Abstract
Chromosome numbers are reported for 112 endemic or indigenous vascular plants from New Zealand. Ninety one of these are new and the remainder provide confirmation of previous counts. Many of the counts fill gaps in the available record of chromosome numbers of New Zealand plants and a summary table provides a list of the genera where numbers remain to be determined. With the publication of these numbers, c. 85% of the indigenous vascular flora of New Zealand now has a documented chromosome number.
Introduction
Chromosome numbers are a valuable indicator of biodiversity as well as providing characters that are of use in systematic and evolutionary studies (Stace Citation2000) and also help in the understanding of issues of genetic diversity in the conservation of rare and endangered species (de Lange et al. Citation2008; Young & Murray Citation2000). Following the publication of comprehensive indices of chromosome numbers of indigenous vascular plants (Dawson Citation2000; Dawson et al. Citation2000), the gaps in the records for New Zealand were greatly clarified. Subsequent papers by Dawson et al. (Citation2007), de Lange & Murray (Citation2002), de Lange et al. (2004) and Murray et al. (Citation2005) have filled many of the gaps but chromosome numbers are still lacking for c. 20% of the species. Using these indices and the recent papers of new counts, the checklist of New Zealand indigenous plants (de Lange & Rolfe Citation2010) and this publication we estimate that chromosome numbers are now known for c. 85% of the native vascular plants of New Zealand. Nevertheless, there remain whole genera, such as Euphrasia and Gahnia, for which no chromosome numbers are known for New Zealand species and several large genera, such as Carex, Dracophyllum, Lepidium, Myosotis and Pimelea, for which counts are lacking for many of the species. In addition, many of the published chromosome counts have been obtained from single representatives of the species and as there are now several significant examples of intraspecific chromosome variation, for example Raoulia australis Hook.f. and R. hookeri Allan (Dawson et al. Citation1993) Hebe odora (Hook.f.) Cockayne (Dawson & Beuzenberg. Citation2000), Lobelia angulata (G.Forst.) Hook.f. (treated as Pratia angulata) (Murray et al. Citation2004) and Crassula ruamahanga A.P.Druce (de Lange et al. Citation2008), it is valuable to have counts made from several plants throughout their geographical range.
Here we report the chromosome numbers for 110 taxa from 36 families, 90 of them for the first time. Two counts are also provided, one for a Rarotongan (Cook Islands) plant of Alternanthera sessilis, a species not present in New Zealand (Heenan et al. Citation2009) and the other for Plantago lanceolata, a common naturalised plant in New Zealand.
Materials and methods
The plants used in this study are listed in . They were collected from the wild and grown either at the University of Auckland or at Oratia Native Plant Nursery, Oratia, Auckland. Voucher specimens are deposited in the herbaria of the Auckland Institute and Museum (AK), Museum of New Zealand Te Papa Tongarewa (WELT), Victoria University of Wellington (WELTU), Canterbury University (CANU) and the Allan Herbarium (CHR). Chromosomes were observed at mitotic metaphase or meiotic metaphase I using the methods described in de Lange et al. (Citation2004a). The taxonomic treatment follows Allan (Citation1961), the Angiosperm Phylogeny Group (APG III Citation2009), Cheeseman (Citation1925) and de Lange & Rolfe (Citation2010). Threatened and uncommon plant ratings follow de Lange et al. (Citation2009b). Authorities for plants for which chromosome counts are presented here are given in , otherwise they are given in the text.
Table 1 Documented chromosome numbers of New Zealand (and Rarotongan) plants: miscellaneous genera.
We have not provided an exhaustive list of all the previously reported chromosome numbers in the genera that we have studied as this would have resulted in an excessively long reference list. However, extensive background information is available at ‘The Index to Plant Chromosome Numbers’ (IPCN) at www.tropicos.org/Project/IPCN and for New Zealand, a comprehensive listing of chromosome numbers is available on Ngā Tipu o Aotearoa New Zealand Plants (http://nzflora.landcareresearch.co.nz).
Results and discussion
contains a list of the chromosome numbers of all the plants studied and micrographs of the chromosomes of selected species are shown in Figs. 1–3. The following notes provide additional information about the plants that were studied. The taxonomic classes follow the recent phylogenetic classification proposed by Chase and Reveal (Citation2009).
LYCOPODIIDAE
Lycopodiaceae
Huperzia and Lycopodium
As currently accepted (de Lange & Rolfe Citation2010), there are four genera of Lycopodiaceae in New Zealand, Huperzia, Isoetes, Lycopodiella and Lycopodium. Counts have previously been reported for the two species of Isoetes by Marsden (Citation1979) and for one species of Lycopodiella (L. lateralis (R.Br.) B.Øllg.) and one Lycopodium (L. scariosum G.Forst.) by de Lange et al. (Citation2004a). Here, we report new counts for New Zealand material of Huperzia varia (R. Br.) Trevis. (2n=256), (the form previously treated by Allan (Citation1961) as Lycopodium billardierei Spring), and for two species of Lycopodium, L. deuterodensum (2n=68) and L. fastigiatum (2n=60) (A). Huperzia australiana (Herter) Holub and Lycopodium volubile G.Forst. remain to be counted. Published counts for Huperzia from elsewhwere include H. miyoshina (Makino) Ching (2n=134), H. occidentalis (Clue) Kartesz et Gandhi (2n=134) and H. prolifera (Blume) Trevis. n=c.278 (IPCN) show no obvious relationship to that of H. varia. However, a recent biosystematic investigation has shown that Huperzia is paraphyletic, and that those species widely known as the ‘tassel ferns’ of which H. varia s.l. is one, are distinct at genus level from Huperzia s.s. (A. Field, James Cook University, pers. comm. 2011). The count obtained for L. fastigiatum is the same as that obtained previously for L. scariosum but neither shows any obvious numerical relationship to that of L. deuterodensum, indicating cytological variability in the genus.
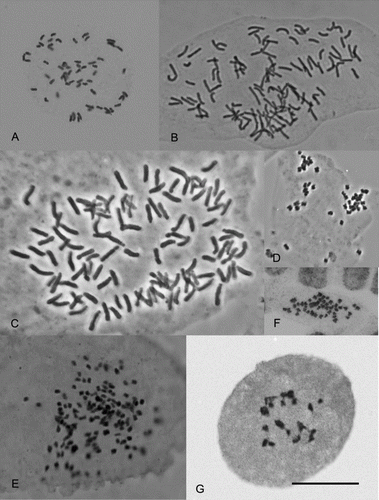
Figure 1 Chromosomes of New Zealand vascular plants. A, Lycopodium fastigatum, 2n = 60. B, Blechnum colensoi, 2n = 122. C, Adiantum viridescens, 2n = 116. D, Daucus glochidiatus, 2n = 44. E, Astelia aff. nervosa, 2n = 140. F, Leptinella dispersa subsp. rupestris, 2n = 52. G, Senecio aff. dunedinensis, n = 20II. Scale bar = 10 µm.
POLYPODIIDAE
Blechnaceae
Three new counts have been obtained for Blechnum which add to the 15 already reported for other species in New Zealand by various authors (Dawson Citation2000). Both B. fraseri and New Zealand material of B. minus had 2n=56, whereas B. colensoi had 2n=122 (B). Here, following advice from B.P.J. Molloy and J.D. Lovis (pers. comm.) we accept B. minus as present in New Zealand contrary to the treatment of Chambers & Farrant (Citation1998). The New Zealand species of the genus appear to have two basic numbers, x=28 and x=33. Shepherd et al. (Citation2007) provided a molecular phylogeny of the New Zealand Blechnaceae which showed that B. colensoi was sister to the morphologically similar B. patersonii (R.Br.) Mett. (2n=66, 132 (Tindale & Roy Citation2002)); an interesting result given the difference in chromosome number between B. colensoi and B. patersonii. The other two numbers reported, for B. fraseri and B. minus, fit within the range of chromosome numbers previously established for the majority of the New Zealand species.
Cyatheaceae
Published counts of n=69 have been recorded for Cyathea colensoi (Hook.f.) Domin, C. dealbata (G.Forst.) Sw. (for which a count of n=72 has also been reported (Brownlie Citation1954)), C. medullaris (G.Forst.) Sw. and C. smithii Hook.f. (Dawson Citation2000). Here, we report counts of 2n=138 for two further species, the indigenous C. cunninghamii and the Raoul Island endemic C. kermadecensis. Only C. milnei Hook.f., another Kermadec Island endemic, remains uncounted.
Dryopteridaceae
A plant of Lastreopsis kermadecensis, a newly described endemic species from Raoul Island (Perrie & Brownsey Citation2012), had 2n=164, equivalent to the meiotic counts reported for L. glabella (A. Cunn.) Tindale and L. microsora subsp. pentangularis (Colenso) Tindale. This is the tetraploid number for the genus with the other two New Zealand species L. hispida (Sw.) Tindale and L. velutina (A.Rich.) Tindale being diploid (n=41) (Dawson et al. Citation2000).
Pteridaceae
With the count of 2n=116 (C) reported here for Adiantum viridescens all seven indigenous species have now been counted. Our count of 2n=116 for Cheilanthes distans is clearly different from the count of 2n=84 reported by Tindale & Roy (Citation2002) for Australian plants named C. distans. It is, however, close to the 2n=c.110 reported by Quirk et al. (Citation1983) for their Australian specimens. Brownlee (Citation1957) also recorded n=87 for New Zealand specimens of C. sieberi Kunze subsp. sieberi which is also different from the 2n=56 and 84 reported for Australian specimens (Tindale & Roy Citation2002). Chromosome numbers in this genus are variable and apomixis has also been reported (Tindale & Roy Citation2002).
MAGNOLIIDAE
Amaranthaceae
Rarotonga (Cook Islands) material of Alternanthera sessilis had 2n=28. This is the same number as that reported for New Zealand examples of A. denticulata R.Br. and A. nahui Heenan et de Lange (treated as A. sessilis (L.) Roem et Schult. in de Lange et al. (Citation2004a)). Five other species in the genus have counts of n=17, n=20, 2n=96 and 2n=100 (IPCN), suggesting no obvious pattern.
