Abstract
A comprehensive review of the morphology, anatomy, taxonomy, chemistry and ecology of the endemic New Zealand facultative shrub epiphyte Pittosporum cornifolium (Pittosporaceae) is presented. Strong habitat specificity restricts this species to lowland forest and coastal habitats, which are widely yet discontinuously distributed north of latitude 42°15′S. Pittosporum cornifolium is typically associated with old growth forest systems and low nutrient substrates, with low mean daily temperatures in the coldest month (<0.6 °C) and high mean October vapour pressure deficits (>0.5 kPa) apparently restricting its distribution. Significant morphological variability is evident in leaves and flowers, especially with respect to plants from the Poor Knights Islands. Genetic analyses of five mainland populations and individuals representing Poor Knights Islands populations revealed relatively low genetic diversity at the population level which is likely to be the result of geographic isolation. Molecular phylogenetic studies suggest a New Caledonian origin for the species with close affinities to both P. pimeleoides subspecies. Several lines of evidence suggest recognition of the Poor Knights Islands entity as a new taxon. However, analysis of additional morphological, reproductive and molecular data across the full geographic range will be required to confirm current inferences. Although populations have declined, P. cornifolium is not currently threatened, however, it should be considered for reintroduction to sites in districts where its range has been severely reduced.
Introduction
Pittosporum cornifolium A. Cunn. ex Hook. (Pittosporaceae) is an endemic shrub epiphyte that typically perches on forest canopy trees, but also grows on the forest floor (terrestrial lifestyle) or on rocks (rupestral). This species is one of four shrub epiphytes endemic to New Zealand, the others being P. kirkii, Brachyglottis kirkii and Griselinia lucida (Oliver Citation1930). Pittosporum cornifolium has a distinctive whorled leaf arrangement, reddish brown (Hooker 1832) to yellow flowers (Cooper Citation1956), which are typically unisexual in function (Petrie Citation1921), and capsules characteristic of the genus with seeds embedded in a sticky pitch substance (Gaertner Citation1788). The primary habitats of P. cornifolium are lowland and coastal ecosystems which, in recent times (<200 years), have been subjected to widespread clearance and fragmentation, resulting in major reductions to the species’ potential population range (F.M. Clarkson Citation2011). Despite habitat discontinuity, P. cornifolium occupies a wide geographic range which extends throughout the North Island, including numerous offshore islands, to the northern reaches of the South Island (Cooper Citation1956). Targeted scientific research specific to this inconspicuous epiphyte species has been very limited. Early morphological and anatomical research has been restricted to a few individuals from a single geographic region (Kirk Citation1871; Petrie Citation1921; Oliver 1930; Cooper Citation1956; Wilkinson Citation1992). More recent research by F.M. Clarkson (Citation2011) highlights the morphological and anatomical variability and genetic diversity of the species based on a study of five North Island populations and individuals from the Poor Knights Islands, but comparative research is yet to be conducted more broadly across its natural range. Basic information regarding pollination and dispersal modes, seed viability and germination is lacking. Hence, a review of the available biological research is presented to summarize the current state of knowledge, but also to highlight information gaps and encourage future research which will be of value in aiding the conservation and restoration of this distinctive species.
Morphology
We base our morphological description on Cooper's (1956) work, which is the most comprehensive description of P. cornifolium. Pittosporum cornifolium is a perennial, evergreen shrub reaching up to 2.5 m in height, with a distinctive whorled architecture. Branches are dark reddish brown, glabrous and grow in a forked or whorled–verticillate arrangements. Leaves are simple, entire with slightly revolute margins and grow in a whorled arrangement. They are ciliate when young, but soon become glabrate, coriaceous, with a glossy cuticle and have a well-raised midrib above (immersed below), and distinct secondary veins below. Leaves are elliptic–lanceolate to obovate (acute to subacuminate at apex and acute to obtuse at base), 2–10 cm long and 1–5 cm wide. Petioles are broad and glabrous, c. 0.5–3.0 mm long and 0.5–2.0 mm wide. Inflorescences are terminal, 1–10 flowered [depending on functional sex of flowers (Petrie Citation1921)], usually umbelliform. Flowers () are a reddish brown (type form described by Hooker Citation1832) to yellow in colour. Flower pedicels are 2–15 mm long and are subtended by a whorl of leaves with associated caducous bud scales. Flower sepals are non-overlapping, of narrow–lanceolate shape, acute at apex and are 4–7 mm long. Petals are broad, coherent in a tube basally with reflexed tips apically, linear–lanceolate, acute to acuminate at the apex, and 8–12 mm in length. Stamens are 4–6 mm long, anthers sagittiform to elliptic–oblong, 1–2 mm long and 0.5–1 mm broad. Gynoecia are of similar length to stamens, the ovaries are broad and covered with villous hairs, 1.5–3 mm long and 0.5–2 mm broad, styles are 2.5–4 mm long and stigmas range from capitate (two-lobed) to truncate. Petrie (Citation1921) noted that although flowers are perfect, they are generally unisexual in function; male flowers have robust stamens and a withered/aborted ovary, whereas female flowers have an inflated ovary and apparently aborted stamens (). Capsules are two-valved (occasionally three) with a persistent style, c. 1 cm in diameter and ovoid to ellipsoid. Capsule valves are < 1 mm thick and have vermilion-stained interiors. The placentae bare thick strap-like funicles up to 5 mm in length. Seeds are glossy, usually black and irregular (3.1–6.5 mm long), embedded in a viscid substance (Webb & Simpson Citation2001), with four to eight seeds per capsule.
Figure 1 Pittosporum cornifolium individuals in flower. A, Light red colouring (photo: Kerry Jones, Hauturu/Little Barrier Island). B, Reddish brown colouring (photo: Rob L. Suisted, Wilton's Bush Reserve, Wellington). C, Yellow colouring (individual from the Poor Knights Islands).
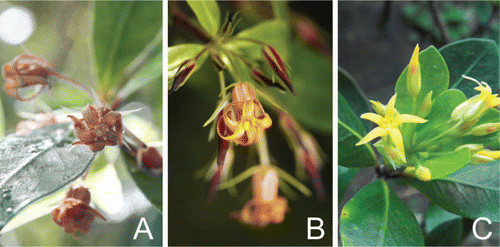
Figure 2 Pittosporum cornifolium flowers showing reproductive structures. A, Young female flower showing large ovary and reduced stamen. B, Young male flower showing large stamen and reduced ovary.
