Abstract
Sticherus tener is confirmed as being present on Resolution Island, Fiordland, extending from the northern half of Five Fingers Peninsula through to the centre of the island. It is also present on nearby Anchor Island in Dusky Sound, and on the Denniston and Stockton Plateaus. Sticherus urceolatus is newly recorded for New Zealand, with populations on the Stockton Plateau and near Takaka, and what appears to be a hybrid population on Indian Island, Dusky Sound. Plants of S. tener and S. urceolatus from northern South Island were previously identified as Sticherus flabellatus. Diagnostic characters for distinguishing the five species of Sticherus occurring in southern Australia and New Zealand, and a key to the New Zealand species, are provided. The distribution, ecology and conservation status of S. tener and S. urceolatus are outlined, and their biogeographic and evolutionary origins are discussed.
Introduction
The fern family Gleicheniaceae comprises six genera (Dicranopteris, Diplopterygium, Gleichenella, Gleichenia, Sticherus and Stromatopteris) and about 125 species worldwide (Smith et al. Citation2006). It is pantropical in distribution but extends also into south temperate regions.
Three genera, Dicranopteris, Gleichenia and Sticherus, are indigenous to New Zealand. All are characterised by a distinctive growth form in which the rachis repeatedly forks, producing a bud at the end of the old branch between the two new branches. Each bud is potentially capable of growing out to form a new tier of branches. The nature of the branching, and the pattern of new tiers of fronds, produce distinctive growth forms. In Gleichenia, lateral tiering and extensive lateral branching at wide angles give rise to a thicket-forming habit, reflected in the vernacular name of tangle fern. In Sticherus, narrower branch angles give rise to fan-shaped or parasol-shaped fronds, known as umbrella ferns, sometimes in two or more vertical tiers (Brownsey & Smith-Dodsworth Citation2000).
Dicranopteris is represented in New Zealand by a single pantropical species, Dicranopteris linearis (Burm.f.) Underw., which is confined to thermal areas of the North Island. It is recognised by having leafy ultimate segments only on the distal costae (i.e. the α costae), veins in the ultimate segments branching at least twice, and sori comprising 6–10 sporangia (Brownsey & Smith-Dodsworth Citation2000; Shaw & Ranker Citation2011: D).
Gleichenia has about 12 species worldwide, mostly distributed in southeast Asia and Australasia. The genus is represented in New Zealand by four currently recognised species: Gleichenia alpina R.Br., Gleichenia dicarpa R.Br. and Gleichenia microphylla R.Br., all of which also occur in Australia (Chinnock & Bell Citation1998), and one endemic species, Gleichenia inclusisora Perrie, L.D.Sheph. & Brownsey (Perrie et al. Citation2012). They are distinguished from species of Sticherus and Dicranopteris by the wide branch angles of the costae, leafy ultimate segments arising from all costae, and the sori comprising two to four sporangia. However, DNA sequence analysis indicates that the taxonomy of New Zealand Gleichenia may be more complex than presently recognised (Perrie et al. Citation2007, Citation2012).
Sticherus is pantropical with about 95 species worldwide and the greatest diversity in tropical America (Gonzales & Kessler Citation2011). It is represented in New Zealand by four species, and is distinguished by the narrow branch angles of the costae, relatively long (7–40 mm) leafy ultimate segments on all costae (except sometimes the proximal ones), and the sori comprising four or five sporangia. Two species, Sticherus cunninghamii (Heward ex Hook.) Ching and Sticherus flabellatus (R.Br.) H.St.John, have long been recognised in New Zealand. Sticherus cunninghamii is an endemic species widely distributed throughout New Zealand, although less common in the east and south of the South Island. Sticherus flabellatus also occurs in Australia, New Caledonia and New Guinea. In New Zealand, it is confined to areas from North Cape to the Bay of Plenty. Previous reports of S. flabellatus from the South Island (e.g. Given Citation1982) are now considered erroneous (see below). These two species are distinguished by the form of the frond, which is umbrella-like in S. cunninghamii and fan-shaped in S. flabellatus. The leafy ultimate segments are up to 18 mm long and entire in S. cunninghamii, but up to 40 mm long and at least distally serrate in S. flabellatus.
A third species in New Zealand, Sticherus tener (R.Br.) Ching, was first reported by Garrett et al. (Citation1998). This was based on a single collection made by Alan Mark in mountain beech forest on Five Fingers Peninsula, Dusky Sound, Fiordland (Mark s.n., 13 November 1984, OTA 41949). In their investigation of Sticherus in southeast Australia, Garrett et al. (Citation1998) recognised four species—S. flabellatus, Sticherus lobatus N.A.Wakef., S. tener and one new species, Sticherus urceolatus M.Garrett & Kantvilas, which they segregated from S. tener. This treatment was followed in Flora of Australia by Chinnock & Bell (Citation1998) who also reported the presence of S. tener in Fiordland based on the solitary collection made by Alan Mark. Brownsey & Smith-Dodsworth (Citation2000) recognised the species in New Zealand, but noted that better material was required to confirm the identity and determine the extent of its distribution.
