Abstract
Bryoclaviculus campylopi, a tiny, beautiful inoperculate discomycete fungus found only in association with the moss Campylopus acuminatus, is described as a new genus and species from New Zealand. Phylogenetically it has a sister relationship with the northern hemisphere moss-associated species Bryoglossum gracile. It is distinguished macroscopically from Bryoglossum by its cup-shaped fruit body and microscopically by features of the asci and paraphyses.
Introduction
The bryophilous lifestyle has evolved independently many times among the fungi, resulting in a highly diverse set of species growing in association with mosses, including mushroom-like basidiomycetes, operculate and inoperculate discomycetes and perithecial ascomycetes (e.g. Döbbeler Citation2002; Davey & Currah Citation2006; Eckstein & Eckstein Citation2009; Stenroos et al. Citation2010). The moss-associated species have a diverse range of relationships with their hosts (Davey & Currah Citation2006) but are typically specialized to that habitat (Stenroos et al. Citation2010). Few reports have been published on bryophilous fungi from New Zealand. Examples include a series of papers on tiny ascomycete species in a range of genera (Döbbeler Citation1978, Citation1981, Citation1996, Citation2001, 2003; Döbbeler & Triebel Citation2000), the usually Sphagnum-associated, lichenized mushroom Lichenomphalia (Galloway Citation2007; Geml et al. Citation2012), and the putatively pathogenic, tiny, mushroom-like Mniopetalum bryophilum (Segedin Citation1994).
Here we report a bryophilous species of Leotiomycetes, Bryoclaviculus campylopi, as a new genus and species from New Zealand.
Material and methods
Specimens were examined macroscopically when both fresh and dried. Dried specimens were rehydrated in 3% KOH, hymenial elements were examined in Melzer's reagent, and vertical sections were cut at a thickness of about 10 µm using a freezing microtome and were mounted in lactic acid. Specimens have been deposited in the Otago Regional Herbarium, University of Otago Botany Department (OTA) and the New Zealand Fungal and Plant Disease Collection, Landcare Research (PDD). Coordinates of the collecting sites use the WGS84 datum and geographic districts within New Zealand follow Crosby et al. (Citation1998).
DNA was extracted from three apothecia removed from dried specimen PDD 101074 using REDExtract-N-Amp Plant PCR Kit (Sigma, St Louis, MO, USA). The apothecia were ground in an extraction buffer with a plastic pestle in the Eppendorf tube. Following this, DNA extraction and polymerase chain reaction were carried out following the manufacturer's instructions. DNA sequences were generated to allow the New Zealand fungus to be incorporated into the phylogeny of moss-inhabiting Leotiomycetes provided by Stenroos et al. (Citation2010). Internal transcribed spacer (ITS), partial large subunit (LSU), partial mitochondrial small subunit (mtSSU) and partial small subunit (SSU) sequences were obtained using the same primers as Stenroos et al. (Citation2010). RPB2 amplification was not successful. The alignment of Stenroos et al. (Citation2010) was downloaded from TreeBase (http://treebase.org) and the sequences generated from PDD 101074 (GenBank numbers JX393084–JX393087) were concatenated and incorporated into the alignment using Geneious (Drummond et al. Citation2011). Data from isolates selected to represent the diversity across the Leotiomycetes as treated by Stenroos et al. (Citation2010), excluding RPB2, were analysed with Bayesian phylogenetic methods using MrBayes 3.1.2 (Huelsenbeck & Ronquist Citation2001; Ronquist & Huelsenbeck Citation2003) with gaps treated as missing data, applying the GTR+I+G model for SSU and LSU, SYM+I+G for 5.8S and HKY+I+G for mtSSU, the models selected using the Akaike information criterion method in Mr ModelTest 2.3 (Nylander Citation2004). The data set was run with two chains for 10 million generations, trees sampled every 1000 generations with a burn-in of 25%. Bayesian posterior probabilities were obtained from 50% majority rule consensus trees.
Taxonomy
Bryoclaviculus L. Ludw., P.R. Johnst. & Steel gen. nov.
Mycobank. # MB 803178
Type species. Bryoclaviculus campylopi L. Ludw., P.R. Johnst. & Steel
Diagnosis. Differs from Bryoglossum in having a cupulate receptacle, paraphyses swollen apically, and ascus wall undifferentiated at apex.
Etymology. Derived from -bryon (Greek for moss) and the diminutive of clavus (Latin for nail).
Description. Apothecia stipitate, receptacle cupulate, excipulum of cylindrical cells arranged at low angle to surface of receptacle, cell walls thickened, gelatinous; paraphyses swollen, knob-like at apex; ascus wall thinner across apex but with no refractive or amyloid apical apparatus; ascospores hyaline, 0-septate.
