severe ecological disturbance to native communities (Bax et al. Citation2001; Piriz & Casas Citation2001; Grosholz Citation2002), and specifically seaweed assemblages, where competition for light and substrate can lead to the local exclusion of native species (DeWreede Citation1996). For this reason, introduced algae have been mentioned as one of the major problems throughout the world's oceans, altering natural communities and causing significant economic losses (Mathieson et al. Citation2003). The heteromorphic life cycle of S. dubyi enables this species to be a potential successful occupant of different ecological niches, since the crustose phase (tetrasporophyte thallus) can avoid herbivory (Megan & Steneck Citation2001), and the foliose phase (gametophyte thallus) settles and survives easily, which probably explains its wide distribution.
It has been documented that 31 exotic species have become established on the Argentinian coast (Orensanz et al. Citation2002). In the case of the algae Undaria pinnatifida, an important decrease in the specific richness and diversity of the local algal community has been shown (Casas et al. Citation2004), while Irigoyen et al. (Citation2011) observed a reduced abundance of some fish species and a higher benthic invertebrate richness and diversity attributable to the provision of new habitat structures. Further local algal introduction cases in Argentina include the red alga Anotrichium furcellatum (Boraso de Zaixso & Akselmann Citation2005) and the brown alga Sporochnus pedunculatus (Boraso & Negri Citation1997; Boraso de Zaixso Citation1999).
Although there is little information on the abundance and dynamics of S. dubyi populations in its native areas, Gorostiaga et al. (Citation2004) reported that this species occurs in isolated clusters and is common when compared with other algae in the north of Spain. In this study, gametophytes of S. dubyi showed a patchy distribution with an average of 38.75 plants per m2 at the lower intertidal zone. We have observed individuals of these species during spring tides in the lower intertidal but we were unable to elucidate their distribution pattern. This report will allow future monitoring of S. dubyi in order to understand not only the original establishment of this population, but also its impact on the local indigenous algal community.
Acknowledgements
The work was partially supported by the project PICT 2007-01398 of the National Agency of Scientific and Technological Promotion. We would like to thank NM Chiaradia for his help during the sampling. We also thank the two anonymous reviewers for their kind and helpful suggestions and specially to Dr TM Murphy of the Central Veterinary Research Laboratory of County Kildare, Ireland, for correcting the final version of the manuscript.
References
- Bax , N , Carlton , JT , Mathews-Amos , A , Haedrich , RL , Howarth , FG , Purcell , JE , Rieser , A and Gray , A . 2001 . The control of biological invasions in the world's oceans . Conservation Biology , 15 : 1234 – 1246 .
- Boraso , AL and Negri , R . 1997 . Presencia de Sporochnus pedunculatus (Sporochnales, Phaeophycofita) en la costa argentina . Phycis Seccion A , 54 : 23 – 24 .
- Boraso de Zaixso , AL . 1999 . Cladophora falklandica (Hook. Et Harv.) Hooker et Harvey en la costa argentina . Phycis , 57 : 55 – 59 .
- Boraso de Zaixso , AL and Akselmann , R . 2005 . Anotrichium furcellatum (Ceramiaceae, Rhodophyta) en Argentina. Una posible especie invasora . Boletín Sociedad Argentina de Botánica , 40 : 207 – 213 .
- Boudouresque CF , Verlaque M 2002 . Assessing scale and impact of ship-transported alien macrophytes in the Mediterranean Sea . In: CIESM Workshop Monography no. 20 Istanbul, Turkey 6–9 November 2002 . Pp. 53 – 61 .
- Caram , B and Jónsson , S . 1972 . Nouvelle inventaire des algues marines de l'Islande . Acta Botanica Islandica , 1 : 5 – 31 .
- Casas , G , Scrosati , R and Piriz , ML . 2004 . The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina) . Biological Invasions , 6 : 411 – 416 .
- Castilla , JC , Uribe , M , Bahamonde , N , Clarke , M and Desqueyroux-Faundez , R . 2005 . Down under the southeastern Pacific: marine nonindigenous species in Chile . Biological Invasions , 7 : 213 – 232 .
- DeWreede , RE . 1996 . The impact of seaweed introductions on biodiversity . Global Biodiversity , 6 : 2 – 9 .
- Felsenstein , J . 1985 . Confidence limits on phylogenies: an approach using the bootstrap . Evolution , 39 : 783 – 791 .
- Gabriel , D , Parente , MI , Neto , AI , Raposo , M , Schils , T and Fredericq , S . 2010 . Phylogenetic appraisal of the genus Platoma (Nemastomatales, Rhodophyta), including life history and morphological observations on P. cyclocolpum from the Azores . Phycologia , 49 : 2 – 21 .
- Gabriel , D , Schils , T , Parente , MI , Draisma , SGA , Neto , AI and Fredericq , S . 2011 . Taxonomic studies in the Schizymeniaceae (Nemastomatales, Rhodophyta): on the identity of Schizymenia sp. in the Azores and the generic placement of Nemastoma confusum . Phycologia , 50 : 109 – 121 .
- Gavio , B , Hickerson , E and Fredericq , S . 2005 . Platoma chrysymenioides sp. nov. (Schizymeniaceae), and Sebdenia integra sp. nov. (Sebdeniaceae), two new red algal species from the northwestern Gulf of Mexico, with phylogenetic assessment of the Cryptonemiales complex (Rhodophyta) . Gulf of Mexico Science , 1 : 38 – 57 .
