Abstract
Nothofagus solandri is regarded as more tolerant to drought than Nothofagus menziesii in the field. However, the physiology of responses to water limitation in these species is not well understood. In this study, the thermal sensitivity of leaf respiration and its underlying metabolism in response to drought were investigated in mature trees and saplings. Respiration (R d) and photosynthesis (A max) were measured during drying and re-wetting cycles. In addition, respiratory pathway changes were evaluated by oxygen isotope fractionation and protein analyses. Under drought treatment in the glasshouse, both species showed similar photosynthetic performance, but under mild water stress N. solandri was able to increase A max. Under moderate water deficit (around −2 MPa), N. solandri increased respiration at a base temperature of 10°C (R 10) but then decreased it to initial values after re-watering. In N. menziesii, R 10 did not respond significantly to water-stress treatment. The temperature sensitivity of R d (Q 10 and E o) was unchanged for both species during the gradual deficit water treatment in the glasshouse. Although respiratory electron flow was mainly via the cytochrome pathway under all conditions, an increase in alternative oxidase/cytochrome oxidase protein content suggests that the alternative pathway is involved in modulating respiratory metabolism during the recovery after drought.
Introduction
Approximately half of daily net photosynthetic carbon fixation is released through respiration (R) the following evening (Turnbull et al. Citation2001; Flexas et al. Citation2006). Respiration by leaves plays an important role in determining growth and survival of plants as it produces energy and carbon skeletons required for biosynthesis and cellular maintenance. In spite of its recognized importance, the effects of water stress on respiration at the physiological level are poorly understood. The available experimental evidence does not support a clear pattern of R d response to water stress, with different studies showing increased (Zagdanska Citation1995; Bartoli et al. Citation2005; Galmés et al. Citation2007), unaffected (Lawlor & Fock Citation1975; Flexas et al. Citation2005) or decreased (González-Meler et al. Citation1999; Ribas-Carbó et al. Citation2005b) rates of respiration. Irrespective of how respiration responds to water stress, the ratio of R to assimilation (A) generally increases in response to increasing water stress, and decreases in response to re-watering (Atkin & Macherel Citation2009).
Plant respiration comprises a suite of biochemical reactions that are sensitive to temperature. This thermal response may vary in response to environmentally induced changes in substrate supply (e.g. due to shade-induced or drought-induced changes in photosynthesis) and energy demand (e.g. resulting from water-stress-induced increases in protein turnover or maintenance of ion gradients; Slot et al. Citation2008; Atkin & Macherel Citation2009). In spite of its well-recognized importance to plant carbon balance, regulation of respiration by drought at the metabolic level is not well understood. It has been indicated that in dry sites, respiration increases in some tree species while photosynthesis decreases—this shifts the balance of carbon gain and loss, limiting carbon acquisition in drier sites with respect to wetter sites (Turnbull et al. Citation2001).
The action on plants of various adverse factors, including drought, disturbs electron transport along the main cytochrome-mediated oxidation pathway (CP) and so reduces ATP synthesis. The disturbance of electron transport dramatically increases the potential for the generation of superoxide and other reactive oxygen species (González-Meler et al. Citation1999; Noguchi et al. Citation2005; Ribas-Carbó et al. Citation2005b; Armstrong et al. Citation2006; Shugaeva et al. Citation2007). Fortunately, plants possess an alternative non-phosphorylating pathway (AP) that channels the electrons directly from ubiquinone to O2 through the alternative oxidase protein (AOX). Several positive roles are attributed to the AP: it may assist in the maintenance of redox balance in electron transport when the CP is restricted (Millenaar et al. Citation2000; Florez-Sarasa et al. Citation2007), allow carbon flux through the tricarboxylic acid cycle when ADP supply limits CP activity, or may protect against harmful reactive oxygen generation (Atkin et al. Citation2002; Bartoli et al. Citation2005; Kumar et al. Citation2007; Flexas et al. Citation2006). It has been reported that severe water stress induces a sharp decrease in CP activity, concomitantly with a similar increase in the AP rate, so that total respiration rate remains relatively constant (Ribas-Carbó et al. Citation2005b). Increases in AP respiration have been shown to be accompanied by increases in AOX protein abundance under stress conditions (Taylor et al. Citation2002; Bartoli et al. Citation2005).
Beech forest in New Zealand usually grows on hilly or mountainous terrain, where the weather is colder and wetter, growing seasons are shorter and soils are less fertile than low altitudes, where mixed conifer–broadleaf forest dominates (Orwin Citation2008). Nothofagus solandri commonly dominates the forests at high altitudes in the drier inland mountain regions of the South Island, while Nothofagus menziesii dominates or co-dominates with N. solandri at timberlines with high rainfall and improved soil conditions (Wardle Citation1984). Sun et al. (Citation1995) relate this difference in distribution of N. solandri and N. menziesii to differences between the two species in tolerance to water stress, with N. solandri much more tolerant of water stress than N. menziesii. In this study, we conducted studies quantifying changes in leaf respiration (and oxidase activity) in response to the gradual onset of water deficit and during recovery from drought. An overarching notion was that the drought-tolerant N. solandri is capable of greater adjustment in respiration in response to drought, being able to maintain or decrease its overall respiration with respect to photosynthesis in comparison to its less drought-tolerant counterpart N. menziesii. This would allow N. solandri to cope with lower water availability in its habitat, especially during the summer. We further hypothesize that N. solandri responds to severe drought by increasing the respiratory flux through the AP and decreasing the flux through the CP. In contrast, N. menziesii has limited ability to change electron partitioning between both pathways under severe water stress.
Materials and methods
Plant material and treatments
Mature trees of both study species (500–700 cm in height) growing under open conditions were selected to determine the respiratory response to seasonal variation in temperature and water availability. We selected individuals of Nothofagus solandri var. cliffortioides (Mountain Beech) and Nothofagus menziesii (Silver Beech) growing on the campus of the University of Canterbury, New Zealand (43°32′S; 172°37′E) with heights between 500 and 700 cm growing in open sites or at the edge of other trees. Seasonal measurements of respiration, photosynthesis and shoot water potential were made during April 2010 (late summer, the drier season) and in mid-August 2010 (mid-winter). Temperature data were obtained from the weather station of the Geography Department, University of Canterbury.