Apiaceae
We obtained a count of 2n=22 for Anisotome lyallii, the same number as that recorded for 10 other New Zealand species (Dawson Citation2000). Counts are still needed for seven taxa to complete the New Zealand representatives of the genus. We found 2n=14 in South Island specimens of Chaerophyllum colensoi var. delicatulum, this is the same as that recorded for C. colensoi (Hook.f.) K.F.Chung var. colensoi and for the overseas C. temulum L. and C. temulentum L., whereas all other species in the genus for which counts have been published, including the New Zealand endemic C. ramosum (Hook.f.) K.F.Chung, have either n=11 or 2n=22 (IPCN). We report 2n=44 (D) for the sole indigenous species of Daucus, D. glochidiatus. This is the same number as that reported by Iovene et al. (Citation2008) for Australian plants.
Asteliaceae
Chromosome numbers of New Zealand species of Astelia range from n=35/2n = 70 (four species) to 2n=140 (one species) to n=105/2n=210 or c. 210 (two species) (Dawson Citation2000). Our count of 2n=140 (F) for an undescribed northern New Zealand Astelia, allied to A. nervosa, which has 2n=210, is the second report of a tetraploid number for the genus in New Zealand.
Asteraceae
Leptinella
We obtained a count of 2n=104 for the recently described Leptinella conjuncta (Heenan Citation2009) which is the same as that recorded for the allied members of the L. pectinata (Hook.f.) D.G.Lloyd et C.J.Webb group (Dawson Citation2000) from which L. conjuncta was segregated. We also report 2n=52 for L. dispersa subsp. rupestris (F), the same number as that recorded for subsp. dispersa (Dawson Citation2000).
Microseris
A plant of Microseris scapigera from the Old Man Range had 2n=36, the same as three previous counts for the species (Dawson Citation2000).
Olearia
As currently circumscribed, there are c. 41 species and varieties of Olearia in New Zealand (de Lange & Rolfe Citation2010). Six of these are uncounted (de Lange & Rolfe Citation2010) so here we report counts for four of them, O. lacunosa, O. rani var. rani, O. telmatica and O. townsonii. All have 2n=108, the same number as that reported for 31 other Olearia, polyploids with 2n=216, 288, 324, c. 432 and > 400 have also been reported in the genus (Dawson Citation2000; de Lange et al. Citation2004a; de Lange & Rolfe Citation2010). Olearia is now considered to be paraphyletic (Wagstaff et al. Citation2011) and it is interesting that the genera that cluster with it, such as Celmisia, Damnamenia and Pachystegia, all also have chromosome numbers based on x=54 (Dawson Citation2000; de Lange & Rolfe Citation2010).
Picris
We obtained 2n=10 for Picris angustifolia subsp. angustifolia which is the same number as that reported from mitotic counts for P. burbidgeae S.Holzapfel and a meiotic count (n=5II) for P. angustifolia subsp. merxmuelleri Lack et S.Holzapfel by Murray & de Lange (Citation1999).
Senecio
Senecio, with 30 named and widely accepted taxa (de Lange & Rolfe Citation2010) has been well investigated cytologically, all taxa having at least one chromosome count (Dawson Citation2000). Nevertheless, there are a number of informally recognised entities, two of which we report counts for here. The first of these is allied to S. dunedinensis Belcher from which it consistently differs by its dark maroon to brown–black, narrow leaves with deeply crenate (scalloped) margins. To date, this race is known from scattered sites in the eastern South Island from the Leatham, Marlborough, south to the Ahuriri. It usually grows on base-rich substrates such as limestone, basalt or within the apatite-rich facies of the partially metamorphosed sedimentary greywacke rocks common in the South Island. Plants of this race from Mt Cass, North Canterbury had n=20II (G) and 2n=40, the same number as reported for S. dunedinensis s.s. (Dawson Citation2000). We obtained counts of 2n=60 (A), for a second informally recognised entity, S. aff. glomeratus treated as provisionally distinct and endemic to the Chatham Islands (de Lange et al. Citation2011a). This number is the same as that recorded for New Zealand specimens of S. glomeratus (Dawson Citation2000). The Chatham Islands race was briefly discussed and illustrated by Webb et al. (Citation1988) and it is the race most commonly found on those islands. However, in some places there it grows with S. glomeratus s.s., its status is currently under investigation (P.J. de Lange, unpubl. data; Heenan et al. Citation2010). Aside from these two informally recognised entities, we also record counts of 2n=40 for another Chatham Island endemic S. radiolatus subsp. radiolatus and also for the Southern Kermadec Islands endemic S. lautus subsp. esperensis. A further confirmatory count of 2n=60 was obtained for Raoul Island specimens of the Kermadec endemic S. kermadecensis.
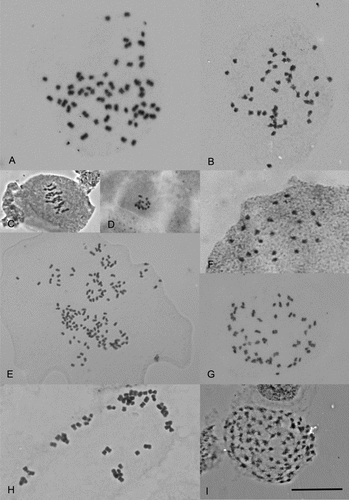
Figure 2 Chromosomes of New Zealand vascular plants. A, Senecio aff. glomeratus, 2n=60. B, Myosotis laeta, 2n=46. C, Drosera hookeri, n=16II. D, Drosera stenopetala, 2n=16. E, Acrothamnus colensoi, 2n=146. F, Gaultheria paniculata, 2n=22. G, Geranium (c) (CHR 546319; Von), 2n=52. H, Selliera microphylla, 2n=56. I, Juncus holoschoenus var. holoschoenus, 2n=106. Scale bar = 10 µm.
Boraginaceae
New Zealand representatives of the cosmopolitan genus Myosotis are currently under taxonomic investigation (H. Meudt, Museum of New Zealand Te Papa Tongarewa, pers. comm. 2012). Currently some 42 indigenous taxa are accepted for the genus and most lack chromosome counts (see comments by de Lange & Murray Citation2002). Here we report three new counts for Myosotis laeta (2n=46) (B), M. aff. drucei (n=22II) and n=22II for M. pygmaea. The M. aff. drucei plant matched M. drucei (L.B.Moore) de Lange et Barkla in flower colour, leaf pigmentation and hair distribution (see de Lange et al. Citation2010), but its leaves are narrower than usual for that species and further, it grew under Chionochloa pallens Zotov subsp. pallens in an alpine flush. Ecologically, this is not the usual habitat for M. drucei and if the leaf shape is considered significant then this gathering might be better placed with an unnamed segregate of M. pygmaea Colenso known as M. aff. pygmaea (Volcanic Plateau). However, our gathering differs in flower colour (cream vs. white) and leaf hair distribution from this latter plant. Myosotis in New Zealand is chromosomally variable (e.g., 2n=36, 40, 44, 46 and 48) with 2n=44 or 46 the most common number (de Lange & Rolfe Citation2010).
Brassicaceae
With the recent recognition of the Australian Rorippa laciniata as naturally occurring in New Zealand (de Lange et al. 2009a) there are now three indigenous New Zealand representatives of the genus. New Zealand plants of R. laciniata had n=48II which differs from that reported for R. palustris (L.) Besser (n=16) and the endemic R. divaricata (Hook.f.) Garn.-Jones et Jonsell (2n=48) (Dawson Citation2000). Based on these results, R. laciniata is probably 12-ploid if we assume that the basic number for the genus is x=8 (Schranz et al. Citation2006).
Convolvulaceae
Currently, New Zealand has two indigenous species of Dichondra, D. repens J.R.Forst et G.Forst and the endemic D. brevifolia Buchanan. However, it is widely accepted that there are further, as yet unnamed, entities present within the variable D. brevifolia (Webb et al. Citation1988). Here, we present a count of 2n=30 for a Chatham Island sample of the D. brevifolia agg. this is the same number as that recorded for D. brevifolia s.s. and D. repens. Two seedlings raised from seeds of Ipomoea pes-caprae subsp. brasiliensis collected in beach drift on the Ripirō Beach, North Auckland (de Lange Citation2012a) had 2n=30, the same number as that reported previously using Raoul Island material (de Lange et al. Citation2004a).
Coriariaceae
Examples from Raoul Island of the endemic Coriaria arborea var. kermadecensis had 2n=40, the same as that reported for var. arborea. The counts for these two varieties are the lowest chromosome number recorded for the five New Zealand species, and indeed for the genus (Dawson Citation2000, IPCN) that have been counted.
Crassulaceae
A plant of the recircumscribed species Crassula ruamahanga (de Lange et al. Citation2004a, Citation2008) from Waitutu Forest, Southland, South Island had 2n=47II. This is the same number as that obtained from several other locations in the North and Chatham Islands (de Lange et al. Citation2008).
Cyperaceae
Bolboschoenus
There are no previously published chromosome counts that we know of for the three New Zealand species of Bolboschoenus. Here we report an approximate count of 2n=c. 110 for B. fluviatilis. This count is different from those reported for purportedly the same species from the USA (Harriman Citation1981; Löve & Löve Citation1981) where 2n=c. 94 and 2n=104 have been obtained, but it agrees with a meiotic count (n=55II) reported by Hicks (Citation1928) from Canadian material. Counts are still required for B. caldwellii (V.J.Cook) Soják and B. medianus (V.J.Cook) Soják to complete the New Zealand representatives of the genus.
Carex
New counts from five indigenous Carex species are reported here: C. astonii (2n=c. 60), C. calcis (2n=c. 68), C. cremnicola (2n=c. 60), C. edgariae (2n=c. 60), C. kirkii var. kirkii (2n=68–70) and C. trachycarpa (2n=66–68). Carex chromosomes are small, poorly staining and holocentric, which we have found previously (de Lange & Murray Citation2002; de Lange et al. Citation2004a) make it difficult to obtain definitive chromosome counts. The approximate numbers that we have obtained from this sample of species are within the range now reported for the majority of the 44 indigenous carices already counted from the New Zealand flora. Counts are still required for 29 taxa to complete the indigenous representatives of the genus of New Zealand (de Lange & Rolfe Citation2010).
