Abstract
Deceptive orchids typically employ food or sexual deception of male insects. Brood-site deception, when flowers are pollinated by female flies fooled into attempting oviposition, is less well characterized for orchids but is probably common among New Zealand terrestrial orchid species. Helmet orchids (Corybas, Nematoceras and related genera) are considered specialist brood-site deceivers that mimic mushrooms and are pollinated by ovipositing female fungus gnats (Mycetophilidae). We monitored pollination in the endemic spurred helmet orchid, Corybas cheesemanii, and trapped insects visiting orchids and sympatric mushrooms. Flowering occurred from mid-May to early July, each flower lasted 23.14 d, 78.5% of the population flowered (147/194 plants), and c.25% of these set seed naturally (50% of bagged orchids set seed via autonomous self-pollination). Traps caught mostly flies (Mycetophilidae, Anisopodidae and Lauxaniidae), but orchid, mushroom and control traps did not attract significantly different fauna. Seven mycetophilids (of both sexes) visited both orchids and mushrooms, but we found no compelling evidence that C. cheesemanii was pollinated by these. Only some mycetophilid individuals caught above orchids were small enough to enter orchids, but none carried orchid pollinia. If C. cheesemanii is fungus gnat-pollinated, the range of species and sexes attracted suggests a more generalist pollination strategy than assumed.
Introduction
Deceptive pollination systems attract pollinators without providing rewards (Jersáková et al. Citation2006). Pollinators may be attracted by signals that mimic resources such as food or mates, or those that exploit pollinator preferences for certain features, e.g. ultraviolet reflectance (Schaefer & Ruxton Citation2009; Gaskett Citation2013). Brood-site deceptive pollination relies on insects such as Diptera and Coleoptera that usually oviposit in decaying organic matter, and occurs in at least 10 angiosperm families (Urru et al. Citation2010, Citation2011; Policha & Roy Citation2012). Pollinators are lured with signals such as odours that mimic oviposition substrates including carrion or fungi (Jürgens et al. Citation2006; Kaiser Citation2006; Urru et al. Citation2011; van der Niet et al. Citation2011). The flowers can be very large (e.g. titan arum Amorphophallus titanum) and generally dark coloured, often with a contrasting light-coloured trap chamber (Beaman et al. Citation1988; Urru et al. Citation2011).
Brood-deceptive orchids
Orchids are well-known for their unusual deceptive pollination systems. The best studied systems are food deception, when nectarless orchids are pollinated by foraging insects, and sexual deception, when orchids are pollinated by male insects fooled into sexual behaviour (recent reviews include Phillips et al. Citation2009; Schiestl & Schlüter Citation2009; Vereecken et al. Citation2010; Gaskett Citation2011).
Brood-site deception is less common among orchids than food or sexual deception, but is reported or suspected in several groups. There is strong evidence for brood-site mimicry in the foul-smelling South African terrestrial endemic orchid Satyrium pumilum (Diseae: Satyriinae; van der Niet et al. Citation2011). This species is pollinated by female flies from the family Sarcophagidae that usually mate and oviposit on carcasses. Neoptropical Dracula orchids (Epidendreae: Pleurothallidinae) were suspected brood-site mimics because their red-brown colours, fungal aromas and gill-like structures resemble mushrooms (Kaiser Citation2006; Dentinger & Roy Citation2010; Endara et al. Citation2010). Observations confirm that at least two species, Dracula felix and Dracula lafleurrii, are predominantly visited by drosophilid flies that usually reproduce in fungi (Endara et al. Citation2010). Charles Darwin noted preliminary evidence for brood-site mimicry in another pleurothallid orchid. He observed numerous insect eggs laid in flowers in a Kew hothouse (in Zootrophion atropurpureum, as Masdevallia fenestrata; Darwin Citation1885).
Odours are not mandatory for brood-site deception. Southeast Asian Paphiopedilum (Cypripedioideae: Cypripedieae) are faintly scented or scentless. However, as is typical for brood-site deceivers, they are mostly pollinated by female insects (Syrphidae; hoverflies) that sometimes lay eggs in the flowers (Bänziger Citation1996; Shi et al. Citation2007, Citation2009; Bänziger et al. Citation2012). Pollinator attraction may be visual in Paphiopedilum with some species exploiting insects' innate attraction to yellow, whereas others may attract insects with dark spots that mimic aphids (Shi et al. Citation2009).
Fly-pollinated orchids
Most orchids are pollinated by insects. After Hymenoptera, the Diptera (true flies) are the most important group (Christensen Citation1994). Flies are vital and sometimes sole pollinators for a range of rewarding, food-deceptive and sexually deceptive orchids. For example, Chinese Cypripedium pollinated by scathophagids and drosophilids (Li et al. Citation2012), various South African species pollinated by long-tongued flies (Nemestrinidae; e.g. Anderson & Johnson Citation2009) and South and Central American species whose pollinators include tachinids, sciarids, chloropids and phorids (e.g. Borba & Semir Citation2001; Singer et al. Citation2006). Fly pollination is particularly important in New Zealand, which has a wide diversity of flies and fly-pollinated plants, and lacks diversity in some important pollinator guilds such as social hymenoptera and butterflies (Newstrom & Robertson Citation2005). Many New Zealand orchids are likely to be fly-pollinated, e.g. rewarding epiphytes Earina and Winika (Lehnebach & Robertson Citation2004) and deceptive terrestrials such as the greenhoods Pterostylis and Diplodium and related and synonymous genera (Lehnebach et al. Citation2005).