Saplings of N. solandri (100–130 cm high) and N. menziesii (160–190 cm high) were maintained in 5 L pots that contained a mixture of bark and chip (coarse organic material) with a ratio of 8 : 2, respectively. The potting mix contained a base level of slow release fertilizer (N : P : K in the proportions 8.8 : 5.5 : 10.6 plus trace elements). Individuals of both species were maintained for approximately 2 months at temperatures between 10 and 20°C, light intensities between 500 and 800 µmol photons m−2 s−1 and watered every other day, maintaining water potentials of 0.5–0.9 MPa for N. solandri and 0.8–0.9 MPa for N. menziesii. To evaluate the respiratory response under severe water deficit, groups of individuals of both species were moved to a glasshouse facility at the University of Canterbury, where they were subjected to gradual water deficit treatment by suspending irrigation. Temperature and light intensity variations inside the glasshouse were measured every 10 min and stored in a datalogger (Campbell Scientific CR21X; Logan, UT, USA). The levels of water deficit were characterized at different times during the imposition of the drought treatment by measurements of shoot xylem water potential at midday using a pressure chamber (Scholander et al. Citation1965; model PSI System 1100). The drought treatment was applied until plants reached a water potential of around −3 MPa (severe water deficit) and then the plants were re-irrigated. Recovery was measured 3–4 and 10 days after re-watering.
Photosynthesis and respiration measurements
A max and R d were measured using a portable infrared gas analysis system (Li-Cor 6400; Li-Cor, Lincoln, NE, USA). For mature trees, gas exchange was measured from excised secondary branches that were re-cut under water and kept in the dark. Previous studies have found no difference in respiration rates between in situ leaves and leaves from detached branches (Turnbull et al. Citation2003, Citation2005). In the glasshouse, gas exchange was measured in leaves on branches from the upper third of each plant.
Photosynthesis was measured as maximum assimilation rate (A max). For excised branches from mature trees, the A max was measured at 600 ppm of CO2 (we used more CO2 to avoid photosynthetic limitations by stomatal closure in excised branches) while potted saplings in the glasshouse were measured at 400 ppm CO2. The photon flux density (Q) used was 1700 µmol photons m−2 s−1. In addition, chlorophyll fluorescence yield (F v/F m) was measured on dark-adapted samples during each measurement period according to Maxwell & Johnson (Citation2000) with a Mini-PAM (Pulse Amplitude Modulation; Walz, Effeltrich, Germany). For R d measurements, leaves were dark-adapted for 30 min before measurement. Measurements were made at 15, 18, 21, 24 and 27 °C for the seasonal study and at 12, 16, 20, 24 and 28 °C for the glasshouse drought experiment. Temperature responses of R d were measured inside growth cabinets at the University of Canterbury, which allowed us to control instantaneous measurement temperature of both the plant and the foliage within the gas exchange cuvette. The temperature response of respiration was modelled using a modified Arrhenius equation (Lloyd & Taylor Citation1994; Turnbull et al. Citation2003; Kruse & Adams Citation2008):
Where R is the respiration rate, R 10 is the respiration rate at a reference temperature (T o) of 10 °C (273K), T a is the measurement temperature of R, g is the ideal gas constant (8.314 J mol−1 K−1) and E 0 is a parameter related to the overall energy of activation. Non-linear curve fitting was performed using the Marquardt–Levenberg algorithm (Sigma Plot, v8. SPSS Inc. Chicago, IL, USA).
Oxygen isotope measurements
Electron partitioning between the CP and AP was evaluated in vivo by determining the discrimination against the heavier isotope of oxygen (18O) during respiratory O2 consumption (Guy et al. Citation1989; Ribas-Carbó et al. Citation2005a). We used an incubation method previously adopted by Searle et al. (Citation2011) and more recently described in detail by Kornfeld et al. (Citation2012). Approximately 0.7 g for N. solandri and 1 g for N. menziesii of leaf material was placed in a 12 mL septum-topped Exetainer® (Labco, High Wycombe, UK) with a pellet of KOH to reduce CO2 build-up. Four Exetainers were filled with varying amounts of leaf material from each sample to achieve a range of oxygen consumption. The Exetainers were incubated at 20 °C for 4 h and gas samples were taken by displacing the air in the Exetainer with water using gas-tight syringes. The samples were analysed on a ThermoScientific Delta V Plus Isotope Ratio mass spectrometer with a Finnigan Gas Bench II and a Varian fused silica 5A molecular sieve gas chromatography capillary column. The ratio of O2/N2 was used to determine the rate of oxygen consumption. Isodat software (Thermo Scientific, Bremen, Germany) was used to calculate the area under the oxygen and nitrogen peaks, and the 18O/16O ratio. The change in the O2/N2 ratio, relative to the O2/N2 ratio of the air samples taken on the sampling day, was used to calculate the fraction of oxygen consumed, or – ln(f). Calculations of oxygen isotope fractionation were made as described by Guy et al. (Citation1989) and Ribas-Carbó et al. (Citation2005a) with modifications to yield a value of discrimination against 18O/16O. The change in this ratio relative to the 18O/16O ratio in air samples taken on the sampling day was used to calculate ln(R/R o)*1000. The slope of a regression between – ln(f) and ln(R/R o)*1000 can be interpreted as the discrimination value (D, ‰).
We were unable to determine the end points of oxygen isotope fractionation for AOX andcytochrome oxidase (COX) as we could not sufficiently inhibit N. solandri or N. menziesii tissue with salicylhydroxamic acid and KCN, despite using a range of concentrations and incubation times. Despite exhaustive efforts, none of the methods used for infiltrations of inhibitors resulted in < 30% residual respiration rate when both inhibitors were applied. As a consequence, in this investigation, changes in D are used to indicate the relative changes in electron partitioning through the CP and AP (Searle et al. Citation2011; Kornfeld et al. Citation2012).