Despite the fact that the presence of a distinctive species of Sticherus had been known about in Fiordland for over 20 years, the extreme isolation and inaccessibility of Five Fingers Peninsula prevented the plant from being re-collected until very recently. The search for more material of that species resulted in the completely unexpected finding of what appeared to be the fourth species, S. urceolatus, in the same general area. It was first identified from material collected on Indian Island in Dusky Sound by Brian Rance. This prompted an examination of all South Island collections of Sticherus, especially those reported as S. flabellatus by Given (Citation1982) and Nichol & Overmars (Citation2008). It quickly became apparent that specimens previously identified as S. flabellatus from Stockton Plateau, Denniston Plateau and near Takaka were actually a mixture of S. urceolatus and S. tener, and that there was no evidence of S. flabellatus from the South Island.
The purpose of this paper is to confirm the identity and report on the occurrence of S. tener and S. urceolatus in Fiordland, northern Westland and northwest Nelson; to provide a key and diagnosis for identification purposes; and to report on the ecology, distribution and threat status of these two species. Full descriptions of all the New Zealand species will be prepared for the electronic Flora of New Zealand http://www.nzflora.info/.
Materials and methods
Field collections and observations of Sticherus species in Fiordland were made on Resolution Island by Brian Rance, Richard Ewans and George Ledgard in March 2008, on Indian Island by Brian Rance in May 2010, on both islands by Richard Ewans and Donna Worthy in October 2011, and on Anchor Island by Richard Ewans, Alex Fergus and Donna Worthy in April 2012. Comparable collections and observations were made by Simon Walls near Takaka in April 2012, and by Leon Perrie and Patrick Brownsey on Stockton Plateau and Denniston Plateau in June 2012. Herbarium specimens are lodged at WELT (herbarium abbreviations follow Thiers Citation2012).
Collections of all New Zealand species of Sticherus, including all those cited by Given (Citation1982), were compared with material of S. flabellatus, S. lobatus, S. tener and S. urceolatus from southern Australia. Collections in AK, CHR, HO, MEL, OTA and WELT were used for this purpose.
In light of the polyploidy of Sticherus urceolatus (Garrett et al. Citation1998), and the frequent correlation of spore size with polyploidy (Barrington et al. Citation1986), spore size in New Zealand material was investigated by calculating mean exospore length and width from a sample of 30 spores for each specimen measured. Spores were mounted in 1 : 1 glycerol : water, and measured at×1000 magnification.
Identification of the Sticherus species in New Zealand was further tested using phylogenetic analyses of DNA sequences of the chloroplast locus trnL-trnF (trnL intron trnL 3′-exon, and trnL-trnF intergenic spacer). These were generated using the methods in Perrie et al. (Citation2007) from silica-gel-preserved, field-collected samples identified initially as S. cunninghamii (n=3), S. tener from Five Fingers Peninsula, Fiordland (n=1), Denniston (n=2), Stockton (n=2) and Tasmania (n=1), and S. urceolatus from Indian Island, Fiordland (n=1), Stockton (n=5), Rangihaeata, Takaka (n=1) and Little Onahau River, Takaka (n=1). These were compared with sequences published by Perrie et al. (Citation2007) for two New Zealand samples of each of S. cunninghamii and S. flabellatus, and with unpublished sequences of Daniel Ohlsen (The University of Melbourne) of Australian S. flabellatus from New South Wales, S. tener from Tasmania and S. urceolatus from Victoria. The result for the sample initially identified as S. urceolatus from Indian Island was verified by independent re-extraction. Sample details are given in the Appendix. Chilean Sticherus cryptocarpus (Hook.) Ching was used as an outgroup.
ClustalX 2.1 (Larkin et al. Citation2007) was used to align the DNA sequences. PAUP* 4.0b10 (Swofford Citation2002) was used to perform a maximum parsimony analysis, with a branch and bound search. Because of the low level of homoplasy (see Results), model-based analyses (e.g. maximum likelihood, Bayesian analysis) were superfluous.
Results
Key to New Zealand species of Sticherus
| 1. | a) Ultimate segments arising at near right angles to the costa; segments always present on proximal costae (primary branches) ………………………… S. tener b) Ultimate segments arising at an acute angle to the costa; segments lacking on proximal costae, or only one or two present …………………………………… 2 | ||||
| 2. | a) Longest segments on ultimate leaflets (ultimate branches) usually < 15 mm long; undersides of segments white/glaucous, bearing abundant, broad, bicoloured scales ………………… S. cunninghamii b) Longest segments on ultimate leaflets usually > 15 mm long; undersides of segments green, bearing occasional, narrow, brown scales. ……………………… 3 | ||||
| 3. | a) Ultimate leaflets > 10 times longer than proximal costae; segments on ultimate leaflets 1.5–2 mm wide, margins serrate throughout ……………… . S. flabellatus b) Ultimate leaflets <10 times longer than proximal costae; segments on ultimate leaflets 2–3 mm wide, margins entire or minutely serrate only at apices ……… S. urceolatus | ||||
Morphology
Descriptions of S. flabellatus, S. lobatus, S. tener and S. urceolatus from southern Australia are given by Garrett et al. (Citation1998) and Chinnock & Bell (Citation1998), and for S. cunninghamii and S. flabellatus from New Zealand by Allan (Citation1961) and Brownsey & Smith-Dodsworth (Citation2000). Diagnostic characters for distinguishing the five species are presented in , and short descriptions are given below (data taken primarily from Garrett et al. Citation1998). We have used the recent terminology of Shaw & Ranker (Citation2011) for frond parts, with that of Garrett et al. (Citation1998) added in brackets.