New Zealand and Australian Helmet orchids (Corybas, Nematoceras, Singularybas and related genera) are widely believed to attract female fungus gnats as pollinators (Mycetophilidae: Diptera) via brood deception and mimicry of fungal scents and colours (Jones Citation1970; Fuller Citation1994; Jersáková et al. Citation2006; Scanlen Citation2006; Clements et al. Citation2007; St George Citation2007). Chance sightings indicate that fungus gnats do visit, remove pollinia from, and sometimes oviposit in, the flowers from at least some helmet orchid species (Miller Citation1918; Fuller Citation1994; Scanlen Citation1996, Citation2006, Citation2008; St George Citation2001). However, fungal mimicry is untested, and it is not known whether only females are attracted. Furthermore, taxonomic revisions mean that these reports are for species that are now mostly accepted as Nematoceras rather than Corybas (for old and new names, see Clements et al. Citation2007). After revisions, New Zealand retains just one Corybas species, the endemic spurred helmet orchid Corybas cheesemanii (Hook. f. ex Kirk). This species is presumed to be brood-deceptive and pollinated by fungus gnats, but spectral and gas chromatography–mass spectroscopy analyses reveal strong ultraviolet reflectance, and no evidence of the fungal mimicry expected of a brood-site mimic (Kelly & Gaskett unpublished data).
Mycetophilid fungus gnats (Nematocera: Diptera) are traditionally considered inefficient pollinators in comparison to more recent fly groups that tend to be larger-bodied and more densely haired (Mesler et al. Citation1980; Okuyama et al. Citation2004). However, fungus gnats are known to be efficient and specialist pollinators of small, dark, sometimes foul-smelling flowers, including the orchid Listera cordata (Mesler et al. Citation1980; Goldblatt et al. Citation2004; Okuyama et al. Citation2004). They may well pollinate New Zealand's unrewarding Corybas and Nematoceras orchids, which are similarly small and dark, but rarely scented. New Zealand has a diverse range of endemic fungus gnats available to act as pollinators (Tonnoir & Edwards Citation1927; Jaschhof & Didham Citation2002; Toft & Chandler Citation2004). Some families are relict lineages from the Jurassic, such as Ditomyiidae and the recently discovered endemic family Rangomaramidae. Also present are the globally abundant families Keroplatidae and Mycetophilidae, which probably diverged more recently but remain basal to many other Dipteran taxa.
There are currently no published data on whether fungus gnats do pollinate New Zealand's only Corybas species, C. cheesemanii, and some report that it is predominantly self-pollinating (Molloy Citation1990). Here we survey the phenology and fruit-set of C. cheesemanii, test for autonomous self-pollination and evaluate evidence for brood-site deception of fungus gnats by comparing insects trapped above C. cheesemanii flowers and sympatric mushrooms, quantifying the dimensions of the orchids and the sizes of the potential pollinators, and searching for evidence of oviposition in flowers. If C. cheesemanii is a classic brood-site deceiver, we predict that orchids and mushrooms will be visited by the same species of fungus gnats, that there will be more female than male fungus gnats attracted, that insects attracted will be small enough to fit within orchids to access pollinia, and that orchids will contain insect eggs.
Materials and methods
Study species and site
Corybas cheesemanii grows in lowland to montane scrub or forest shady leaf litter below Kunzea ericoides (kānuka), Pinus radiata, Beilschmiedia tarairi (taraire) and Nothofagus (beech) spp. (Smith Citation2009; Scanlen & St George Citation2010). Each plant produces only a single flower comprising a pearlescent ultraviolet-reflective helmet-like dorsal sepal (Kelly & Gaskett, unpublished data) and small lateral petals above a white labellum with a pair of nectarless spurs (de Lange et al. Citation2007; Scanlen & St George Citation2010). Corybas cheesemanii is not considered threatened and is distributed widely across the Three Kings, North, South and Chatham Islands (de Lange et al. Citation2006). Our study site (c.210 m2) was in Oratia, southwest of Auckland, New Zealand (latitude – 36.919499, longitude 174.611635). The orchids grew close to a stream, in a gully, in leaf litter below Cyathea dealbata (silver fern), Myrsine australis (red matipou), Coprosma lucida (shiny karamu) and Hedycarya arborea (pigeonwood).
Phenology and floral measurements
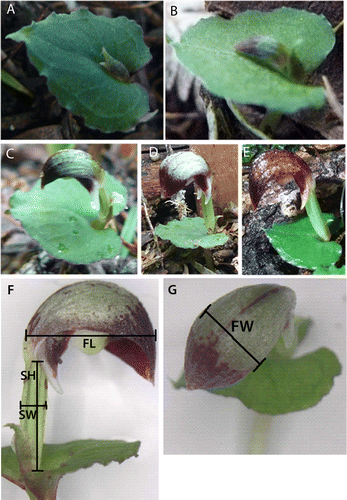
The study site was comprehensively surveyed for C. cheesemanii plants from April until mid-December 2011. We searched the entire site with care during the lead up to flowering and are confident that we found every individual plant that produced an above-ground leaf during the season. Plants in 10 clusters or subsites were labelled individually and monitored before, during and after flowering. Developmental stages recorded were: leaf only, emerging stem (A), bud (B), flowering imminent (C), open flower (D), mature flower (purple/brown in colour; E), shrivelled flower, senesced, long stem with swelling ovary (indicating fruit set) and seed set. Surveys were conducted once a week before flowering commenced (April–May), then twice a week during flowering (May–June), and then once every second week during fruit and seed set (August–December). Measurements of flower length and width, and stem height and width (after floral senescence to assess ovary swelling and fruit set; F,G) were taken every 1–2 wk over the flowering season for 20 individually tagged orchids. Measurements of stem width and height continued after floral senescence until seed set for those flowers that had an elongating stem that indicated fruit set. We dissected 16 mature flowers to measure the width of the narrowest internal diameter a pollinator would have to pass to access the pollinia and stigma. We also checked for the presence of nectar or exudate by probing the nectary-shaped spurs at the labellum base.