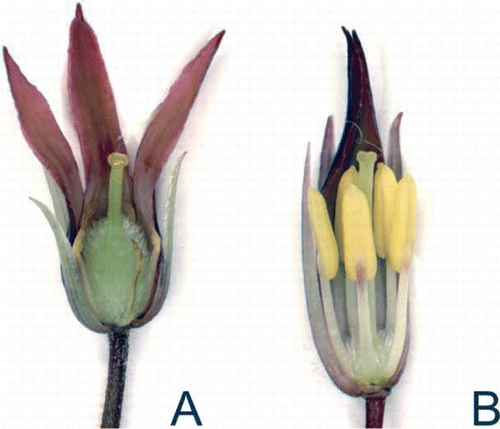
Morphological variants of P. cornifolium have been recognized previously. In comparison with mainland (‘type’) plants, individuals from the Poor Knights Islands have yellow flowers (Smith Citation2004), and larger, thicker, more coriaceous leaves that are obovate to rhomboid (subacuminate to obtuse at apex and acute to obtuse at base) (F.M. Clarkson Citation2011).
Illustrations
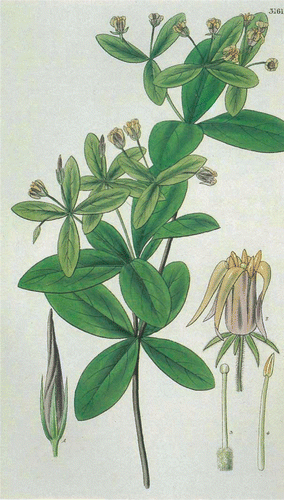
Pittosporum cornifolium is illustrated by Hooker (1832, pl. 3161; ), Osborne (in Goulding Citation1983, pl. 35), Eagle (Citation2006, p. 217) and Jones (cover of New Zealand Botanical Society Newsletter 96, 2009).
Anatomy
The stem and root anatomies of P. cornifolium appear typical of xerophytic dicotyledons. Both have thick peridermal layers, comprising phellogen (cork cambium), phelloderm (secondary cortex) and phellem (cork) (F.M. Clarkson Citation2011). A ring of lysigenous secretory ducts is present in the stem between the primary phloem and cortex tissue layers (F.M. Clarkson Citation2011).
The leaf anatomy of P. cornifolium has previously been described from transverse sections by Oliver (Citation1930) and Wilkinson (Citation1992) and more recently by F.M. Clarkson (Citation2011). Leaf tissue layers adjacent to the leaf midrib comprise an upper cuticle, upper epidermis, a thick ‘aqueous layer’/hypodermis, a closely packed mesophyll layer composed of palisade and spongy tissue, lower epidermis and lower cuticle. Pittosporum cornifolium leaf anatomy is consistent with a xeromorphic form, particularly due to the presence of hypodermal leaf tissue layers, because this layer may function as both a water-storing tissue and a supporting tissue when cell walls become considerably thickened (Fahn Citation1982; Cutler et al. Citation2008). In addition, schizogenous secretory ducts have been noted in the midrib and scattered vascular bundles throughout the leaf (Wilkinson Citation1992; F.M. Clarkson Citation2011).
Comparative leaf anatomy of the Poor Knights Islands form and the mainland form revealed a trend towards thicker leaf blades and leaf tissue layers in the Poor Knights Islands form (F.M. Clarkson Citation2011).
Cytology
The chromosome number of P. cornifolium is 2n=24 (de Lange et al. Citation2004). Chromosome counts of 2n=24 have also been reported in all members of New Zealand Pittosporum (de Lange et al. 2004).
Taxonomy and relationships
Pittosporum
Pittosporum Banks ex Gaertn, the type genus of Pittosporaceae, is represented in New Zealand and Australia and more widely throughout the Pacific (as far east as the Hawaiian Islands), extending west to eastern Africa and north into Asia (Haas Citation1977; Cayzer Citation1997). The genus name is derived from the Greek words ‘pitta’ meaning resin and ‘spora’ meaning seed (Gaertner 1788), referring to the characteristic resinous seeds of most species within the genus. Pittosporum comprises over 100 species of evergreen shrubs or trees (up to 30 m high) (Cooper Citation1956).
Early classification systems within Pittosporum were based on morphological characteristics such as leaf size and shape, number of valves per capsule, inflorescence type, placenta size (Cooper Citation1956) and various anatomical features such as leaf tissue layers (Wilkinson Citation1992). Relationships between species were postulated based on capsule valve number – and grouped within either bivalved or trivalved groups (Gowda Citation1951). However, morphological studies within the genus have been complicated by the abundance of phenotypic plasticity (ecophenetic or heteroblastic) (Chandler et al. Citation2007), and both hybridism and introgression, which skew well-defined discriminating characteristics (Allan Citation1961). Hence species delimitations and interspecific relationships remain unresolved for some taxa.
Although Pittosporum is abundant in Australia (Gowda Citation1951), the highest levels of endemism occur in New Caledonia (45 species; Tirel & Veillon Citation2002), New Zealand (21 species; de Lange et al. Citation2010) and the Hawaiian Islands (11 species; Wagner et al. Citation1999).
New Zealand Pittosporum
All of the 21 New Zealand species of Pittosporum are endemic (de Lange et al. 2010). Nine of these are endemic to the North Island, and two to the South Island (Eagle Citation1982, 2006). There are two further entities that require resolution: P. aff. crassifolium from Raoul Island (see Eagle Citation2006) and the Pittosporum found on Stephens Island, which is intermediate in appearance between P. crassifolium and P. tenuifolium. Historic relationships among New Zealand Pittosporum were inferred using flower position and inflorescence type (Kirk Citation1899; Cheeseman Citation1925) and development (heteroblastic vs. monoblastic; Allan Citation1961). However, recent molecular data do not support this grouping (Hathaway Citation2001; Chandler et al. Citation2007). Phylogenetic relationships among the genera of Pittosporaceae, including New Zealand Pittosporum, have been assessed using the internal transcribed spacer (ITS) region of nuclear ribosomal DNA (nrDNA), supporting an Australian origin of the family, with all other colonization events from Australia or from subsequent island hopping (Chandler et al. Citation2007). New Zealand taxa formed two distinct clades; one clade included P. cornifolium, P. pimeleoides subsp. pimeleoides and P. pimeleoides subsp. majus with affinities to New Caledonian taxa, and the second clade comprised all other taxa with Australian affinities.