Bryoclaviculus campylopi L. Ludw., P.R. Johnst. & Steel sp. nov.,

Mycobank. # MB 803179
Holotype. New Zealand: Otago, vic. Dunedin, Swampy Summit, western slope along access road (45°47′28.1′′ S, 170°28′31.9′′ E, c.707 m above sea level [asl]), on Campylopus acuminatus, coll. L.R. Ludwig, 17 June 2011 (OTA 062197; isotype PDD 101074).
Diagnosis. Differs from Bryoglossum gracile because of its cupulate receptacle, paraphyses swollen apically and longer ascospores.
Etymology. Refers to host.
Description. Apothecia arise from a mass of disorganized fungal tissue at the base of leaf axils. The infected shoots of the host are symptomless. No fungal hyphae were observed within the host leaves, although a few host cells near the base of the infected leaf axils were disrupted. Apothecial cup about 0.5–1 mm diameter, pale yellow to bright orange when fresh, pale orange-brown when dry, stipe about 3–4 mm long, narrow, translucent white when both fresh and dry. Ectal excipulum comprising cylindric to long-cylindric cells 5–10 µm diameter, with hyaline, gelatinous walls about 2 µm wide between adjacent cell lumens, cells arranged at a low angle to the surface of the receptacle; outermost cells of excipulum extend into narrow hypha-like elements, 3 µm diameter, embedded in thick gelatinous matrix. Medullary excipulum comprising long cylindrical cells 4.5–6 µm diameter, with hyaline, slightly thickened walls. Paraphyses 2 µm diameter, swelling suddenly to 6.5–7.5 µm at knob-like apex. Asci 80–95×9–10.5 µm, cylindrical, apex broadly rounded, wall slightly thinner across apex than at sides of ascus immediately adjacent to apex, nonamyloid, croziers at base, eight-spored. Ascospores 15–20.5×2.5–3(−3.5) µm (average 17.9×2.9 µm, n=23), cylindrical, not tapering to rounded ends, straight, 0-septate, hyaline.
Habitat and distribution. Known only in association with living Campylopus acuminatus plants growing in montane to subalpine localities in the central and southern part of the South Island, New Zealand.
Additional specimens examined. New Zealand: Otago, vic. Dunedin, Swampy Summit, about 100 m south of Doppler Station, c.45°47′44′′S, 170°28′38′′E, c.730 m asl, coll. L.R. Ludwig & J. Steel, 6 July 2012 (PDD 102833); Swampy Summit, coll. J. Steel, 22 July 2012 (PDD 102832); west slope of Swampy Summit, moss cushion under flax, 45°47′28.1′′S, 170°28′31.9′′E, c.707 m asl, coll. L.R. Ludwig, 31 May 2011 (OTA 062204); Swampy Summit access road, southern part, 45°48′38.0′′S, 170°29′56.3′′E, c.545 m asl, coll. L.R. Ludwig, 11 June 2011 (OTA 062198); Swampy Summit, west slope along access road, 45°47′30.5′′S, 170°28′35.6′′E, c.720 m asl, coll. L.R. Ludwig, 7 September 2012 (OTA 062199); Mt Cargill, near summit, south facing slope along access road, 45°48′48.1′′S, 170°33′ 11.5′′E, c.660 m asl, coll. L.R. Ludwig, 8 July 2011 (OTA 062203); tramping track between top of Mt Cargill and Buttars Peak 45°48′44.1′′S, 170°33′28.0′′E, c.612 m asl, coll. L.R. Ludwig, 8 July 2011 (OTA 062202); Silver Peaks, along tramping track, c.350 m north of Pulpit Rock, 45°44′35.7′′S, 170°27′07.3′′E, c.725 m asl, coll. L.R. Ludwig, 4 August 2011 (OTA 062200); Silver Peaks, junction of track from Pulpit Rock to Jubilee Hut and track towards Mt Allan, 45°44′23.6′′S, 170°26′53.4′′E, c.727 m asl, coll. L.R. Ludwig, 4 August 2011 (OTA 062201). Mid Canterbury, Banks Peninsula, Hinewai Reserve, southern slope of North Hioi, 43°49′35.5′′S, 172°59′54.6′′E, c.723 m asl, coll. L.R. Ludwig, 18 July 2011 (OTA 062087); Hinewai Reserve, South Hioi, 43°49′37.0′′S, 172°59′53.1′′E, c.710 m asl, coll. L.R. Ludwig, 18 July 2011 (OTA 062089); Hinewai Reserve, summit of Stony Bay Peak/Taraterehu, 43°49′14.1′′S, 173°00′21.3′′E, c.780 m asl, coll. L.R. Ludwig, 18 July 2011 (OTA 062088); Hinewai Reserve, Sou'West Tor, 43°49′24.8′′S, 173°00′01.2′′E, c.685 m asl, coll. L.R. Ludwig, 18 July 2011 (OTA 062085); Banks Peninsula, Flag Peak, on southern slope, near summit, 43°50′08.1′′S, 172°59′17.2′′E, c.800 m asl, coll. L.R. Ludwig, 18 July 2011 (OTA 062086).