Figure 1 Stages of floral development in Corybas cheesemanii. A, emerging flower stem; B, bud; C, flowering imminent; D, open flower; E, mature open flower. Phenological measurement locations for Corybas cheesemanii flowers: F, FL, flower length; SH, stem height; SW, stem width; and G, FW, flower width.
Pollinator exclusion
To test for self-pollination, enclosures made from tulle netting (2-mm mesh), wire and garden staples were placed over 18 C. cheesemanii orchids as soon as flower buds appeared to prevent pollinator access to flowers. The nets were checked regularly for damage or holes. After flower senescence, the plants were assessed regularly for fruit set by monitoring stem length and ovary width.
Insect trapping
Insects visiting C. cheesemanii flowers and any sympatric mushrooms (Basidiomycetes) growing within 100 m of flowers were sampled with traps constructed from plastic bottles cut in half with the neck then inverted inside the base to form a funnel. The trap was then placed above the specimen with the bottom of the bottle pointing upwards, and held in place 10 cm above the ground using metal stakes (as per Marino Citation1991). Trapping commenced in April 2011, before C. cheesemanii began to flower, when traps were placed above mushrooms (n=10) or above nearby leaf litter (mushroom controls, n=10). As each mushroom senesced, usually within 4 d, all traps were moved to new mushrooms and a total of 43 individuals were surveyed. Once flowering began in mid-June, additional traps were set above mature C. cheesemanii flowers (n=10) and nearby C. cheesemanii leaves, i.e. plants that were not flowering (orchid controls, n=10), giving a total of 40 traps. Before the flowering season, traps were checked and emptied weekly. During peak flowering, traps were emptied every 1–2 d for 2 wk. As flowers senesced or disappeared, traps were relabelled and moved to new flowers until the end of the flowering period (n=17 in total). Insects were taken to the laboratory to be pinned and identified and checked for orchid pollinia. Insect specimens were first identified to order, and then all flies were identified to at least family level. Mycetophilidae were identified to species and gender. The width of each insect caught was measured using callipers. The composition of insects visiting the four treatments was compared with non-parametric analyses of similarity (Hammer et al. Citation2001). Analysis of variance was used to compare floral dimensions of orchids that did and did not set seed (SPSS 20). The observed and expected numbers of male versus female mycetophilids visiting mushrooms and orchids were compared with chi-square test. Values are presented as mean±SEM.
Results
Phenology
For all the subsites combined, there were 194 C. cheesemanii plants, 75.8% of these produced a flower. Floral buds appeared during early May, with just over 100 orchids in bud. The flowers commenced opening 24.36 d later and each flower remained open for 23.14 d. Peak flowering occurred in early June when c.62% of the orchids were in flower, and all flowering had finished by early July. Death or disappearance of flowers (perhaps by herbivory) occurred throughout flowering and fruiting, with the majority gone by mid-December. Of the 147 orchids that flowered, 43 (29.25%) commenced post-pollination stem elongation, and of those 37 (25.17%) were confirmed to have set seed.
Floral measurements
Corybas cheesemanii flowers were 9.55±0.23 mm long and 5.92±0.18 mm wide. After flower senescence, stem width was not significantly different between flowers that were pollinated and set fruit (2.55±0.20 mm) and those that did not set fruit (2.48±0.10 mm; ANOVA d.f.=1, F=3.029, P=0.10). However, stem elongation differed markedly between flowers that set fruit (53.69±7.58 mm, max.=172 mm, n=42) and flowers that did not (7.73±0.33 mm, n=17; ANOVA d.f.=1, F=9.536, P<0.005). The width of the flower opening in a mature flower was 1.81±0.09 mm and the narrowest part of the flower was 0.84±0.04 mm. No nectar was found in the orchid spurs. No insect eggs were found in any of the flowers.
Pollinator exclusion
Of the 18 bagged flowers, 12 developed a long stalk with a swollen ovary, indicating pollination, and of those, nine (50%) were confirmed to have set seed. The remaining six orchids either died before flowering finished, or their flowers senesced without setting seed.