Eleocharis
New Zealand has five species of Eleocharis, only one of which, E. neozelandica Kirk is endemic (de Lange & Rolfe Citation2010). Here, we report a further confirmatory count for E. acuta R.Br. of 2n=20, the same number as that recorded for E. acuta and A. gracilis R.Br. The other New Zealand species have either 2n=30 (E. neozelandica, E. pusilla R.Br.) or 2n=100 (E. sphacelata R.Br.) (de Lange et al. Citation2004a).
Ficinia
Currently, New Zealand has two species of Ficinia, F. nodosa and F. spiralis (A.Rich.) Muasya et de Lange. However, as the only distinction between Ficinia and closely related Isolepis is the presence of a gynophore, and as a few Isolepis are known to possess a gynophore (Muasya & de Lange Citation2010) it is likely that Ficinia will at some stage be merged with Isolepis (M. Muasya, pers. comm.). Here we report 2n=30 in F. nodosa the same number as that recorded for F. spiralis (de Lange et al. Citation2004a). This number shows no obvious relationship to the numbers obtained for New Zealand species of Isolepis (Dawson Citation2000 and below) and there do not appear to be any other published counts of Ficinia that the New Zealand chromosome numbers can be compared with.
Gahnia
A count of 2n=100 was obtained for Gahnia setifolia. This is the first count for a New Zealand species of the genus with a further five New Zealand species left to be counted (de Lange & Rolfe Citation2010). Gahnia beecheyi H.Mann from Hawaii has a published count of 2n=c. 96 (Skottsberg Citation1955).
Isolepis
Of the 15 Isolepis taxa indigenous to New Zealand published counts are available for two species. Isolepis aucklandica Hook.f. has n=21/2n=42 and there are counts of 2n=24, 30, 48 and 60 for non-New Zealand plants of I. cernua (Vahl) Roem. et Schult. var. cernua (Dawson Citation2000). Here, we present a new count of 2n=c.64 for I. crassiuscula. As in Carex (above), Isolepis chromosomes are difficult to study but from the existing counts the genus would appear to be chromosomally variable.
Oreobolus
New Zealand has three indigenous species of Oreobolus none of which have previously published counts. Here we report a count of 2n=38 for O. pectinatus, probably the most common and widespread of the three New Zealand species. One other species, the Hawaiian O. furcatus H.Mann has 2n=42 (Seberg Citation1988).
Scirpus
Following several revisions of Scirpus, New Zealand now has only the one indigenous species Scirpus polystachyus (de Lange & Rolfe Citation2010) which appears to be a relatively recent natural trans-Tasman coloniser (P.D. Champion, pers. comm., 2012; de Lange Citation2012b; de Lange et al. Citation2004b, 2009b) to the west coast of the South Island. Healy & Edgar (Citation1980) treat it as naturalised. A plant of this species from Lake Ianthe had 2n=60. As the overseas chromosome literature does not appear to have caught up entirely with the taxonomic changes in Scirpus it is impractical for us to relate this count to chromosome numbers available in overseas literature.
Uncinia
Counts for all the named indigenous Uncinia in New Zealand are complete (Dawson Citation2000; de Lange & Rolfe Citation2010). However, there are still a number of potentially new and as yet undescribed Uncinia plants awaiting formal description. Here we report a count of 2n=88 for one these, a race of Uncinia uncinata (L.f.) Kük. seemingly endemic to the Chatham Islands (de Lange et al. Citation2011a). This count is the same as that obtained for U. uncinata, and for 30 of the 34 accepted species in New Zealand though species with 2n=94 and 2n=132 also occur (Beuzenberg Citation1970; Heenan & de Lange Citation2001).
Droseraceae
New Zealand has seven indigenous species of Drosera, one species, D. stenopetala is endemic and the other six are shared with Australia (de Lange & Rolfe Citation2010; Gibson et al. Citation2010; Salmon Citation2001). One of these indigenous species is now known as D. hookeri, having previously been confused with the Australian endemic D. peltata (Gibson et al. Citation2010). Previously counts of n=10 and 2n=20 had been published for only one New Zealand species D. spatulata (Dawson Citation2000). Here we present new counts of n=16II for D. auriculata, D. binata, D. hookeri (C) and D. spatulata and 2n=16 for D. stenopetala (D). The count obtained for D. spatulata is different from those reported previously from New Zealand (n=10, 2n=20) (Dawson Citation2000). However, as Salmon (Citation2001) notes, this species is very variable in New Zealand and our count suggests that there may be a genetic basis to this variation worthy of further investigation. If x=8 is taken as the basic number, then D. stenopetala, the only New Zealand endemic is diploid, whereas the other, indigenous species are tetraploid, but as other Drosera species have numbers as low as 2n=10 (Sheikh & Kondo Citation1995) and the chromosomes of the genus are holocentric, assumptions about ploidy levels need careful evaluation.
Ericaceae
Following the merging of the Epacridaceae with the Ericaceae (Kron et al. Citation2002), New Zealand now has 63 taxa spread through 11 genera, only one Androstoma of which is endemic (de Lange & Rolfe Citation2010; Quinn et al. Citation2005). Counts are available for some species in all genera except Sprengelia. However, there are only a few counts in some genera, most notably Dracophyllum, which with 35 named taxa (de Lange & Rolfe Citation2010; Venter Citation2009) is the largest indigenous genus of the family in New Zealand and for which there are still only 16 species counted (Dawson Citation2000; de Lange et al. Citation2004a; de Lange & Rolfe Citation2010). Here, we present four further counts for New Zealand Ericaceae in Acrothamnus (one new count), Dracophyllum (one new count, one confirmatory) and Gaultheria (one new count). The sole New Zealand representative of Acrothamnus, A. colensoi had 2n=146 (E), this is about the same number as the 2n=c. 140 count published by Dawson (Citation2000) as Leucopogon colensoi Hook.f. Of the two counts obtained for Dracophyllum, the count of 2n=26 for D. latifolium is new for that species, whereas that of n=13II accords with the published mitotic count for D. strictum (de Lange et al. Citation2004a). Currently, the counts published for Dracophyllum suggest that despite the wide morphological diversity (Wagstaff et al. Citation2010), chromosomally the genus is rather uniform. A count of 2n=22 was obtained for the previously uncounted Gaultheria paniculata (F), the same number as that reported for five other New Zealand Gaultheria (Dawson Citation2000).
Gentianaceae
Published chromosome counts are available for 24 of the 38 New Zealand taxa of Gentianella (Dawson Citation2000; Glenny Citation2004). Here we add a further count of 2n=36 for the north Westland endemic G. scopulorum. With one exception of a plant of uncertain status with 2n=18 (Dawson & Beuzenberg Citation2000), this is the same number as that reported for the other published New Zealand counts for the genus.
Geraniaceae
Chromosome counts are now published for all eight indigenous Geranium species (Dawson Citation2000; de Lange et al. Citation2004a; de Lange & Rolfe Citation2010). However, it has long been recognised that there are a number of undescribed informally recognised entities some of which probably warrant formal recognition (Mitchell et al. Citation2009). Here we provide a count for one of these, a Geranium of uncertain affinity so far known only from cultivated material said to have arisen from within a clod of soil collected from the Von Valley, Eyre Range by the late A.P. Druce (R. Smith, Wellington Regional Council, pers. comm. 2010). To date, despite intensive field surveys, no further examples of this plant have been seen in the wild, however, material derived from the original Druce garden plant is now reasonably widespread in cultivation and this is currently the subject of an on-going taxonomic investigation (P.B. Heenan, pers. comm.). We report a count of 2n=52 for this plant (G), the same number as that recorded for the majority of the New Zealand species.
Goodeniaceae
New Zealand has two well-defined species of Selliera, S. radicans Cav. and S. rotundifolia Heenan, however, a third species, S. microphylla is also accepted by some botanists (de Lange & Rolfe Citation2010). The circumscription of this third species has always been problematic (Allan Citation1961; Colenso Citation1890) resulting in the traditional view that S. microphylla is a montane to alpine upland species distinguished primarily by its smaller vegetative and floral parts. This view is perhaps a little naïve because even with the segregation of S. rotundifolia (Heenan Citation1997), S. radicans remains a notoriously variable species with respect to size, stature and habitats, such that plants referred to as S. microphylla could be regarded as simply a reduced mountain race of S. radicans. Nevertheless, we counted the chromosomes of two samples, one from the North Island and the other from the South Island that in the field matched the description of S. microphylla. Both of these plants had, over time in cultivation, greatly increased in size and stature such that they were indistinguishable from the majority of the range of forms currently attributed to S. radicans. We found two chromosome numbers, with the North Island gathering of S. microphylla having 2n=56 (H) chromosomes and the South Island plant having 2n=16 chromosomes. Published counts for the other two Selliera species are also 2n=16 (Dawson Citation2000). Thus, while no direct taxonomic conclusion can be made from this result it does suggest that Selliera would repay further systematic and cytological study.
Haloragaceae
We obtained a count of 2n=42 for Myriophyllum triphyllum which now completes the counts for the indigenous representatives of the genus in New Zealand. Based on previous New Zealand counts (de Lange & Murray Citation2002; de Lange et al. Citation2004a) where it was proposed that x=7, M. triphyllum is hexaploid. In addition, we present here a new count of n=7II for M. votschii (A) which is different from the 2n=21 that we reported previously (de Lange & Murray Citation2002).
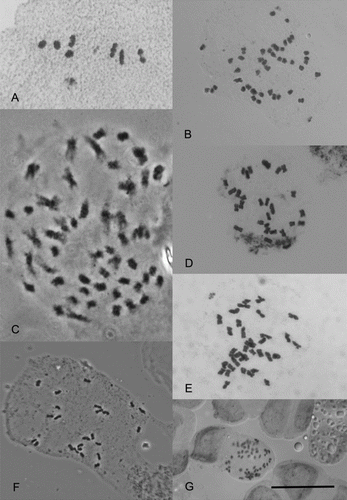
Figure 3 Chromosomes of New Zealand vascular plants. A, Myriophyllum votschii, n = 7II. B, Parahebe jovellanoides, 2n = 40. C, Lepturus repens, 2n = 52–54. D, Puccinellia walkeri subsp. chathamica, 2n = 28. E, Puccinellia walkeri subsp. walkeri, 2n = 35. F, Rytidosperma nudum, 2n = 24. G, Mida salicifolia, 2n = 66. Scale bar = 10 µm.