Western blot analysis of respiratory proteins and carbohydrate analysis
Freeze-dried ground leaf samples were mixed with Laemmli buffer. Proteins were separated by sodium dodecyl sulphate—polyacrylamide gel electrophoresis using 4–12% gradient polyacrylamide gels and then transferred to a nitrocellulose membrane using the iBLOT system (Invitrogen, San Diego, CA, USA). Immunoblotting was performed using the Snap i.d.™ system (Millipore, Billerica, MA, USA). The AOX protein was detected using an anti-AOX monoclonal antibody (Elthon et al. Citation1989) at a dilution of 1 : 300 and then using an anti-mouse horseradish peroxidase conjugate and non-commercial enhanced chemiluminescence solution (Haan & Behrmann Citation2007). The same blots were then incubated in a 15% H2O2 solution to deactivate the anti-mouse horseradish peroxidase conjugate before an application of anti-COX antibody at a dilution of 1:600 (Agrisera, Vannas, Sweden). The blots were photographed with a cryo-cooled digital camera (Chemigenius, Syngene, Cambridge, UK) and the bands were quantified using densitometry (Image, National Institutes of Health, Bethesda, MD, USA). The intensities of the bands were corrected for the background. A standard sample of each species was run on each blot and all samples are reported normalized to this standard. Protein abundance for each sample was then divided by the mean abundance of all analysed samples for each protein and species to equalize the distribution for AOX and COX (Searle et al. Citation2011). Relative protein abundances for AOX and COX were divided to calculate the AOX/COX ratio—when this value is 1 it represents the mean AOX/COX ratio for each species (N. solandri and N. menziesii). For analysis of carbohydrates, leaves were dried and ground in a ball mill. Soluble sugar and starch were analysed following the method of Tissue & Wright (Citation1995).
Statistical analysis
Reported values of all measurements correspond to the means of five replicates in each treatment. The results of the experiments were subjected to two-way analysis of variance (ANOVA) for mature trees (factors: species and season) and one-way ANOVA for glasshouse experiments (factor: water potential). Before ANOVA analysis, data were checked for normality and homogeneity of variances. Fisher least significant difference test was applied for comparison between treatments. Differences between the values were considered significant at P ≤0.05. All the statistical analyses were performed with STATISTICA v6.0 (Statsoft, Tulsa, OK).
Results
Seasonal study
During April, which corresponded to the driest period and the end of the growing season, maximum temperatures ranged between 20 and 25 °C (). During winter (mid-August) the maximum temperatures in the study site were around 10 °C during the period of measurements. The midday shoot xylem water potential was statistically different between seasons (P<0.05) and species (P<0.001) (). Both species showed lower water potentials in April (N. solandri, −1.86±0.18 MPa and N. menziesii, −1.43±0.13 MPa) than in August (N. solandri, −0.74±0.05 MPa and N. menziesii, −0.44±0.04 MPa). Maximum carbon assimilation was considerably higher for N. solandri than N. menziesii during April (14.40±1.03 and 6.08±1.75 µmol CO2 m−2 s−1, respectively) and August (14.97±2.59 and 4.80±0.80 µmol CO2 m−2 s−1, respectively) without a significant seasonal effect (P =0.837). Nothofagus solandri also displayed higher respiratory rates than N. menziesiii during April (0.96±0.09 and 0.24±0.03 µmol CO2 m−2 s−1, respectively) and August (0.48±0.05 and 0.38±0.04 µmol CO2 m−2 s−1, respectively). Nothofagus solandri, which had higher A max and R 10 throughout the year, was able to maintain a lower foliar R/A ratio (0.07±0.01 and 0.03±0.00 for April and August, respectively) than N. menziesii (0.14±0.04 and 0.10±0.03, respectively) ().
Figure 1 Maximum and minimum daily temperatures at the University of Canterbury study site (Geography Department, University of Canterbury). The values correspond to late summer (April 2010) and winter (mid-August 2010). The hatched band indicates the days of measurement.
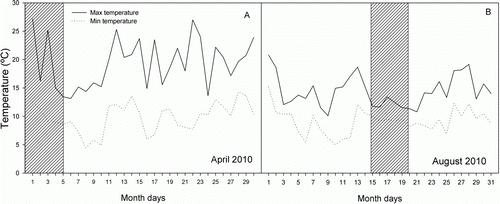
Table 1 Physiological, respiratory and photosynthetic parameters for leaves of Nothofagus solandri and Nothofagus menziesii during late summer and winter.
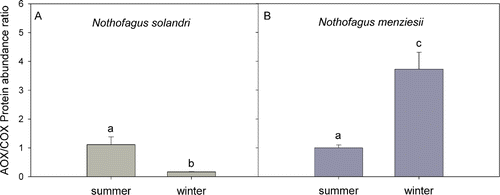
Respiratory thermal-sensitivity parameters obtained from the temperature response curves, E o and Q 10, were significantly higher in August (67,720±6571 and 2.58±0.24, respectively) than April (39,875±1229 and 1.65±0.09, respectively) for N. solandri, whereas N. menziesii did not display any difference between seasons for either parameter (). Discrimination (D), as measured by oxygen isotope fractionation, was higher for N. solandri than N. menziesii in April (19.89±0.18 and 17.34±0.64, respectively) and August (20.36±0.19 and 17.97±0.85, respectively) (). The D values obtained for both species indicate that the mitochondrial electron flow is primarily through the CP. However, when we analysed the abundance of proteins, we did not find any relationship between partitioning of electrons to the CP and COX protein. During April, both species showed a similar AOX/COX protein ratio (). Nothofagus menziesii increased significantly the amount of AOX relative to COX during August, whereas N. solandri increased COX protein abundance relative to AOX. Total non-structural carbohydrate contents were significantly different between species. Soluble sugar percentage was similar for both species during August () but decreased in N. menziesii in April. Starch concentrations where higher for N. solandri than N. menziesii but did not change with the season (P=0.402).
Figure 2 Relative abundance of alternative oxidase (AOX) and cytochrome oxidase (COX) proteins during summer and winter for Nothofagus solandri and Nothofagus menziesii in the seasonal study. Values shown are mean±SEM. Two-way analysis of variance was performed including the factors species (P<0.001) and season (P≤0.05). Different lower case letters show statistical differences (Fisher least significant difference test).
Glasshouse water stress study
Plants were maintained in the glasshouse for 3 months (from May to July). During this period the temperature fluctuated between 24 and 28 °C (midday) and between 14 and 16 °C (night). The maximum natural light received during this period was 400–700 µmol photons m−2 s−1 at midday. Over 9 days, plants of both species were irrigated every other day (Control). Then the drought treatment was applied by suspending irrigation. Different lengths of treatment reflected the different rates at which actual tissue water potential responded to the removal of watering. Hence, we used tissue response as a measure of actual drought impact, and waited until each species had attained a similar level. Maximum water stress was reached in N. solandri (−3.02±0.10 MPa) after 33 days and in N. menziesii (−3.11±0.14 MPa) after 12 days. Changes in specific leaf area and water use efficiency (data not showed) during the treatment indicated that N. solandri had better control in the regulation of water status than N. menziesii and therefore the different drought periods were mainly attributed to their differential drought tolerance. After the plants reached the maximum water stress they were re-irrigated thereafter and recovery was assessed after 3 days (R1) and 10 days (R2) days in N. solandri and after 4 days (R1) and 10 days (R2) days in N. menziesii.