Insect trapping
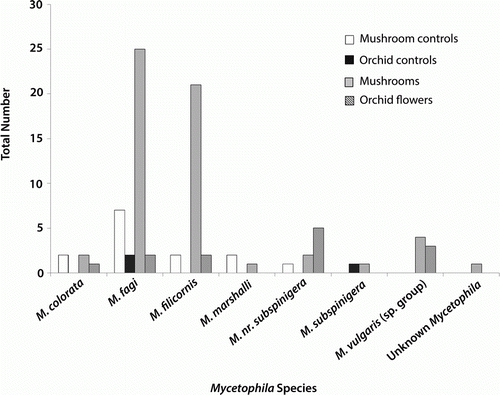
Across all the treatments, Diptera were the most commonly trapped insect group (93.3% of all specimens). The most commonly caught Diptera families were Anisopodidae, Lauxaniidae and Mycetophilidae (). An analysis of similarity found no significant difference in the composition of the fly families caught by the different trap treatments (orchid, orchid control, mushroom, mushroom control; R=−0.0008, P=0.46; ). The orchid traps did catch a subset of those mycetophilids caught above mushrooms, especially Mycetophila colorata, Mycetophila fagi, Mycetophila filicornis, Mycetophila nr. subspinigera and Mycetophila vulgaris sp. group (), but overall the composition of mycetophilids was not significantly different between the traps (R=0.06, P=0.0596).
Figure 2 Number of flies per fly family trapped above mushrooms, near mushrooms (mushroom controls), above flowering Corybas cheesemanii (orchids) and above non-flowering C. cheesemanii plants (orchid controls).
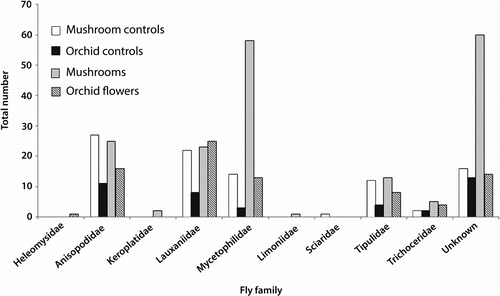
Figure 3 Flies of the genus Mycetophila trapped above mushrooms, mushroom controls, Corybas cheesemanii orchids and orchid controls.
Male Mycetophila were slightly more abundant than females in both mushroom and orchid traps (), but this difference was not statistically significant when compared between these treatments for all Mycetophilidae (χ 2=1.261, d.f.=1, P=0.26) or each Mycetophila species (all P values>0.05). Measurements of flowers (see data above) and trapped insects indicate that very few insects were small enough to be able to enter a flower and access the pollinia and stigma (). Microscope examinations found no pollinia on any of the insects trapped.
Figure 4 Number of male and female Mycetophila trapped above mushrooms, mushroom controls, Corybas cheesemanii orchids and orchid controls.
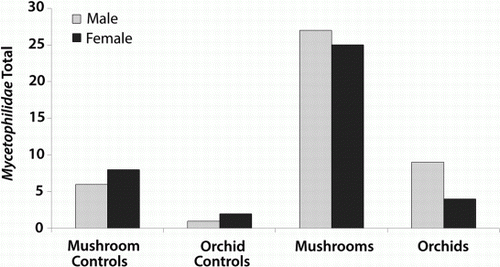
Table 1 Dimensions inside Corybas cheesemanii orchids, and the widths of insect taxa trapped above orchids; insects were measured at the widest part of the thorax.
Discussion
Although a subset of the mycetophilids visiting mushrooms also visited orchids, the range of insects attracted, the lack of significant differences between control and treatment traps, and the lack of pollinia observed on any insect, prevents any conclusions being drawn about the identity of the pollinator or pollinators of C. cheesemanii. There may have been no C. cheesemanii pollinator species at our study site, or insect pollination may be extremely rare for this orchid. Self-pollination is estimated to be present in c.60% of New Zealand orchids, compared with c.31% of orchids internationally (Molloy Citation1990; Peter & Johnson Citation2009). However, C. cheesemanii is unlikely to be exclusively self-pollinated because the fruit-set rates we observed in the wild (c.25%) and bagged individuals (50%) are much lower than expected for obligate selfing in orchids. Autonomous self-pollination in orchids results in almost complete pollination success, e.g. c.95% for Australian Epipogium roseum (Zhou et al. Citation2012) and 100% for South African Eulophia clavicornis var. nutans (Peter & Johnson Citation2009). However, the pollination rates we observed in C. cheesemanii are still higher than expected for completely insect-pollinated deceptive orchids. The global mean for rewarding orchids is 52.9%, for all deceptive orchids it is 25.03% (Neiland & Wilcock Citation1998), and for only sexually deceptive orchids it is 18.4% (Gaskett Citation2011). We suggest that C. cheesemanii has a mixed strategy involving both insect pollination and autonomous self-pollination. This strategy tends to result in intermediate pollination rates similar to those we observed, e.g. 31.5% for Satyrium pumilium (van der Niet et al. Citation2011); 26.3% for Paphiopedilum barbigerum and 58.5% for Paphiopedilum dianthum (Shi et al. Citation2009). A mixed strategy prioritizing insect pollination but allowing self-pollination in the absence of pollinators is likely to be adaptive when pollinators are rare or orchid populations are isolated (Molloy Citation1990; Lehnebach et al. Citation2005; Bernhardt & Edens-Meier Citation2010).
Interestingly, twice as many C. cheesemanii set seed when bagged than in natural conditions (50% versus 25%). This may be because pollinators were visiting unbagged flowers, but so infrequently that pollinia were removed more often than they were deposited. As orchids have their pollen bundled into pollinia, one pollinator visit is sufficient to remove the entire pollen load (Johnson & Edwards Citation2000). Orchids that had pollinia removed but not deposited would therefore be unable to self-pollinate. Unfortunately, the tiny, enclosed flowers of C. cheesemanii meant that we were unable to check for pollinia removal in unbagged flowers without permanently damaging the flowers.