Table 1 Diagnostic characters for southern Australian and New Zealand species of Sticherus (from Garrett et al. 1998, supplemented with data for New Zealand material of S. cunninghamii and S. flabellatus).
Sticherus cunninghamii has fronds that tend to droop in the shape of an umbrella. There is a narrow angle (mean c.44°) between paired proximal costae (primary branches). The ultimate leaflet (ultimate branch) is much longer (mean ratio 15 : 1) than the proximal costa (primary branch). The proximal costa either lacks, or has only one or two, leafy ultimate segments. The ultimate leaflet is narrowly ovate in outline, with the ultimate segments arising at a mean angle of c.65° to the costa. The segments of the ultimate leaflet are longest at or below the middle, 7–18 mm long, 1–3 mm wide, entire, and usually glaucous/white on the underside. There are abundant, broad, fimbriate, bicoloured scales (brown in the centre, fading to colourless on the margins) along the costae, and long hair-like scales on the undersurfaces. Spores measure 29–31 µm by 14–15 µm (four samples from four populations).
Sticherus lobatus has a spreading frond with broad angles (c.75–80°) between paired proximal costae. The ultimate leaflet is somewhat longer than the proximal costa (mean ratio 6 : 1). The proximal costa always bears leafy ultimate segments. The ultimate leaflet is narrowly oblong to narrowly elliptic in outline with the segments arising at almost a right angle (c.80–85°) to the axis. The segments of the ultimate leaflet tend to be uniform in length, or slightly longer near the middle, 20–30 mm long, 2–3 mm wide, entire and green on the underside. The undersides are glabrous or sometimes bear hair-like scales.
Sticherus tener (A) also has a spreading frond with broad angles (c.70°) between paired proximal pinnae. The ultimate leaflet is somewhat longer than the proximal costa (mean ratio 4.3 : 1). The proximal costa always bears leafy ultimate segments. The ultimate leaflet is linear or narrowly oblong in outline with the segments arising at almost a right angle (c.80–85°) to the costa. The segments of the ultimate leaflet are more or less uniform in length, decreasing only at the apex, 8–15 mm long, 2–3 mm wide, entire, and green on the undersides. There are narrow, fimbriate, brown scales along the costae, and hair-like scales on the undersurfaces. Spores measure 33–37 µm by 17–18 µm (four samples from Denniston and one from Stockton).
Figure 1 A, Sticherus tener (same site as Perrie 6636-6639 & Gemmell, 26 May 2012, Denniston, WELT P023789). B, Sticherus urceolatus (Perrie 6613 et al., 25 May 2012, Stockton, WELT P023791). The yellowish colour of this S. urceolatus plant is a reflection of its exposed habitat.

Sticherus urceolatus (B) has fronds that are held partially upright in the form of a fan. There is a somewhat narrow angle (c.50°) between paired proximal pinnae. The ultimate leaflet is somewhat longer than the proximal costa (mean ratio 5.7 : 1). The proximal costa either lacks, or has only one or two, leafy ultimate segments. The ultimate leaflet is narrowly elliptic in outline with the segments arising at 55–65° to the costa. The segments of the ultimate leaflets are longest near the middle, 15–27 mm long, 2–3 mm wide, entire or sometimes minutely serrate near the apices, and green on the undersides. There are occasional, narrow, fimbriate, brown scales along the costae, and hair-like scales on the undersurfaces. Spores measure 38–43 µm by 19–23 µm (four samples from Stockton and one from Rangihaeata, Takaka).
Sticherus flabellatus has fronds that are held partially upright in the form of a fan, rather than drooping like an umbrella. There is a very narrow angle (mean 30°) between paired proximal costae. The ultimate leaflet is much longer than the proximal costa (mean ratio 15.5 : 1). The proximal costa usually lacks leafy ultimate segments. The ultimate leaflet is narrowly elliptic in outline with the segments arising at 50–60° to the costa. The segments of the ultimate leaflet are longest near the middle, 20–40 mm long, 1.5–2 mm wide, minutely serrate along the margins, and green on the underside. There are occasional, very narrow, fimbriate, brown scales along the costae, and hair-like scales on the undersurfaces. Spores measure 32–35 µm by 17–19 µm (four samples from four populations).