- Gorostiaga , JM , Santolari , A , Secilla , A , Casares , C and Diez , I . 2004 . Check-list of the Basque coast benthic algae (north of Spain) . Anales del jardín Botánico de Madrid , 61 : 155 – 180 .
- Grosholz , E . 2002 . Ecological and evolutionary consequences of coastal invasions . Trends Ecology & Evolution , 17 : 22 – 27 .
- Irigoyen , AJ , Eyras , C and Parma , AM . 2011 . Alien algae Undaria pinnatifida causes habitat loss for rocky reef fishes in north Patagonia . Biological Invasions , 13 : 17 – 24 .
- John , DM , Prud'homme van Reine , WF , Lawson , GW , Kostermans , TB and Price , JH . 2004 . A taxonomic and geographical catalogue of the seaweeds of the western coast of Africa and adjacent islands . Beihefte zur Nova Hedwigia , 127 : 1 – 339 .
- Kolar , CS and Lodge , DM . 2001 . Progress in invasion biology: predicting invaders . Trends in Ecology & Evolution , 16 : 199 – 204 .
- Levring , T . 1974 . The marine algae of the Archipelago of Madeira . Boletim Museo Municipale Funchal , 28 : 5 – 111 .
- Maggs , CA and Stegenga , H . 1999 . Red algal exotics on North Sea coasts . Helgoland Marine Research , 52 : 243 – 258 .
- Mathieson , AC , Dawes , CJ , Harris , LG and Hehre , EJ . 2003 . Expansion of the Asiatic green alga Codium fragile ssp. tomentosoides in the Gulf of Maine . Rhodora , 105 : 1 – 53 .
- Megan , ND and Steneck , RS . 2001 . Growth and persistence of diverse intertidal crusts: survival of the slow in a fast-paced world . Marine Ecology Progress Series , 223 : 89 – 100 .
- Neto , AI , Cravo , DC and Haroun , RT . 2001 . Checklist of the benthic marine plants of the Madeira Archipelago . Botanica Marina , 44 : 391 – 414 .
- Nylander JAA 2004 . MrModeltest 2.1 . Program distributed by the author. Evolutionary Biology Centre, Uppsala University .
- Orensanz , JM , Schwindt , E , Pastorino , G , Bortolus , A , Casas , G , Darrigran , G , Elias , R , Lopez Gappa , JJ , Obenat , S , Pascual , M , Penchaszadeh , P , Piriz , ML , Scarabino , F , Spivak , D and Vallarino , EA . 2002 . No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic . Biological Invasions , 4 : 115 – 143 .
- Piriz ML , Casas G 2001 . Introducción de especies y su impacto en la biodiversidad. El caso Undaria pinnatifida (Phaeophyta, Laminariales) . In: Alveal K , Antezana T Sustentabilidad de la biodiversidad. Un problema actual. Bases científico-técnicas. Teorizaciones y proyecciones, Universidad de Concepción, Chile . Pp. 679 – 692 .
- Ramírez , ME . 1982 . Nuevos registros de algas marinas para Antofagasta . Boletín del Museo Nacional de Historia Natural, Chile , 39 : 11 – 26 .
- Ribera Siguan MA 2003 . Pathways of biological invasions of marine plants . In: Ruiz GM , Carlton JT Invasive species: vectors and management strategies . Washington , DC , Island Press . Pp. 183 – 226 .
- Richardson , DM , Pysek , P , Rejmánek , M , Barbour , MG , Panetta , FD and West , CJ . 2000 . Naturalization and invasion of alien plants: concepts and definitions . Diversity Distribution , 6 : 93 – 107 .
- Ronquist , F and Huelsenbeck , JP . 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Scelzo , MA , Elias , R , Vallarino , E , Charrier , M , Lucero , N and Alvarez , F . 1996 . Variación estacional de la estructura comunitaria del bivalvo intermareal Brachidontes Rodríguez (D'Orbigny 1846) en sustratos artificiales (Mar del Plata, Argentina) . Neritica , 10 : 87 – 102 .
- Stamatakis A 2006 RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models Bioinformatics 22 2688 – 2690
- Suzuki , M , Hashimoto , T , Nakayama , T and Yoshizaki , M . 2010 . Morphology and molecular relationships of Leptofauchea rhodymenioides (Rhodymeniales, Rhodophyta), a new record for Japan . Phycological Research , 58 : 116 – 131 .
- Swofford DL 2002 . Paup*. Phylogenetic analysis using parsimony (* and other methods) . Version 4 . Sunderland , MA , Sinauer Associates .
- Thompson , JD , Gibson , TJ , Plewniak , F , Jeanmougin , F and Higgins , DG . 1997 . The CLUSTAL x–Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools . Nucleic Acids Research , 25 : 4876 – 4882 .
- Womersley HBS , Kraft GT 1994 . Family Nemastomataceae Schmitz 1892: 2, nomen conservandum . In: Womersley HBS . The marine benthic flora of southern Australia. Part IIIA. Bangiophyceae and Florideophyceae (Acrochaetiales, Nemaliales, Gelidiales, Hildenbrandiales and Gigartinales sensu lato). Canberra, Australian Biological Resources Study . Pp. 270 – 285 .