The low pollination rates typical for orchids are generally attributed to pollinator limitation, i.e. the absence or rarity of pollinators (Wilcock & Neiland Citation2002; Tremblay et al. Citation2005). The long-lived flowers typical of orchids and observed here for C. cheesemanii (23 d) may be an adaptation to counteract this (Primack Citation1985; Neiland & Wilcock Citation1998; Ashman Citation2004). Corybas cheesemanii flowers in June when there are reduced numbers of flying insects, but this does coincide with peak abundances of many mycetophilid species (Tonnoir & Edwards Citation1927; Toft & Beggs Citation1995). In contrast, most brood-site deceptive flowers are ephemeral and last only a few days (Stensmyr et al. Citation2002; Urru et al. Citation2010). The flowers of the brood-deceptive orchids Satyrium pumilum and Dracula spp. are similarly surprisingly short-lived for orchids, despite abundant pollinators (c.11 d; Endara et al. Citation2010; van der Niet et al. Citation2011). Flowers of the brood-deceptive orchid Paphiopedilum barbigerum last for a similar period when pollinated (c.13 d), whereas unpollinated flowers last for a similar period to those reported here for C. cheesemanii (c.21 d).
Although we were unable to demonstrate any clear differences between the treatments, the trapping method proved effective for sampling mycetophilids and other flies generally associated with leaf litter and fungi (Peterson Citation2007; Evenhius & Okadome Citation2011; Matile Citation2011). The surprisingly large number of insects caught in mushroom control traps perhaps suggests that they were set too close to mushrooms and we would design any further studies to avoid this issue. However, the smaller numbers of insects caught in orchid control traps compared with the other treatments suggests that the insects were not completely ubiquitous at all locations in the field site.
If C. cheesemanii does require insect pollinators, it may have several pollinator species. Although sexually deceptive orchids are usually highly species specific in their pollinator attraction, food and brood-site deception can involve more generalist pollinator attraction (for deceptive orchid pollinator lists, see Cozzolino et al. Citation2005; Singer et al. Citation2006; Phillips et al. Citation2009; Johnson Citation2010; Gaskett Citation2011). For example, brood-deceptive Cretan Arum cyrenaicum and Arum concinnatum attract 11 Diptera families, with flies of several species appearing in just a few individual flowers (Urru et al. Citation2010). A wide diversity of flies pollinate brood-deceptive stapeliads, and many appear to share pollinator species (Meve & Liede Citation1994; Jürgens et al. Citation2006). In contrast, both Rafflesia pricei and the dead horse arum, Helicodiceros muscivorus, predominantly attract flies of just two calliphorid species (Beaman et al. Citation1988; Stensmyr et al. Citation2002). Among brood-deceptive orchids, Satyrium pumilium is pollinated exclusively by sarcophagid flies (especially Sarcophaga spp.; van der Niet et al. Citation2011), Paphiopedilum barbigerum by the syrphid Episyrphus balteatus (Shi et al. Citation2009) and Dracula by drosophilids (especially Zygothrica spp.; Endara et al. Citation2010). This variation in the degree of generalism or specialism is consistent with the fungal preferences of mycetophilids; while some are generalists, others are much more host specific in their choice of oviposition sites (Jakovlev Citation2012).
Surprisingly, we caught equal numbers of males and females of all mycetophilid taxa in control traps and above mushrooms and orchids. Most flowers confirmed to be brood-site deceptive attract predominantly female insects (Urru et al. Citation2011), but attraction of both sexes is reported for some fungi (Jakovlev Citation2012). Our study also suggests mushrooms attract both sexes of fungus gnats, perhaps indicating that the use of mushrooms in fungus gnat reproduction is not limited solely to oviposition by females. Data about mating, courtship and oviposition, and the specific role of fungi in these behaviours, are surprisingly limited for any species of Mycetophilidae (Okuyama et al. Citation2004), although there are many reports of mycetophilid larvae in fungi (Jakovlev Citation2012). Correspondingly, it is difficult to predict the role of a putative fungal mimic such as C. cheesemanii in the mating system of adult fungus gnats that may be potential pollinators. However, if C. cheesemanii is pollinated by mycetophilids (as suggested by anecdotal observations), it may be via the provision of a rendezvous site where both sexes congregate rather than by mimicking a simple oviposition site for females only. Pollination attraction by the provision or mimicry of rendezvous sites may well pre-date the evolution of floral rewards (Fishman & Lilach Citation2013). It is still present in some extant species such as Dracula lafluerrii orchids, which mimic fungi and are pollinated by courting and mating female and male drosophilids, and red Israeli poppies and anemones, which are pollinated by mating glaphyrid beetles (Scarabaeoidea; Endara et al. Citation2010; Keasar et al. Citation2010). The presence of fly eggs in other New Zealand helmet orchids (Nematoceras spp.; Scanlen Citation2006) suggests that the full repertoire of meeting, courtship, mating and oviposition can indeed take place on orchids. Whether these behaviours result in orchid pollination is yet to be confirmed.
Despite the inconclusive nature of our data, we are still able to recommend that a broader definition of brood-site deception be considered when testing for this phenomenon in the New Zealand flora. Bänziger et al. (Citation2012) suggest that two forms of brood-site deception exist in Paphiopedilum, classic chemical attraction of female insects with entrapment, oviposition and pollination, and a perhaps more basal form in which visual attractants replace chemical lures, some food deception occurs and both female and male insects are attracted. Similar variation may occur in New Zealand where mixed and generalist pollination strategies are common (Newstrom & Robertson Citation2005).