Genetics
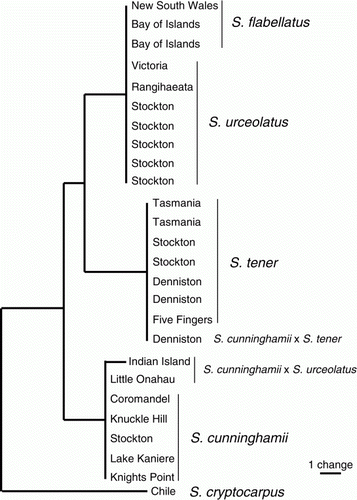
The trnL-trnF alignment comprised 26 samples and 844 characters, of which eight were parsimony-informative and an additional 14 were variable but parsimony-uninformative. Four haplotypes were found among the Australasian samples. Sticherus cunninghamii, S. flabellatus, and S. tener each had distinct haplotypes, differing from one another by between five and six substitutions. Samples of S. tener from Five Fingers Peninsula, Denniston, and Stockton had the same haplotype as those from Tasmania. Samples of S. urceolatus from Rangihaeata and Stockton had the same haplotype as a sample from Victoria, Australia; this haplotype was also found in Australian and New Zealand S. flabellatus. A sample from Little Onahau River, identified initially as S. urceolatus, had the haplotype of S. cunninghamii. The fourth haplotype occurred in a sample from Indian Island identified initially as S. urceolatus. This haplotype differed from that of S. cunninghamii by a single autapomorphic substitution. It is likely that both of these samples are S. cunninghamii×S. urceolatus (see below). Similarly, a sample of S. cunninghamii×S. tener from Denniston has the haplotype of S. tener.
The maximum parsimony analysis recovered two most parsimonious trees of score 23. One of these is shown in , with S. tener sister to S. flabellatus (and S. urceolatus). The other tree differs in having S. cunninghamii sister to S. flabellatus (and S. urceolatus).
Discussion
Distribution and ecology
Plants of Sticherus tener, 30–60 cm tall, were collected at an altitude of 200–300 m from several sites in the northern half of Five Fingers Peninsula (Ewans s.n., 9 Mar. 2008, WELT P023592-P023595, P023615; Ewans & Worthy s.n., 6 Oct. 2011, WELT P023586-P023587), from Cormorant Cove Stream near the centre of Resolution Island (Rance s.n., 8 Mar. 2008, WELT P023585), and from two sites on Anchor Island (Fergus & Ewans s.n., 18 Apr. 2012, WELT P023736; Worthy & Ewans s.n., 18 Apr. 2012, WELT P023737). Alan Mark's original collection (Mark s.n., 13 Nov. 1984, OTA 41949) was made from a transect somewhere near the middle of Five Fingers Peninsula, indicating that the species extends over a distance of at least 12 km. None of the specimens in WELT has spores suitable for investigation, but their morphology and the sequencing results are consistent with S. tener, and there is little suggestion that any of them might be hybrids. Observations made from the recent collections indicate that plants occurred mostly around ridge tops in the northern half of Five Fingers Peninsula on slightly poorer soils under a low, semi-open canopy. The vegetation was usually low, mixed forest or scrub with Leptospermum scoparium, tall Halocarpus biformis, Lepidothamnus intermedius, Metrosideros umbellata and Nothofagus solandri var. cliffortioides, but S. tener also extended into taller beech/podocarp vegetation. Sticherus tener was never found in better soils on slopes. On Five Fingers Peninsula, it was locally common (i.e. patchy), although never abundant. The two collections from Anchor Island were from small patches (possibly a single sprawling individual each). One site was near sea level in low, thick Halocarpus biformis and Lepidothamnus intermedius scrub with heavy moss ground cover, whereas the other site was more similar to the majority of Five Fingers Peninsula sites in altitude and vegetation type.
A specimen collected in 1922 from ‘Dusky Sound’ (Poppelwell s.n., Dec. 1922, WELT P005403) had been identified as S. cunninghamii. Although it has no spores to check, its intermediate frond morphology indicates that it is almost certainly S. cunninghamii×S. tener (or otherwise unusually abaxially glaucous S. tener). This points to a longer presence of S. tener in the region than previously appreciated.
Three collections of S. tener (Given 8784 & Park, 2 July 1975, CHR 276253/B, C; Given 10527 & Crompton, 22 Sept. 1977, CHR 322979; Given 11389 & Lovis, 15 Sep. 1978, CHR 356345) were made between 450 and 600 m on the Stockton Plateau, near Westport by David Given but were reported as S. flabellatus (Given Citation1982). Two populations were also located by Pat Brownsey and Leon Perrie (Perrie 6618 et al., 25 May 2012, WELT P023786; Perrie 6622 et al., 25 May 2012, WELT P023787); the first of these is almost certainly the same as Given 8784 & Park. Plants 20–60 cm tall occurred under overhangs on steep cliffs and along the sides of streams. Associated species included S. urceolatus, S. cunninghamii and Gleichenia inclusisora, with surrounding vegetation including Halocarpus bidwillii, Leptospermum scoparium, Quintinia serrata and Lepidothamnus intermedius.