Maximum quantum yield of PSII (F v/F m) was measured during the drought treatment period for both species. The values were within the range 0.83–0.85, indicating that there was no damage in the photosynthetic machinery as a result of water stress treatment (data not shown). For N. solandri, A max increased significantly initially under mild water deficit (−1.6 MPa) but then decreased significantly as more severe water stress developed (C). In N. menziesii, A max was highly sensitive to water deficit, decreasing significantly under moderate water stress (−2.4 MPa, 7 days from the start of the treatment) and then decreasing further to near zero under severe water stress, 12 days from suspension of irrigation (D). After re-watering, both species showed a similar capacity to recover their photosynthesis to initial values within 10 days ().
Figure 3 Variation in gas exchange parameters in Nothofagus solandri and Nothofagus menziesii during drought treatment and recovery. Dark respiration at 10°C in response to water-deficit treatment and subsequent re-watering was calculated from fitted temperature–response curves using a modified Arrhenius equation (A, B). A max represents the maximum CO2 assimilation measured at 20°C and light saturation (C, D). Foliar R/A is an indicator of the carbon balance at the foliar level (E, F). Values shown are mean±SEM. One-way analysis of variance (P≤0.05) was performed where the factor was xylem water potential during treatment being C (control) and R1-2 (recovery). Different lower case letters show significantly different means (Fisher least significant difference test).
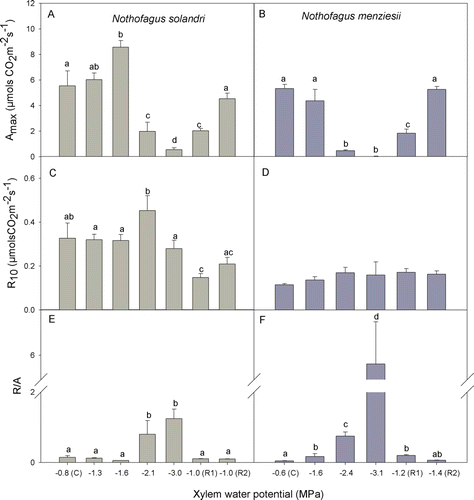
As in the mature trees, N. solandri showed higher leaf respiration rates than N. menziesii (D,E). In N. solandri the respiration rate (R 10) increased in response to moderate water deficit (−2.1 MPa). Under severe water stress the initial rate was re-established, but declined significantly during the first recovery period (R1; A). Ten days after re-watering, N. solandri partially recovered initial respiration rates (A). On the other hand, N. menziesii did not change R 10 during the drought treatment or the recovery period (B). The relationship between R d and A max (R/A; indicating foliar carbon balance) for both species started to increase under moderate water deficit (around −2 MPa) and the maximum imbalance was reached under severe water deficit (E,F). Values in N. menziesii reached considerably higher than in N. solandri, indicating a greater loss of carbon (F). However, after re-watering, both species were able to restore the foliar carbon balance close to initial values (0.10±0.01 and 0.07±0.00 for N. solandri and N. menziesii, respectively). The thermal sensitivity of R, as shown by the parameter E o, did not show a clear response during water stress treatment and recovery in either N. solandri or N. menziesii. Similarly, Q 10 was unchanged for N. solandri (P=0.060) and N. menziesii (P=0.439) despite the severe water stress ().
Table 2 Dark respiration parameters calculated from fitted temperature response curves using a modified Arrhenius equation for leaves of Nothofagus solandri and Nothofagus menziesii during water deficit treatment and recovery.
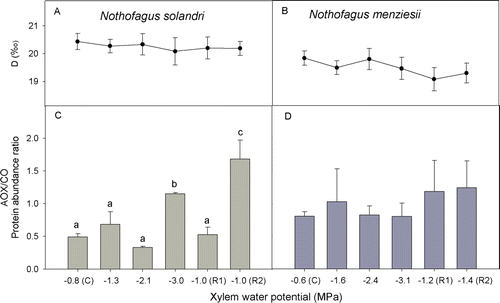
The results obtained from the gradual water deficit treatment suggest that electron flow for both species was primarily and consistently through the CP rather than through the AP, even under the most severe water stress (A,B). The AOX/COX protein abundance indicates that in N. solandri the amount of AOX relative to COX increased under severe water stress and then in late recovery (10 days after re-watering) (C). However, in N. menziesii, the AOX/COX protein ratio did not change across the drought treatment and recovery period (D). Concentrations of soluble sugars and starch were maintained within a fairly narrow range (data not shown) and were not related to changes in respiration and photosynthesis.
Figure 4 Changes in oxygen isotope discrimination (A, B) and protein abundance ratio of alternative oxidase and cytochrome oxidase (AOX/COX) (C, D) during water deficit treatment and recovery for Nothofagus solandri and Nothofagus menziesii. Values shown are mean (± SE). One-way analysis of variance (P≤0.05) was performed where the factor was xylem water potential during treatment being C (control) and R1-2 (recovery). Different lower case letters show significantly different means (Fisher least significant difference test).
Discussion
The response of respiration to temperature under drought was studied in two Nothofagus species with differential drought tolerance (N. solandri more tolerant to drought than N. menziesii). This enabled us to examine the extent to which changes in R d allow the two species to maintain a positive carbon balance under water deficit conditions. Moreover, we evaluated the thermal sensitivity of leaf respiration and a possible role for changes in metabolism (i.e. the alternative pathway) in the respiratory adjustment under drought. Our results show that the responses of respiration at a basal reference temperature of 10 °C (R 10) and A max differ significantly between the two species when they are submitted to a gradual water deficit. The more drought tolerant N. solandri showed more flexibility in its respiratory response (both in mature trees and in glasshouse-grown plants) and the alternative pathway appears to have some role during the recovery of metabolism after a drought period. This work is significant because there are very few studies that have evaluated drought affects on the physiology of respiration. Moreover, few investigations have reported the respiratory response of Nothofagus species in New Zealand to seasonal variations in water availability including a controlled water deficit under glasshouse conditions. Finally, to the best of our understanding this is the first investigation evaluating the respiratory response under water deficit in two tree species with differing drought tolerance.