Acknowledgements
We thank Oratia Church Trust and Geoff Davidson for access to the study site. Field assistance and support was provided by John and Linda Kelly and Velimir Gayevskiy.
References
- Anderson B , Johnson SD 2009 . Geographical covariation and local convergence of flower depth in a guild of fly-pollinated plants . New Phytologist 182 : 533 – 540 . doi: 10.1111/j.1469-8137.2009.02764.x
- Ashman T-L 2004 . Flower longevity . In : Nooden LD Plant cell death processes . San Diego , Elsevier Academic Press . 349 – 362 .
- Bänziger H 1996 . The mesmerizing wart: the pollination strategy of epiphytic lady slipper orchid Paphiopedilum villosum (Lindl) Stein (Orchidaceae) . Botanical Journal of the Linnean Society 121 : 59 – 90 .
- Bänziger H , Pumikong S , Srimuang K-O 2012 . The missing link: bee pollination in wild lady slipper orchids Paphiopedilum thaianum and P. niveum (Orchidaceae) in Thailand . Mitteilungen der Schweizerischen Entomologischen Gesellschaft 85 : 1 – 26 .
- Beaman RS , Decker PJ , Beaman JH 1988 . Pollination of Rafflesia (Rafflesiaceae) . American Journal of Botany 75 : 1148 – 1162 . doi: 10.2307/2444098
- Bernhardt P , Edens-Meier R 2010 . What we think we know vs. what we need to know about orchid pollination and conservation: Cypripedium L. as a model lineage . Botanical Reviews 76 : 204 – 219 . doi: 10.1007/s12229-010-9042-z
- Borba EL , Semir J 2001 . Pollinator specificity and convergence in fly-pollinated Pleurothallis (Orchidaceae) species: a multiple population approach . Annals of Botany 88 : 75 – 88 . doi: 10.1006/anbo.2001.1434
- Christensen DE 1994 . Fly pollination in the orchidaceae . In : Arditti J Orchid biology: reviews and perspectives VI . John Wiley and Sons Inc , New York . 415 – 449 .
- Clements M , Mackenzie A , Copson G , Molloy B , Carmichael N , Skotnicki M , Selkirk P 2007 . Biology and molecular phylogenetics of Nematoceras sulcatum, a second endemic orchid species from subantarctic Macquarie Island . Polar Biology 30 : 859 – 869 . doi: 10.1007/s00300-006-0246-y
- Cozzolino S , Schiestl FP , Müller A , Castro OD , Nardella AM , Widmer A 2005 . Evidence for pollinator sharing in Mediterranean nectar-mimic orchids: absence of premating barriers? Proceedings of the Royal Society B 272 : 1271 – 1278 . doi: 10.1098/rspb.2005.3069
- Darwin C 1885 . The various contrivances by which orchids are fertilised by insects. , 2nd edn . London , John Murray . 300
- de Lange P , Rolfe J , St George I , Sawyer J 2007 . Wild orchids of the lower North Island . Wellington , , New Zealand , New Zealand Department of Conservation . 194
- de Lange PJ , Sawyer JWD , Rolfe JR 2006 . New Zealand indigenous vascular plant checklist . Wellington , , New Zealand , New Zealand Plant Conservation Network .
- Dentinger BTM , Roy BA 2010 . A mushroom by any other name would smell as sweet: Dracula orchids . McIlvainea 19 : 1 – 13 .
- Endara L , Grimaldi DA , Roy BA 2010 . Lord of the flies: pollination of Dracula orchids . Lankesteriana 10 : 1 – 11 .
- Evenhius N , Okadome T 2011 . Family Lauxaniidae . In : Evenhius N Catalog of the Diptera of the Australasian and Oceanian Regions . 576 – 589 . (online version). http://hbs.bishopmuseum.org/aocat/ (accessed 2 February 2012) .
- Fishman MA , Lilach H 2013 . Pollinators' mating rendezvous and the evolution of floral advertisement . Journal of Theoretical Biology 316 : 99 – 106 . doi: 10.1016/j.jtbi.2012.09.006
- Fuller G 1994 . Observation of the pollination of Corybas “A” . Journal of the New Zealand Native Orchid Group 52 : 18 – 22 .
- Gaskett AC 2011 . Orchid pollination by sexual deception: pollinator perspectives . Biological Reviews 86 : 33 – 75 . doi: 10.1111/j.1469-185X.2010.00134.x
- Gaskett AC 2013 . Colour and sexual deception in orchids: progress towards understanding the functions and pollinator perception of floral colour . In : Bernhardt P , Meyer R Darwin's orchids: then and now . Chicago , University of Chicago Press .
- Goldblatt P , Bernhardt P , Vogan P , Manning JC 2004 . Pollination by fungus gnats (Diptera: Mycetophilidae) and self-recognition sites in Tolmiea menziesii (Saxifragaceae) . Plant Systematics and Evolution 244 : 55 – 67 . doi: 10.1007/s00606-003-0067-1
- Hammer Ø , Harper DAT , Ryan PD 2001 . PAST: Paleontological statistics software package for education and data analysis . Palaeontologia Electronica 4 : 9 .
- Jakovlev J 2012 . Fungal hosts of mycetophilids (Diptera: Sciaroidea excluding Sciaridae): a review . Mycology 3 : 11 – 23 .