Both of the proposed New Zealand radiations appear to be relatively recent events due to the limited level of ITS sequence divergence. An average of 1.4% sequence divergence between species in the main radiation make it an estimated 22 million years old. These estimates are consistent with the arrival of Pittosporum in the fossil record (Hathaway Citation2001). The clade containing P. cornifolium and P. pimeleoides was unexpected as these species have not previously been grouped based on morphology. However, this clade grouping was consistent due to identical sequences and an average of 8.3% (47.3 bases) sequence divergence from all other New Zealand taxa (Hathaway Citation2001). Hathaway (Citation2001) proposed that P. cornifolium and both P. pimeleoides subspecies are the result of a more recent colonization into New Zealand from New Caledonia, due to their close affinities with P. gatopenese and three other New Caledonian taxa. Some four interspecific wild hybrids are known within the New Zealand Pittosporum (Druce Citation1977; Ecroyd Citation1994; B.D. Clarkson & Clarkson Citation1994), but no hybrid involving P. cornifolium has been recorded and, in particular, no hybrid with P. pimeleoides.
Nomenclature
Pittosporum cornifolium A. Cunn. ex Hook. was described and illustrated (pl. 3161) by W.J. Hooker in 1832 in Curtis's Botanical Magazine, volume 59 (). According to Cooper (Citation1956, p. 163),
the species was described by W. J. Hooker from material grown at the Royal Botanical Gardens, Kew, and from Allan Cunningham's specimens and notes made by him in New Zealand in 1826. Two ‘type’ sheets in the herbarium of the Royal Botanic Gardens, Kew, bear five labels, two sterile specimens, a fruiting specimen and fragments of flowers. One label is dated 1826, one 1833, two 1838, and one is undated. As the species was described in 1832 only part of the material can have been available to W. J. Hooker.
Allan (Citation1961, p. 316) gives the type locality as ‘in humid woods on the banks of the Kanakana [Kawakawa] and other rivers, Bay of Islands, &c.’ and the type specimen as ‘British Museum, A. Cunningham, 1826’. The specific epithet cornifolium refers to leaves resembling the cornel or dogwood tree belonging to the genus Cornus (Hooker 1832).
Common English names used are cornel-leaved pittosporum (Hooker 1832; Cooper Citation1956; Laing & Blackwell Citation1957), perching pittosporum (Beever Citation1991) and straggling pittosporum (Andersen 1926). Common Māori names used are tāwhiri karo, karo and wharewhareatua (Beever Citation1991). Both perching kohuhu and perching kohukohu are common combined English–Māori names that have been applied (Cockayne Citation1967; Landcare Research Citation2010).
Intraspecific variation
Pittosporum cornifolium demonstrates significant morphological and anatomical variability, as well as genetic variation across widely distributed populations, but especially with respect to differences between the mainland populations and Poor Knights Islands individuals.
Capsule valve numbers within this species are inconsistent, with capsules typically being two-valved, but occasionally producing three (Petrie Citation1921; Allan Citation1961). Terminal umbels vary in flower number from 2 to 10 flowers, and occasionally to single terminal flowers (Petrie Citation1921; Cooper Citation1956). Petrie (Citation1921) notes that functionally male plants have higher numbers of flowers per inflorescence than functionally female plants. Flower colour is variable, with reddish brown (Hooker 1982), light red (Allan Citation1961) and yellow forms (Cooper Citation1956) having been recorded. From our observations, fully yellow forms appear to be confined to the Poor Knights Islands plants while pinks, reds and partial yellow colourations occur on mainland New Zealand and other offshore islands (). Glasshouse collections (2009–2010) showed that mainland plants sourced from the Central North Island flowered earlier (early July to late September) than plants from the Poor Knights Islands (mid August to late October) (F.M. Clarkson Citation2011). The Poor Knights Islands form is also more susceptible to frost, and unlike the mainland form it does not cease growth in winter months (Smith Citation2004).
Continuous variation of mean leaf length and width measures were observed from smaller mainland leaf forms (across nine North Island locations and one South Island location) (34.5–62.6 mm and 12.4–27.1 mm, respectively) to the larger Poor Knights Islands leaf forms (53.7–70.8 mm 27.1–36.7 mm, respectively) (F.M. Clarkson Citation2011). The Poor Knights Islands individuals are mainly distinguished by their greater mean width. Furthermore, maximum leaf length and width measurements were significantly lower for mainland individuals (76 and 35 mm) when compared with Poor Knights Islands individuals (104 and 50 mm, respectively) (F.M. Clarkson Citation2011).
Mean leaf blade thickness measures were significantly greater in the Poor Knights Islands individuals when compared with mainland individuals sourced from two North Island populations (720 and 501 µm, respectively).
Population genetic analyses were conducted on five North Island populations () and eight propagated individuals sourced from the Poor Knights Islands using inter-simple sequence repeats (ISSRs) (F.M. Clarkson Citation2011). Results indicated that genetic diversity was extremely high overall at the species level (90% polymorphic loci) but much lower at the population-level (16.8%–60.9% polymorphic loci). The outcrossing dioecious breeding system of P. cornifolium is likely to be one of the most important factors influencing the observed high species-level diversity (F.M. Clarkson Citation2011). Lower population-level genetic diversity (relative to intra-specific genetic diversity) is likely to result from geographic isolation. On the mainland, population isolation is due to the clearance and fragmentation of lowland ecosystems that host P. cornifolium, whereas the Poor Knights Islands are isolated from the mainland by ocean. Overall, the Poor Knights Islands plants exhibited the lowest levels of genetic diversity compared with mainland populations and were by far the most genetically distinct population (F.M. Clarkson Citation2011).
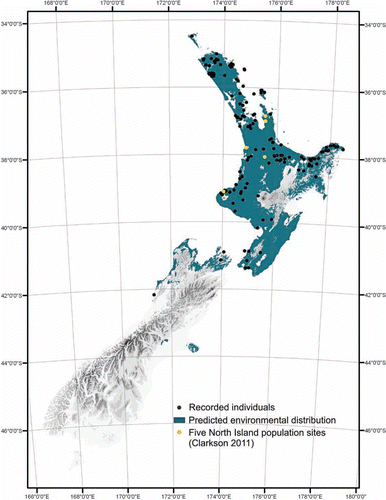
Figure 4 Observed distribution (recorded individuals; n=221) of Pittosporum cornifolium including the five North Island population sites researched by F.M. Clarkson (Citation2011), and predicted environmental distribution based on environmental variables (total annual rainfall, mean October vapour pressure deficits at 0900 h, mean annual temperature, mean minimum daily temperature of the coldest month, elevation, mean annual solar radiation and mean minimum daily solar radiation in June).
Mantel test results revealed a significant correlation between genetic and geographic distance (r = 0.647, p = 0.004). This further supports the main hypothesis that geographic isolation is the main contributing factor to population-level differentiation in P. cornifolium populations (F.M. Clarkson Citation2011).