Phylogeny
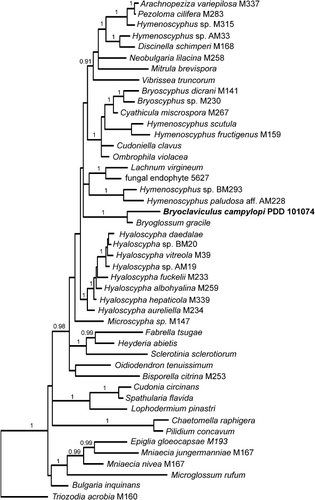
Among the bryophyte-associated species treated by Stenroos et al. (Citation2010), Bryoclaviculus campylopi forms a sister relationship with Bryoglossum gracile (). The same sister relationship between Bryoglossum and Bryoclaviculus is resolved when the Bryoclaviculus sequences are incorporated into the Leotiomycetes alignment of Wang et al. (Citation2006) (unpubl. data).
Discussion
Although Bryoglossum and Bryoclaviculus are closely related phylogenetically and share a bryophilous lifestyle, they are distinguished at the generic level because of differences in macromorphology (Bryoglossum lacking a cupulate receptacle), and micromorphology (Bryoglossum having unswollen paraphyses and asci with a thickened apex and amyloid pore). Bryoclaviculus is known only from cool, high-altitude habitats in southern New Zealand, whereas Bryoglossum is a high-latitude Arctic genus (Kankainen Citation1969, as Mitrula gracilis). Two morphologically very similar species have been described in Bryoglossum, Bryoglossum gracilis and Bryoglossum rehmii (Ohenoja Citation2000), with molecular data available only for the type species, Bryoglossum gracilis.
The nature of the association between Bryoclaviculus campylopi and its host is uncertain. Damage to host tissue is very limited, and minimal, if any, fungal growth occurs inside the host cells. Despite this, there appears to be a true host preference, the fungus so far found only in association with Campylopus acuminatus, even at sites where several mosses grow together. Campylopus acuminatus grows in a variety of conditions, but the fungus has only been observed on cushions in montane to subalpine altitudes (550–800 m), which are sheltered from excessive sunlight and desiccation, such as shading by overhanging rocks or shrubs. Campylopus acuminatus has a wide austral distribution across New Zealand, Australia, Chile and the Falkland Islands (GBIF Citation2013), and further collecting might show Bryoclaviculus campylopi to have a similarly widespread distribution.
Bryophilous Leotiomycetes are phylogenetically diverse and include species in the genera Bryoscyphus, Discinella, Epiglia, Hyaloscypha, Microscypha, Mniacea, Pezoloma and Trizodia (Stenroos et al. Citation2010), Bryoglossum (Redhead Citation1977), Potridiscus (Döbbeler Citation2003), and Roseodiscus (Baral & Krieglsteiner Citation2006; Weischollek et al. Citation2011). All but Roseodiscus and Potridiscus are treated in the phylogeny presented in this paper. Roseodiscus has an ITS sequence available (AJ430395, as Hymenoscyphus rhodoleucus), and this places the type species Roseodiscus rhodoleucus close to the Myxotrichaceae, whereas, with ITS sequences alone, Bryoclaviculus has a relationship similar to that in the full data set used in this paper, as sister to Bryoglossum, and these two genera collectively have a sister relationship with Hyaloscypha (unpubl. data). The morphologically very distinct Potridiscus (Döbbeler Citation2003) has no DNA sequence data available.
Acknowledgements
L. Ludwig thanks Hugh Wilson, manager of the Hinewai Reserve, for his kind hospitality, and is grateful for scholarship funding by the University of Otago and its Botany Department. P.R. Johnston was supported through the Landcare Research Systematics Portfolio, with Core funding support from the Science and Innovation Group of the New Zealand Ministry of Business, Innovation and Employment.
References
- Baral HO, Krieglsteiner L 2006. Hymenoscyphus subcarneus, a little known bryocolous discomycete found in the Bialłowieża National Park. Acta Mycologica 41: 11–20.