Response of assimilation and respiration to drought
In mature trees, N. solandri experienced more negative water potentials than N. menziesii during April (−1.86 versus −1.43 MPa), which could indicate greater water stress. Despite this, rates of photosynthesis in N. solandri were higher than in N. menziesii and did not differ significantly between April and August. Nothofagus menziesii also did not display a significant seasonal response in A max. These responses could indicate that the late-summer ‘drought’ in the study site was not strong enough to suppress photosynthesis in these evergreen species. For this reason, the gradual water deficit treatment was applied in the glasshouse and this enabled us to induce a more severe water deficit. Both species possessed similar A max values (around 6 µmol CO2 m−2 s−1) under control conditions. These rates of photosynthesis were similar to those reported by Sun & Sweet (Citation1996), who studied the responses of photosynthesis to light and temperature in individuals of both species from three different origins. The more drought-tolerant species was able to increase A max under moderate water deficits (from 5.54 to 8.56 µmol CO2 m−2 s−1). This increase in photosynthesis under moderate water deficit has been reported in other species (Scharder & Gould Citation2005) and could indicate that a slight drought may improve carbon gain. An almost complete inhibition of photosynthesis was evident by day 12 of the drought treatment in N. menziesii and after 33 days in N. solandri. Both Nothofagus species were able to fully recover initial photosynthetic rate 10 days after re-watering. Several authors have reported that plants subjected to severe water stress recover only 40–60% of the maximum photosynthetic rate during the day after re-watering, and maximum photosynthesis rates are not always regained (Flexas et al. Citation2006). Our results show that, despite the difference in their drought tolerance, both evergreen Nothofagus species are able to recover their A max despite the occurrence of a severe drought event.
During April, there was a significant increase in R 10 for N. solandri compared with August, but lower temperature sensitivity (lower E o and Q 10). This increase in respiration during the active period may reflect reductions in the adenylate restrictions of respiratory metabolism, via increased rates of ATP turnover or increased ADP (Campbell et al. Citation2007). Also, under the gradual drought treatment, N. solandri was more flexible in its respiratory response than N. menziezii. Nothofagus solandri maintained its R 10 during the 15 days in which it was under mild water deficit (from −1.3 to −1.6 MPa), but under moderate water deficit R 10 increased significantly. A similar response was found in Quercus humilis, which showed an initial increase under mild water stress, followed by a large decrease under severe water stress (Gulías et al. Citation2002). Other authors have reported that this increase of respiration under water stress is probably in response to an increase in energy demand as leaves cope with drought (Flexas et al. Citation2006; Zaragoza-Castells et al. Citation2008). Ten days after re-watering, N. solandri was able to re-establish its initial respiratory rates. The thermal sensitivity of respiration under the glasshouse drought treatment was similar in both Nothofagus species () and Q 10 was unchanged during the water deficit treatment. Maintenance of Q 10 through rapid and adenylate control or enzymatic regulation may be important for the regulation of respiration under drought (Ribas-Carbó et al. Citation2005b; Campbell et al. Citation2007; Searle et al. Citation2011).
The balance between photosynthesis and respiration determines leaf carbon balance, and here we use the ratio of net carbon assimilation to dark respiration (R/A) as a simple estimate of leaf carbon balance that allows a qualitative comparison between species (Pattison et al. Citation1998; Galmés et al. Citation2005). Here, as in other studies, development of water stress resulted in a progressively increased R/A, affecting the foliar carbon balance (Flexas et al. Citation2005, Citation2006). The higher photosynthetic rate in N. solandri mentioned above contributed to maintaining a positive foliar carbon balance over the year, despite the increased R 10 during summer. Under the glasshouse drought treatment, the R/A ratio of N. menziesii increased to a value of 6.5. When compared with the maximum value of 1.25 in the R/A ratio in N. solandri, this indicates that N. menziesii pays a significant cost for its sensitivity to water stress—this is a cost that could clearly underpin its lower competitive ability in drier sites in the field. In addition, the ability of N. solandri to increase its photosynthetic performance under mild drought and maintain its respiratory activity even under severe water stress contributes to the maintenance of a more positive carbon balance in comparison to N. menziesii. These findings clearly support our hypothesis in this work.
Changes in COX and AOX in response to drought
Environmental changes such as low temperature (Watanabe et al. Citation2008) and drought (Ribas-Carbó et al. 2005a,b) may generate changes in the electron partitioning between each of the mitochondrial respiratory pathways. In this investigation, we evaluated electron partitioning by the isotopic discrimination technique to determine if, during a drought period, there was an increase in electron flux toward the AP. The oxygen isotope discrimination values reported here range from 17 to 20‰. It has been reported that D values lower than 19‰ may be uncommon (Ribas-Carbó et al. Citation2005a). However, values of 17‰ and lower have been previously reported for various plant tissues (Guy et al. Citation1989; Millar et al. Citation1998; Nagel et al. Citation2001; Armstrong et al. Citation2006, Citation2008; Searle et al. Citation2011). In both species and in both mature trees and in the gradual water deficit experiment, under drought lower values of D obtained were interpreted as relatively constant electron flux and maintained primarily through the CP.
Although the apparent partitioning between the oxidative pathways (as measured by D) did not change, protein abundance did (AOX/COX ratio). During winter, mature N. menziesii trees synthesized considerably more AOX—although in this case it was not associated with low water availability. In the glasshouse, in N. solandri the changes in R 10 during the gradual water deficit treatment were accompanied by changes in protein contents. An increase in AOX protein content (relative to COX) was displayed during severe water stress and 10 days after re-watering (c). The results found here and in other studies confirm that there is no clear positive correlation between the AOX concentration and its activity (Lennon et al. Citation1997; Guy & Vanlerberghe Citation2005; Ribas-Carbó et al. Citation2005b; Vidal et al. Citation2007; Grant et. al Citation2008; Florez-Sarasa et al. Citation2011). We suggest that this increase in the amount of AOX protein indicates participation of the AP, but probably could not be determined by isotopic discrimination because AOX might have been inactivated. Several studies suggest that post-translational regulation of AOX protein is responsible for regulating activity, either through the reduction status of a disulphide bond or via an effector, such as pyruvate or other α-ketoacids (Grant et al. Citation2008; Vanlerberghe et al. Citation2009). AOX protein has a slow turnover rate and a half-life estimated at 18 h (Millenaar et al. Citation2000). This suggests that the protein is not generated de novo but may rather be biochemically activated. Armstrong et al. (Citation2008) postulated that an increase in the activation of pre-existing AOX protein is very fast, occurring within minutes to hours. They showed that in Arabidopsis thaliana, an increase in AP activity is a short-lived response that subsides once the plant is able to re-establish flux via the CP. Assuming that AOX protein is not generated de novo but is biochemically activated, we suggest that this activation may occur during the night. In the present study, R d and associated metabolism were measured during the day, but some authors have postulated that respiration measured in the dark during the day might change substrate supply and sink demand compared with nocturnal conditions (Ribas-Carbó et al. (Citation2005b). This leaves open the question of how electron flow via the alternative pathway might change during the night period. For this reason we suggest that the AP may be activated during the night period, when respiration dominates plant carbon metabolism. This would be a fruitful line of study in future investigations on the regulation of the AOX. We suggest making R d measurements with isotopic discrimination and protein analyses during the night period.