- Jaschhof M , Didham R 2002 . Rangomaramidae fam. nov. from New Zealand and implications for the phylogeny of the Sciaroidea (Diptera: Bibionomorpha) . Studia Dipterologica—Suppl . 11 : 1 – 60 .
- Jersáková J , Johnson SD , Kindlmann P 2006 . Mechanisms and evolution of deceptive pollination in orchids . Biological Reviews 81 : 219 – 235 . doi: 10.1017/S1464793105006986
- Johnson SD 2010 . The pollination niche and its role in the diversification and maintenance of the southern African flora . Philosophical Transactions of the Royal Society B 365 : 499 – 516 . doi: 10.1098/rstb.2009.0243
- Johnson SD , Edwards TJ 2000 . The structure and function of orchid pollinaria . Plant Systematics and Evolution 222 : 243 – 269 . doi: 10.1007/BF00984105
- Jones DL 1970 . The pollination of Corybas diemenicus (H.M.R.) Rupp and W.H. Nicholls ex H.M.R . Rupp. Victorian Naturalist 87 : 373 – 374 .
- Jürgens A , Dötterl S , Meve U 2006 . The chemical nature of fetid floral odours in stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae) . New Phytologist 172 : 452 – 468 . doi: 10.1111/j.1469-8137.2006.01845.x
- Kaiser R 2006 . Flowers and fungi use scents to mimic each other . Science 311 : 806 – 807 . doi: 10.1126/science.1119499
- Keasar T , Harari AR , Sabatinelli G , Keith D , Dafni A , Shavit O , Zylbertal A , Shmida AVI 2010 . Red anemone guild flowers as focal places for mating and feeding by Levant glaphyrid beetles . Biological Journal of the Linnean Society 99 : 808 – 817 . doi: 10.1111/j.1095-8312.2010.01384.x
- Lehnebach CA , Robertson AW 2004 . Pollination ecology of four epiphytic orchids of New Zealand . Annals of Botany 93 : 773 – 781 . doi: 10.1093/aob/mch097
- Lehnebach CA , Robertson AW , Hedderley D 2005 . Pollination studies of four New Zealand terrestrial orchids and the implication for their conservation . New Zealand Journal of Botany 43 : 467 – 477 . doi: 10.1080/0028825X.2005.9512968
- Li P , Pemberton R , Zheng G , Luo Y 2012 . Fly pollination in Cypripedium: a case study of sympatric C. sichuanense and C. micranthum . Botanical Journal of the Linnean Society 170 : 50 – 58 . doi: 10.1111/j.1095-8339.2012.01259.x
- Marino P 1991 . Dispersal and coexistence of mosses (Splachnaceae) in patchy habitats . Journal of Ecology 79 : 1047 – 1060 . doi: 10.2307/2261097
- Matile L 2011 . Family Mycetophilidae . In : Evenhius N Catalog of the Diptera of the Australasian and Oceanian Regions . 135 – 145 . (online version). http://hbs.bishopmuseum.org/aocat (accessed 2 February 2012).
- Mesler MR , Ackerman JD , Lu KL 1980 . The effectiveness of fungus gnats as pollinators . American Journal of Botany 67 : 564 – 567 . doi: 10.2307/2442297
- Meve U , Liede S 1994 . Floral biology and pollination in stapeliads — new results and a literature review . Plant Systematics and Evolution 192 : 99 – 116 . doi: 10.1007/BF00985911
- Miller D 1918 . A new fungus-gnat which fertilizes Corysanthes oblonga Hook . New Zealand Journal of Science and Technology 1 : 4 .
- Molloy BPJ 1990 . Pollination systems of New Zealand native orchids . In : St George I , McCrae D The New Zealand orchids: natural histroy and cultivation . Dunedin , New Zealand Native Orchid Group . 36 36– 56
- Neiland MRM , Wilcock CC 1998 . Fruit set, nectar reward, and rarity in the Orchidaceae . American Journal of Botany 85 : 1657 – 1671 . doi: 10.2307/2446499
- Newstrom L , Robertson A 2005 . Progress in understanding pollination systems in New Zealand . New Zealand Journal of Botany 43 : 1 – 59 . doi: 10.1080/0028825X.2005.9512943
- Okuyama Y , Kato M , Murakami N 2004 . Pollination by fungus gnats in four species of the genus Mitella (Saxifragaceae) . Botanical Journal of the Linnean Society 144 : 449 – 460 . doi: 10.1111/j.1095-8339.2003.00259.x
- Peter CI , Johnson SD 2009 . Autonomous self-pollination and pseudo-fruit set in South African species of Eulophia (Orchidaceae) . South African Journal of Botany 75 : 791 – 797 . doi: 10.1016/j.sajb.2009.07.007
- Peterson B 2007 . Family Anisopodoidea . In : Evenhius N Catalog of the diptera of the Australasian and Oceanian regions . 180 – 181 . (online version). http://hbs.bishopmuseum.org/aocat/ (accessed 2 February 2012).
- Phillips R , Faast R , Bower C , Brown G , Peakall R 2009 . Implications of pollination by food and sexual deception for pollinator specificity, fruit set, population genetics and conservation of Caladenia (Orchidaceae) . Australian Journal of Botany 57 : 287 – 306 . doi: 10.1071/BT08154
- Policha TJ , Roy BA 2012 . Fungi, Plants, and Pollinators: Sex, Disease, and Deception . In : Southworth D Biocomplexity of plant–fungal interactions . Hoboken , , USA , Wiley-Blackwell . 165 – 183 .