Comparisons of ITS sequence data for both mainland and Poor Knights Islands P. cornifolium individuals revealed a single point mutation at 583 base pairs (F.M. Clarkson Citation2011). This was an unexpected result, especially considering that identical ITS sequences were observed between mainland P. cornifolium and both P. pimeleoides subspecies. It is hypothesized that sequence divergence in this offshore island variant would have a maximum age that is consistent with the isolation of the offshore island group from the mainland (F.M. Clarkson Citation2011), that is, less than one million years (Hayward Citation1986).
Together these data on leaf anatomy, morphology, population genetic structure and ITS sequence divergence of the Poor Knights Islands individuals may warrant the delineation of a new subspecies, or even species (F.M. Clarkson Citation2011).
Chemistry
Early phytochemichal investigation revealed saponin and tannin in the leaves of the New Zealand species P. cornifolium, P. crassifolium, P. eugenioides and P. huttonianum (Greshoff Citation1909). Jay (Citation1969) observed flavonoids such as quercertin and kampferol throughout members of Pittosporum including the New Zealand taxa P. crassifolium, P. eugenioides, P. dallii and P. tenuifolium. In addition, he found the flavonoid isorhamnetin in P. eugenioides and the flavone apigenin in P. tenuifolium. Phytosterols have been isolated from the bark of P. colensoi and P. eugenioides (Cambie & Parnell Citation1969) and polyacetylenes from the root of P. crassifolium (Bohlmann & Zdero 1975 as cited in Nemethy & Calvin Citation1982).
Reproductive biology
Flowering
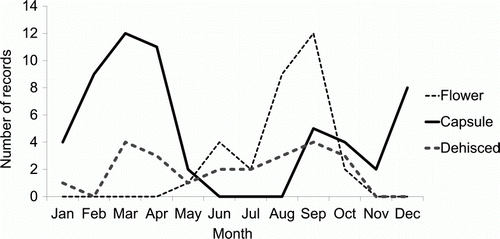
Pittosporum cornifolium flowers annually and has an autumn–spring flowering season extending from May to October with peak flowering in September () (F.M. Clarkson Citation2011). Petrie (Citation1921) noted the tendency for male plants to produce more flowers than female plants, with male plants producing terminal umbels with up to 10 flowers (usually six to eight), and female plants producing single terminal flowers.
Pollination and seeding
Individual plants are typically dioecious (Petrie Citation1921). However, plants grown from cuttings off an apparently dioecious male plant have produced infrequent capsules (F.M. Clarkson Citation2011). Godley (Citation1979) has described this ‘inconstant male’ trait among other dioecious genera in New Zealand. Thus, P. cornifolium appears to be subdioecious, outcrossing is likely to be the preferential mode of fertilization but self-fertilization may also be possible (controlled studies have yet to be undertaken to confirm this) (F.M. Clarkson Citation2011). Capsules can be found on female plants all year round and often green capsules are present from the current flowering year together with dehisced capsules from the previous year ().
Although no records of specific insect pollinators exist, P. cornifolium is thought to be entomophilous due to its small flower size and the absence of features adapted to pollination by birds (Webb et al. Citation1999). These characteristics are consistent throughout New Zealand Pittosporum taxa (Webb et al. Citation1999). Insect visitations have been recorded for P. tenuifolium by beetle species, Erirhinus limbatus and Tigones caudata (Thomson Citation1926), and numerous Diptera (fly) species (Heine Citation1937); for P. crassifolium by fly species, Calliphora stygia and Syrphus novae-zelandiae, members of families Tachinidae and Opomyzidae, the introduced bee Apis mellifera (Heine Citation1937) and insect orders Coleoptera, Hymenoptera and Hemiptera (Anderson Citation2003); and for P. eugenioides by introduced flies (Thomson Citation1926).
However, Anderson (Citation2003) has also recorded bird visitations to P. crassifolium by the endemic honey eaters, tui (Prosthemadera novaeseelandiae) and bellbird (Anthornis melanura); this association was previously underestimated and suggests birds may be active pollinators among other members of the New Zealand Pittosporum. Castro and Robertson (Citation1997) document visitation of P. cornifolium flowers by endemic honeyeaters hihi (Notiomystis cincta) and bellbird; this further suggests birds may have a larger influence in the active pollination of Pittosporum than first suspected.
After fertilization, the ovules develop into black seeds (see morphology). Mature capsules open to reveal vermilion stained interiors with seeds immersed in a sticky resin (Cooper Citation1956; Poole & Adams Citation1994). Pittosporum cornifolium is thought to be bird dispersed due to its resinous seeds (Oliver Citation1930; Burrows Citation1994), the vermilion red inner capsule coating may also act as an attractant. Oliver (Citation1930) records P. cornifolium seeds being accidentally attached to feathers which would serve as a dispersal mode, and Powlesland (Citation1987) has recorded consumption of the seed by native kōkako (Callaeas cinerea), however, tests to determine whether seeds are viable once passed through the gut have yet to be conducted.
No information is available on seed viability and seed germination rates but results for P. obcordatum (B.D. Clarkson & Clarkson Citation1994) show that seed from female plants germinated readily but seed collected from inconstant males failed to germinate.
Lifespan and population structure
No published data on lifespan have been found. One individual growing on the University of Waikato campus in Hamilton is known from planting records to be at least 34 years of age. An estimated age of c. 42 years and a diameter growth rate of 0.09 cm per year were extrapolated for the main trunk (3.9 cm) by counting 12 assumed annual growth rings on a 1.1 cm diameter side branch. This specimen is in good health and actively growing, indicating that P. cornifolium may live for 50 years or more.
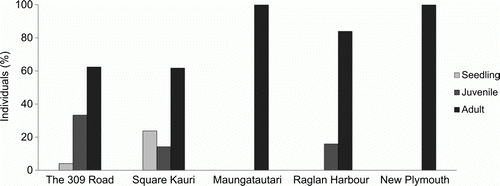
In a study of five North Island populations (), height and width estimates and reproductive status of P. cornifolium individuals were obtained to determine population (life stage) structure () (F.M. Clarkson Citation2011). The five populations displayed a range of life-stage structures. Only two of the populations exhibited recent regeneration (Square Kauri and The 309 Road) and of the remaining three populations, only Raglan Harbour had individuals in the juvenile size class (). The remaining two populations, Maungatautari and New Plymouth, were entirely composed of adult individuals (). The combined sex ratio of these populations was slightly skewed with a higher proportion of male to female individuals (61: 39 respectively). However, all of the populations contained reproductively mature female plants which flower regularly, producing capsules and seed (F.M. Clarkson Citation2011).