Two populations of S. tener were also found at the southern margin of the Denniston Plateau. One of the two GPS coordinates given by Nichol & Overmars (Citation2008) for ‘S. flabellatus’ appeared to be for a plant of S. cunninghamii, but the other located a plant of S. tener (Perrie 6631-6633 et al., 26 May 2012, WELT P023788). Another population was found about 150 m away (Perrie 6636-6639 & Gemmell, 26 May 2012, WELT P023789; A). Both populations were within rock overhangs on a sandstone hill, with plants growing in the colluvium and on the lower walls. Associated species included Empodisma minus, Gleichenia dicarpa and Gleichenia inclusisora, with surrounding vegetation including Weinmannia racemosa, Metrosideros umbellata, Phyllocladus alpinus and Leptospermum scoparium. A patch of S. cunninghamii×S. tener (Perrie 6634-6635 et al., 26 May 2012, WELT P023783-P023784) occurred on a wet rock wall below one of the above populations. That it is a hybrid is indicated by its clearly intermediate frond and scale morphology and its abnormally formed spores. It provides definitive evidence that Sticherus hybrids do occur in New Zealand. Gleicheniaceae hybrids have not previously been recorded in New Zealand, but Sticherus hybrids have been reported from elsewhere (Duek Citation1974; Jermy & Walker Citation1985; Gonzales & Kessler Citation2011).
Plants initially identified by us as S. urceolatus, 50–70 cm tall, were collected from a single 15×15 m site at the outlet of the lake in the centre of Indian Island, at an altitude of c.120 m (Rance & West s.n., 22 May 2010, WELT P023265; Ewans & Worthy s.n., 5 Oct. 2011, WELT P023588-P023591). They grew commonly on small hummocks raised above the wet, swampy, semi-permanent stream outlet in low (4–5 m), scrubby, podocarp-dominated forest with Leptospermum scoparium, Nothofagus solandri var. cliffortioides, Dacrydium cupressinum, Halocarpus biformis, Leptecophylla juniperina, Elaeocarpus hookerianus, Metrosideros umbellata, Weinmannia racemosa, Pimelea gnidia and Dracophyllum menziesii. Ground cover included many mosses and Blechnum procerum. The chloroplast DNA sequence obtained from this population was unexpectedly allied to S. cunninghamii (rather than S. flabellatus/S. urceolatus). A possible explanation is that the sequenced plant was actually S. cunninghamii×S. urceolatus. The abaxial lamina of this sample is slightly glaucous (consistent with the involvement of S. cunninghamii) but that is the only morphological discrepancy, with it otherwise matching S. urceolatus. None of the material from Indian Island has spores suitable for investigation. In the absence of specimens with normally formed and large spores, and given the sequencing result, there is presently no definitive evidence that S. urceolatus remains on Indian Island (cf. S. cunninghamii×S. urceolatus), but it has clearly grown there in the past.
Several collections of S. urceolatus were made from the Stockton Plateau by David Given (Given 8784 & Park, 2 July 1975, CHR 276253/A; Given 9634, 29 Sep. 1976, CHR 355870; Given 9635, 29 Sep. 1976, CHR 355869; Given 10518 & Crompton, 22 Sep. 1977, CHR 322983; Given 11382 & Lovis, 15 Sep. 1978, CHR 356343; Given 11826, 17 July 1979, CHR 498317), but reported as S. flabellatus (Given Citation1982). Additional collections have been made from Stockton (Ballin s.n., 10 Jan. 1976, CHR 280272; Bartlett s.n., Jan 1979, WELT P010361; Given 13281, 15 Feb. 1983, CHR 404072) and between the Stockton and Denniston plateaus (Simpson 8526, shallow sandstone cave at 900 m on Mount Augustus, CHR 440087). During June 2012, five populations were located by Pat Brownsey and Leon Perrie around the Mangatini Stream at Stockton (Perrie 6613 et al., 25 May 2012, WELT P023791; Perrie 6614 et al., 25 May 2012, WELT P023792; Perrie 6619 et al., 25 May 2012, WELT P023793; Perrie 6621 et al., 25 May 2012, WELT P023794; Perrie 6624 et al., 25 May 2012, WELT P023795; B). Plants 30–75 cm tall occurred under overhangs on steep, north-facing cliffs, and along streamsides, track cuttings and roadsides, and in more open areas on sloping river banks, at 300–600 m altitude (see Given Citation1982: figure 3). Associated species included Gleichenia dicarpa, Empodisma minus and S. tener, with surrounding vegetation including Halocarpus bidwillii, Quintinia serrata and Leptospermum scoparium. From our observations, S. urceolatus is much more abundant at Stockton than S. tener.