Juncaceae
Of the 16 Juncus accepted as indigenous to New Zealand (de Lange & Rolfe Citation2010) chromosome counts of 2n=40 have been published for three species, J. edgariae L.A.S.Johnson et K.L.Wilson (Sect. Genuini), J. novae-zelandiae (Sect. Septati) and J. scheuchzerioides Gaudich. (Sect. Septati). Here we present new counts of 2n=106 (I) for J. holoschoenus var. holoschoenus and 2n=34 for J. novae-zelandiae. This latter count differs from the previous published count of 2n=40 (Dawson Citation2000).
Nanodeaceae
The Nanodeaceae is a new family proposed as part of the re-circumscription of the Santalaceae (Nickrent et al. Citation2010). The new family comprises two genera: the Southern American and Falkland Islands monotypic Nanodea (N. muscosa C.F.Gaertn.) and New Zealand monotypic Mida (de Lange Citation2011b; Nickrent et al. Citation2010). Here we report 2n=66 for Mida salicifolia (G), which is the first count for this endemic plant and also completes counts for the sole indigenous New Zealand representative of the family. As far as we are aware there are not published counts for Nanodea muscosa.
Orchidaceae
The recent discovery of a species of Taeniophyllum indigenous to northern New Zealand was documented by Renner & Beadel (2011). In that paper, one of our mitotic counts of 2n=38 was published. Here in addition to that count we present a further meiotic count of n=19II. Although Renner & Beadel (2011) treated the Taeniophyllum as T. norfolkianum D.L.Jones, B.Gray et M.A.Clem., the relationship of the New Zealand plant to that Norfolk Island endemic and indeed the Australia T. muelleri Benth. and T. wilkianum T.E.Hunt needs further critical evaluation (M.A.M. Renner, Sydney University, pers. comm.). Taeniophyllum scaberulum Hook. f. has 2n=36 (Chatterji Citation1978) but no other species appear to have been counted.
Oxalidaceae
New Zealand has four indigenous species of Oxalis (de Lange & Rolfe Citation2010) and at least one unnamed plant that may warrant species rank (Murray & de Lange Citation1999 as Oxalis aff. rubens). For New Zealand, currently published counts are available for O. magellanica G.Forst. (n=10) and O. aff. rubens (2n=24) (Dawson Citation2000). Here we present new counts of 2n=22 for O. exilis and 2n=24 for O. thompsoniae.
Piperaceae
Three species of Peperomia are accepted as indigenous to the New Zealand Botanical Region as defined by Allan (Citation1961). Counts of 2n=44 have been published for P. tetraphylla (G.Forst.) Hook. et Arn. and P. urvilleana A.Rich. (Dawson Citation2000; Murray & de Lange Citation1999) leaving P. blanda var. floribunda (Miq.) H.Huber uncounted. Within the New Zealand Botanical Region, P. blanda var. floribunda is known only from the Kermadec Islands where it is locally common on Raoul Island. Our plants of this species from Raoul Island had 2n=66 but Samuel & Morawetz (Citation1989) found 2n=22 in plants of this species, but did not report on the origin of their plant.
Plantaginaceae
Notwithstanding the nomenclatural tumult that indigenous New Zealand representatives of this family have received over the last decade, we favour retention of the New Zealand genera merged by some into an expanded Veronica (Garnock-Jones et al. Citation2007). Thus we present here two counts for Parahebe, in P. jovellanoides we found n=20II, 2n=40 (B) these were published previously (with permission) as Veronica jovellanoides Garn.-Jones et de Lange by Davidson et al. (Citation2009). This number is unusual for Parahebe being shared only with the distantly related P. decora Ashwin (Davidson et al. Citation2009). By contrast, P. senex (2n=42) has the same basic number as all other Parahebe for which counts have been published (Dawson Citation2000).
New Zealand indigenous representatives of Plantago have recently been revised by Meudt (Citation2012) and a new species P. udicola was described. That species has 2n=96 chromosomes and is regarded as 16-ploid based on x=6 (Murray et al. Citation2010). Meudt (Citation2012) made reference to our previously unpublished count of 2n=96 from Lake Sarah, Canterbury; that number is published here. We have also counted the chromosomes of P. lanceolata, a commonly naturalised species in New Zealand, obtaining 2n=12, confirming it as diploid though there are a few reports of tetraploid plants of this species (Brullo et al. Citation1985; Murín Citation1997).
Poaceae
Previously we have undertaken a major survey of the chromosomes of the indigenous New Zealand Poaceae (de Lange & Murray Citation2002; Murray et al. Citation2003, Citation2005). Counts are now available for 94% of the New Zealand indigenous grass flora (Edgar & Connor Citation2010). Here we document several counts that are either new or were published with our permission in Edgar & Connor (Citation2010).
Deschampsia
New Zealand has six species of Deschampsia, five of these are indigenous, D. flexuosa (L.) Trin. is naturalised. Counts have previously been published for four of the five indigenous taxa (Murray et al. Citation2005) which left only the high alpine Central Otago endemic D. pusilla uncounted. Here we report a count of 2n=26 for that species, the same number as that recorded for the other four indigenous species.
Hierochloe
The latest publication on Hierochloe leaves the genus with seven indigenous species (Edgar & Connor Citation2010). Counts are available for five of these (Murray et al. Citation2005) leaving H. cuprea Zotov and H. recurvata uncounted. Here we report 2n=28 for H. recurvata. Assuming a basic number of x=7, this species is tetraploid as is the allied H. novae-zelandiae Gand. (Murray et al. Citation2005), the three other species (H. brunonis Hook.f., H. fusca Zotov and H. redolens (Vahl) Roem. et Schult.) with 2n=84 are 12-ploid, and H. equiseta Zotov is hexaploid (2n=42).
Lachnagrostis
Here we report a count of 2n=56 for a specimen of Lachnagrostis tenuis. Previously our count had been cited as L. glabra in Edgar & Connor (Citation2010). However, the voucher specimen for the count has since been redetermined as L. tenuis by Lachnagrostis expert A. Brown (University of South Australia, pers. comm. 2010).
Lepturus
The tropical grass Lepturus repens reaches its southern limit on the remote Herald Islets off the north-eastern coastline of Raoul Island (de Lange Citation2011a). Plants collected from North Chanter in 2005 had a chromosome number in the range 2n=52–54 (C) which is within the range reported for this species from Sri Lanka (Tateoka Citation1958). Currently the New Zealand examples of this widespread tropical grass are referred to var. cinereus (Burcham) Fosberg (Edgar & Connor Citation2010) because of its ‘semi-prostrate habit’ (Sykes & West Citation1996). However, this condition is (at least for the Kermadec plants) environmentally induced by the extremely exposed situation from where Sykes collected his plants, material which, notably, he did not cultivate back in New Zealand. Our cultivated plant of this grass from North Chanter rapidly developed an erect bunched growth habit typical of L. repens var. repens plants we have seen growing in sheltered situations elsewhere in the Pacific, and further, the same growth habit was also seen in wild plants observed by one of us (PdL) in May 2011 while visiting other better vegetated islets within the Herald Islets (de Lange Citation2011a). On this basis, we think that the Kermadec Islands plants are better referred to L. repens sensu lato rather than continuing to recognise them as var. cinereus or indeed recognising any of the other named varieties in this variable species. This opinion accords with the views of other botanists who have studied and/or collected L. repens from the wider Pacific and who regard it as an extremely plastic and variable tropical grass species (R.O. Gardner & W. A. Whistler, pers. comm. 2011).
Poa
Chromosome numbers were previously known from all but six (Poa aucklandica ssp. rakiura Edgar, P. celsa Edgar, P. cookii (Hook.f.) Hook.f., P. maia Edgar, P. senex Edgar and P. tonsa Edgar) of the 39 indigenous Poa species (Dawson Citation2000; Murray et al. Citation2005). Here we report new counts of 2n=28 for P. celsa, P. senex and P. tonsa, and confirmatory counts of 2n=28 for P. intrusa and P. subvestita.
Puccinellia
Counts for all the indigenous New Zealand Puccinellia, except P. walkeri subsp. antipoda and P. walkeri subsp. walkeri were reported by de Lange & Murray (Citation2002). Here we report new counts of 2n=42 for the Antipodes Island endemic P. walkeri subsp. antipoda and 2n=35 for P. walkeri subsp. walkeri from plants from coastal Otago and Southland. Puccinellia walkeri is chromosomally variable with P. walkeri subsp. antipoda being hexaploid, whereas plants of subsp. chathamica comprises hexaploids from the Chatham and tetraploids (reported here, D) and hexaploids from the Auckland Islands (Murray et al. Citation2005). Puccinelia walkeri subsp. walkeri is pentaploid (E). This chromosome variation suggests that their treatment as subspecies by Edgar (Citation1996) may need re-evaluation and that perhaps they may be better treated again as species (de Lange & Rolfe Citation2010). Populations of subsp. chathamica from the Auckland Islands clearly warrant further investigation as does the origin of the pentaploid subsp. walkeri. We also obtained a further count of 2n=56 for P. raroflorens confirming a previous report by de Lange & Murray (Citation2002).
Rytidosperma
If one accepts the merger of Pyrrhanthera exigua (Kirk) Zotov into Rytidosperma (Linder & Verboom Citation1996; Linder et al. Citation2010) and rejects R. tenue (Petrie) Connor et Edgar as a sterile hybrid (see below) then New Zealand has 20 indigenous species of Rytidosperma. Counts have been obtained from all but two species of the genus, R. merum Connor et Edgar and R. nudum (Dawson Citation2000; de Lange & Murray Citation2002; Murray et al. Citation2005). Here we report a count of 2n=24 for R. nudum (F). This grass had erroneously been treated as a sterile hybrid (de Lange et al. Citation2009a, de Lange & Rolfe Citation2010) but it has now been found in sufficient quantity and over such a wide part of the main axial ranges of the North Island that, despite the fact that viable seeds have never been found, it is unlikely to be a hybrid. This is in marked contrast to R. tenue apparently also sterile, and known only from the type and two other gatherings, and which appears to be a naturally occurring uncommon hybrid between R. buchananii (Hook.f.) Connor et Edgar and R. gracile (Hook.f.) Connor et Edgar (B.P.J. Molloy, pers. comm. 2009; see also de Lange et al. Citation2009a, de Lange & Rolfe Citation2010).