The AP may play an important role both during and after a drought period, working together with the CP to increase the energy, regenerating and osmoregulatory mechanisms that may have been damaged during the water stress. Under stress conditions, such as drought, the activity of the AOX pathways allows the tricarboxylic acid cycle to continue providing carbon skeletons for metabolism and this is particularly important in the synthesis of compatible solutes (Bartoli et al. Citation2005). Moreover, severe drought lowers photosynthetic rate, decreasing carboxylation efficiency. Under these conditions, high light intensity may result in photoinhibition and photo-oxidative destruction of the photosynthetic apparatus. Photosynthetic activity may be inhibited through an imbalance between light capture and use which may produce excessive reduction of the electron transport chain generating reactive oxygen species (, O2, H2O2, OH−) leading to oxidative damage (Gulías et al. Citation2004). During recovery, when the plant begins to readjust its metabolism after severe water stress, the AP will probably act to sustain the redox state of the ubiquinone pool, limiting oxygen radical species production (Millar et al.
Citation1998; Florez-Sarasa et al. Citation2007) and also maintaining chloroplast function by avoiding the over-reduction of electron carriers (Bartoli et al. Citation2005).
Concluding remarks
The aim of the present study was to evaluate the effect of drought on the temperature response of respiration in two species with differing drought tolerance. When saplings of each species were submitted to a gradual water deficit period, the capacity of N. solandri to adjust respiration under the progressive water deficit and maintain photosynthetic activity even under severe drought contributed to a better carbon balance compared with the less tolerant species N. menziesii. A similar response has been found in Nothofagus dombeyi, which also has been described as a drought-tolerant and cold-tolerant species (Zuñiga et al. Citation2006; Pipper et al. Citation2007) and for other evergreen species from Mediterranean regions with seasonal droughts (Galmés et al. Citation2007; Atkin & Macharel Citation2009). Regarding the mechanisms underlying the respiratory response, we postulate that in N. solandri, variations in respiration may be associated with activation of the AP (reflected in an increase in the amount of AOX during severe water stress and the recovery period) which is post-translationally regulated and probably activated during the night. Our results suggest that these differential respiratory and photosynthetic responses are a consequence of their differential drought tolerance, which together with other factors will be determining the ecological distribution of these two tree species in New Zealand forests.
Acknowledgements
Thanks to Jenny Ladley and Mauricio Urbina for technical assistance, Stephanie Searle and Sam Thomas for training with immunoblotting, and Ari Kornfeld for advice and help with isotopic analysis. We gratefully acknowledge the Marsden Fund of the Royal Society of New Zealand and the School of Biological Sciences of the University of Canterbury for supporting this project. Finally, thanks to MECESUP UCO0708 for financing the internship of CS in the laboratory of MHT.
References
- Armstrong , AF , Logan , D , Tobin , AK , O'Toole , P and Atkin , OK . 2006 . Heterogeneity of plant mitochondrial responses underpinning respiratory acclimation to the cold in Arabidopsis thaliana leaves . Plant, Cell and Environment , 29 : 940 – 949 . doi: 10.1111/j.1365-3040.2005.01475.x
- Armstrong , AF , Badger , MR , Day , DA , Barthet , MM , Smith , PMC , Millar , AH , Whelan , J and Atkin , OK . 2008 . Dynamic changes in the mitochondrial electron transport chain underpinning cold acclimation of leaf respiration . Plant Cell and Environment , 31 : 1156 – 1169 . doi: 10.1111/j.1365-3040.2008.01830.x
- Atkin , OK , Zhang , QS and Wiskich , JT . 2002 . Effect of temperature on rates of alternative and cytochrome pathway respiration and their relationship with the redox poise of the Quinone Pool . Plant Physiology , 128 : 212 – 222 . doi: 10.1104/pp.010326
- Atkin , OK and Macherel , D . 2009 . The crucial role of plant mitochondria in orchestrating drought tolerance . Annals of Botany , 103 : 581 – 597 . doi: 10.1093/aob/mcn094
- Bartoli , CG , Gomez , F , Gergoff , G , Guiamét , JJ and Puntarulo , S . 2005 . Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions . Journal of Experimental Botany , 56 : 1269 – 1276 . doi: 10.1093/jxb/eri111
- Campbell , C , Atkinson , L , Zaragoza-Castells , J , Lundmark , M , Atkin , OK and Hurry , V . 2007 . Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group . New Phytologist , 176 : 375 – 389 . doi: 10.1111/j.1469-8137.2007.02183.x
- Elthon , TE , Nickels , RL and McIntosh , L. 1989 . Monoclonal antibodies to the alternative oxidase of higher plant mitochondria . Plant Physiology , 89 : 1311 – 1317 . doi: 10.1104/pp.89.4.1311
- Flexas J , Galmés J , Ribas-Carbó M , Medrano H 2005 . The effects of drought in plant respiration . In : Lambers H , Ribas-Carbó M Advances in photosynthesis and respiration. Plant respiration: from cell to ecosystem Vol. 18 . Dordrecht , Springer . Pp. 177 – 194 .