Sticherus urceolatus has also been collected from Rangihaeata Head near the mouth of the Takaka River (Chinnock s.n., 15 Aug. 1967, WELT P024171-2; Druce s.n., May 1975, CHR 275524-5; Walls s.n., 11 Apr. 2012, WELT P023790). Fourteen individuals were noted by Walls along shoreline bluffs including coal measures. Associated species included Kunzea ericoides (forming a sparse canopy), Leucopogon fasiculatus, Ulex europaeus, Pteridium esculentum and Dianella nigra. Nearby on the Little Onahau River, in sandy granite soil in the flood zone and under a light canopy of Kunzea ericoides, was a 3×2 m patch of what appears to be S. cunninghamii×S. urceolatus (Walls s.n., 10 Apr. 2012, WELT P023785). This identification is based on its somewhat intermediate morphology (superficially like S. urceolatus but the segments are too short and untoothed), abnormally formed spores, and the plant having the chloroplast haplotype of S. cunninghamii. An earlier collection that is probably also from Little Onahau also has abnormally formed spores (Brownlie 2209, 7 Jan. 1973, ‘c.3 miles west of Takaka …100 [feet]’, CHR 397934). Plants in the Takaka area were first reported by Kingsley (Citation1893) as S. flabellatus but appear to have been unsubstantiated by any herbarium specimen until re-discovered by V.M. Scott in June 1955 (Scott s.n., 10 June 1955, ‘Takaka’, CHR 87170) from an undisclosed locality (Given Citation1982).
Norton & Overmars (Citation1990) reported a ‘small colony of Sticherus flabellatus’ from an additional site between Stockton and Denniston, at 760 m on Mount Frederick. Although the voucher specimen, thought to be in CANU, cannot be located (Pieter Pelser, University of Canterbury, personal communication, 29 August 2012), photographs clearly indicate that this is S. urceolatus.
Conservation status
Sticherus tener was listed as a Vagrant in New Zealand by de Lange et al. (Citation1999), a status that has remained unchanged since that time (de Lange et al. Citation2009), and that was appropriate when the species was known only from a single collection. Vagrants are defined as ‘Taxa whose occurrences, though natural, are sporadic and typically transitory. Most (if not all) fail to establish themselves beyond their point of arrival because of reproductive failure or for some specific ecological reasons’. The 1922 Poppelwell specimen from ‘Dusky Sound’ of S. cunninghamii×S. tener and the widespread populations of S. tener indicate that neither the Vagrant nor Coloniser (now established but only arrived since 1950) categories are appropriate.
One of the two Denniston sites seen by us comprised over 200 (small) fronds, but it occupied an area less than 3×2 m and may have been a single individual. The other Denniston site included approximately 15 discrete patches, which may have each been a separate individual. It totalled c.150 fronds, which collectively also would have occupied an area less than 5×5 m. The two sites we saw at Stockton were even smaller, and together would have constituted only 5–20 individuals beyond sporelings. Collections made by Given at Stockton represent an additional one or two populations of unknown size. The populations in the Dusky Sound area are therefore critical for the conservation assessment of S. tener. There, it occurs over at least half the length of Five Fingers Peninsula, and extends to the centre of Resolution Island (i.e. a distance of at least 12 km) and to Anchor Island. Fronds are numerous at some sites. However, because Sticherus can spread extensively via its creeping rhizome, establishing the number of individuals is difficult. It is doubtful whether the number of individuals in the Dusky Sound area is sufficient, even when added to those at Stockton and Denniston, to exceed the 250 threshold of individuals for the Nationally Critical category. If it does, the correct category is Naturally Uncommon, as the populations are not known to have declined. We note, however, that the populations at Denniston are in a proposed mining site (Nichol & Overmars Citation2008).
The conservation status of S. urceolatus in New Zealand has not previously been considered but its situation is similar to S. tener. The Kingsley (Citation1893) report indicates a long presence of S. urceolatus in New Zealand, and it occurs (or has occurred) in widespread populations. The Indian Island and Little Onahau River populations are discounted because they appear to be S. cunninghamii×S. urceolatus; in any case they are very small. The Rangihaeata population comprises c.14 individuals. Sticherus urceolatus at Stockton is known from no more than 12 populations. There can be many fronds at some sites, but this does not necessarily mean there are many individuals. It is doubtful whether the number of individuals of S. urceolatus around Stockton is sufficient, even when added to those from Rangihaeata, to exceed the 250 threshold of the Nationally Critical category. If it does, then the correct category is Naturally Uncommon, as the populations are not known to have declined.
The qualifiers of Range Restricted (scattered populations, of possibly specialist habitat), Secure Overseas (in Australia), and Data Poor are appropriate for both species. We note the uncertainty over the number of individuals, and that Townsend et al. (Citation2008) recommend a precautionary approach of opting for the higher threat category when deciding between two categories (i.e. in the present cases, Nationally Critical versus Naturally Uncommon). On seeing the data presented here, the Indigenous Vascular Plant Threat Listing Panel sponsored by the New Zealand Department of Conservation have adopted a conservation status of Nationally CriticalDP,RR,SO for both S. tener and S. urceolatus (Peter de Lange, Chair, Indigenous Vascular Plant Threat Listing Panel, personal communication, 6 September 2012).