Simplicia
The chromosome numbers of the endemic ditypic genus Simplicia have been well documented (Dawson Citation2000; de Lange & Murray Citation2002; Murray et al. Citation2005). The genus is, as first noted by Zotov (Citation1971), most closely related to the south-east Asian genus Aniselytron Merr. (R. Soreng, Smithsonian Instution, pers. comm. 2011). Here we correct one count reported previously by de Lange & Murray (Citation2002, p. 8) as Simplicia buchananii. The specimen on which that count was based (AK 252968) is in fact an unusual westerly outlier of S. laxa (Smissen et al. Citation2011). However, we obtained a count of 2n=28 for plants of S. buchananii from near Gordons Pyramid, Kahurangi National Park and the same number was also obtained for North Island (upper Rangitīkei Valley) material of S. laxa. Smissen et al. (Citation2011) note that North Island populations of S. laxa are genetically distinct from South Island S. laxa and that this may warrant further taxonomic investigation.
Trisetum
Edgar (Citation1998) in her treatment of Trisetum spicatum documented its worldwide variation noting that there are diploid, tetraploid and hexaploid cytotypes, and further that there was considerable taxonomic disagreement over the number of subspecies and varieties. As such, she accepted the one variable species and advised (Edgar Citation1998) that ‘it would be unwise to distinguish any infraspecific taxa in the New Zealand Botanical Region without an extensive biosystematic and cytological investigation as well as comparison with European and North and South American material’. Here we report 2n=28 (tetraploid based on x=7) for a Rēkohu (Chatham Island) example of T. spicatum. Some Chatham Islands plants of T. spicatum differ from the usual South Island race by their larger stature and glabrous rather than pubescent or villous culms. However, our voucher specimen when collected had glabrous culms but after two years of cultivation the culms became villous, as described for South Island plants (Edgar Citation1998). Murray et al. (Citation2005) obtained a C-value for a Campbell Island plant of T. spicatum which suggested a chromosome number of 2n=56 (octoploid). However, that plant died before a chromosome count could be obtained. Irrespective, we agree with Edgar (Citation1998) that further study of T. spicatum is still necessary, and as the species is easily grown it would be an ideal candidate for a thorough cytological investigation.
Polygonaceae
New Zealand has 15 species of Persicaria, one of these, P. decipiens (R.Br.) K.L.Wilson is accepted as indigenous, while a second P. prostrata is either treated as naturalised (Webb et al. Citation1988) or as indigenous (P.D. Champion, pers. comm. 2012; de Lange et al. Citation2004a, 2009b; de Lange & Rolfe Citation2010). In New Zealand P. prostrata is a scarce species of lake turf habitats recorded mostly from the Rotorua Lakes District and Te Urewera in the North Island and from several stations along the West Coast of the South Island. These sites accord with the known trans-Tasman movement of grey teal (Anas gracilis) which are known vectors of wetland plant propagules (de Lange et al. Citation2011b) such that inferring an indigenous status for a plant with a known indigenous avian disperser and which has been recorded from New Zealand since at least 1882 (Webb et al. Citation1988) seems reasonable. Irrespective of its biostatus, plants of P. prostrata from Lake Okataina had 2n=c. 20. Persicaria decipiens has previously been counted with n=20 (Dawson Citation2000; as Polygonum decipiens).
Potamogetonaceae
New Zealand has six indigenous representatives of the Potamogetonaceae in four genera, Lepilaena (L. bilocularis Kirk), Potamogeton (P. cheesemanii A.Benn., P. ochreatus Raoul, P. suboblongus), Stuckenia (S. pectinata) and Zannichellia (Z. --palustris L.) (de Lange & Rolfe Citation2010). Counts have previously been published for these New Zealand species; Lepilaena (2n=12), Zannichellia (2n=24) and one species of Potamogeton (P. cheesemanii, 2n=c. 28) (Dawson Citation2000; de Lange et al. Citation2004a). Here we report a new count of 2n=28 for the endemic P. suboblongus, the same number as that reported by de Lange et al. (Citation2004a) for the indigenous P. cheesemanii. We also obtained counts of 2n=c. 78 for New Zealand material of Stuckenia pectinata which is the same number that has been reported from most of its range overseas (Holub Citation1997; Les & Haynes Citation1996).
Ranunculaceae
New Zealand has the one indigenous mousetail, Myosurus minimus subsp. novae-zelandiae (Garnock-Jones Citation1986) for which a single count of 2n=16 has been published previously (Dawson Citation2000). Here we provide a confirmatory count of 2n=16.
Restionaceae
New Zealand has five indigenous Restionaceae in three genera (Apodasmia, Empodisma and Sporadanthus) and counts have been published for all of these (Dawson Citation2000; Wagstaff & Clarkson Citation2012). Here we present a new count of 2n=48 for a Rēkohu (Chatham Island) plant allied to Apodasmia similis (Edgar) Briggs et L.A.S.Johnson and discussed by Heenan et al. (Citation2010) and de Lange et al. (Citation2011a) on the basis of its larger stature and subtle differences in details of the inflorescence morphology (P.B. Heenan, Landcare Research, pers. comm. 2012). The count we obtained is the same as that recorded from South Island (Fiordland) plants of A. similis (Dawson Citation2000).
Scrophulariaceae
New Zealand has three indigenous Myoporum, M. laetum G.Forst., M. rapense subsp. kermadecense (W.R.Sykes) Chinnock and the newly described M. semotum (Heenan & de Lange Citation2011). A plant of M. semotum from Rangatira (South East Island) in the Chatham Islands group had 2n=108, the same number as that obtained for the other two species (Dawson Citation2000; de Lange et al. Citation2004a).
Tetrachondraceae
The Tetrachondraceae is monogeneric with type species, the New Zealand endemic Tetrachondra hamiltonii and South American T. patagonica Skottsb. (Wagstaff et al. Citation2000). Plants of T. hamiltonii, from the Mākirikiri Tarns, Ruahine Ranges, North Island had 2n=72.
Thymeleaeaceae
Since 2008 there have been a series of papers revising New Zealand Pimelea (Burrows Citation2008, Citation2009a, Citation2009b, Citation2011a, Citation2011b) and in the process some 53 taxa have been recognised, many of which are new to the New Zealand flora. Chromosome numbers are known for only nine of these taxa (Burrows Citation2009a; Dawson Citation2000; de Lange & Rolfe Citation2010). Here we report 12 counts, nine new, from ten taxa and one putative wild hybrid.
The count of 2n=36 we obtained for a plant identified by C.J. Burrows as Pimelea prostrata subsp. prostrata matches one of the three published counts reported for P. prostrata s.l. (n=18, 36, 2n=36, 90) (Beuzenberg & Hair Citation1983; Burrows Citation2009a; Dawson Citation2000; Dawson & Beuzenberg Citation2000). The current treatment of P. prostrata recognises five subspecies and counts have been published for three of these (subsp. prostrata, subsp. seismica C.J.Burrows and subsp. vulcanica C.J.Burrows). Both P. prostrata subsp. seismica and subsp. vulcanica have counts of 2n=36 (see discussion in Burrows Citation2009a). Burrows (Citation2009a) suggests that P. prostrata subsp. prostrata may comprise a polyploid series and that this needs further investigation. To that we would add the comment that the treatment offered by Burrows (Citation2009a) for P. prostrata is still far from satisfactory resulting in confusion as subspecies are often sympatic and/or syntopic and their defining characters often merge across all named subspecies. Burrows (Citation2009a) suggests that P. prostrata is a species complex and has been subjected to repeated past and on-going introgression. While this may account for some of the problems we have had with using his treatment we feel that this species complex warrants further critical investigation using modern molecular and cytological techniques to not only test the taxa erected by Burrows (Citation2009a) but also the claims of widespread introgression between them.
A similar situation arises for P. urvilleana, where two subspecies, subsp. nesica C.J.Burrows and subsp. urvilleana were distinguished by Burrows (Citation2009a) with past introgression being suggested for the merging of distinguishing characters across parts of the subspecies’ range. We obtained new counts of 2n=36 for both P. urvilleana subsp. nesica and subsp. urvilleana (as defined by Burrows Citation2009a) from North Cape.
A count of 2n=72 was also obtained for the newly described, endemic P. mimosa (Burrows Citation2011b). Of those counted from New Zealand only P. oreophila (probably subsp. oreophila as circumscribed by Burrows Citation2011a) has been reported to have the same number (Dawson & Beuzenberg Citation2000).
We also investigated the two subspecies of P. orthia, finding 2n=36 in both subsp. orthia and subsp. protea. Again we feel that further taxonomic investigation into the status of P. orthia subsp. protea is warranted. The plants we have grown from the type locality (collected for us by one of naming authors M.J. Thorsen) have little in common with P. orthia subsp. orthia resembling rather a semi-erect to erect state of P. urvilleana s.l.
The other five counts of 2n=36 for P. microphylla, P. pseudolyallii, P. telura, P. tomentosa and P. villosa are all new. The count reported here for P. villosa is from a Rēkohu (Chatham Island) plant. Burrows (Citation2009b) treated P. villosa as comprising two subspecies, subsp. villosa and subsp. arenaria (A.Cunn.) C.J.Burrows. Burrows (Citation2009b) stated that both subspecies were present on the Chatham Islands. However, our observations on the Chatham Islands suggest that there is only one taxon on the islands that corresponds to his P. villosa subsp. villosa (de Lange et al. Citation2011a) and Burrow's examples of hybrids including ones collected by one of us (PdL) are merely shade forms of this. Resolution of this is beyond the scope of this paper and we recommend further taxonomic investigation. Until then, we prefer to recognise one species P. villosa and within it a potentially distinct race P. aff. villosa for which the name P. dasyantha Colenso probably applies (M.J. Thorsen, pers. comm. 2010).