Distribution
Geographic range
The known range of P. cornifolium extends from the North Cape (North Island) to the Marlborough Sounds and Paparoa Range (South Island) with a southern limit near Barrytown on the West Coast (latitude 42°15′S; Cooper Citation1956). It also occurs on numerous northern offshore islands such as Tawhiti Rahi/Poor Knights Islands, Taranga/Hen Island, Hauturu/Little Barrier Island, Aotea/Great Barrier Island, Kawau Island and Waiheke Island (Cooper Citation1956) () (F.M. Clarkson Citation2011).
Environmental range
Throughout its geographic range, P. cornifolium can be found in a variety of rupestral and forest ecosystems within an elevation range of 0–786 m above sea level and a mean elevation of 248 m above sea level (F.M. Clarkson Citation2011). A predicted environmental distribution map was developed from recorded Pittosporum cornifolium locations (n=221) which were overlain on Land Environments of New Zealand (LENZ) environmental surfaces (Leathwick et al. 2003) to obtain minimum and maximum values of selected environmental variables (, ) (F.M. Clarkson Citation2011). Pittosporum cornifolium is distributed widely in the coastal and lowland zones of the North Island. However, there are some obvious gaps in both its observed and predicted distribution (). Gaps in the observed distribution that are inconsistent with potential environmental distribution include the Waikato basin where environmental variables are favourable but the area was formerly dominated by extensive wetland systems and thus lacks appropriate habitat (B.R. Clarkson Citation2002). The environment is suitable south of the Wairoa lowlands in the Wairarapa but there are no current records, probably because nearly all lowland forest in the area has been cleared (Nicholls Citation1980). Similarly, much of the Taupo Volcanic Zone is favourable in terms of the predicted environmental distribution of P. cornifolium, but observed absences are likely to be due to the ecological impact caused by the 232 AD (Hogg et al. 2011) Taupo eruption. Both Mahia in the North Island and Banks Peninsula in the South Island are also environmentally favourable locations where P. cornifolium has not been recorded. The often companion obligate epiphyte Griselinia lucida is present at both of these localities: Banks Peninsula (Laing Citation1919) and Mahia (Whaley et al. Citation2001). Additionally, many predominantly North Island warm–temperate species such as nikau (Rhopalostylis sapida) are found growing in the Banks Peninsula area (Wilson Citation2002). Pittosporum cornifolium is largely unrecorded from the drier eastern side of the North Island where native forests have been almost completely removed, and the low rainfalls are not conducive to rich epiphyte floras (B.R. Clarkson & Clarkson Citation1991). Absence from the Wairoa, Gisborne and Waiapu lowlands was predicted due to the high mean October vapour pressure deficits exceeding 0.47 kPa (F.M. Clarkson Citation2011). Pittosporum cornifolium was absent from the Kaimanawa and Ikawhenua ranges in the Central North Island in both observed and predicted distribution maps. Mean daily temperature minimums for the coldest month are lower than 0.6 °C in the area. Pittosporum cornifolium has both restricted observed distributions and predicted distributions in the South Island; these limits are set primarily by low mean daily temperature minimums of the coldest month (< 0.6 °C), but also from a combination of low mean annual temperature (< 9.6 °C), low mean annual rainfall (< 937 mm), low mean annual solar radiation (< 13.1 kJ/m2/day), low mean minimum solar radiation in June (< 4.2 kJ/m2/day) and high elevation (> 786 m) () (F.M. Clarkson Citation2011).
Table 1 Summary statistics of environmental variables from known plot locations of Pittosporum cornifolium. Environmental variables include: total annual rainfall (r), mean October vapour pressure deficits at 0900 hours (vpd), mean annual temperature (mat), mean minimum daily temperature of the coldest month (t min), elevation, mean annual solar radiation (mas) and mean minimum daily solar radiation in June (junes).
Plant communities
Pittosporum cornifolium is represented in a range of lowland and coastal forest, and rupestral ecosystems. Within this range, it exhibits three distinct lifestyles; epiphytic, terrestrial and rupestral, the most common being the epiphytic lifestyle. The overall lifestyle statistics obtained from herbarium records (n=92) and an ecological survey of five North Island populations (n=110) were 107 (53%) epiphytic, 52 (26%) terrestrial and 43 (21%) rupestral (F.M. Clarkson Citation2011). A combination of field survey data from the North Island and herbarium data sets (n=142) revealed P. cornifolium were recorded in 20 different vegetation types (), all of which were forest except for rupestral shrubland (F.M. Clarkson Citation2011). The three most common types included kauri (Agathis australis) forest, rupestral shrubland and rimu/tawa (Dacrydium cupressinum/Beilschmiedia tawa) forest. Although it is never a dominant component of these types, P. cornifolium occasionally attains co-dominant status in the rupestral communities of Raglan Harbour.
Table 2 Vegetation types containing Pittosporum cornifolium based on North Island field survey and data from herbarium records (n=142).
Lowland and coastal forest communities
Pittosporum cornifolium occurs in several New Zealand lowland forest types including kauri, mixed podocarp, coastal pōhutukawa (Metrosideros excelsa) and broadleaved forest types. Within these lowland forest systems, where it exhibits both epiphytic and terrestrial lifestyles, the species appears to have an affinity to older growth forest and/or remnant old growth trees (F.M. Clarkson Citation2011).
The tall kauri forests of the North Island, which usually occur on well-drained hill sides (Oliver Citation1930), host P. cornifolium of both terrestrial and epiphytic lifestyles. Within this forest type, P. cornifolium has been found growing abundantly in the crowns and directly beneath the crowns of large old growth kauri trees, on the trunks of tree fern species Cyathea dealbata and C. cunninghamii, and in association with Collospermum hastatum, Astelia solandri, A. trinervia and Brachyglottis kirkii (F.M. Clarkson Citation2011).
Podocarp-dominant lowland forests, with their dense canopy of foliage, contain the greatest diversity of epiphytes (Oliver Citation1930). Within these forests, P. cornifolium can be found on a variety of host trees including: rimu (Dacrydium cupressinum), tōtara (Podocarpus totara), mataī (Prumnopitys taxifolia) and kahikatea (Dacrycarpus dacrydioides) and grow primarily in association with C. hastatum and A. solandri (F.M. Clarkson Citation2011).
Broadleaved dominant lowland forests also provide a range of canopy hosts. These include tawa (Beilschmiedia tawa), taraire (Beilschmiedia taraire), pukatea (Laurelia novae-zelandiae), pūriri (Vitex lucens) and tītoki (Alectryon excelsus), and again P. cornifolium grows primarily in association with C. hastatum and A. solandri (F.M. Clarkson Citation2011).
The main coastal forest hosts are pōhutukawa (Metrosideros excelsa) and pūriri (F.M. Clarkson Citation2011). Kirk (Citation1872) noted abundant epiphytic Griselinia lucida, P. cornifolium and A. solandri on Tarawera lake shore pōhutukawa prior to the 1886 eruption. Large pūriri growing around the Raglan Harbour, Waikato, also host epiphytic P. cornifolium (F.M. Clarkson Citation2011).