Biogeographic and evolutionary origins
Sticherus tener is widespread only in Tasmania, with outlying populations in southern Victoria. Sticherus urceolatus occurs in eastern New South Wales, southern Victoria and throughout Tasmania (Chinnock & Bell Citation1998). It seems almost certain that both species have arrived in New Zealand as a result of long-distance dispersal from Australia. It is possible that this has happened within historical time, and that the populations in New Zealand, though established and reproducing, have not had time to spread very far from their points of entry. In both cases, it seems likely that there have been two or three separate dispersal events from Australia to different parts of the South Island. These examples add to the growing body of evidence for trans-Tasman dispersals in both directions (see Perrie et al. Citation2010).
Sticherus urceolatus was only recognised as a species distinct from both S. tener and S. flabellatus by Garrett et al. (Citation1998). It overlaps in some morphological characters with both species, and is known to be a tetraploid. However, S. tener and S. flabellatus are diploid species, and the overlap in morphology suggests that S. urceolatus could be an allotetraploid derived from these two parent species. The distributions of the three species in Australia lend some support to this hypothesis, with S. tener largely confined to Tasmania, S. urceolatus present in Tasmania, Victoria and southern New South Wales, and S. flabellatus extending from the eastern tip of Victoria to north Queensland. More detailed genetic analyses could provide further evidence for the evolutionary origin of S. urceolatus and whether it has become established in New Zealand following one or more dispersal events.
Acknowledgements
We thank Donna Worthy and Alex Fergus (DOC) for assistance with fieldwork in Fiordland; Carol West (DOC) for preparing the specimen from Indian Island; Barry Walker and Michelly Carvalho (Solid Energy) for assistance with fieldwork on Stockton Plateau; Michael Gemmell and Wendy Hogg for assistance with fieldwork at Denniston; and Fred Overmars for information on the Stockton and Denniston populations. This research was supported by Core funding for Crown Research Institutes from the Ministry of Business, Innovation and Employment's Science and Innovation Group.
References
- Allan , HH . 1961 . Flora of New Zealand , Vol. 1 , Wellington : Government Printer .
- Barrington , DS , Paris , CA and Ranker , TA . 1986 . Systematic inferences from spore and stomate size in the ferns . American Fern Journal , 76 : 149 – 159 . doi: 10.2307/1547723
- Brownsey , PJ and Smith-Dodsworth , JC . 2000 . New Zealand ferns and allied plants , Auckland : Bateman .
- Chinnock , RJ and Bell , GH . 1998 . Gleicheniaceae . Flora of Australia , 48 : 148 – 162 .
- de Lange , PJ , Heenan , PB , Given , DR , Norton , DA , Ogle , CC , Johnson , PN and Cameron , EK . 1999 . Threatened and uncommon plants of New Zealand . New Zealand Journal of Botany , 37 : 603 – 628 . doi: 10.1080/0028825X.1999.9512657
- de Lange , PJ , Norton , DA , Courtney , SP , Heenan , PB , Barkla , JW and Cameron , EK . 2009 . Threatened and uncommon plants of New Zealand (2008 revision) . New Zealand Journal of Botany , 47 : 61 – 96 . doi: 10.1080/00288250909509794
- Duek , JJ . 1974 . A newly recognized Gleichenia hybrid from Cuba . American Fern Journal , 64 : 74 – 76 . doi: 10.2307/1547001
- Garrett , M , Kantvilas , G and Laws , H . 1998 . Sticherus urceolatus (Gleicheniaceae), a new fern species from southern Australia . Muelleria , 11 : 101 – 111 .
- Given , DR . 1982 . Records of Sticherus flabellatus (R.Br.) H.St John (Pteridophyta-Gleicheniaceae) from South Island, New Zealand . New Zealand Journal of Botany , 20 : 381 – 385 . doi: 10.1080/0028825X.1982.10428507
- Gonzales , J and Kessler , M . 2011 . A synopsis of the Neotropical species of Sticherus (Gleicheniaceae), with descriptions of nine new species . Phytotaxa , 31 : 1 – 54 .
- Jermy , AC and Walker , TG . 1985 . Cytotaxonomic studies of the ferns of Trinidad . Bulletin of the British Museum (Natural History), Botany , 13 : 133 – 276 .
- Kingsley , RI . 1893 . Botanical notes from Takaka district . Transactions and Proceedings of the New Zealand Institute , 25 : 304 – 305 .
- Larkin , MA , Blackshields , G , Brown , NP , Chenna , R , McGettigan , PA , McWilliam , H , Valentin , F , Wallace , IM , Wilm , A , Lopez , R , Thompson , JD , Gibson , TJ and Higgins , DG . 2007 . Clustal W and Clustal X version 2.0 . Bioinformatics , 23 : 2947 – 2948 . doi: 10.1093/bioinformatics/btm404
- Nichol R , Overmars F 2008 . Vegetation and Flora Baseline Survey . L & M Coal Ltd Escarpment Mine Project, Denniston Plateau . Westport , Resource and Environmental Management Ltd . http://www.wcrc.govt.nz/escarpment/Application/Volume%202%20-%20Technical%20Reports/9%20-%20Vegetation%20and%20Flora.pdf (accessed 22 March 2012) .