The last count comes from a cultivated plant collected from Pipinui Point, on the coast west of Wellington. These plants have been treated by botanists as an unnamed race of P. aridula Cheeseman but they were treated as a northerly outlier of P. pseudolyallii by Burrows (Citation2011a). The plant we examined was pollen sterile and had a chromosome number of 2n=54, a markedly different number to that we obtained from a P. pseudolyallii plant from Little Peel, Canterbury (2n=36). The Pipinui race of P. pseudolyallii probably warrants further study, it seems unlikely that all plants of that race are hybrids, indeed plants raised by cuttings from that site and held at Percy Reserve, Petone, Wellington between 1991 and 1997 produced viable seed that was successfully germinated, the seedlings of which showed no divergence from the parent plants (P.J. de Lange, unpubl. data). However, we were unable to examine those plants as they are no longer extant. Other Pimelea recorded from the Pipinui site include members of the P. prostrata complex (plants mostly matching P. prostrata subsp. seismica (2n=36)) and P. cryptica C.J.Burrows et Enright which remains uncounted. If P. pseudolyallii plants are all uniformly 2n=36 then hybrids between it and P. prostrata subsp. seismica are unlikely to have 2n=54 chromosomes. Possibly our plant is a hybrid involving P. cryptica. Further study into this northerly population of P. pseudolyallii is needed to resolve the apparent chromosome variation and taxonomic status of that population.
Counts are now available for about one third of New Zealand's Pimelea taxa and although many taxa remain to be counted, some patterns are emerging. It would seem that 2n=36 is the most common number, and that P. prostrata s.l. might be chromosomally variable. A similar situation was also described for P. oreophila which was reported to have diploid and polyploid races (Rattenbury Citation1957, Burrows Citation1958, Dawson & Beuzenberg Citation2000). However, these comments will need to be reviewed in light of the recent treatment of P. oreophila and its allies offered by Burrows (Citation2011a).
Winteraceae
Two plants of the Northland endemic Pseudowintera insperata as reported by Heenan & de Lange (Citation2006) had 2n=86, the same number as that recorded for the other three species in the genus (Dawson Citation2000).
Conclusions and future work
With the publication of this, the 40th in the series of chromosome ‘Contributions’ it is pertinent to reflect on what more needs to be done. We now have at least one count for c. 85% of the angiosperm flora, which means that there are c. 350 species or subspecies that remain to be examined. The majority of these are in the Magnoliidae with 17 of 188 Polypodiidae and 5 of 13 Lycopodiidae uncounted. In we have attempted to compile a list of the families and genera where these gaps occur and it is clear that the distribution of ‘missing’ numbers is not random. In the great majority of families there are just one or two genera each with just a few species where counts are missing but the Cyperaceae, Asteraceae, Orchidaceae and Poaceae stand out as the families where most work remains to be done. To some extent this reflects the contribution that these families make to the total number of species in the New Zealand flora although some families make a disproportionate contribution. The most obvious of these is the Cyperaceae, which has the greatest number of uncounted species, 58 of a total of c. 177 (de Lange & Rolfe Citation2010) which contrasts with the Asteraceae with more than twice as many species but only 12 uncounted. The Apiaceae also contains a large number of uncounted species, 29 of 131, with, for example, 13 species of Aciphylla still to be counted. The reasons for the gaps are various. In some taxa, small chromosome size coupled with a large number of chromosomes make counting particularly difficult. We have also found that the mitotic chromosomes of many members of the Cyperaceae and Juncaceae do not stain readily with standard methods so observations on meiosis may prove to be a better source of chromosome numbers for these plants. However, these species often do not flower when grown out of their natural range so this is a further impediment to meiotic analysis. Many of the other gaps are for plants from remote and or poorly accessible habitats so obtaining seed or living material is often difficult. There is also the problem that some groups are taxonomically ‘difficult’ and may for that reason be under represented in the current chromosome record.
Table 2 Summary of the families and genera that contain species and subspecies that have not had their chromosomes counted.
Chromosome studies of New Zealand plants are now entering the end of the alpha stage with information available for the great majority of native plants. However, despite pioneering studies on chromosome evolution in genera such as Hebe (Frankel & Hair Citation1937), the development of molecular methods of chromosome analysis using in situ hybridisation with specific gene or sequence probes or whole genomes have not been widely used even though they offer the potential to unravel the evolution of polyploid complexes that are so prevalent in the New Zealand flora. A recent example of this is the work of Wong (Citation2011) on Plantago where the alloploid ancestry of several hexaploids and the 16-ploid P. udicola has been clearly demonstrated using whole genomic probes of putative diploid progenitors to ‘paint’ the chromosomes of the polyploids. Chromosome painting can also be useful to identify interspecific hybrids as demonstrated in the naturally occurring hybrids between Lobelia angulata (as Pratia angulata) and L. perpusilla Hook.f. (Murray et al. Citation2004) and, to some extent, in species within the Kunzea species complex (de Lange et al. Citation2005).
Chromosome surveys have also provided useful insights into the population structure of both rare and common plants. Several surveys (Dawson & Beuzenberg Citation2000; Dawson et al. Citation1993; de Lange et al. Citation2008; Murray et al. Citation2010) have found that more extensive sampling has in some cases revealed quite extensive intraspecific chromosome number variation. An extreme example of this is Crassula ruamahanga where plants from 16 localities spanning much of the range of the species had 11 different chromosome numbers that ranged from 2n=42 to 2n=100 (de Lange et al. Citation2008). This sort of variation has implications for both practical conservation and in systematic and evolutionary studies.
Acknowledgements
We are grateful to the following people: Sarah Beadel, Ewen Cameron, Paul Champion, Bev Clarkson, Murray Dawson, Rhys Gardner, Peter Heenan, Graeme Jane, the late Phil Knightbridge, Brian Molloy, Muthama Muasya, Matt Renner, Robyn Smith, Mike Thorsen, Steve Wagstaff, Kath Walker, Art Whistler, for the provision of plants, the maintenance of these in cultivation, and/or for their specialist comments on the various plants discussed in this paper.
References
- Allan H H 1961 . Flora of New Zealand . Vol. I . Wellington , Government Printer . 1085 p.
- APG III 2009 . An update of the Angiosperm Phylogeny Group classification of the orders and families of flowering plants: APG III . Botanical Journal of the Linnean Society 161 : 105 – 121 . doi: 10.1111/j.1095-8339.2009.00996.x
- Beuzenberg , EJ . 1970 . Contributions to a chromosome atlas of the New Zealand flora – 14. Miscellaneous species . New Zealand Journal of Botany , 8 : 260 – 263 . doi: 10.1080/0028825X.1970.10429127
- Beuzenberg , EJ and Hair , JB . 1983 . Contributions to a chromosome atlas of the New Zealand flora – 25. Miscellaneous species . New Zealand Journal of Botany , 21 : 13 – 20 .
- Brownlie , G . 1954 . Introductory notes to cyto-taxonomic studies of New Zealand ferns . Transactions of the Royal Society of New Zealand , 82 : 665 – 666 .
- Brownlie , G . 1957 . Cyto-taxonomic studies on New Zealand Pteridaceae . New Phytologist , 56 : 207 – 209 . doi: 10.1111/j.1469-8137.1957.tb06967.x
- Brullo , S , Pavone , P and Terrasi , MC . 1985 . Considerazioni cariologiche sul genere Plantago in Sicilia . Candollea , 40 : 217 – 230 .
- Burrows CJ 1958 . Variation in some species of the genus Pimelia . Unpublished MSc thesis, University of Canterbury . 141 p.
- Burrows , CJ . 2008 . Genus Pimelea (Thymelaeaceae) in New Zealand 1. The taxonomic treatment of seven endemic glabrous-leaved species . New Zealand Journal of Botany , 45 : 127 – 176 . doi: 10.1080/00288250809509760
- Burrows , CJ . 2009a . Genus Pimelea (Thymelaeaceae) in New Zealand 2. The endemic Pimelea prostrata and Pimelea urvilliana species complexes . New Zealand Journal of Botany , 47 : 163 – 229 . doi: 10.1080/00288250909509804
- Burrows , CJ . 2009b . Genus Pimelea (Thymelaeaceae) in New Zealand 3. The taxonomic treatment of six endemic hairy-leaved species . New Zealand Journal of Botany , 47 : 325 – 354 . doi: 10.1080/00288250909509813
- Burrows , CJ . 2011a . Genus Pimelea (Thymelaeaceae) in New Zealand 4. The taxonomic treatment of ten endemic abaxially hairy-leaved species . New Zealand Journal of Botany , 49 : 41 – 106 . doi: 10.1080/0028825X.2010.536558
- Burrows , CJ . 2011b . Genus Pimelea (Thymelaeaceae) in New Zealand 5. The taxonomic treatment of five endemic species with both adaxial and abaxial leaf hair . New Zealand Journal of Botany , 49 : 367 – 412 . doi: 10.1080/0028825X.2011.577437
- Chambers , TC and Farrant , PA . 1998 . The Blechnum procerum (‘capense’) (Blechnaceae) complex in New Zealand . New Zealand Journal of Botany , 36 : 1 – 19 . doi: 10.1080/0028825X.1998.9512544
- Chase , MW and Reveal , JL . 2009 . A phylogenetic classification of the angiosperms to accompany APG III . Botanical Journal of the Linnean Society , 161 : 122 – 127 . doi: 10.1111/j.1095-8339.2009.01002.x
- Chatterji , AK . 1978 . Chromosome numbers and karyotypes of some Sarcanthinae orchids . Proceedings of the Indian Science Congress , 65 : 94 – 95 .
- Cheeseman , TF . 1925 . Manual of the New Zealand flora , 1163 Wellington : Government Printer .
- Colenso , W . 1890 . A description of some newly-discovered Phænogamic plants, being a further contribution towards the making-known the botany of New Zealand . Transactions and Proceedings of the New Zealand Institute , 22 : 459 – 493 .