Other associated species in lowland and coastal forests include Metrosideros fulgens, Asplenium polyodon and Microsorum pustulatum (F.M. Clarkson Citation2011).
Rupestral communities
Pittosporum cornifolium can be found growing as a rupestral in coastal rock communities including Maunganui Bluff (G. Bowden, Tawapou Nursery, pers. comm. 2010), Waiheke Island, Poor Knights Islands (Cooper Citation1956), and Raglan Harbour where P. cornifolium grows as a co-dominant species amongst scrub communities of A. banksii and Griselinia lucida (F.M. Clarkson Citation2011).
Succession
Oliver (Citation1930) describes the general pattern of epiphyte succession in New Zealand forests as follows: primary colonization of bark by small lichens and mosses, which facilitate (by substrate alteration) the establishment of ferns or orchid species. The increase in community composition encourages further establishment (i.e. lichens and mosses adhere to fern rhizomes or aerial roots of orchids), while exfoliated bark and fallen leaves are caught and decay to produce a fine, low nutrient, gritless soil that collects in large quantities (vertical branches may support soil layers as thick as a third of the branch diameter). Moisture is retrieved directly via rainfall or indirectly via trunk runoff, while evaporation is retarded by the covering of foliose lichens (Oliver Citation1930).
Epiphytic P. cornifolium is late successional as it commonly colonizes already established nest hosts A. solandri and Collospermum hastatum (Oliver Citation1930; B.D. Clarkson Citation1985; Burns & Dawson Citation2005; F.M. Clarkson Citation2011). Nest epiphytes facilitate the establishment of larger shrub epiphytes like P. cornifolium by providing a source of organic matter and moisture, thereby enhancing microclimate suitability (see Dickinson et al. Citation1993).
Rupestral individuals at Raglan Harbour were also found growing in association with nest host species (A. banksii), where facilitation by the nest host is likely, as P. cornifolium individuals were notably absent from raw rock surfaces (F.M. Clarkson Citation2011).
The detritus substrate of terrestrial individuals growing beneath kauri stands in the Coromandel Peninsula is composed of mostly brown loam soils, which are typically low nutrient and acidic (Molloy Citation1998). Detritus substrates are a result of long-term soil development by gradual erosion and the build up of organic matter provided by old growth kauri forest systems. Therefore, the build up of detritus soils in old growth systems may facilitate the establishment of terrestrial P. cornifolium as does substrate development in canopy and rupestral systems (F.M. Clarkson Citation2011).
Conservation and restoration
Conservation status
The loss of lowland and coastal habitat in the Waikato region (Leathwick et al. Citation1995), and in the majority of ecological regions around New Zealand (McGlone Citation1989), has meant that the historical range of P. cornifolium is significantly reduced and its populations depleted. Additionally, the strong habitat specificity of P. cornifolium restricts it to habitats of very limited extent (F.M. Clarkson Citation2011).
Although P. cornifolium can be locally uncommon, it does not currently meet the specific criteria (see de Lange et al. Citation2009) to be considered nationally threatened (F.M. Clarkson Citation2011). It is found in numerous protected natural areas including national parks, scenic reserves and QE II open space covenants. However, there is no doubt that its range has been reduced and that it has been lost from a number of sites where it was previously collected (F.M. Clarkson Citation2011). Observations also suggest that it is palatable to possums, and possum browsing may have contributed to the reduction of some populations (Ravine Citation1995; Mitcalfe & Horne Citation2005). Its epiphytic cogener, P. kirkii is classified as declining (de Lange et al. 2009). Surveillance of P. cornifolium populations may be warranted to detect gradual or sudden decline.
Restoration
To date, only a few restoration projects around New Zealand appear to have considered the reintroduction of P. cornifolium. These include Karori Sanctuary (Karori Sanctuary Trust Citation2008), Matakohe Island (Ritchie Citation2000) and Motuihe Island (Hawley Citation2005).
Conservation efforts should be focused on retaining substantial populations in large reserves while restoration is required where numbers are reduced or the species has been recently lost.
The significant correlation between geographic distance and genetic differentiation among five North Island populations of P. cornifolium highlights the importance of plant provenance and associated ecotypes when sourcing seed for restoration (F.M. Clarkson Citation2011). The concept of ecological microhabitats is also important, especially in consideration of the different lifestyles of P. cornifolium in a range of lowland and coastal ecosystems (epiphytic, rupestral and terrestrial). Ideally, source sites would be both the closest to the restored site (to maintain provenance) and be a similar ecosystem to the one in need of restoring (F.M. Clarkson Citation2011). Furthermore, seeds should be collected from as many individuals as possible to maintain levels of genetic diversity similar to that of source populations (F.M. Clarkson Citation2011).
Conclusion
Pittosporum cornifolium has been described by Oliver (Citation1930) as a typical epiphyte in that the species is habitually epiphytic. However, although it is more commonly epiphytic, it can also be found growing in abundance in terrestrial and rupestral lifestyles. With this diversity of lifestyles, P. cornifolium may be considered a facultative epiphyte as defined by Benzing (Citation2004). Using the lifestyle statistics presented (see Plant Communities), the null model for Benzing's classifications developed by Burns (Citation2010) places P. cornifolium as a facultative epiphyte. Epiphytes become increasingly facultative as environmental conditions in tree canopies converge on terrestrial environmental conditions (Benzing Citation2004). The strong habitat specificity of P. cornifolium restricts it to lowland and coastal habitats of very limited extent, which are widely yet discontinuously distributed. Environmental factors that may restrict distribution include high vapour pressure deficits, cooler temperatures, limited rainfall, limited solar radiation and higher elevations. Edaphic range is characterized by low nutrient substrate types. The distinctive leaf morphology and anatomy, as well as the differentiated population genetic structure and ITS sequence divergence, in the Poor Knights Islands individuals suggest that recognition of a new taxon may be warranted. This requires detailed examination of the taxon across its mainland range, the Poor Knights Islands, and other northern offshore islands where the species is present. Although P. cornifolium can be locally uncommon across its known range, it does not currently meet the specific criteria to be considered regionally or nationally threatened. However, there is no doubt that its range has been reduced and that it has been lost from a number of sites where it was previously collected. Key considerations for the conservation and restoration of this species in areas in which populations have been depleted or completely displaced include sourcing for local provenance and specific microhabitats and associated lifestyles. Finally, future research should focus on baseline ecological research including deciphering pollination and dispersal modes, as well as germination and seed viability trials, as this is fundamental to developing informed conservation and restoration strategies.