- Norton , DA and Overmars , FB . 1990 . A new record for Sticherus flabellatus in Buller . Canterbury Botanical Society Journal , 24 : 36 – 38 .
- Perrie , LR , Ohlsen , DJ , Shepherd , LD , Garrett , M , Brownsey , PJ and Bayly , MJ . 2010 . Tasmanian and Victorian populations of the fern Asplenium hookerianum result from independent dispersals from New Zealand . Australian Systematic Botany , 23 : 387 – 392 . doi: 10.1071/SB10028
- Perrie , LR , Bayly , MJ , Lehnebach , CA and Brownsey , PJ . 2007 . Molecular phylogenetics and molecular dating of the New Zealand Gleicheniaceae . Brittonia , 59 : 129 – 141 . doi: 10.1663/0007-196X(2007)59[129:MPAMDO]2.0.CO;2
- Perrie , LR , Shepherd , LD and Brownsey , PJ . 2012 . Gleichenia inclusisora, a new and uncommon tangle fern from New Zealand . New Zealand Journal of Botany , 50 : 401 – 410 . doi: 10.1080/0028825X.2012.724015
- Shaw , SW and Ranker , TA . 2011 . New and improved terminology for Gleicheniaceae . American Fern Journal , 101 : 117 – 124 . doi: 10.1640/0002-8444-101.2.117
- Smith , AR , Pryer , KM , Schuettpelz , E , Korall , P , Schneider , H and Wolf , PG . 2006 . A classification for extant ferns . Taxon , 55 : 705 – 731 . doi: 10.2307/25065646
- Swofford , DL . 2002 . PAUP* phylogenetic analysis using parsimony (and other methods) 4.0 Beta , Sunderland , MA : Sinauer Associates .
- Thiers B 2012 . Index Herbariorum: a global directory of public herbaria and associated staff . New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/ (accessed 1 July 2012) .
- Townsend , AJ , de Lange , PJ , Duffy , CAJ , Miskelly , CM , Molloy , J and Norton , DA . 2008 . New Zealand Threat Classification System manual , Wellington : Department of Conservation .
Appendix
Samples used in genetic analysis, with GenBank numbers
Novel sequences are underlined.
Sticherus cryptocarpus: Lehnebach s.n., Chile, VALD, DQ910528.
S. cunninghamii : Perrie 3463 & Shepherd, 21 Dec. 2004, Coromandel, WELT P021164, DQ910529; Perrie 3354, 27 Oct. 2004, Knuckle Hill, WELT P020795, DQ910530; Perrie et al. 6623, 25 May 2012, Stockton, WELT P023780, JX569713; Brownsey, Perrie 6713 et al., 29 May 2012, Lake Kaniere, WELT P023781, JX569714; Hogg, Perrie 6750 et al., 31 May 2012, Knights Point, WELT P023782, JX569715.
S. cunninghamii × S. tener : Perrie 6634 et al., 26 May 2012, Denniston, WELT P023783, JX569716.
S. cunninghamii × S. urceolatus : Walls s.n., 10 Apr. 2012, Little Onahau, WELT P023785, JX569717; Ewans & Worthy s.n., 5 Oct. 2011, Indian Island, WELT P023588, JX569718.
S. flabellatus : Perrie 3435 & Shepherd, 19 Dec. 2004, Bay of Islands, WELT P021165, DQ910531; Perrie 3436 & Shepherd, 19 Dec. 2004, Bay of Islands, WELT P021166, DQ910532; Ohlsen s.n., New South Wales, MELU, n.a.
S. tener : Perrie 6618 et al., 25 May 2012, Stockton, WELT P023786, JX569719; Perrie 6622 et al., 25 May 2012, Stockton, WELT P023787, JX569720; Perrie 6631 et al., 26 May 2012, Denniston, WELT P023788, JX569721; Perrie 6636 & Gemmell, 26 May 2012, Denniston, WELT P023789, JX569722; Ewans & Worthy s.n., 6 Oct. 2011, Five Fingers Peninsula, WELT P023586, JX569723; Ohlsen s.n., Tasmania, MELU, n.a.; Brownsey TAS 22, 5 Dec. 2007, Tasmania, WELT P022158, JX569724.
S. urceolatus : Walls s.n., 10 Apr. 2012, Rangihaeata Head, WELT P023790, JX569725; Perrie 6613 et al., 25 May 2012, Stockton, WELT P023791, JX569726; Perrie 6614 et al., 25 May 2012, Stockton, WELT P023792, JX569727; Perrie 6619 et al., 25 May 2012, Stockton, WELT P023793, JX569728; Perrie 6621 et al., 25 May 2012, Stockton, WELT P023794, JX569729; Perrie 6624 et al., 25 May 2012, Stockton, WELT P023795, JX569730; Ohlsen s.n., Victoria, MELU, n.a.