- Davidson , GR , Garnock-Jones , PJ and de Lange , PJ . 2009 . Two additional indigenous species of Veronica (Plantaginaceae) from northern New Zealand: V. jovellanoides, a new and highly endangered species, and V. plebeia R.Br . New Zealand Journal of Botany , 47 : 271 – 279 . doi: 10.1080/00288250909509809
- Dawson , MI . 2000 . Index of chromosome numbers of indigenous New Zealand spermatophytes . New Zealand Journal of Botany , 38 : 47 – 150 . doi: 10.1080/0028825X.2000.9512673
- Dawson , MI and Beuzenberg , EJ . 2000 . Contributions to a chromosome atlas of the New Zealand flora – 36. Miscellaneous families . New Zealand Journal of Botany , 38 : 1 – 23 . doi: 10.1080/0028825X.2000.9512671
- Dawson , MI , Ward , JM , Groves , BE and Hair , JB . 1993 . Contributions to a chromosome atlas of the New Zealand flora – 32. Raoulia (Inuleae-Compositae (Asteraceae)) . New Zealand Journal of Botany , 31 : 97 – 106 . doi: 10.1080/0028825X.1993.10419537
- Dawson , MI , Brownsey , PJ and Lovis , JD . 2000 . Index of chromosome numbers of indigenous New Zealand pteridophytes . New Zealand Journal of Botany , 38 : 25 – 46 . doi: 10.1080/0028825X.2000.9512672
- Dawson , MI , Molloy , BPJ and Beuzenberg , EJ . 2007 . Contributions to a chromosome atlas of the New Zealand flora – 39. Orchidaceae . New Zealand Journal of Botany , 45 : 611 – 684 . doi: 10.1080/00288250709509743
- de Lange , PJ . 2011a . Kermadec Biodiscovery Expedition 2011 (Part III – The Herald Islets, Napier and Nugent) . Trilepidea , 94 : 3 – 11 .
- de Lange , PJ . 2011b . What's up in the Santalales? . Trilepidea , 91 : 1 – 5 .
- de Lange , PJ . 2012a . Beach morning glory (Ipomoea pes-caprae subsp. brasiliensis) in beach drift on Ripiro Beach, Omamari Stream Mouth, Northland . Wellington Botanical Society Bulletin , 54 : 7 – 11 .
- de Lange PJ 2012b . Scirpus polystachyus . New Zealand Plant Conservation Network http://www.nzpcn.org.nz/flora_details.asp?ID=730 (updated continuously, accessed 18 June 2012) .
- de Lange , PJ and Murray , BG . 2002 . Contributions to a chromosome atlas of the New Zealand flora – 37. Miscellaneous families . New Zealand Journal of Botany , 40 : 1 – 23 . doi: 10.1080/0028825X.2002.9512767
- de Lange , PJ and Rolfe , JR . 2010 . New Zealand indigenous vascular plant checklist , 131 Wellington : New Zealand Plant Conservation Network .
- de Lange , PJ , Murray , BG and Datson , PM . 2004a . Contributions to a chromosome atlas of the New Zealand flora – 38. Counts for 50 families . New Zealand Journal of Botany , 42 : 873 – 904 . doi: 10.1080/0028825X.2004.9512936
- de Lange , PJ , Norton , DA , Heenan , PB , Courtney , SP , Ogle , BPJ , Rance , BD , Johnson , PN and Hitchmough , R . 2004b . Threatened and uncommon plants of New Zealand . New Zealand Journal of Botany , 42 : 45 – 76 . doi: 10.1080/0028825X.2004.9512890
- de Lange , PJ , Datson , PM , Murray , BG and Toelken , HR . 2005 . Hybridism in the Kunzea ericoides complex (Myrtaceae): an analysis of artificial crosses . Australian Systematic Botany , 18 : 117 – 131 . doi: 10.1071/SB04043
- de Lange , PJ , Heenan , PB , Keeling , DJ , Murray , BG , Smissen , R and Sykes , WR . 2008 . Biosystematics and conservation: a case study with two enigmatic and uncommon species of Crassula from New Zealand . Annals of Botany , 101 : 881 – 899 . doi: 10.1093/aob/mcm294
- de Lange , PJ , Heenan , PB and Townsend , AJ . 2009a . Rorippa laciniata (Brassicaceae), a new addition to the flora of New Zealand . New Zealand Journal of Botany , 47 : 133 – 137 . doi: 10.1080/00288250909509800
- de Lange , PJ , Norton , DA , Courtney , SP , Heenan , PB , Barkla , JW , Cameron , EK , Hitchmough , R and Townsend , AJ . 2009b . Threatened and uncommon plants of New Zealand (2008 revision) . New Zealand Journal of Botany , 47 : 61 – 96 . doi: 10.1080/00288250909509794
- de Lange , PJ , Heenan , PB , Norton , DA , Rolfe , JR and Sawyer , JWD . 2010 . Threatened plants of New Zealand , 471 Christchurch : Canterbury University Press .
- de Lange , PJ , Heenan , PB and Rolfe , JR . 2011a . Checklist of vascular plants recorded from the Chatham Island Islands , 57 Wellington : Department of Conservation .
- de Lange , PJ , Townsend , AJ and Rolfe , JR . 2011b . Crassula natans var. minus (Crassulaceae) a new trans-Tasman natural weed arrival to northern New Zealand . New Zealand Journal of Botany , 49 : 361 – 366 . doi: 10.1080/0028825X.2011.574708
- Edgar , E . 1996 . Puccinellia Parl. (Gramineae: Poeae) in New Zealand . New Zealand Journal of Botany , 34 : 17 – 32 . doi: 10.1080/0028825X.1996.10412688
- Edgar , E . 1998 . Trisetum Pers. (Gramineae: Aveneae) in New Zealand . New Zealand Journal of Botany , 36 : 539 – 564 . doi: 10.1080/0028825X.1998.9512594
- Edgar E , Connor HE 2010 . Flora of New Zealand . Vol. V . Grasses. 2nd edition . Lincoln , Manaaki Whenua Press . 650 .
- Frankel , OH and Hair , JB . 1937 . Studies on the cytology, genetics, and taxonomy of New Zealand Hebe and Veronica . New Zealand Journal of Science and Technology , 18 : 669 – 687 .
- Garnock-Jones , PJ . 1986 . A new status for the New Zealand mousetail (Myosurus, Ranunculaceae) . New Zealand Journal of Botany , 24 : 351 – 354 . doi: 10.1080/0028825X.1986.10412683
- Garnock-Jones , PJ , Albach , D and Briggs , BG . 2007 . Botanical names in Southern Hemisphere Veronica (Plantaginaceae): sect. Detzneria, sect. Hebe, and sect. Labiatoides . Taxon , 56 : 571 – 582 .
- Gibson , RP , Conn , BJ and Conran , JG. 2010 . Drosera hookeri R.P.Gibson, B.J. Conn & Conran, a replacement name for Drosera foliosa Hook.f. ex Planch. nom. illeg. (Droseraceae) . Journal of the Adelaide Botanic Gardens , 24 : 39 – 42 .
- Glenny , D . 2004 . A revision of the genus Gentianella in New Zealand . New Zealand Journal of Botany , 42 : 361 – 530 . doi: 10.1080/0028825X.2004.9512910
- Harriman , NA . 1981 . Cyperaceae. In: Löve Á. Chromosome number reports LXXI . Taxon , 30 : 508 – 519 .
- Healy AJ , Edgar E 1980 . Flora of New Zealand . Vol. III . Adventive cyperaceous, petalous and spathaceous monocotyledons . Wellington , Government Printer . 220 p.
- Heenan , PB . 1997 . Sellieria rotundifolia (Goodeniaceae), a new, round-leaved, species from New Zealand . New Zealand Journal of Botany , 35 : 133 – 138 . doi: 10.1080/0028825X.1997.10414148
- Heenan , PB . 2009 . A diminutive new species of Leptinella (Asteraceae) from arid habitats of the South Island, New Zealand . New Zealand Journal of Botany , 47 : 127 – 132 . doi: 10.1080/00288250909509799
- Heenan , PB and de Lange , PJ . 2001 . A new dodecaploid species of Uncinia (Cyperaceae) from ultramafic rocks, Surville Cliffs, Northland, New Zealand . New Zealand Journal of Botany , 39 : 373 – 380 . doi: 10.1080/0028825X.2001.9512743
- Heenan , PB and de Lange , PJ . 2006 . Pseudowintera insperata (Winteraceae), an overlooked and rare new species from northern New Zealand . New Zealand Journal of Botany , 44 : 89 – 98 . doi: 10.1080/0028825X.2006.9513008
- Heenan , PB and de Lange , PJ . 2011 . Myoporum semotum (Scrophulariaceae), a new tree species from the Chatham Islands, New Zealand . New Zealand Journal of Botany , 49 : 17 – 26 . doi: 10.1080/0028825X.2010.526767
- Heenan , PB , de Lange , PJ and Keeling , J . 2009 . Alternanthera nahui, a new species of Amaranthaceae indigenous to New Zealand . New Zealand Journal of Botany , 47 : 97 – 105 . doi: 10.1080/00288250909509795
- Heenan , PB , Mitchell , AD , de Lange , PJ , Keeling , J and Paterson , AM . 2010 . Late Cenozoic origin and diversification of Chatham Islands endemic plant species revealed by analyses of DNA sequence data . New Zealand Journal of Botany , 48 : 83 – 136 . doi: 10.1080/0028825X.2010.494337
- Hicks , GC . 1928 . Chromosome studies in the Cyperaceae, with special reference to Scirpus . Botanical Gazette , 86 : 295 – 317 . doi: 10.1086/333901
- Holub , J . 1997 . Stuckenia Börner 1912 – the correct name for Coleogeton (Potamogetonaceae) . Presilia , 69 : 361 – 366 .