- Flexas , J , Bota , J , Galmés , J , Medrano , H and Ribas-Carbó , M . 2006 . Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress . Physiologia Plantarum , 127 : 343 – 352 . doi: 10.1111/j.1399-3054.2006.00621.x
- Florez-Sarasa , ID , Bouma , TJ , Medrano , H , Azcon-Bieto , J and Ribas-Carbó , M . 2007 . Contribution of the cytochrome and alternative pathways to growth respiration and maintenance respiration in Arabidopsis thaliana . Physiologia Plantarum , 129 : 143 – 151 . doi: 10.1111/j.1399-3054.2006.00796.x
- Florez-Sarasa , ID , Flexas , J , Rasmusson , AG , Umbach , AL , Siedow , JN and Ribas-Carbó , M . 2011 . In vivo cytochrome and alternative pathway respiration in leaves of Arabidopsis thaliana plants with altered alternative oxidase under different light conditions . Plant, Cell and Environment , 34 : 1373 – 1383 . doi: 10.1111/j.1365-3040.2011.02337.x
- Galmés , J , Cifre , J , Medrano , H and Flexas , J . 2005 . Modulation of relative growth rate and its components by water stress in Mediterranean species with different growth forms . Oecologia , 145 : 21 – 31 . doi: 10.1007/s00442-005-0106-4
- Galmés , J , Ribas-Carbó , M , Medrano , H and Flexas , J . 2007 . Response of leaf respiration to water stress in Mediterranean species with different growth forms . Journal of Arid Environments , 68 : 206 – 222 . doi: 10.1016/j.jaridenv.2006.05.005
- González-Meler , MA , Ribas-Carbó , M , Giles , L and Siedow , JN . 1999 . The effect of growth and measurement temperature on the activity of the alternative respiratory pathway . Plant Physiology , 120 : 765 – 772 . doi: 10.1104/pp.120.3.765
- Grant , NM , Miller , RE , Watling , JR and Robinson , SA . 2008 . Synchronicity of thermogenic activity, alternative pathway respiratory flux, AOX protein content, and carbohydrates in receptacle tissues of sacred lotus during floral development . Journal of Experimental Botany , 59 : 705 – 714 . doi: 10.1093/jxb/erm333
- Gulías , J , Flexas , J , Abadia , A and Medrano , H . 2002 . Photosynthetic responses to water deficit in six Mediterranean sclerophyll species: possible factors explaining the declining distribution of Rhamnus ludovici-salvatoris, an endemic Balearic species . Tree Physiology , 22 : 687 – 697 . doi: 10.1093/treephys/22.10.687
- Gulías J , Galmés J , Cifre J , Medrano H , Flexas J 2004 . Ecofisiología de la fotosíntesis y la eficiencia en el uso del agua en la vegetación de la cuenca mediterranea . In : Cabrera HM Fisiología ecológica en plantas . Ediciones Universitarias de Valparaíso , Valparaíso . Pp. 157 – 172 .
- Guy , RD , Berry , JA , Fogel , ML and Hoering , TC . 1989 . Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide sensitive respiration in plants . Planta , 177 : 483 – 491 . doi: 10.1007/BF00392616
- Guy , RD and Vanlerberghe , GC . 2005 . Partitioning of respiratory electrons in the dark in leaves of transgenic tobacco with modified levels of alternative oxidase . Physiologia Plantarum , 125 : 171 – 180 . doi: 10.1111/j.1399-3054.2005.00557.x
- Haan , C and Behrmann , I . 2007 . A cost effective non-commercial ECL solution for Western blot detections yielding strong signals and low background . Journal of Immunological Methods , 318 : 11 – 19 . doi: 10.1016/j.jim.2006.07.027
- Kornfeld , A , Horton , TW , Yakir , D , Searle , SY , Griffin , KL , Atkin , OK , Subke , J-A and Turnbull , MH . 2012 . A field-compatible method for measuring alternative respiratory pathway activities in vivo using stable O2 isotopes . Plant Cell and Environment , 35 : 1518 – 1532 . doi: 10.1111/j.1365-3040.2012.02507.x
- Kruse , J and Adams , MA . 2008 . Three parameters comprehensively describe the temperature response of respiratory oxygen reduction . Plant, Cell & Environment , 31 : 954 – 967 . doi: 10.1111/j.1365-3040.2008.01809.x
- Kumar , N , Vyas , D and Kumar , S . 2007 . Plants at high altitude exhibit higher component of alternative respiration . Journal of Plant Physiology , 164 : 31 – 38 . doi: 10.1016/j.jplph.2005.11.001
- Lawlor , DW and Fock , H . 1975 . Photosynthesis and photorespiratory CO2 evolution of water-stressed sunflower leaves . Planta , 126 : 247 – 258 . doi: 10.1007/BF00388966
- Lennon , AM , Neuenschwander , UH , Ribas-Carbó , M , Giles , L , Ryals , JA and Siedow , JN . 1997 . The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco . Plant Physiology , 115 : 783 – 791 .
- Lloyd , J and Taylor , JA . 1994 . On the temperature dependence of soil respiration . Functional Ecology , 8 : 315 – 323 . doi: 10.2307/2389824
- Maxwell , K and Johnson , G . 2000 . Chlorophyll fluorescence—a practical guide . Journal of Experimental Botany , 51 : 659 – 668 . doi: 10.1093/jexbot/51.345.659
- Millar , AH , Atkin , OK , Menz , RI , Henry , B , Farqhuar , G and Day , DA . 1998 . Analysis of respiratory chain regulation in roots of soybean seedlings . Plant Physiology , 117 : 1083 – 1093 . doi: 10.1104/pp.117.3.1083
- Millenaar , FF , Roelofs , R , Gonzalez-Meler , MA , Siedow , JN , Wagner , AM and Lambers , H . 2000 . The alternative oxidase in roots of Poa annua after transfer from high-light to low-light conditions . Plant Journal , 23 : 623 – 632 . doi: 10.1046/j.1365-313x.2000.00832.x
- Nagel , OW , Waldron , S and Jones , HG . 2001 . An off-line implementation of the stable isotope technique for measurements of alternative respiratory pathway activities . Plant Physiology , 127 : 1279 – 1286 . doi: 10.1104/pp.010372
- Noguchi , K , Taylor , NL , Millar , AH , Lambers , H and Day , DA . 2005 . Response of mitochondria to light intensity in the leaves of sun and shade species . Plant, Cell and Environment , 28 : 760 – 771 . doi: 10.1111/j.1365-3040.2005.01322.x
- Orwin J 2008 . Southern beech forest . The encyclopedia of New Zealand . http://www.teara.govt.nz/en/southern-beech-forest (accessed 29 January 2013) .