Acknowledgements
We gratefully acknowledge access provided by Landcare Research to the National Vegetation Survey Databank (NVS) and Land Environments of New Zealand (LENZ). We thank the following herbaria for access to their records, University of Waikato (WAIK), Allan (CHR), Auckland Museum (AK), Museum of New Zealand Te Papa Tongarewa (WELT) and New Zealand Forest Research Institute (NZFRI). This project was aided by funding gratefully received from the University of Waikato Masters Scholarship and the George Mason Charitable Trust. We also appreciate comments and contributions from Dr Bev Clarkson, Dr Jake Overton, Toni Cornes and Catherine Bryan.
References
- Allan , HH . 1961 . Flora of New Zealand , Vol. 1 , Wellington : Government Printer .
- Andersen , JC . 1926 . Popular names of New Zealand plants . Transactions and Proceedings of the New Zealand Institute , 56 : 659 – 714 .
- Anderson , SH . 2003 . The relative importance of birds and insects as pollinators of the New Zealand flora . New Zealand Journal of Ecology , 27 : 83 – 94 .
- Beever , J . 1991 . A dictionary of Maori plant names . Auckland Botanical Society Bulletin , 20 : 1 – 75 .
- Benzing , DH . 2004 . “ Vascular epiphytes ” . In Forest canopies , Edited by: Lowman , MD and Rinker , HB . 175 – 211 . Berlin : Springer Verlag .
- Burns , KC . 2010 . How arboreal are epiphytes? A null model for Benzing's classifications . New Zealand Journal of Botany , 48 : 185 – 191 .
- Burns , KC and Dawson , J . 2005 . Patterns in the diversity and distribution of epiphytes and vines in a New Zealand forest . Austral Ecology , 30 : 883 – 891 .
- Burrows , CJ . 1994 . Fruit types and seed dispersal modes of woody plants in Ahuriri Summit Bush, Port Hills, western Banks Peninsula, Canterbury, New Zealand . New Zealand Journal of Botany , 32 : 169 – 181 .
- Cambie , RC and Parnell , JC . 1969 . A New Zealand phytochemical survey. Part 7. Constituents of some dicotyledons . New Zealand Journal of Science , 12 : 453 – 466 .
- Castro , I and Robertson , AW . 1997 . Honeyeaters and the New Zealand forest flora: the utilization and profitability of small flowers . New Zealand Journal of Ecology , 21 : 169 – 179 .
- Cayzer LW 1997 . Revision of the systematics and biogeography of the family Pittosporaceae in Australia . Unpublished PhD thesis Australian National University Canberra .
- Chandler , GT , Plunkett , GM , Pinney , SM , Cayzer , LW and Gemmill , CEC . 2007 . Molecular and morphological agreement in Pittosporaceae: phylogenetic analysis with nuclear ITS and plastid trnl–trnf sequence data . Australian Systematic Botany , 20 : 390 – 401 .
- Cheeseman , TF . 1925 . Manual of the New Zealand flora , 2nd edition , Wellington : Government Printer .
- Clarkson , BD . 1985 . The vegetation of the Kaitake Range, Egmont National Park, New Zealand . New Zealand Journal of Botany , 23 : 15 – 31 .
- Clarkson , BD and Clarkson , BR . 1994 . Ecology of an elusive endemic shrub, Pittosporum obcordatum Raoul . New Zealand Journal of Botany , 32 : 155 – 168 .
- Clarkson , BR . 2002 . “ Swamps, fens and bogs ” . In Botany of the Waikato , Edited by: Clarkson , BD , Merrett , M and Downs , T . 47 – 55 . Hamilton : Waikato Botanical Society .
- Clarkson , BR and Clarkson , BD . 1991 . Turanga Ecological District. New Zealand Protected Natural Areas Programme No. 14 , 131 Gisborne : Department of Conservation .
- Clarkson FM 2011 . Population genetics and autecology of the endemic shrub epiphyte Pittosporum cornifolium . Unpublished MSc thesis, University of Waikato. Hamilton .
- Cockayne , L . 1967 . New Zealand plants and their story , 4th edition , Wellington : Government Printer .
- Cooper , RC . 1956 . The Australian and New Zealand species of Pittosporum . Annals of the Missouri Botanical Garden , 43 : 87 – 184 .
- Cutler , DF , Botha , T and Stevenson , DW . 2008 . Plant anatomy: an applied approach , Oxford : Blackwell .
- de Lange , PJ , Heenan , PB , Norton , DA , Rolfe , J and Sawyer , J . 2010 . Threatened plants of New Zealand , Christchurch : Canterbury University Press .
- de Lange , PJ , Murray , BG and Datson , PM . 2004 . Contributions to a chromosome atlas of the New Zealand flora – 38. Counts for 50 families . New Zealand Journal of Botany , 42 : 873 – 904 .
- de Lange , PJ , Norton , DA , Courtney , SP , Heenan , PB , Barkla , JW , Cameron , EK , Hitchmough , R and Townsend , AJ . 2009 . Threatened and uncommon plants of New Zealand (2008 revision) . New Zealand Journal of Botany , 47 : 61 – 96 .
- Dickinson , KJM , Mark , AF and Dawkins , B . 1993 . Ecology of lianoid/epiphytic communities in coastal podocarp rain forest, Haast ecological district, New Zealand . Journal of Biogeography , 20 : 687 – 705 .
- Druce , AP . 1977 . Trees, shrubs, and lianes of New Zealand (including wild hybrids) , Lower Hutt : Botany Division, DSIR .
- Eagle A 1982 . Eagle's trees and shrubs of New Zealand . Second series . Auckland , Collins .
- Eagle , A . 2006 . Eagle's trees and shrubs of New Zealand , Revised edition , Wellington : Te Papa Press .
- Ecroyd , CE . 1994 . Regeneration of Pittosporum turneri communities. Conservation Advisory Science Notes 99 , Wellington : Department of Conservation .
- Fahn , A . 1982 . Plant anatomy , 3rd edition , Oxford : Pergamon Press .
- Gaertner J 1788 . Pittosporum Banks ex Sol . De Fructibus et Seminibus 1 : 286 .
- Godley , EJ . 1979 . Flower biology in New Zealand . New Zealand Journal of Botany , 17 : 441 – 466 .
- Goulding , JH . 1983 . Fanny Osborne's flower paintings , Auckland : Heinemann .
- Gowda , M . 1951 . The genus Pittosporum in the Sino-Indian region . Journal of the Arnold Arboretum , 32 : 263 – 343 .
- Greshoff , M . 1909 . Phytochemical investigation at Kew . Kew Bulletin , 10 : 397
- Haas , JE . 1977 . The pacific species of Pittosporum Banks ex Gaertn . Allertonia , 1 : 73 – 167 .