- Iovene , M , Grzebelus , E , Carputo , D , Jiang , J and Simon , PW . 2008 . Major cytogenetic landmarks and karyotype analyses in Daucus carota and other Apiaceae . American Journal of Botany , 97 : 793 – 804 . doi: 10.3732/ajb.0700007
- Kron , KA , Judd , WS , Stevens , PF , Crayn , DM , Anderberg , AA , Gadek , PA , Quinn , CJ and Luteyn , JL . 2002 . A phylogenetic classification of Ericaceae: molecular and morphological evidence . Botanical Review , 68 : 335 – 423 . doi: 10.1663/0006-8101(2002)068[0335:PCOEMA]2.0.CO;2
- Les , DH and Haynes , R . 1996 . Coleogeton (Potamogetonaceae), a new genus of pondweeds . Novon , 6 : 389 – 391 . doi: 10.2307/3392046
- Linder , PH and Verboom , GH . 1996 . Generic limits in Rytidosperma (Danthonieae, Poaceae) complex . Telopea , 6 : 597 – 627 .
- Linder , PH , Baeza , M , Barker , NP , Galley , C , Humphreys , AM , Lloyd , KM , Orlovich , DA , Pirie , MD , Simon , BK , Walsh , N and Verboom , GA . 2010 . A generic classification of the Danthonioideae (Poaceae) . Annals of the Missouri Botanical Garden , 97 : 306 – 364 . doi: 10.3417/2009006
- Löve , Á and Löve , D . 1981 . Cyperaceae. In: Löve Á IOPB chromosome number reports LXXII . Taxon , 30 : 829 – 861 .
- Marsden CR 1979 . Morphology and taxonomy of Isoetes in Australasia, India, north-east and south-east Asia, China and Japan . Unpublished PhD thesis, University of Adelaide , Adelaide , Australia . 185 p.
- Meudt , HM . 2012 . A taxonomic revision of native New Zealand Plantago (Plantaginaceae) . New Zealand Journal of Botany , 50 : 101 – 178 . doi: 10.1080/0028825X.2012.671179
- Mitchell , AD , Heenan , PB and Paterson , AM . 2009 . Phylogenetic relationships of Geranium species indigenous to New Zealand . New Zealand Journal of Botany , 47 : 21 – 31 . doi: 10.1080/00288250909509789
- Muasya , AM and de Lange , PJ . 2010 . Ficinia spiralis (Cyperaceae) a new genus and combination for Desmoschoenus spiralis . New Zealand Journal of Botany , 48 : 31 – 39 . doi: 10.1080/00288251003660703
- Murín , A . 1997 . Karyotaxonomy of some medicinal and aromatic plants . Thaiszia , 7 : 75 – 88 .
- Murray , BG and de Lange , PJ . 1999 . Contributions to a chromosome atlas of the New Zealand flora – 35. Miscellaneous families . New Zealand Journal of Botany , 37 : 511 – 522 . doi: 10.1080/0028825X.1999.9512650
- Murray , BG , Weir , I , Ferguson , AR and de Lange , PJ . 2003 . Variation in DNA C-value and genome size in New Zealand native grasses . New Zealand Journal of Botany , 41 : 63 – 69 . doi: 10.1080/0028825X.2003.9512832
- Murray , BG , Datson , PM , Lai , ELY , Sheath , KM and Cameron , EK . 2004 . Polyploidy, hybridization and evolution in Pratia (Campanulaceae) . New Zealand Journal of Botany , 42 : 905 – 920 . doi: 10.1080/0028825X.2004.9512937
- Murray , BG , de Lange , PJ and Ferguson , AR . 2005 . Nuclear DNA variation, chromosome numbers and polyploidy in the endemic and indigenous grass flora of New Zealand . Annals of Botany , 96 : 1293 – 1305 . doi: 10.1093/aob/mci281
- Murray , BG , Meudt , HM , Tay , ML and Garnock-Jones , PJ . 2010 . New chromosome counts in New Zealand species of Plantago (Plantaginaceae) . New Zealand Journal of Botany , 48 : 197 – 204 . doi: 10.1080/0028825X.2010.515598
- Nickrent , DL , Malécot , V , Vidal-Russell , R and Der , JP . 2010 . A revised classification of Santalales . Taxon , 59 : 538 – 558 .
- Perrie , LR and Brownsey , PJ . 2012 . Lastreopsis kermadecensis, a new fern species from Raoul Island in the Kermadec Islands, New Zealand, with notes on L. pacifica . New Zealand Journal of Botany , 50 : 29 – 36 . doi: 10.1080/0028825X.2011.640336
- Quinn , CJ , Brown , EA , Heslewood , MM and Crayn , DM . 2005 . Generic concepts in Styphelieae (Ericaceae): the Cyathodes group . Australian Systematic Botany , 18 : 439 – 454 . doi: 10.1071/SB05005
- Quirk , H , Chambers , TC and Regan , M . 1983 . The fern genus Cheilanthes in Australia . Australian Journal of Botany , 31 : 501 – 553 . doi: 10.1071/BT9830501
- Rattenbury , JA . 1957 . Chromosome numbers in New Zealand angiosperms . Transactions of the Royal Society of New Zealand , 84 : 936 – 938 .
- Renner , MAM and Beadel , SM . 2012 . Taeniophyllum norfolkianum: a second genus of Vandeae (Orchidaceae) indigenous to New Zealand . New Zealand Journal of Botany , 49 : 435 – 439 . doi: 10.1080/0028825X.2011.580766
- Salmon B 2001 . Carnivorous plants of New Zealand . Manurewa , Ecosphere Publications . 303 p.
- Samuel , R and Morawetz , W . 1989 . Chromosomal evolution within Piperaceae . Plant Systematics and Evolution , 166 : 105 – 117 . doi: 10.1007/BF00937879
- Schranz , ME , Lysak , MA and Mitchell-Olds , T . 2006 . The ABC's of comparative genomics in the Brassicaceae: building blocks of the crucifer genomes . Trends in Plant Science , 11 : 535 – 542 . doi: 10.1016/j.tplants.2006.09.002
- Seberg , O . 1988 . Taxonomy, phylogeny, and biogeography of the genus Oreobolus R.Br. (Cyperaceae), with comments on the biogeography of the South Pacific continents . Botanical Journal of the Linnean Society , 96 : 119 – 195 . doi: 10.1111/j.1095-8339.1988.tb00632.x
- Shepherd , LD , Perrie , LR , Parris , BS and Brownsey , PJ . 2007 . A molecular phylogeny for the New Zealand Blechnaceae ferns from analyses of chloroplast trnL–trnF DNA sequences . New Zealand Journal of Botany , 45 : 67 – 80 . doi: 10.1080/00288250709509703
- Sheikh , SA and Kondo , K. 1995 . Differential staining with orcein, Giemsa, CMA, and DAPI for comparative chromosome study of 12 species of Australian Drosera (Droseraceae) . American Journal of Botany , 82 : 1278 – 1286 . doi: 10.2307/2446251
- Skottsberg , C . 1955 . Chromosome numbers in Hawaiian flowering plants . Arkiv för Botanik , 3 : 63 – 70 .
- Smissen , RD , de Lange , PJ , Thorsen , MJ and Ogle , CC . 2011 . Species delimitation and conservation genetics in the rare New Zealand endemic grass genus Simplicia . New Zealand Journal of Botany , 49 : 187 – 199 . doi: 10.1080/0028825X.2010.525244
- Stace , C . 2000 . Cytology and cytogenetics as a fundamental taxonomic resource for the 20th and 21st centuries . Taxon , 49 : 451 – 477 . doi: 10.2307/1224344
- Sykes , WR and West , CJ . 1996 . New records and other information on the vascular flora of the Kermadec Islands . New Zealand Journal of Botany , 34 : 447 – 462 . doi: 10.1080/0028825X.1996.10410126
- Tateoka , T . 1958 . Notes on some grasses. VII. Cytological evidence for the phylogenetic difference between Lepturus and Monerma . Cytologia , 23 : 447 – 451 . doi: 10.1508/cytologia.23.447
- Tindale , MD and Roy , SK . 2002 . A cytotaxonomic survey of the Pteridophyta of Australia . Australian Systematic Botany , 15 : 839 – 937 . doi: 10.1071/SB00034
- Venter , S. 2009 : A taxonomic revision of the genus Dracophyllum Labill . (Ericaceae). Unpublished PhD Thesis, Victoria University of Wellington , Wellington . 551 p.
- Wagstaff , SJ and Clarkson , BR . 2012 . Systematics and ecology of the Australasian genus Empodisma (Restionaceae) and description of a new species from peatlands in northern New Zealand . PhytoKeys , 13 : 39 – 79 . doi: 10.3897/phytokeys.13.3259
- Wagstaff , SJ , Martinsson , K and Swenson , U . 2000 . Divergence estimates of Tetrachondra hamiltonii and T. patagonica (Tetrachondraceae) and their implications for austral biogeography . New Zealand Journal of Botany , 38 : 587 – 596 . doi: 10.1080/0028825X.2000.9512707
- Wagstaff , SJ , Dawson , MI , Venter , S , Munzinger , J , Crayn , DM , Steane , DA and Lemson , KL . 2010 . Origin, diversification, and classification of the Australasian genus Dracophyllum (Richeeae, Ericaceae) . Annals of the Missouri Botanical Garden , 97 : 235 – 258 . doi: 10.3417/2008130
- Wagstaff , SJ , Breitweiser , I and Ito , M . 2011 . Evolution and biogeography of Pleurophyllum (Astereae, Asteraceae), a small genus of megaherbs endemic to the subantarctic islands . American Journal of Botany , 98 : 62 – 75 . doi: 10.3732/ajb.1000238
- Webb CJ , Sykes WR , Garnock-Jones PJ 1988 . Flora of New Zealand . Vol. IV . Christchurch , DSIR Botany Division . 1365 .
- Wong C 2011 . Molecular cytogenetics and genome evolution in New Zealand Plantago species . Unpublished MSc thesis, The University of Auckland , New Zealand . 132 p.
- Young , AG and Murray , BG . 2000 . Genetic bottlenecks and dysgenic gene flow into re-esyablished populations of the grassland daisy, Rutidosis leptorrhynchoides . Australian Journal of Botany , 48 : 409 – 416 . doi: 10.1071/BT98083
- Zotov , VD . 1971 . Simplicia T. Kirk (Gramineae) . New Zealand Journal of Botany , 9 : 539 – 544 . doi: 10.1080/0028825X.1971.10430200