- Crosby TK, Dugdale JS, Watt JC 1998. Area codes for recording specimen localities in the New Zealand subregion. New Zealand Journal of Zoology 25: 175–183. 10.1080/03014223.1998.9518148
- Davey ML, Currah RS 2006. Interactions between mosses (Bryophyta) and fungi. Canadian Journal of Botany 84: 1509–1519. 10.1139/b06-120
- Döbbeler P 1978. Moosbewohnende Ascomyceten I. Die Pyrenocarpen, den Gametophyten besiedelnden Arten. Mitteilungen der Botanischen Staatssammlung München 14: 1–360.
- Döbbeler P 1981. Moosbewohnende Ascomyceten V. Die auf Dawsonia vorkommenden Arten der Botanischen Staatssammlung München. Mitteilungen der Botanischen Staatssammlung München 17: 393–474.
- Döbbeler P 1996. Potriphila navicularis gen. et sp. nov. (Ostropales, Ascomycetes), ein bipolar verbreiteter Parasit von Polytrichum alpinum. Nova Hedwigia 62: 61–77.
- Döbbeler P 2001. Polytrichadelphus magellanicus sensu lato (Musci) and its ascomycetes – different fungi on different hosts. Sendtnera 7: 35–45.
- Döbbeler P 2002. Microniches occupied by bryophilous ascomycetes. Nova Hedwigia 75: 275–306. 10.1127/0029-5035/2002/0075-0275
- Döbbeler P 2003. Ascomycetes on Dendroligotrichum (Musci). Nova Hedwigia 76: 1–44. 10.1127/0029-5035/2003/0076-0001
- Döbbeler P, Triebel D 2000. Potridiscus polymorphus (Leotiales) – a new ascomycete on Polytrichaceae (Musci) with palaeoaustral distribution. Hoppea 61: 71–83.
- Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, Field M, Heled J, Kearse M, Markowitz S, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A 2011. Geneious v5.4. http://www.geneious.com/ (accessed 15 November 2012).
- Eckstein J, Eckstein G 2009. Bryoparasitische Pezizales (Ascomycetes) der Gattungen Lamprospora, Octospora und Neottiella im Alten Botanischen Garten von Göttingen (Deutschland, Niedersachsen). Herzogia 22: 213–228.
- Galloway DJ 2007. Flora of New Zealand: Lichens. Revised second edition. Lincoln, Manaaki Whenua Press.
- GBIF 2013. Global Biodiversity Information Facility http://data.gbif.org/occurrences/ (accessed 2 February 2013, using search term “Campylopus acuminatus”).
- Geml J, Kauff F, Brochmann C, Lutzoni F, Laursen GA, Redhead SA, Taylor DL 2012. Frequent circumarctic and rare transequatorial dispersals in the lichenised agaric genus Lichenomphalia (Hygrophoraceae, Basidiomycota). Fungal Biology 116: 388–400. 10.1016/j.funbio.2011.12.009
- Huelsenbeck JP, Ronquist F 2001. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. 10.1093/bioinformatics/17.8.754
- Kankainen E 1969. On the structure, ecology and distribution of the species of Mitrula s. lat. (Ascomycetes, Geoglossaceae). Karstenia 9: 23–34.
- Nylander JAA 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
- Ohenoja E 2000. Geoglossaceae Corda. In: Hansen L, Knudsen H eds. Nordic macromycetes, Volume 1, Ascomycetes. Copenhagen, Nordsvamp. Pp. 177–183.
- Redhead SA 1977. The genus Mitrula in North America. Canadian Journal of Botany 55: 307–325. 10.1139/b77-042
- Ronquist F, Huelsenbeck JP 2003. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. 10.1093/bioinformatics/btg180
- Segedin BP 1994. Studies in the Agaricales of New Zealand: new records and new species of the genera Cheimonophyllum, Mniopetalum, and Anthracophyllum (Tricholomataceae, Collybieae). New Zealand Journal of Botany 32: 61–72. 10.1080/0028825X.1994.10410407
- Stenroos S, Laukka T, Huhtinen S, Döbbeler P, Myllys L, Syrjanend K, Hyvonene J 2010. Multiple origins of symbioses between ascomycetes and bryophytes suggested by a five-gene phylogeny. Cladistics 26: 281–300. 10.1111/j.1096-0031.2009.00284.x
- Wang Z, Johnston PR, Takamatsu S, Spatafora JW, Hibbett DS 2006. Toward a phylogenetic classification of the Leotiomycetes based on rDNA data. Mycologia 98: 1065–1075. 10.3852/mycologia.98.6.1065
- Wieschollek D, Helleman S, Baral HO, Richter T 2011. Roseodiscus formosus spec. nov. – ein bryophiler Pionier mit falschem Namen. Zeitschrift für Mycologique 7: 161–174.