- Pattison , RR , Goldstein , G and Ares , A . 1998 . Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species . Oecologia , 117 : 449 – 459 . doi: 10.1007/s004420050680
- Pipper , FI , Corcuera , LJ , Alberdi , M and Lusk , C . 2007 . Differential photosynthetic and survival responses to soil drought in two evergreen Nothofagus species . Annals of Forest Science , 64 : 447 – 452 . doi: 10.1051/forest:2007022
- Ribas-Carbó M , Robinson SA , Giles L 2005a . The application of the oxygen-isotope technique to assess respiratory pathway partitioning . In : Lambers H , Ribas-Carbó M Advances in photosynthesis and respiration: plant respiration: from cell to ecosystem . Urbana , IL , Springer .
- Ribas-Carbó , M , Taylor , NL , Giles , L , Busquets , S , Finnegan , PM , Day , DA , Lambers , H , Medrano , H , Berry , JA and Flexas , J . 2005b . Effects of water stress on respiration in soybean leaves . Plant Physiology , 139 : 466 – 473 . doi: 10.1104/pp.105.065565
- Scholander , P , Hammel , H , Bradstreet , E and Hemmingsen , E . 1965 . Sap pressure in vascular plants. Negative hydrostatic pressure can be measured in plants . Science , 148 : 339 – 346 . doi: 10.1126/science.148.3668.339
- Schrader , M and Gould , SJ . 2005 . Assay and functional analysis of dynamin-like protein 1 in peroxisome division . Methods in Enzymology , 404 : 586 – 597 .
- Searle , SY , Thomas , S , Griffin , K , Horton , T , Kornfeld , A , Yakir , D , Hurry , V and Turnbull , MH . 2011 . Leaf respiration and alternative oxidase in field-grown alpine grasses respond to natural changes in temperature and light . New Phytologist , 189 : 1027 – 1039 . doi: 10.1111/j.1469-8137.2010.03557.x
- Shugaeva , NA , Vyskrebentseva , EI , Orekhova , SO and Shugaeva , G . 2007 . Effect of water deficit on respiration of conducting bundles in leaf petioles of sugar beet . Russian Journal of Plant Physiology , 54 : 329 – 335 . doi: 10.1134/S1021443707030065
- Slot , M , Zaragoza-Castells , J and Atkin , OK . 2008 . Transient shade and drought have divergent impacts on the temperature sensitivity of leaf dark respiration . Functional Plant Biology , 35 : 1135 – 1146 . doi: 10.1071/FP08113
- Sun , OJ , Sweet , GB , Whitehead , D and Buchan , GD . 1995 . Physiological responses to water stress and waterlogging in Nothofagus species . Tree Physiology , 15 : 629 – 638 . doi: 10.1093/treephys/15.10.629
- Sun , OJ and Sweet , GB . 1996 . Comparison of frost tolerance of Nothofagus solandrivar. Cliffortioides (Hook.f.) Poole and Nothofagus menziesii (HooW.i.) Oerst . New Zealand Journal of Botany , 34 : 273 – 278 . doi: 10.1080/0028825X.1996.10410691
- Taylor , NL , Day , DA and Millar , AH . 2002 . Environmental stress causes oxidative damage to plant mitochondria leading to inhibition to glycine decarboxylase . Journal of Biological Chemistry , 277 : 42663 – 42668 . doi: 10.1074/jbc.M204761200
- Tissue , DT and Wright , SJ . 1995 . Effect of seasonal water availability on phenology and the annual shoot carbohydrate cycle of tropical forest shrubs . Functional Ecology , 9 : 518 – 527 . doi: 10.2307/2390018
- Turnbull , MH , Whitehead , D , Tissue , DT , Schuster , WSF , Brown , KJ and Griffin , KL . 2001 . Responses of leaf respiration to temperature and leaf characteristics in three deciduous tree species vary with site water availability . Tree Physiology , 21 : 571 – 578 . doi: 10.1093/treephys/21.9.571
- Turnbull , MH , Whitehead , D , Tissue , DT , Schuster , WSF , Brown , KJ and Griffin , KL . 2003 . Scaling foliar respiration in two contrasting forest canopies . Functional Ecology , 17 : 101 – 114 . doi: 10.1046/j.1365-2435.2003.00713.x
- Turnbull , MH , Tissue , DT , Griffin , KL , Richardson , SJ , Peltzer , DA and Whitehead , D . 2005 . Respiration characteristics in temperate rainforest tree species differ along a long-term soil-development chronosequence . Oecologia , 143 : 271 – 279 . doi: 10.1007/s00442-004-1803-0
- Vanlerberghe , GC , Cvetkovska , M and Wang , J . 2009 . Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase? . Physiologia Plantarum , 137 : 392 – 406 . doi: 10.1111/j.1399-3054.2009.01254.x
- Vidal , G , Ribas-Carbó , M , Garmier , M , Dubertret , G , Rasmusson , AG , Mathieu , C , Foyer , CH and De Paepe , R . 2007 . Lack of respiratory chain complex I impairs alternative oxidase engagement and modulates redox signaling during elicitor-induced cell death in tobacco . Plant Cell , 19 : 640 – 655 .
- Wardle JA 1984 . The New Zealand beeches ecology, utilization and management . Christchurch , , New Zealand , New Zealand Forest Service . 447 p.
- Watanabe , CK , Hachiya , T , Terashima , I and Noguchi , K . 2008 . The lack of alternative oxidase at low temperature leads to a disruption of the balance in carbon and nitrogen metabolism, and to an up-regulation of antioxidant defence system in Arabidopsis thaliana leaves . Plant, Cell and Environment , 31 : 1190 – 1202 . doi: 10.1111/j.1365-3040.2008.01834.x
- Zagdanska , B . 1995 . Respiratory energy demand for protein turnover and ion transport in wheat leaves upon water deficit . Physiologia Plantarum , 95 : 428 – 436 . doi: 10.1111/j.1399-3054.1995.tb00859.x
- Zaragoza-Castells , J , Sánchez-Gómez , D , Hartley , IP , Matesanz , S , Valladares , F , Lloyd , J and Atkin , OK . 2008 . Climate dependent variations in leaf respiration in a dry-land, low productivity Mediterranean forest: the importance of acclimation in both high-light and shaded habitats . Functional Ecology , 22 : 172 – 184 .
- Zúñiga , R , Alberdi , M , Reyes-Diaz , M , Olivares , E , Hess , S , Bravo , L and Corcuera , LJ . 2006 . Seasonal changes in the photosynthetic performance of two evergreen Nothofagus species in south central Chile . Revista Chilena de Historia Natural , 79 : 489 – 504 .