- Hathaway LA 2001 . Phylogenetic relationships between the New Zealand Pittosporum (Pittosporaceae) inferred from ITS sequences of rDNA . Unpublished MSc thesis University of Waikato Hamilton .
- Hawley , J . 2005 . Motuihe restoration plan , Auckland : Department of Conservation .
- Hayward , BW . 1986 . “ Origin of the offshore islands of northern New Zealand and their landform development ” . In The 43 offshore islands of northern New Zealand , Edited by: Wright , AE and Beever , RE . Wellington : Department of Lands and Survey .
- Heine EM 1937 . Observations on the pollination of New Zealand flowering plants . Transactions and Proceedings of the Royal Society of New Zealand 67 : 131 – 148 .
- Hogg A Lowe D Palmer J Boswijk G Ramsey C 2011 . Revised calendar date for the Taupo eruption derived by 14C wiggle-matching using a New Zealand kauri 14C calibration data set . The Holocene 22 : 1 – 11 doi: 10.1177/0959683611425551 .
- Hooker W 1832 . Plate 3161: Pittosporum cornifolium . Cornel-leaved pittosporum. Curtis's Botanical Magazine 59 .
- Jay , M . 1969 . Chemotaxonomic researches on vascular plants. XIX. Flavonoid distribution in the Pittosporaceae . Botanical Journal of the Linnean Society , 62 : 423 – 429 .
- Karori Sanctuary Trust 2008 . Forest flora restoration . http://www.sanctuary.org.nz/Site/Conservation_and_Research/Restoration/Forest_flora_and_fauna/Forest_flora_restoration_.aspx. (accessed 15 January 2010) .
- Kirk T 1871 . On the New Zealand species of Pittosporum, with descriptions of new species . Transactions and Proceedings of the Royal Society of New Zealand 4 : 260 – 267 .
- Kirk T 1872 . Notes on the flora of the Lake District of the North Island . Transactions and Proceedings of the Royal Society of New Zealand 5 : 322 – 345
- Kirk , T . 1899 . The students’ flora of New Zealand and the outlying islands , Wellington : Government Printer .
- Laing RM 1919 . The vegetation of Banks Peninsula, with a list of species (flowering-plants and ferns) . Transactions and Proceedings of the Royal Society of New Zealand 51 : 356 – 407
- Laing , RM and Blackwell , EW . 1957 . Plants of New Zealand , Christchurch : Whitcombe and Tombs .
- Landcare Research 2010 . Pittosporum cornifolium . Ngā Tipu o Aotearoa-New Zealand Plants. http://nzflora.landcareresearch.co.nz/ (accessed 5 December 2010) .
- Leathwick J , Clarkson BD , Whaley P 1995 . Vegetation of the Waikato region: current and historical perspectives . Landcare Research Contract Report: LC956/022 . Envrionment Waikato , Hamilton .
- Leathwick J Morgan F Wilson G Rutledge D McLeod M Johnston K 2003 Land environments of New Zealand: a technical guide David Bateman Auckland 237
- McGlone , MS . 1989 . The Polynesian settlement of New Zealand in relation to environmental and biotic changes . New Zealand Journal of Ecology , 12 : 115 – 129 .
- Mitcalfe BJ , Horne JC 2005 . A botanical survey of the indigenous forest remnants in Wellington Botanic Garden, Glenmore Street, Wellington . Unpublished report to Friends of Wellington Botanic Garden Inc, Wellington .
- Molloy , L . 1998 . Soils in the New Zealand landscape: the living mantle , Lincoln : New Zealand Society of Soil Science .
- Nemethy , EK and Calvin , M . 1982 . Terpenes from Pittosporaceae . Phytochemistry , 21 : 2981 – 2982 .
- Nicholls , JL . 1980 . The past and present extent of New Zealand's indigenous forests . Environmental Conservation , 7 : 309 – 310 .
- Oliver , WRB . 1930 . New Zealand epiphytes . Journal of Ecology , 8 : 1 – 50 .
- Petrie D 1921 . Descriptions of new native flowering-plants, with a few notes . Transactions and Proceedings of the Royal Society of New Zealand 53 : 365 – 371 .
- Poole , AL and Adams , NM . 1994 . Trees and shrubs of New Zealand , Lincoln : Manaaki Whenua Press .
- Powlesland , RG . 1987 . The foods, foraging behaviour and habitat use of North Island kokako in Puketi State Forest, Northland . New Zealand Journal of Ecology , 10 : 117 – 128 .
- Ravine DA 1995 . Manawatu Plains Ecological District. NZ Protected Natural Areas . Programme No. 33. Wanganui, Department of Conservation .
- Ritchie J 2000 . Matakohe/Limestone Island Scenic Reserve Restoration Plan . Prepared for the Friends of Matakohe/Limestone Island Incorporated Society. Auckland, Natural Logic Environmental Management Limited .
- Smith , P . 2004 . Pittosporum, the misunderstood genus . New Zealand Garden Journal , 7 : 14 – 18 .
- Thomson GM 1926 . The pollination of New Zealand flowers by birds and insects . Transactions and Proceedings of the Royal Society of New Zealand 56 : 106 – 125 .
- Tirel , C and Veillon , JM . 2002 . Flore de la Nouvelle-Calédonie 24. Pittosporaceae , Paris : Museum National d'Histoire Naturelle .
- Wagner WL , Herbst DR , Sohmer SH 1999 . Manual of the flowering plants of Hawai'i, revised edition with supplement by WL Wagner and DR Herbst . Bishop Museum Special Publication 97 . Honolulu : University of Hawai'i Press . Pp. 1855 – 1918 .
- Webb , CJ , Lloyd , DG and Delph , LF . 1999 . Gender dimorphism in indigenous New Zealand seed plants . New Zealand Journal of Botany , 37 : 119 – 130 .
- Webb , CJ and Simpson , MJA . 2001 . Seeds of New Zealand gymnosperms and dicotyledons , Christchurch : Manuka Press .
- Whaley KJ , Clarkson BD , Emmett DK , Innes JG , Leathwick JR , Smale MC , Whaley PT 2001 . Tiniroto, Waihua, Mahia and Matawai Ecological Districts: survey report for the Protected Natural Areas Programme . New Zealand Protected Natural Areas Programme. Gisborne, Department of Conservation .
- Wilkinson , HP . 1992 . Leaf anatomy of the Pittosporaceae . Botanical Journal of the Linnean Society , 110 : 1 – 59 .
- Wilson , HD . 2002 . Hinewai: the journal of a New Zealand naturalist , Christchurch : Shoal Bay Press .