- Primack RB 1985 . Longevity of Individual Flowers . Annual Review of Ecological Systematics 16 : 15 – 37 . doi: 10.1146/annurev.es.16.110185.000311
- Scanlen E 1996 . Flies on New Zealand native orchids . Journal of the New Zealand Native Orchid Group 59 : 12 – 13 .
- Scanlen E 2006 . Flies' eggs in Nematoceras triloba agg Journal of the New Zealand Native Orchid Group 98 : 34 .
- Scanlen E 2008 . Nematoceras at Te Kauri Lodge . Journal of the New Zealand Native Orchid Group 107 : 35 .
- Scanlen E , St George I 2010 . Colour Field Guide to the Native Orchids of New Zealand . Wellington , , New Zealand , New Zealand Native Orchid Group Inc . 82
- Schaefer HM , Ruxton GD 2009 . Deception in plants: mimicry or perceptual exploitation? Trends in Ecology and Evolution 24 : 676 – 685 . doi: 10.1016/j.tree.2009.06.006
- Schiestl FP , Schlüter PM 2009 . Floral isolation, specialized pollination, and pollinator behavior in orchids . Annual Review of Entomology 54 : 425 – 46 . doi: 10.1146/annurev.ento.54.110807.090603
- Shi J , Cheng J , Luo D , Shangguan F-Z , Luo Y-B 2007 . Pollination syndromes predict brood-site deceptive pollination by female hoverflies in Paphiopedilum dianthum (Orchidaceae) . Acta Phytotaxonomica Sinica 45 : 551 – 560 . doi: 10.1360/aps07025
- Shi J , Luo Y-B , Bernhardt P , Ran J-C , Liu Z-J , Zhou Q 2009 . Pollination by deceit in Paphiopedilum barbigerum (Orchidaceae): a staminode exploits the innate colour preferences of hoverflies (Syrphidae) . Plant Biology 11 : 17 – 28 . doi: 10.1111/j.1438-8677.2008.00120.x
- Singer RB , Marsaioli AJ , Flach A , Reis MG 2006 . The ecology and chemistry of pollination in Brazilian orchids: recent advances . In : Teixeira da Silva JA Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues . Middlesex , Global Science Books . 569 – 582 .
- Smith V 2009 . Thomas Frederic Cheeseman and Corybas cheesemanii Journal of the New Zealand Native Orchid Group 113 : 22 – 23 .
- St George I 2001 . An insect pollinated form of Corybas aff. tribolus? Journal of the New Zealand Native Orchid Group 79 : 4 .
- St George I 2007 . The pollination of Nematoceras iridescens . Journal of the New Zealand Native Orchid Group 102 : 10 .
- Stensmyr MC , Urru I , Collu I , Celander M , Hansson BS , Angioy A-M 2002 . Rotting smell of dead-horse arum florets . Nature 420 : 625 – 626 . doi: 10.1038/420625a
- Toft RJ , Beggs JR 1995 . Seasonality of crane flies (Diptera: Tipulidae) in South Island beech forest in relation to the abundance of Vespula wasps (Hymenoptera: Vespidae) . New Zealand Entomology 18 : 37 – 43 . doi: 10.1080/00779962.1995.9722000
- Toft RJ , Chandler PJ 2004 . Three introduced species of Mycetophilidae (Diptera: Sciaroidea) established in New Zealand . New Zealand Entomology 27 : 43 – 50 . doi: 10.1080/00779962.2004.9722123
- Tonnoir A , Edwards F 1927 . New Zealand Fungus Gnats (Diptera, Mycetophilidae) . Transactions of the New Zealand Institute 57 : 747 – 878 .
- Tremblay RL , Ackerman JD , Zimmerman JK , Calvo RN 2005 . Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification . Biological Journal of the Linnean Society 84 : 1 – 54 . doi: 10.1111/j.1095-8312.2004.00400.x
- Urru I , Stensmyr MC , Hansson BS 2011 . Pollination by brood-site deception . Phytochemistry 72 : 1655 – 1666 . doi: 10.1016/j.phytochem.2011.02.014
- Urru I , Stökl J , Linz J , Krügel T , Stensmyr MC , Hansson BS 2010 . Pollination strategies in Cretan Arum lilies . Biological Journal of the Linnean Society 101 : 991 – 1001 . doi: 10.1111/j.1095-8312.2010.01537.x
- van der Niet T , Hansen DM , Johnson SD 2011 . Carrion mimicry in a South African orchid: flowers attract a narrow subset of the fly assemblage on animal carcasses . Annals of Botany 107 : 981 – 992 . doi: 10.1093/aob/mcr048
- Vereecken NJ , Dafni A , Cozzolino S 2010 . Pollination syndromes in Mediterranean orchids – implications for speciation, taxonomy and conservation . Botanical Reviews 76 : 220 – 240 . doi: 10.1007/s12229-010-9049-5
- Wilcock C , Neiland R 2002 . Pollination failure in plants: why it happens and when it matters . Trends in Plant Science 7 : 270 – 277 . doi: 10.1016/S1360-1385(02)02258-6
- Zhou X , Lin H , Fan X-L , Gao J-Y 2012 . Autonomous self-pollination and insect visitation in a saprophytic orchid, Epipogium roseum (D.Don) Lindl . Australian Journal of Botany 60 : 154 – 159 .